SUMMARY
SETTING:
The ototoxic effects of aminoglycosides (AGs) lead to permanent hearing loss, which is one of the devastating consequences of multidrug-resistant tuberculosis (MDR-TB) treatment. As AG ototoxicity is dose-dependent, the impact of a surrogate measure of AG exposure on AG-induced hearing loss warrants close attention for settings with limited therapeutic drug monitoring.
OBJECTIVE:
To explore the prognostic impact of cumulative AG dose on AG ototoxicity in patients following initiation of AG-containing treatment for MDR-TB.
DESIGN:
This prospective cohort study was nested within an ongoing cluster-randomized trial of nurse case management intervention across 10 MDR-TB hospitals in South Africa.
RESULTS:
The adjusted hazard of AG regimen modification due to ototoxicity in the high-dose group (≥75 mg/kg/week) was 1.33 times higher than in the low-dose group (<75 mg/kg/week, 95% CI 1.09–1.64). The adjusted hazard of developing audiometric hearing loss was 1.34 times higher than in the low-dose group (95%CI 1.01–1.77). Pre-existing hearing loss (adjusted hazard ratio [aHR] 1.71, 95%CI 1.29–2.26) and age (aHR 1.16 per 10 years of age, 95%CI 1.01–1.33) were also associated with an increased risk of hearing loss.
CONCLUSION:
MDR-TB patients with high AG dose, advanced age and pre-existing hearing loss have a significantly higher risk of AG-induced hearing loss. Those at high risk may be candidates for more frequent monitoring or AG-sparing regimens.
Keywords: survival analysis, cumulative hazard, ototoxicity
Multidrug-resistant tuberculosis (MDR-TB; defined as Mycobacterium tuberculosis resistant to both isoniazid and rifampicin) is often treated with injectable aminoglycosides (AGs) such as kanamycin (KM) or amikacin (AMK).1 To improve treatment outcomes and patients’ quality of life, the World Health Organization recently proposed the option to replace AGs with oral alternatives (e.g., bedaquiline); however, the standardized and most affordable short-course regimens continue to use AMK and streptomycin for at least 4 months.2
During the injectable treatment phase, a large proportion of MDR-TB patients develop permanent hearing loss due to irreversible apoptotic hair cell damage in the cochlea.3,4 AG-induced hearing loss begins with the highest, often inaudible, frequencies and may occur with or without tinnitus prior to presentation of hearing loss in audible lower frequencies.5,6 AG ototoxicity may cause early AG regimen modification (i.e., a reduction of dose or discontinuation of therapy),7 which may lead to negative treatment outcomes due to the attenuated bactericidal efficacy of AG.8–11 This is a particularly salient issue in resource-limited settings with limited options to substitute an AG.
Therapeutic drug monitoring (TDM) of AGs—the measurement of AG peaks and troughs in the blood—provides objective information for clinicians to determine dose;12 however, this is not feasible in many resource-limited settings.13 Although the exact mechanism of AG ototoxicity is not fully understood, it is believed to be dose-dependent.14 We therefore hypothesized that patients with a high weekly AG dose—as a surrogate measure of AG exposure—would have a greater risk of developing hearing loss than those with a lower weekly dose.
STUDY POPULATION AND METHODS
Study design and setting
This prospective cohort study was nested within an ongoing cluster-randomized clinical trial of a nurse case management (NCM) intervention to improve MDR-TB treatment outcomes across 10 hospitals in the Eastern Cape and KwaZulu-Natal Provinces of South Africa. Full details regarding the parent study have been reported elsewhere (NCT02129244).15
For this substudy, we included participants (age ≥13 years) enrolled in the parent study from November 2014 to June 2017. We excluded patients with the following conditions: 1) those receiving neither intramuscular KM nor AMK injection, 2) those confirmed to have drug-susceptible or extensively drug-resistant TB based on baseline drug susceptibility testing (DST) that resulted during the injectable phase, and 3) those who transferred to another TB facility during the injectable phase.
Aminoglycoside dosing in MDR-TB treatment
According to contemporary South African National Department of Health guidelines, the standard MDR-TB regimen consisted of at least 4–6 months of injectable phase (or intensive phase) treatment with one intramuscular injectable AG (e.g., KM or AMK) and at least four oral antimycobacterials.1,7 The initial AG dose was based on the patients’ baseline weight—15 mg × weight (kg)—and on the weight band-dosing table, according to which the dose (ml) was selected in practice (Table 1).7 The frequency of AG dosing varied from one to seven times a week, and was determined by the physicians’ clinical judgment, based on the patients’ pre-existing conditions at the baseline evaluation.
Table 1.
