Abstract
Many factors contribute to the poor survival of malignant brain tumor patients, some of which are not easily remedied. However, one contributor to the lack of progress that may be modifiable is poor clinical trial accrual. Surveys of brain tumor patients and neuro-oncology providers suggest that clinicians do a poor job of discussing clinical trials with patients and referring patients for clinical trials. Yet, data from the Cancer Action Network of the American Cancer Society suggest that most eligible oncology patients asked to enroll on a clinical trial will agree to do so. To this end, the Society for Neuro-Oncology (SNO) in collaboration with the Response Assessment in Neuro-Oncology (RANO) Working Group, patient advocacy groups, clinical trial cooperative groups, including the Adult Brain Tumor Consortium (ABTC), and other partners are working together with the intent to double clinical trial accrual over the next 5 years. Here we describe the factors contributing to poor clinical trial accrual in neuro-oncology and offer possible solutions.
Keywords: brain tumors, clinical trial accrual, neuro-oncology
The prognosis for adult patients with malignant brain tumors remains poor, with only minor improvements in survival over the past few decades. While many factors contribute to this lack of progress, a major impediment to improving outcomes is poor clinical trial accrual. In general, when cancer patients are eligible and offered a clinical trial, more than 50% of patients enroll.1–5 Yet, the percentage of patients who actually enroll on clinical trials is much lower. In 2002, Chang et al reported that only 21.3% of malignant glioma patients participated in a clinical trial.6 Unfortunately, accrual to neuro-oncology trials has remained stagnant over the past decade. A 2016 survey of brain tumor patients by the National Brain Tumor Society (NBTS) revealed that only 21% of patients participated in a clinical trial and only 24% of patients were informed of clinical trials at the time of diagnosis.7 Similarly, a 2018 paper on the clinical trials landscape for glioblastoma (GBM) estimated that only 8–11% of newly diagnosed GBM patients enroll in clinical trials.8 Based on this information, the Society for Neuro-Oncology (SNO), NBTS, and the Neuro-Oncology Branch of the National Cancer Institute (NCI) partnered on a survey of over 350 neuro-oncology providers in an effort to identify challenges and barriers to clinical trial referral and participation.9 According to results from this survey, less than 30% of all patients are referred for clinical trials, but over one-third of participants noted that their institution did not track clinical trial referral, making accurate estimates difficult.9
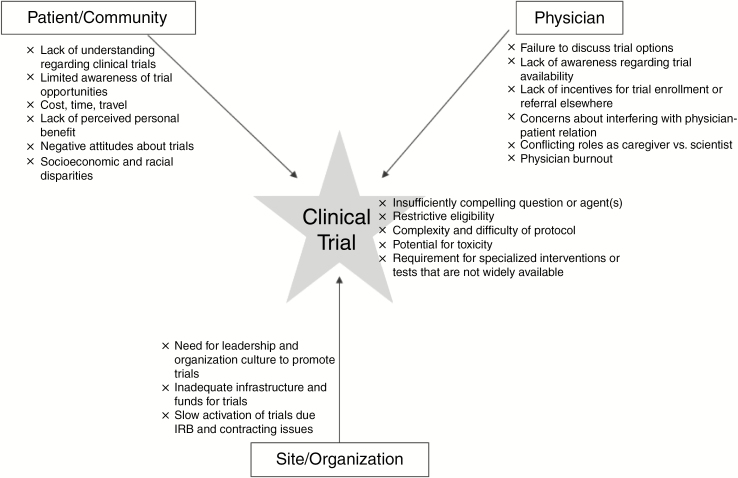
SNO, in collaboration with the Response Assessment in Neuro-Oncology (RANO) Working Group, patient advocacy groups, clinical trial cooperative groups, including the Adult Brain Tumor Consortium (ABTC), and other partners are working together with the intent to double clinical trial accrual over the next 5 years. There have been previous efforts to identify barriers to clinical trial accrual in cancer.10–13 Here, we discuss the factors contributing to poor trial accrual specifically to neuro-oncology trials and offer possible solutions. We will focus on patient and community factors, disparities, physician and provider factors, clinical trial factors, and site and organizational factors (Table 1, Figure 1).
Table 1.