Aminoglycoside weight band-dosing
| Weight (kg) | Dose (mg) | Amount (ml) |
|---|---|---|
| <24 | 333 | 1.0 |
| 25–29 | 416 | 1.25 |
| 30–37 | 500 | 1.5 |
| 38–43 | 583 | 1.75 |
| 44–49 | 666 | 2.0 |
| 50–53 | 750 | 2.25 |
| 54–57 | 833 | 2.5 |
| 58–63 | 915 | 2.75 |
| ≥64 | 1,000 | 3.0 |
Study procedures and measures
The following clinical parameters were abstracted from the parent study: TB diagnostic results (i.e., smear, Xpert® MTB/RIF [Cepheid, Sunnyvale, CA, USA], line-probe assay, sputum culture, and DST), medical history, including previous TB history and human immunodeficiency virus (HIV) status, TB treatment regimen and antiretroviral therapy (ART), weight, height, audiological findings, chest X-ray, serum creatinine, adverse drug reactions, and treatment adherence.7 On the day of admission to the MDR-TB treatment program, patients were interviewed for sociodemographic history and self-reported symptoms. Additional data were also collected through medical chart review and the National Health Laboratory System (NHLS) online laboratory portal. Weekly data from baseline to the end of the injectable phase of MDR-TB treatment, including regimen changes and audiological findings based on chart review and patient interviews, were recorded at all the sites.
Several study variables not captured by the parent study were additionally collected for this analysis. Serum albumin levels were collected from the NHLS, and audiograms were captured by medical chart review to achieve specific audiological data at each frequency to define study outcomes of hearing loss. Hearing threshold—the lowest intensity of sound in decibels (dB) that the person could hear—was tested at baseline, monthly, and whenever the patient’s hearing condition worsened, using an audiometer in a standard audio booth or KUDUwave™ (eMoyo, Johannesburg, South Africa),16 a computer-based portable audiometer, at frequencies ranging from 250 to 8,000 Hz.17 The hearing threshold was then used to define our primary outcome based on the degree of hearing loss.
The present study defined pre-existing composite hearing loss as 1) a hearing threshold outside of the normal range (above 25dB) in one or both ears at any frequency in the range from 250 to 8,000 Hz, tested by baseline audiometry (i.e., pre-existing audiometric hearing loss), or 2) self-reported auditory symptoms including tinnitus or hearing loss. The outcomes of AG-induced hearing loss were observed during the 6 months of the injectable phase and further defined as: 1) clinically determined hearing loss resulting in a change in treatment (i.e., reduced or stopped AG) due to ototoxicity confirmed by either audiological evaluation or self-reported auditory symptoms, or 2) audiometric hearing loss, defined as a deterioration of at least one category of hearing loss compared to baseline hearing in the same range of frequencies in one or both ears. The proxy measure of cumulative AG exposure following treatment initiation was calculated as 1) weekly AG dose = prescribed daily AG dose (mg) × frequency of dosing (times per week), categorized as high-dose (≥5,000 mg/week), medium-dose (3,000–4,999 mg/week) and low-AG dose (<3,000 mg/week), and 2) standardized weekly AG dose, which was calculated as follows:
which was dichotomized as high-dose (≥75 mg/kg/week) and low-dose (<75 mg/kg/week).
Statistical analysis
Statistical analysis included descriptive and correlational statistics to determine the most appropriate cut-offs for categorical variables of AG dose and to explore the prevalence of hearing loss and the associations with risk factors. To assess the relationships with AG-induced hearing loss, we conducted bivariate analysis on potential confounders, including age, type of AG, pre-existing hearing loss, CD4 count, estimated glomerular filtration rate (eGFR), weight, arm of the parent trial, and demographic variables. A Cox proportional-hazard model was then used to explore hazard ratios (HRs) for the time to developing AG-induced hearing loss, adjusting for other covariates. In this model, we included all variables that were significantly associated with hearing loss (P < 0.05) in bivariate analysis. Two separate analyses were conducted to evaluate associations with clinically determined hearing loss in the full cohort and with audiometrically determined hearing loss in the subgroup of participants with available baseline and follow-up audiograms. Our primary association of interest was between the standardized weekly AG dose and hearing loss (either clinically or audiometrically assessed). All analyses were performed using Stata/IC 15 software (Stata Corp, College Station, TX, USA).18
Ethical approval
The parent study was approved by the Provincial Health Research Committee of the Eastern Cape (Bhisho) and KwaZulu-Natal (Pietermaritzburg, South Africa) Provincial Departments of Health in South Africa. The parent study and this substudy were both approved by the Biomedical Research and Ethics Committee of the University of KwaZulu-Natal, Durban, South Africa, and the Institutional Review Board of the Johns Hopkins Medical Institutions, Baltimore, MD, USA (NA_00078899/CIR00024657).