Factors impacting accrual and potential solutions
| Challenges | Potential Solutions |
|---|---|
| A: Patient and Community Factors | |
| Hindrances to patient’s decision-making ability, including low level of education, limited understanding of clinical trials, and impact of disease on neurocognitive function | • Provider education to improve communication with patients regarding clinical trials (including written and online materials and videos) |
| • Engagement of patients and providers with advocacy groups | |
| • Use of clinical trial patient navigator | |
| Patient’s limited awareness of clinical trial opportunities | • Patient education campaigns focused on reducing barriers to trial participation |
| • Brain tumor registry to help providers identify trial eligible patients and to provide an opportunity for outreach to those patients | |
| • Simplify the ability to identify local and national trials through improved online search tools, smart phone apps, or clinical trial patient navigators | |
| • Engagement with patient advocacy groups, brain tumor support groups, and use of patient navigators | |
| Patient misconceptions about research study involvement including negative personal and family attitudes about clinical trials and perceived lack of personal benefit | • Patient education campaigns focused on demystifying clinical trials and dispelling rumors about them |
| • Engagement with patient advocacy groups which can promote the existence and benefits/ risks of joining a clinical trial | |
| Suboptimal (ie, poorly timed or rushed) discussion of clinical trial opportunities with overwhelmed patients/caregivers | • Heighten empathy for patients/caregivers |
| • Incorporate multimedia materials to effectively describe in layman’s terms the study rationale, potential benefits/risks and logistics | |
| • Improve informed consent documents and processes to facilitate greater understanding of the issues involved in clinical trial participation | |
| • Provide enough time for patient/caregivers concerns to be addressed; incorporate additional research team input from nurses, mid-levels, and navigators | |
| • Refer patients to brain tumor patient advocacy organizations for additional support and access to patient navigators | |
| Concerns about the complexity and difficulty of complying with protocols, cost, and time/convenience | • Design trials that are more patient friendly by obtaining their input |
| • Resources to facilitate travel and reduce costs of trial participation such as parking, housing, and absence from work | |
| • Ensure coverage of routine patient care costs in clinical trials by both federal and private payers | |
| • Open larger number of trials at smaller centers (a role especially for the National Clinical Trials Network in the United States) | |
| • Use novel technologies such as telemedicine to minimize trips for clinical trial assessments | |
| • Trial-design changes aimed at reducing the number of clinical trial visits | |
| B: Disparities | |
| Access and referral patterns | • Establish standard paradigm for referral of patients to neuro/medical oncology |
| • Implementation of patient navigators dedicated to support of URM/vulnerable patients | |
| • Establish partnerships with community-based organizations serving URM | |
| Unconscious bias | • Unconscious bias training with regular evaluation of its efficacy and relevance |
| Lack of diversity in oncology workforce | • Strengthen pipeline of URM candidates into neurology, oncology, neurosurgery, and radiation oncology |
| C: Physician and Provider Factors | |
| Failure to discuss clinical trials as an option with patients | Change standard practice to one where clinical trials are always discussed as an option in addition to existing standard therapies, particularly at two timepoints: |
| • When formulating the initial plan of care. | |
| • At the time of disease progression or disease recurrence. | |
| • Early on, dispel potential patient and caregiver attitudes that clinical trials are only for a time when all other options have run out, especially since patients who are heavily pre- treated are less likely to qualify for trials | |
| Time and inconvenience | • Trial navigators and/or electronic tools (eg, apps) to allow for rapid, real-time trial matching |
| • Brief trial summaries pitched at physicians and at patients to provide a high-level overview of the trial rationale, design, risks/benefits, and visit schedule can distill complex information into some of the key elements | |
| • Optimizing and streamlining referral processes on both the referring and receiving ends to reduce the barriers to referring patients to outside institutions for trials. | |
| Lack of knowledge | • For complex cases scheduled in advance, care team members could prepare ahead of the encounter to gather information on the best course of action |
| • If preparing ahead of the encounter is not possible, an alternative could be to acknowledge the complexity of the patient’s case, and explain that further discussion with colleagues will take place (ie, multidisciplinary tumor board) to identify the best course of action | |
| Lack of information about available clinical trials including eligibility criteria | • Take advantage of resources such as a clinical research navigator and on-line matching tools (eg, apps), who can help identify clinical trials available at the institution that may be appropriate for each patient |
| • When a patient is a good candidate for clinical trials and there is not a trial available at the provider’s institution, consider searching for studies available at referring center(s) | |
| • Efforts to develop trial search engines which can provide accurate and appropriate potential trial matches while minimizing manual data input from the provider, and which can filter for key factors (geography, stage, disease status, lines of therapy, relevant biomarkers) are underway and should continue | |
| Lack of willingness to refer a patient to another center for study (including financial incentives) | • Encourage a change in culture to always consider referring patients to centers with trials if one is not available locally, if practically feasible. |
| • Allow patients to receive some of their evaluations and treatments with the referring physician to reduce the sense that the physician is “losing” their patients; to validate the importance of a continued connection between the referring physician and the patient; to reduce the financial disincentive to refer patients; and to support stronger collaborations between oncology teams at the referring and trial sites | |
| Lack of Incentive | • Consider a research RVU system that compensates for clinical trial related activities |
| • Increase possibility of authorship for physicians who enroll patients into clinical trials | |
| Concerns regarding a patient’s interest and ability to participate | • A candid discussion with the patient and the research staff, ideally during the encounter to discuss treatment options, should be conducted to address any source of concerns for participation |
| Concern about the interference in the physician-patient relationship | • Discussions about goals of care, patient’s preferences and expectations should be done during the clinical trial in the same manner as they are done during routine clinical care |
| Conflict between the physician’s role as caregiver versus scientist | There are at least three strategies to help mitigate this concern: |
| • To place the patient’s needs and preferences first | |
| • To enroll or refer to trials which are scientifically valid and designed in a clinically justifiable manner | |
| • To explain the differences between the role of the primary oncologist and the role of the clinical investigator, particularly when they are embodied in the same person | |
| • Ask patients for any source of concerns about conflicts between the roles, and address them | |
| Physician burnout | • Understand and acknowledge the effect that clinical trials can have on physician burnout |
| • Work with institutional leadership to emphasize the value of access to clinical trials and the need for resources to facilitate clinical research. | |
| • Work with the research staff, research nurses, clinical research coordinators and other personnel to address challenges to distribute the burden across the trial team. • Future platforms, such as artificial intelligence may assist with curating clinical trial options. • Shared electronic medical records across institutions may improve access. |
|
| D: Clinical Trial Factors | |
| Patient/caregiver hardships due to frequent study center visits limit enthusiasm for trial participation | • Incorporate patient/caregiver/advocate feedback into early drafts of clinical trial during development |
| • Allow study assessments and treatments, especially those considered “standard of care” to be done locally; | |
| • Require study center visits only when critically relevant to the study therapy; | |
| • Proactively incorporate patient/caregiver considerations into need for regulatory requirements for source documentation | |
| Inefficient clinical trial design features | • Incorporate multi-stage, multi-arm trials with adaptive randomization |
| • Incorporate careful toxicity and efficacy stopping rules | |
| • Consider lower statistical power thresholds for non-registration efficacy trials | |
| • Involve at an early stage of clinical trial design patient advocacy organizations, patients and caregivers | |
| Excessively stringent eligibility criteria limit trial participation | • Limit inclusion/exclusion to criteria critically relevant to study primary endpoint |
| • Ensure eligibility criteria do not preferentially exclude a demographic or racial group, eg, upper or lower age limits, or excluding comorbidities more highly associated with demographic or socioeconomic subgroup unless specific rationale for exclusion exists. | |
| • Include patients with primary and metastatic brain tumors in early phase oncology clinical trials | |
| Technology trials ignore clinical equipoise challenges and incorporate traditional trial endpoints | • Allow non-traditional primary endpoint/s that addresses key clinically meaningful objective of technology being assessed |
| E: Site and Organizational Factors | |
| Clinical research requires specialized personnel, training, infrastructure, resources | • Effective leadership of a multidisciplinary team and organization culture to promote accrual |
| • Adequate infrastructure to allow clinical research | |
| • Cancer care accreditation to incentivize trial enrollment | |
| • Hire physicians and research staff committed to clinical research | |
| Slow activation of trials due to IRB issues, contracting issues, etc. | • Centralize IRB operations with harmonization across countries |
| Limited resources at community centers to support clinical research | • Greater partnership between academic and community oncology centers |
| • Enhanced incentives for patient enrollment in clinical trials or referrals to academic centers for clinical trials | |
| • Education programs for community physicians emphasizing the importance of clinical trials |
Abbreviations: IRB institutional review board, RVU relative value unit, URM underrepresented minority
Figure 1.
Potential barriers to clinical trial accrual.
Patient and Community Factors
Patient and community factors impeding clinical trial participation of brain tumor patients are poorly understood. Indeed, disease-specific research regarding these factors is critically important due to the influence of the illness on cognitive function, behavior, and motor skills, which can impair patients’ medical decision making, employment, and sense of control. This section evaluates patient and community factors that might impede trial participation with the goal of identifying opportunities for developing interventions to improve participation.
Patient-specific factors
A wide range of brain tumor patient factors could influence clinical trial participation. Some of the factors which negatively affect participation in brain tumor trials, such as social disparities,14,15 language or cultural barriers, and older age,6 are common across oncology. Some factors are unique to the population given the impact of the disease on neurocognitive function.
The timing and way in which clinical trials are discussed with patients may impact accrual, particularly depending on the patient’s level of understanding and the health care provider’s ability to communicate trial information. Lower educational achievement is a known barrier to clinical trial enrollment; previous studies have identified associations between education and patients’ ability to make decisions about clinical trial participation in other cancer types.1,16–19 Regardless of their educational background, the explanation of complex trial-related procedures by providers and lengthy consent forms may overwhelm patients and caregivers with no prior knowledge of clinical research methodology or terminology. The nature of clinical research and procedures such as randomization, blinding, and biospecimen collection—as well as the extra time involved as a study participant due to added travel for clinic appointments—all add complications that may be perceived as unwanted interventions beyond routine care. Additionally, clinical trials are typically presented during the emotional and psychosocial upheaval of a new or recurrent cancer diagnosis.
In prior studies, the quality of communication, a strong patient–physician relationship fostering a sense of trust, and a patient-friendly presentation of clinical trial information were factors associated with improved oncology trial participation,4 highlighting the importance of communication and providing appropriate patient education. Verbal, written, video, and online review materials, carefully prepared in layman’s terms, as well as adequate time to address questions and concerns can prove invaluable to patients/caregivers pondering varied and complex treatment options. Sensitive attention to trial complexities, such as use of a placebo or blinded trial design, as well as availability of financial or other supportive resources (such as travel compensation, discounted lodging, etc) can also help encourage trial participation.
Some neurologic symptoms associated with a brain tumor may be negative predictors of trial enrollment. Based on neurocognitive testing and standardized research consent capacity measures, patients with malignant gliomas perform significantly below healthy controls with respect to appreciation, reasoning, and understanding of consents.20 Not surprisingly, patients with a greater degree of cognitive impairment are less likely to enroll onto clinical trials.21 Higher symptom burden may be associated with a lower performance score such as Karnofsky performance status (KPS), precluding enrollment on a trial due to traditional inclusion criteria requiring good performance status. Physicians may also be unwilling to expose patients with a borderline KPS to the burden of a clinical trial. Additionally, patients with cognitive impairment or a greater neurologic symptom burden may feel less independent; loss of autonomy itself has been inversely correlated with participation in clinical trials in other cancers.22 Receptive or global aphasia raise concerns about a patient’s ability to provide informed consent and to understand all treatment information.20 Given the high incidence of cognitive impairment in primary brain tumor patients, additional studies are warranted to investigate the influence of cognitive function and symptom burden on trial enrollment and determine strategies to mitigate this barrier. Of course, some patients may not be suitable for trials, but there should be a relatively low threshold to consider trials for brain tumor patients given the poor results with standard therapies.