RESULTS
Of the 1,279 participants enrolled in the parent trial, 936 were eligible for the present analysis (Figure 1). The median age was 35 years (interquartile range 29–42), 505 (54%) were male, 697 (75%) were co-infected with HIV, and 432 (62%) had known exposure to ART at baseline. Of the 602 patients with a baseline CD4 count available (median 188 cells/mm3), 320 (53%) had a CD4 count of <200 cells/mm3 and 109 (18%) had a CD4 count of ≤50 cells/mm3.
Figure 1.
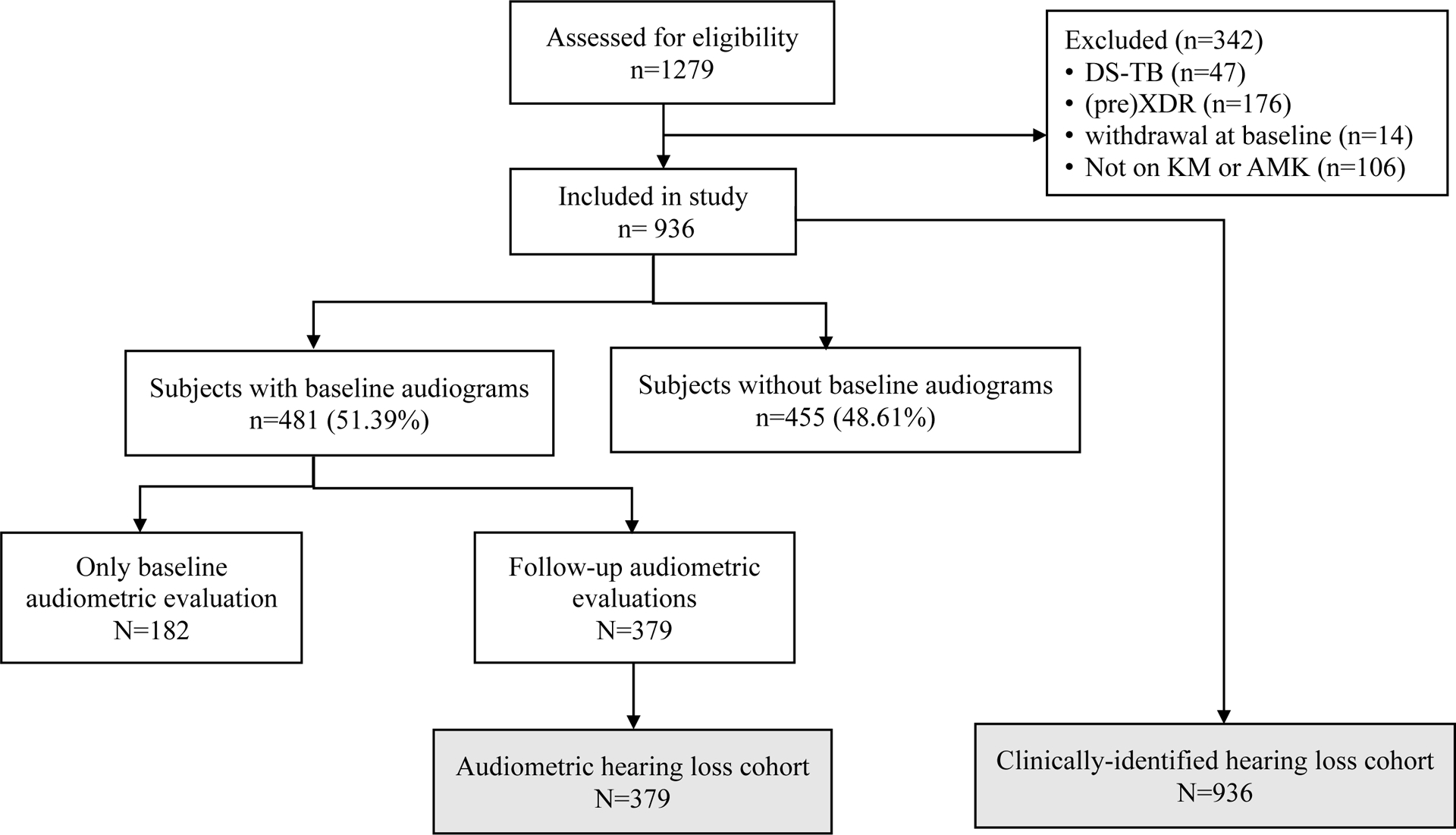
Diagram for Study Flow
Abbreviations. KM: kanamycin; AMK: amikacin; DS-TB: drug-sensitive tuberculosis; XDR: extensively drug-resistant TB
At baseline, only 66 (7%) of the study patients had renal impairment (eGFR < 60 ml/min/1.73m2), 297 (32%) were underweight (body mass index [BMI] < 18.5 kg/m2), 551 (59%) had baseline hypoalbuminemia (serum albumin < 35g/l), and 142 (15%) reported either tinnitus or hearing loss. Among those who were tested for audiometric hearing loss (n = 481) by either audio booth (n = 238) or portable KUDUwave (n = 243), 289 (60%) had at least mild hearing loss (≥26 dB) at any frequency from 250 to 8,000 Hz. Those who had auditory symptoms were more likely to have audiometrically confirmed hearing loss at baseline at any frequency (χ2[degree of freedom, df 1]=14.69, P< 0.001). About 35% of the participants (n = 330) received a high, standardized, weekly AG dose (≥75 mg/kg/week). KM was the most common choice of AG (847 patients, 90%), since nine hospital sites offered KM, whereas only one hospital site offered AMK.