Limited awareness of clinical trials
Another important patient-related barrier to clinical trial enrollment is limited awareness of clinical trials and limited understanding of the overall goals of clinical research. Based on a survey of cancer patients and caregivers, most reported that their physicians did not discuss clinical trials as a treatment option and most believed that their physicians would have recommended clinical trials if trials were appropriate.23 Indeed, in the 2016 NBTS survey of brain tumor patients, only 42% of patients were informed about clinical trials at any time during the course of their illness by their medical team.7 Although many centers do not have trials to offer patients, the crucial importance of clinical research in improving care for brain cancer is a message worthy of discussion with all patients regardless of stage of treatment (thus also helping dispel the myth that clinical trials are only for those with advanced disease). Unfortunately, increased awareness itself may not be adequate to expand patient enrollment in clinical trials.7 The most common incentive for clinical trial participation cited in the NBTS survey was to not only help “me” but also help future brain tumor patients.7 This altruistic motivation is common in cancer patient trial participants, particularly in phase III clinical trials.24 A better understanding of the critical role of clinical research in advancing patient outcomes—in addition to the overall value of clinical research to society—deserves greater public dissemination.
Misconceptions about research study involvement
Another barrier to clinical trial participation is the misconception about risks and benefits of research study involvement. For example, patient randomization to the control arm of a study might be perceived as a lost opportunity to derive clinical benefit, leading to the patient’s non-enrollment. A recent study found that low trial enrollment might be related to a lack of understanding of details and availability of clinical trials by physicians in addition to their lack of familiarity with the principal investigators and sites where trials are available.25 A patient may feel that a physician is offering a clinical trial for his or her own selfish reasons rather than to provide the best care for the patient; this may lead to a perceived lack of personal benefit by the patient and limit engagement in clinical trials. Importantly, the design of a clinical trial can dissuade some patients from enrolling. All efforts to enhance trial enrollment will be enabled by greater involvement of patients and advocacy groups via their input into the design of clinical trials at an early stage of a trial’s development. One uncommon barrier (<10% of respondents) identified by the NBTS survey is the fear of experimental therapy toxicity, including life-threatening events.7 Clear, honest communication of realistic risks and benefits of therapeutic interventions may reduce these barriers and enhance brain tumor clinical trial enrollment.
Patient-related logistical and cost constraints
Another prominent barrier to trial enrollment is the distance required to travel to participate and the cost of participation.21,26,27 Travel can be a barrier for many reasons, including a patient’s inability to drive due to seizures, financial burden linked to travel and lodging expenses, time away from work for patients and caregivers, childcare costs, and overall stress related to travel. Opening trials at more centers, allowing delivery of radiation therapy or other standard of care (SOC) treatments closer to home, greater involvement of local oncologists, use of novel technologies such as telemedicine,28,29 collection of patient-reported data through mobile devices such as the MyStudies app from the US Food and Drug Administration (FDA),30 trial-design changes such as reducing the number of clinical trial visits, and resources to facilitate travel and reduce lost income may all be factors which enhance trial participation. The FDA recently updated its guidance to clinical investigators allowing reimbursements to patients in clinical trials for lodging and travel.31,32 When feasible, investigators should consider budgeting such reimbursements to patients, particularly for complex trials requiring frequent and extended visits.
Education campaigns, brain tumor registries, and social media may reduce barriers to trial participation
Education campaigns should focus on reducing barriers to trial participation. In addition, research is needed to identify the greatest barriers specific to brain tumor patients. The focus should be on the impact of neurologic deficits, symptom burden, cognitive dysfunction, and feelings of loss of control in addition to known general factors such as dispelling myths associated with clinical research (eg, clinical trials are only a last-resort treatment). Development of a brain tumor registry to quickly identify newly diagnosed cases with specific molecular profiles across the country could enable targeted education for these patients and physicians for early intervention, highlighting the potential benefits of precision medicine trials. Similar efforts are in place using the Screening Patients for Efficient Clinical Trial Access (SPECTA) platform by the European Organisation for Research and Treatment of Cancer (EORTC)33 (NCT02307604).
Social media can also help disseminate credible information about clinical trials to a broader audience (including rural communities, younger patients, patients with rare tumors, and underrepresented minorities), improve patient understanding about research, and provide a platform for communication between researchers, clinicians, patients, caregivers, and patient advocates.34,35 Approximately 70% of online adults in the United States are regularly on social media, and usage only continues to increase.36 Social media may help engage patients in clinical research, particularly those from underserved populations. In an examination of recruitment strategies for studies of young adult female cancer survivors, internet-based recruitment resulted in the highest number and yield of participants.37 Because social media is inherently different from traditional print media, including privacy concerns in this less-controlled environment, the US National Institutes of Health (NIH) provides guidance to investigators on social media strategies for clinical trials.38–40
The internet, particularly Clinicaltrials.gov, is a frequent resource for patients and caregivers seeking treatment options, but searches for clinical trials can be cumbersome and overwhelming. Efforts to simplify identification of local studies could include development of easily accessible clinical trial matching tools available through a website or a smartphone app, increased use of clinical trial navigators, and engagement with advocacy groups and provision of patient navigators to help find clinical trial opportunities (Table 2).