Of 936 participants, 379 (40%) were tested for baseline and follow-up audiometric hearing loss and were thereby eligible to be assessed for time to developing audiometric hearing loss (see Table 2 for participant characteristics in each cohort).
Table 2.
Baseline characteristics of participants
| Clinical hearing assessment cohort (n = 936) | Audiometric hearing assessment cohort (n = 379) | |
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Male | 505 (54) | 201 (53) |
| Female | 431 (46) | 178 (47) |
| Age, years | ||
| 13–19 | 45 (5) | 23 (6) |
| 20–29 | 241 (26) | 99 (26) |
| 30–39 | 355 (38) | 151 (40) |
| 40–49 | 172 (18) | 68 (18) |
| ≥50 | 123 (13) | 38 (10) |
| Weekly AG dose, mg/week | ||
| Low (<3000) | 237 (25) | 88 (23) |
| Medium (3000–4999) | 422 (45) | 175 (46) |
| High (≥5000) | 276 (30) | 116 (31) |
| Standardized weekly AG dose, mg/kg/week | ||
| Low (<75) | 596 (64) | 235 (62) |
| High (≥75) | 330 (35) | 144 (38) |
| HIV status and CD4 count, cells/mm3 | ||
| HIV-negative | 239 (26) | 86 (23) |
| HIV-positive with CD4 >200 | 282 (30) | 127 (34) |
| HIV-positive with CD4 <200 | 320 (34) | 130 (34) |
| Unknown CD4 count | 95 (10) | 36 (9) |
| ART status among HIV-infected | (n = 697) | (n = 293) |
| No ART at baseline | 265 (38) | 107 (37) |
| On ART at baseline | 432 (62) | 186 (63) |
| Previous history of DR-TB | ||
| New DR-TB | 478 (51) | 190 (50) |
| Ever had prior TB | 421 (45) | 177 (47) |
| Unknown | 37 (4) | 12 (3) |
| Pre-existing composite hearing loss* | ||
| Normal hearing | 568 (60) | 183 (48) |
| Baseline hearing loss | 366 (39) | 196 (52) |
| Unknown | 2 (1) | 0 (0) |
| BMI, kg/m2 | ||
| Underweight (<18.5) | 329 (35) | 139 (36) |
| Normal (18.5–24.9) | 445 (48) | 184 (48) |
| Overweight or obese (>25) | 153 (16) | 56 (16) |
| Unknown | 9 (1) | 0 (0) |
| Serum albumin, g/l | ||
| Normal (≥35) | 193 (21) | 86 (23) |
| Hypoalbuminemia (<35) | 551 (59) | 210 (55) |
| Unknown | 192 (20) | 83 (22) |
| eGFR, ml/min/1.73 m2 | ||
| ≥90 | 590 (63) | 249 (66) |
| 60–89 | 196 (21) | 82 (21) |
| <60 | 66 (7) | 20 (5) |
| Unknown | 84 (9) | 28 (7) |
| NCM intervention of parent study | ||
| Intervention site | 430 (46) | 205 (54) |
| Control site | 506 (54) | 174 (46) |
Pre-existing composite hearing loss defined as confirmed by either audiometry or self-reported auditory symptoms.
AG = aminoglycoside; HIV = human immunodeficiency virus; CD4 = cluster of differentiation 4; ART = antiretroviral therapy; DR-TB = drug-resistant tuberculosis; BMI = body mass index; eGFR = estimated glomerular filtration rate; NCM = nurse case management.
Clinically determined hearing loss
Initial AG regimens were modified due to clinically determined hearing loss during the injectable phase at least once in 420 participants (44%). After adjusting for age, eGFR, pre-existing composite hearing loss, AG type, and NCM intervention assignment, the hazard of AG regimen modification due to ototoxicity among the high-dose group (≥75 mg/kg/week) was 1.33 times as high as among the low-dose group (<75 mg/kg/week, 95% confidence interval [CI] 1.09–1.64; Table 3 and Figure 2). The two most common choices of regimen modification were reducing AG frequency (41%) and stopping AG (40%). In the low AG dose group (<3,000 mg/week), in particular, providers tended to stop AG when ototoxicity was observed (147/237, 62%); patients tended to reduce the frequency of AG in the medium (3,000–4,999 mg/week; 220/422, 52%) and high (≥5,000 mg/week; 116/276, 42%) AG dose groups (χ2[df 6]= 49.48, P < 0.001).
Table 3.
HRs for HL
| Clinically determined hearing loss (n = 936) | Audiometric hearing loss (n =379) | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | aHR (95% CI) | HR (95% CI) | aHR (95% CI) |
| Standardized weekly AG dose, mg/kg/week* | ||||
| 1 | 1 | |||
| <75 | 1 (Reference) | 1 (Reference) | Reference | Reference |
| ≥75 | 1.25 (1.03–1.53) | 1.33 (1.09–1.64) | 1.21 (0.94–1.57) | 1.34 (1.02–1.77) |
| Age (10 years old) | 1.00 (0.92–1.10) | 1.02 (0.93–1.12) | 1.22 (1.08–1.38) | 1.16 (1.01–1.33) |
| eGFR, ml/min/1.73m2 | 1.00 (1.00–1.00) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) |
| Pre-existing composite HL† | — | — | ||
| Normal hearing | 1 (Reference) | 1 (Reference) | ||
| Baseline HL | 1.67 (1.38–2.02) | 1.68 (1.38–2.07) | ||
| Pre-existing audiometric HL | — | — | ||
| Normal hearing | 1 (Reference) | 1 (Reference) | ||
| Baseline HL | 1.64 (1.25–2.14) | 1.71 (1.29–2.27) | ||
| Type of audiometer | — | — | ||
| Audio booth | 1 (Reference) | 1 (Reference) | ||
| KUDUwave | 1.21 (0.94–1.56) | 1.12 (0.82–1.52) | ||
| Type of AG | ||||
| Amikacin | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Kanamycin | 2.18 (1.43–3.32) | 2.95 (1.68–5.19) | 1.64 (1.03–2.62) | 1.31 (0.70–2.46) |
| NCM intervention | ||||
| Intervention site | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Control site | 1.12 (0.92–1.35) | 1.01 (0.82–1.24) | 1.33 (1.03–1.72) | 1.41 (1.03–1.94) |
| HIV status‡ | — | — | ||
| Negative | 1 (Reference) | 1 (Reference) | ||
| Positive | 1.21 (0.96–1.52) | 1.20 (0.88–1.64) | ||
Dichotomized due to lack of statistical differences between low exposure (<60 mg/kg/week) and intermediate exposure (60–74.9 mg/kg/week) patients if AG dose was <75 mg/kg/week.
Defined as confirmed by either audiometry or self-reported auditory symptoms.
Not retained in the multivariate model.
HR = hazard ratio; HL= hearing loss; CI= confidence interval; aHR = adjusted HR; AG = aminoglycoside; eGFR = estimated glomerular filtration rate; NCM = nurse case management; HIV = human immunodeficiency virus.
Figure 2.