Table 2.
Patient Advocacy Groups
| Name of Organization | Headquarters | Contact Information | Clinical Trial Related Services for Patients/Caregivers |
|---|---|---|---|
| Accelerate Brain Cancer Cure (ABC2) | Washington, DC, USA | abc2.org | Provides links to clinical trial resources through their website |
| 1-202-419-3140 | |||
| American Brain Tumor Association (ABTA) | Chicago, IL, USA | www.abta.org | Clinical trial finder and navigators available through TrialConnect app.emergingmed.com/abta/home or phone 1-877-769-4833 |
| 1-800-886-2282 | |||
| Brain Tumor Network (BTN) | Ponte Vedra Beach, FL, USA | www.braintumornetwork.org | Professional staff conduct personalized clinical trial searches and provide treatment-related navigation throughout the continuum of care |
| 1-844-286-6110 | |||
| International Brain Tumour Alliance (IBTA) | Tadworth, Surrey, UK | theibta.org | Links to international clinical trial portals available through their website |
| +44 (0) 1737 813872 | |||
| Musella Foundation for Brain Tumor Research and Information | Hewlett, NY, USA | virtualtrials.com | Copayment assistance program; Clinical trial finder available through their website |
| 1-888-295-4740 | |||
| National Brain Tumor Society (NBTS) | Newton, MA, USA | braintumor.org | Clinical trial finder and navigators available through their website or phone 1-877-769-4812 |
| 1-617-924-9997 |
Disparities
The 2002 Institute of Medicine report entitled “Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care” first defined the concept of health care disparity as racial or ethnic differences in the quality of health care that are not due to access-related factors or clinical needs, preferences, and appropriateness of intervention.41 Disparities with respect to prevention, early intervention, and survival have been documented in multiple diseases, including cancers42–46 and in clinical trial enrollment for cancer.47–49 There is also evidence that patients from subsets of ethnic minority groups may not be afforded the same opportunity to participate in clinical trials and thus benefit from advanced therapies offered via clinical trials.50–52 Additionally, given the advent and emphasis on advanced genomics and personalized medicine, biomarkers from diverse populations are less likely captured, thus limiting our ability to determine if true differences exist in mutational profiles across various ethnic groups.46
While the incidence of primary brain tumors is higher in non-Hispanic white patients, there is conflicting evidence regarding survival outcomes as they relate to race.53–55 However, as in other cancers, clinical trial enrollment among minority populations remains poor. In this section, we will discuss challenges in accrual to clinical trials for underrepresented ethnic minorities (URM), as well as possible approaches to addressing them. The Association of American Medical Colleges (AAMC) has defined URMs to include people from the following groups: black/African American, Hispanic/Latino, Native American, Pacific Islander, and mainland Puerto Ricans.56 It is important to note that disparities of care also exist for populations based on age, sex, socioeconomic status, and sexual orientation, and the impact of these on care of neuro-oncology patients warrants further investigation.
Access and referral patterns
Patient-specific factors underlying limitations to accessing proper health care for URMs include lack of insurance or underinsurance, transportation issues, cultural and language barriers, and opportunity cost from income lost from work and other practical obstacles.47,48 Lack of insurance or underinsurance can directly impact access to clinical trials, especially if care at larger academic institutions with more trial options such as NCI-designated cancer centers is not covered.48 Perhaps as an extension of insurance status, access to institutions dedicated to cancer care and clinical trials may also be dependent on referral patterns for care, which in turn may be contingent upon geography, the patient’s medical condition and preference, the preferences of the referring medical team, and patients’ awareness of trials, among other factors. Treatment planning may be interrupted and delayed especially in instances where initial surgery or biopsy is obtained at an institution separate from that of the specialist care providers.57 For patients from URM groups with wariness or unfamiliarity of the health care system as well as those with limited English or medical literacy, clinical trials may only be briefly discussed or deferred due to time considerations.48 While patient resources such as patient navigators and advocacy groups such as the American Brain Tumor Association (ABTA), the Brain Tumor Network (BTN), and NBTS are in place to guide patients and families through their treatment process (Table 2), greater attention and support is needed for the most vulnerable patients or for those who may not have an awareness of the existence of these programs.
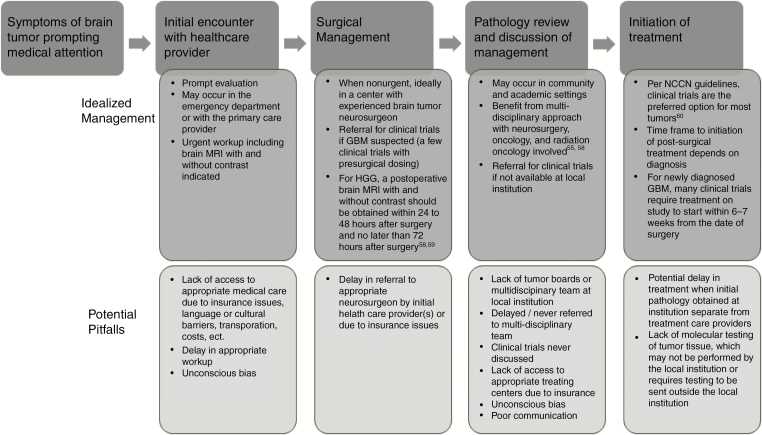
To address larger issues such as insurance coverage and other socioeconomic determinants of health (eg, income, education, habitat), a multidisciplinary effort by stakeholders including physicians, hospital administrators, policy makers, and legislators is needed. These factors are outside the scope of this current paper. A solution to this challenge in the immediate term would be to first establish a standardized management algorithm with proposed timing and steps in care for management of patients with brain tumors (Figure 2).58 Ideally, each step of the algorithm functions as a trigger or reflex, such that this may eliminate any uncertainty or hesitation in moving the patient through treatment in a smooth and uninterrupted manner. The algorithm would provide a framework for treatment planning within and across institutions ranging from large academic centers to smaller community settings and allow patients to better understand the necessary steps in their treatment course.