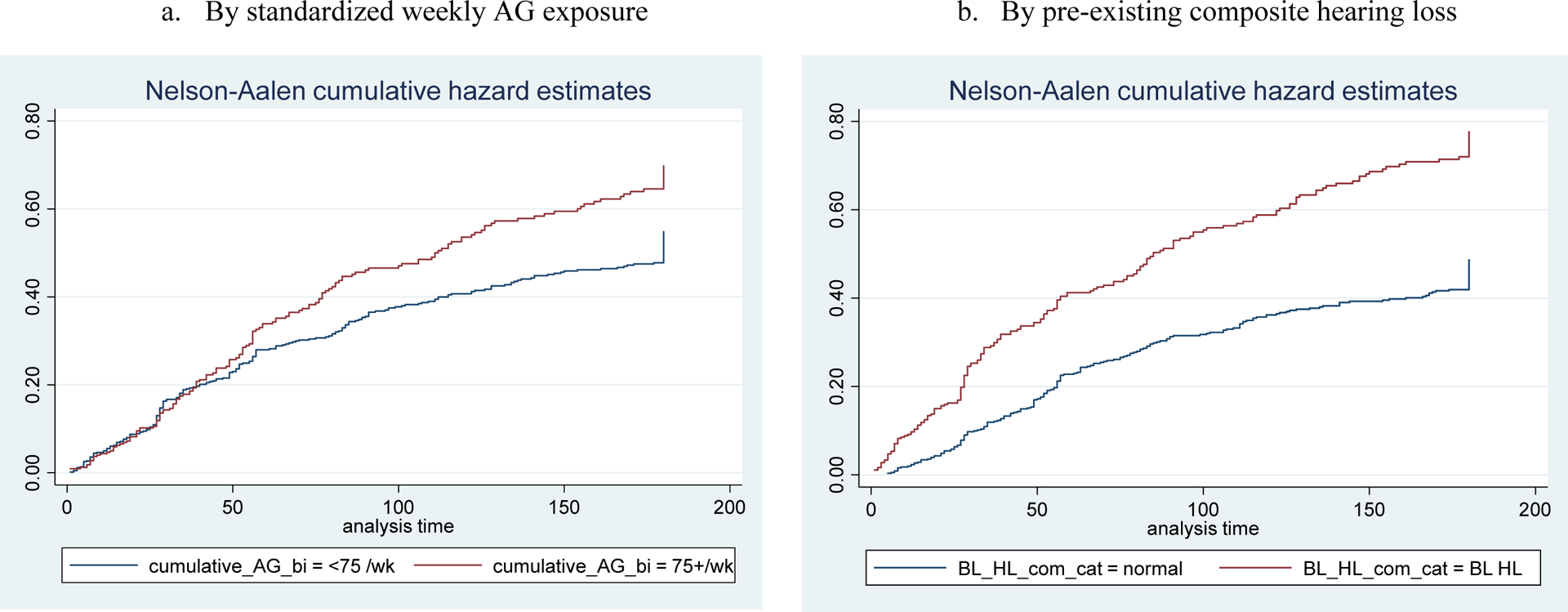
Kaplan-Meier Cumulative Proportion of AG-induced Hearing Loss in Clinically Identified Hearing Loss Cohort
Audiometric hearing loss
Of 379 subjects who were tested with audiometry, 238 (63%) developed any level of hearing loss during the injectable phase. In the final model—after adjusting for age, eGFR, pre-existing audiometric hearing loss, type of audiometer, AG type, and NCM intervention assignment—patients with a high AG dose (≥75 mg/kg/week) had 1.34 times higher adjusted hazard of hearing loss than those with a low dose (95% CI 1.02–1.77). We estimated that patients with baseline audiometric hearing loss had 1.71 times higher adjusted hazard of hearing loss than those with normal hearing (95% CI 1.29–2.26), and that every 10 years of age was associated with an increase in the adjusted hazard of hearing loss of 1.16 (95% CI 1.01–1.33; Table 3 and Figure 3). Audiometric hearing loss was more common among those who received KM than among those who received AMK (58% vs. 5%; aHR 1.64, 95% CI 1.03–2.62).
Figure 3.
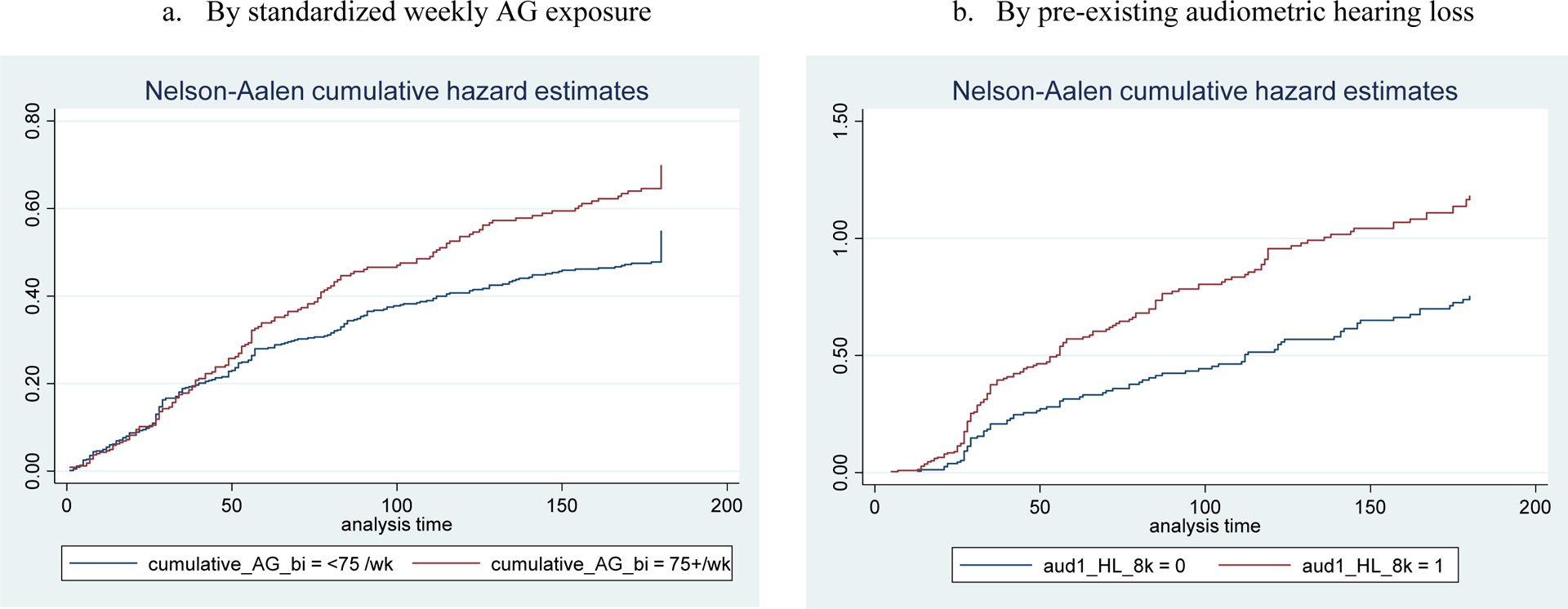
Kaplan-Meier Cumulative Proportion of AG-induced Hearing Loss in Audiometric Hearing Loss Cohort
DISCUSSION
We found that cumulative weekly AG dosage was associated with the hazard of AG-induced hearing loss and AG regimen modification during the injectable phase of MDR-TB treatment. In this study, patients received AG according to the South African national guidelines that stipulated that the standard MDR-TB long-course regimen should consist of one intramuscular injectable AG with an average dose of 15 mg/kg at a maximum frequency of five times per week for at least 6 months.7 The surprising finding in our study is that even within the narrow dosing range provided, based on the weight-banded dosing guidance (e.g., 750 mg/week for someone weighing 50 kg vs. 1000 mg/week for someone weighing >65 kg), it was evident that even a modest increase in dose led to a measurable increase in the risk of hearing loss. This implies that the clinical therapeutic window for AG is very narrow. In this setting, and without a clear target exposure that maximizes efficacy of this drug, avoiding the use of this class of drugs for TB whenever possible is essential. In situations where the drug must be used, care should be taken to avoid doses in excess of 75 mg/kg/week; monitoring is of