Fig. 2.
Recommended management algorithm for newly diagnosed primary brain tumor patients and potential pitfalls, particularly for underrepresented minority populations. HGG = high-grade glioma.
Drawing on work which already exists through internal institutional and patient advocacy groups, dedicated patient navigator programs specific to clinical trials participation can be expanded, perhaps through patient advocacy, for vulnerable patients with limited literacy of health care systems or those most at risk to being lost to follow-up. As noted earlier, a specific patient’s care may cross various institutions; however, at the time of an initial diagnosis, the patient should be provided by the initial treating care provider with an overall framework of the treatment course, including options for treatment, the various roles of the treatment team, and opportunities for psychosocial support.
Bias and mistrust
The history of medicine is notable for transgressions with respect to race and ethnicity, which has laid groundwork for mistrust among URMs toward larger institutions including health care systems.41 In medical and research education, keen attention is paid to training and retraining to avoid errors in designing and carrying out treatment protocols. In a routine clinical encounter, however, unconscious (or implicit) bias has been determined to be a source of discrimination which may impact the treatment relationship. Communication may be poorer in what have been identified as “racially discordant interactions” between a racial minority patient and a non–racial minority physician.61–63 The medical care provider displays uncertainty as a result of misbeliefs about the patient (or his/her identity), and the patient reacts in turn. This may result in shorter patient encounters, with the patient being less willing to follow instructions and the physician less likely to recommend a course of action or clinical trial, based on an assumption that the patient may not choose to participate.41,61 Although the impact of unconscious bias may be more challenging to quantify as it relates to overall survival outcome, this still represents an important key to establishing a treatment relationship between patient and physician.
Whether through larger SNO/RANO-led efforts or at a local/institutional level, there should be a commitment to ensuring that all members of a treatment team (physician, nurse, patient navigators) are trained in unconscious bias and receive updated training at intervals. Similar to medical school programs of cultural competency,64 institutions should establish metrics for success of the program and continually evaluate for efficacy and relevance.
Lack of exposure to diverse workforce
Black/African Americans are 13% of the US population and Hispanic Americans are 18% of the population, yet 2.8% of US oncologists identify as African American and 5.8% as Hispanic.65 The weakening of this training pipeline is likely due to concerns around education, income, and opportunity. However, it is also compounded by a decline in URM physicians and medical students, owing to reduction in medical school recruitment and pipeline programs; limited exposure to oncology and oncology subspecialties in medical school, insufficient URM role models, and implicit bias in candidate selection.61,65,66
Through an American Society of Clinical Oncology (ASCO)‒led taskforce established to increase racial and ethnic diversity in the oncology workforce, a strategic plan was developed to this end. As the functional and daily challenges faced by primary brain tumor patients are unique, SNO and partnering organizations should consider a parallel or separate pipeline program, aimed at recruitment of physicians from URM groups into neuro-oncology. URM student organizations such as the Student National Medical Association (SNMA) may provide a worthy and enthusiastic pool of the next generation of clinicians, researchers, and leaders in neuro-oncology. Similar efforts can be made with respect to resident trainees. Efforts for pairing current medical students and resident trainees with faculty mentors or representation of SNO at national conferences may be a small step in establishing a longitudinal pipeline critical to ensuring equal, high-quality care for our patients.
Physician and Provider Factors
Physicians and clinical providers play a key role in the clinical trial process. This includes the identification of patients for appropriate trials, discussion regarding the option of participation, assessment of patients on study, and compliance with trial conduct. In general, there are two main options for physicians to enroll patients into clinical trials: enrolling onto trials that are open at the physician’s own institution and referring patients for open trials at other institutions. The latter option is particularly important when good SOC options do not exist, when there are no available or appropriate trials at the physician’s own institution, and when patients are motivated to participate in a clinical trial at another institution. In this section, we focus on the interaction between patients and their treating physician. Rogers and colleagues report that the most frequent barriers clinicians face include factors related to trial location (eg, difficulty finding trials in the patient’s geographic area, patient being unable to stay for treatment at the academic site), lack of available slots on an existing trial, patient not meeting eligibility criteria, and limited staff resources.9 The importance of the physician’s role in trial accrual is strongly supported by the variance in accrual between physicians within a practice.67 The difference between “high” accruers and “low” accruers is unlikely due to different patient populations within the same practice and likely due to individual physician-related factors. Here we identify and provide solutions to the common challenges that physicians and providers experience with clinical trials.