critical importance. The practice patterns for providers were clearly impacted in this evaluation: in cases where AG ototoxicity was detected either by audiological evaluation or by the presence of auditory symptoms of ototoxicity, providers tended to discontinue the AG regimen if patients were receiving low dosage (< 3000 mg/week), while they tended to reduce AG frequency rather than the daily dose if patients were receiving medium or higher dosage (≥ 3000 mg/week). Advanced age and pre-existing hearing loss were also significantly associated with the risk of audiometric hearing loss and with the decision of AG regimen modification. These findings highlight the importance of not only baseline screening of hearing as routine practice, but also more frequent audiometric evaluations or the administration of a less ototoxic regimen in elderly patients with pre-existing hearing loss.
As AG ototoxicity is dose-dependent and has a narrow therapeutic window, the AG dosage should be tightly regulated in inpatient settings, with serial measurement of creatinine levels coupled with TDM and adjustment of dosing to remain within targeted therapeutic ranges.13,19 Although AG-induced hearing loss is a common, debilitating, adverse outcome from MDR-TB treatment, strategies to reduce this risk, such as selection of non-ototoxic oral alternatives, conducting TDM, or regular audiological monitoring, are limited in many TB programs in low- or middle -income countries. Because AGs are inexpensive and highly potent,6,14,20 financial considerations may, in part, explain the higher risk of AG-induced hearing loss in resource-limited settings compared to high-resource settings.21 Thus, as this study suggests, the standardized weekly AG dose may be used to identify individuals at high risk for AG-induced ototoxicity and guide healthcare providers in developing personalized interventions to prevent AG-induced hearing loss in medically underserved settings. Specifically, for those at high risk for developing hearing loss, AG-sparing regimens combined with close monitoring of drug safety should be considered. In settings where AG-sparing regimens cannot be used and TDM is impractical, the standardized weekly AG dose may serve as a reasonable proxy measure of AG exposure, and close observation of hearing and dosing adjustment should be planned for those on high dosage at treatment initiation.