Clinical care
The option of participation in a clinical trial is a major part of professional practice guidelines such as those from the National Comprehensive Cancer Network (NCCN).60 As a scientific community, the review and discussion about local and regional clinical trial options should become part of the standard workflow for good clinical practice. If this discussion is missed, patients may not have the opportunity to participate in a trial that may be of interest to them.68 There are several potential barriers that limit this discussion. From a practical perspective, the provider may feel that there is insufficient time in an appointment to discuss clinical trials in addition to the standard therapies.69 Another challenge is the lack of knowledge regarding the potential trials that patients may be eligible for either at the provider’s own institution or at another site.19 This may be due to lack of time to search and identify potential studies for the individual patient. In fact, the burden of the clinical trial process has been identified as a major barrier for referring patients for clinical trials.70 However, most patients are unaware of available clinical trials, and therefore the role of the physician in identifying clinically appropriate studies is critical.71 In addition, provider attitudes toward clinical trials are an essential factor for successful trial enrollment. Patients whose physicians are motivated to present the trial in detail, explain the relevance of the study intervention in their medical management, and encourage them to participate are more likely to enroll. This is also perceived by patients who view their physicians as the most trusted health care professional source of health information.72 Another barrier is the unwillingness to refer patients to an outside institution where a trial is identified. When the referral does not happen, it is a missed opportunity to both enhance patient care through clinical trial participation and collaborate with referral centers.
There are other challenges to clinical trial enrollment. Some physicians may not want to enroll patients in randomized studies if they feel uncomfortable with the presence of a control arm, as discussed further in the clinical trial factors section. In addition, the physician’s perception about risk of toxicity in an individual patient with factors such as older age, worse KPS, extensive comorbidities, and a high level of symptom burden may negatively influence a physician’s decision to recommend a clinical trial. Age is a significant influence on recruitment of patients to clinical trials73 and is a strong independent predictor of trial participation in glioma patients.6 The interaction between age and risk of toxicity perceived by the physician has been shown to influence enrollment of elderly women in breast cancer clinical trials.74 Given the importance of clinical research in improving outcomes, it is critical to minimize physician bias and its influence on clinical trial participant selection.
Some physicians may have the perception that the patient is not interested in participating and may be concerned about an adverse effect on the physician–patient relationship.75 Both of these challenges may be addressed by allocating time for specific discussions with patients. These discussions are actually similar to discussions that clinicians have with patients during standard treatments, where clinicians will discuss with patients about risks, benefits, and candidacy for any standard therapy. Additional time is also spent in the care of patients enrolled on clinical trial, including additional documentation, assessment of adverse events, tumor measurements, and regulatory activities. Whether physicians should be compensated for the additional time required to address clinical trial questions (similar to the billing mechanism in the US that supports the additional time required for end of life discussions) is unclear; however, what is clear is that identifying and presenting clinical trials to patients does take time. Another factor that may also influence trial enrollment that is closely related to the physician–patient relationship is the potential conflict physicians perceive as their role as caregiver versus scientist.69 In addition, the physician may have an interest in enrolling patients on a particular study over another concurrently run trial, which can skew accrual and may or may not be the best fit for the patient. Potential solutions to these challenges are shown in Table 1.
Physician-related logistical barriers
The interest and enthusiasm for research participation by physicians may vary between specialties, and between clinical practice models, such as academic versus private practice versus hospital based. The physician financial compensation model may influence the likelihood of recruitment to trials available within a physician’s practice as well as the likelihood of referral to another institution for trial consideration. In the US, physician compensation is often tied to relative value unit (RVU) production, a measure of value used in the reimbursement formula for physician services. As detailed above, trial accrual and participation increase the time and complexity of patient care that may reduce physician efficiency, with a subsequent reduction in RVU production. Therefore, many physicians regard research participation as a “volunteer” activity. If a patient is referred to another center for trial participation, the result is a loss of revenue for the referring physician and, often more importantly, his or her institution. Allocation of RVUs for research-related activities (research RVUs76,77) may be a strategy to incentivize greater participation, although it requires a concerted effort between physicians, institutions, and payors to provide direct and indirect incentives for participation in research activities. Expanding authorship to more physicians who accrue to trials78 is another strategy, particularly if recognized by institutions for academic promotion.
Clinical operations play an important role to support physicians in enrolling patients in clinical trials. For example, adequate staff effort is critical to secure timely referrals and to ensure safe continuity of care in cases where a trial is not available on site. Potential solutions to encourage trial accrual related to logistical concerns are shown in Table 1.
Physician burnout
Physician burnout related to clinical research is a complex problem.79 As outlined above, physicians can experience significant challenges regarding accrual of patients to clinical trials. These challenges can be frustrating and can harm the motivation of providers to enroll, or even to discuss clinical trials as an option with their patients. Potential solutions can include supporting research navigators who can alleviate the workload of curating and reviewing clinical trials for patients and addressing the financial constraints that physicians face.75 In looking into the future, new technologies may be able to assist in decreasing the physician’s burden—for example, artificial intelligence may help screen patients for eligibility to clinical trials and may help identify clinical trial options that are available at other institutions. However, the affordability and the integration of such technologies will be key elements for their access and use. Electronic medical records, which are widely available today, make clinical information easier to access and may assist in clinical research, particularly for trials that are available at the same institution.