There were several limitations in this study. Substantial missing data for baseline measures limited the power of our analysis. In particular, missing data on audiograms, BMI, creatinine clearance, and CD4 count reflected a lack of adherence to MDR-TB treatment guidelines; our patient population may thus have represented a more adherent population and may not be fully generalizable to all populations of patients undergoing AG-based MDR-TB treatment. We hypothesized that renal function at treatment initiation may directly influence the level of AG accumulation in the inner ear because AGs are excreted by glomerular filtration and are nephrotoxic. However, the impact of renal function was underpowered due to missing data on serum creatinine during follow-up and the low prevalence of renal failure at baseline. Future studies should explore how renal function contributes to the risk of hearing loss. This study only explored the outcome of hearing loss over a 6-month follow-up period. Further analyses using data on long-term hearing loss outcomes along with treatment outcomes could be helpful to determine a threshold dose and/or interval for better clinical guidance and the impact of AG dosing on TB treatment outcomes. Although we focused on hearing loss (cochlear toxicity) as a primary outcome, vestibular ototoxicity—including dizziness, ataxia, or nystagmus—also merits further study. Finally, due to minimal use of AMK in this sample, our results may be less generalizable to settings in which AMK is increasingly used instead of KM.
CONCLUSION
Our analysis found significant impact of a standardized weekly AG dose on AG-induced hearing loss. Also, the presence of pre-existing hearing loss and advanced age were associated with an increased risk of AG-induced hearing loss. Such findings may assist clinicians’ judgment in the selection of MDR-TB regimens.
Acknowledgements
Research reported in this manuscript was supported by the National Institute of Allergy and Infectious Disease (R01 AI104488-01A1 to JF), the National Institute of Nursing Research (F31 NR016910-01A1 to HH) of the National Institutes of Health (Bethesda, MD, USA), Sigma Theta Tau International Global Nursing Research Grant, Sigma Theta Tau International/Association of Nurses in AIDS Care Grant, Global Korean Nursing Foundation Scientific Award, Dr Scholl Foundation Dissertation Scholarship, and the Johns Hopkins Center for Global Health Established Field Placements Grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned organizations/institutions.
H. W. F. is a surgical advisory board member of Advanced Bionics and MedEl Corporations. All other authors report no potential conflicts.
References
- 1.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 updates WHO/HTM/TB/2016.04 Geneva, Switzerland: WHO, 2016. https://apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf?sequence=1. Accessed November 2019. [Google Scholar]
- 2.World Health Organization. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis, 2018 update. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 3.Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res 1996; 98(1–2): 116–124. [DOI] [PubMed] [Google Scholar]
- 4.Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res 1997; 104(1–2): 1–14. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JA, Godfrey-Faussett P, Jacobs K, Ebrahim A, Hesseling AC, Schaaf HS. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J 2012; 40(5): 1277–1286. [DOI] [PubMed] [Google Scholar]
- 6.Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol 2011; 2011: 937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Republic of South Africa Department of Health. Management of drug-resistant tuberculosis: policy guidelines. Pretoria, South Africa: Department of Health, 2013. [Google Scholar]
- 8.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PloS One 2013; 8(5): e63057–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wu S, Xia Y, et al. Adverse events associated with treatment of multidrug-resistant tuberculosis in China: an ambispective cohort study. Int Med J Experimental Clin Res 2017; 23: 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torun T, Gungor G, Ozmen I, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9(12): 1373–1377. [PubMed] [Google Scholar]
- 11.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet (London, England) 2005; 365(9456): 318–326. [DOI] [PubMed] [Google Scholar]
- 12.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74(8): 839–854. [DOI] [PubMed] [Google Scholar]
- 13.Gogtay NJ, Kshirsagar NA, Dalvi SS. Therapeutic drug monitoring in a developing country: an overview. Br J Clin Pharmacol 2001; 52 (Suppl 1): 103S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez LS 3rd, Spencer JP. Aminoglycosides: a practical review. Am Fam Physician 1998; 58(8): 1811–1820. [PubMed] [Google Scholar]
- 15.Farley JE, Kelly AM, Reiser K, et al. Development and evaluation of a pilot nurse case management model to address multidrug-resistant tuberculosis (MDR-TB) and HIV in South Africa. PloS One 2014; 9(11): e111702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanepoel de W, Biagio L. Validity of diagnostic computer-based air and forehead bone conduction audiometry. J Occup Environ Hyg 2011; 8(4): 210–214. [DOI] [PubMed] [Google Scholar]
- 17.Tysome J, Kanegaonkar R. Hearing: an introduction & practical guide. Boca Raton, FL, USA: CRC Press, 2016. [Google Scholar]
- 18.StataCorp. Stata statistical software: release 15. College Station, TX, USA: StataCorp, 2017. [Google Scholar]
- 19.Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Int Med J 2011; 41(6): 441–449. [DOI] [PubMed] [Google Scholar]
- 20.Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med 2016; 6(6): a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong H, Budhathoki C, Farley JE. Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. Int J Tuberc Lung Dis 2018; 22(6): 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]