Clinical Trial Factors
The development and implementation of clinical trials may adversely affect trial accrual and enrollment. Clinical trial factors that serve as barriers include the lack of input from patients, caregivers, and advocates in clinical trial design; lack of engagement with local health care providers in clinical trial implementation; overly stringent participant eligibility, and challenges associated with incorporation of novel technologies into neuro-oncology practice. Here, we discuss general principles across different types of clinical trials. There are additional factors affecting accrual depending on the type of clinical trial (ie, early phase trials versus late phase trials, randomized versus single arm studies, sponsored versus consortium versus investigator-initiated studies) which are worth further evaluation.
Trial factors affecting patient participation and health care provider engagement
Incorporation of feedback from key stakeholders including patients, caregivers, and advocates early in the developmental process of a clinical trial can provide invaluable perspectives that may markedly enhance how user-friendly and attractive a trial is to patients. Often such input addresses practical and logistical issues not appreciated by investigators focused on the trial’s ability to address its key objectives. An important example, repeatedly raised by patients and caregivers, is that clinical trials should incorporate flexibility to allow study assessments that are considered SOC, particularly laboratory, imaging, and physical examination evaluations, to be performed locally whenever possible. Similarly, allowing standard therapies, such as radiation therapy or approved systemic therapies, to be administered locally according to established guidelines rather than at the trial center would ensure continuity of care, enhance quality of life, and reduce financial and logistical burdens of travel. Such considerations will also likely heighten enthusiasm among local clinicians who value remaining key contributors for their patients enrolled on a trial and lessen the financial disincentives of referring to another institution.
On the other hand, evaluations and treatments are typically required to be performed at the study center, even those that are SOC because of perceived and actual regulatory requirements specifying that data be incorporated into a study database, and hence subject to audit, and be obtained from a validated, quality controlled, and certified source. For example, in the US, sponsors/investigators for studies under an investigational new drug agreement are compelled to add laboratories, imaging centers, and local treatment providers to the FDA 1572 form with appropriate credential certifications filed in the participant’s study record. Rather than bother with this significant administrative effort and associated risks of potentially missed or inaccurate data, many sponsors/investigators simply opt to require patients to travel to the study center for all evaluations and treatments. Although such regulatory considerations are ultimately based on ensuring patient safety during trial participation, they can impose a significant burden on patients/caregivers by requiring an inordinate frequency of study center visits. Better clarification of study-specific regulatory requirements between investigators, sponsors, and auditors as well as a willingness to allow standard assessments and treatments to be performed locally by investigators for trials, despite the additional effort, will be important to lessen the impact of this issue on clinical trial accrual. In this way, visits to the study center are reserved for evaluations that are critically relevant to the investigational therapy.
Eligibility criteria
Excessively stringent eligibility criteria may also impede accrual. Although a growing number of trials appropriately restrict eligibility to a subset of patients with tumors exhibiting a specific mutation or biologic feature being targeted by an investigational agent, many other eligibility criteria are unnecessarily restrictive, excluding patients who could effectively contribute to addressing the study’s primary question. For example, many phase I trials of CNS tumors limit participation to patients with histopathologic grade IV GBMs, those at first or second recurrence, and those who have not progressed on prior bevacizumab, while in reality, patients with lower-grade, isocitrate dehydrogenase (IDH) wildtype gliomas or even uncommon primary malignant CNS tumors (for whom clinical trials rarely exist) or those with unlimited number of recurrences or who have progressed on prior bevacizumab could contribute to determine safety and a maximum tolerated dose (MTD)/recommended phase II dose (RP2D). For these early trials where safety is the primary outcome and treatment is not specifically targeted at a specific histology, specified laboratory criteria and performance status should be considered the main eligibility criteria for participation. An exploratory cohort of specific histology or treatment history could be incorporated once the MTD/RP2D is defined.
The definition of the most appropriate SOC for each and every patient is beyond the scope of this article, but concurrent use of tumor treating fields in combination with adjuvant temozolomide for newly diagnosed GBM is a common exclusion criterion in many current trials. This may unnecessarily exclude some patients from trial participation. The decision to exclude concomitant use of tumor treating fields in trials for newly diagnosed GBM should depend on whether there is a scientific reason to do so (ie, potential interference with the therapy being evaluated, with the evaluation of side effects from therapy, or with the endpoints of the study). For randomized clinical trials, if tumor treating field use is to be allowed for patients on study, proper stratification should be utilized to control confounding effects.
Some specific eligibility criteria can also be inappropriate for some phase II and III trials. For example, trials with a primary efficacy endpoint of progression-free survival (PFS) or overall survival (OS) should not exclude participants who have undergone a gross total resection prior to study enrollment, including trials on recurrent glioma. Although patients without measurable disease cannot be included in the determination of the objective response rate,59 they can contribute to evaluation of a PFS or OS primary endpoint.
US regulatory bodies recognize the importance of loosening overly restrictive eligibility criteria when appropriate.80 Indeed, ASCO, Friends of Cancer Research (FOCR), and the FDA examined specific eligibility criteria for population groups historically excluded from trials, including patients with brain metastases, and recommended ways to broaden eligibility criteria for these populations in cancer clinical trials. The RANO Brain Metastases Working Group have made similar recommendations.81 Subsequently, the National Cancer Institute (NCI) pledged to utilize these broadened eligibility criteria in clinical trials going forward. A remaining barrier is the continued exclusion of glioma patients from most phase I trials in cancer based on outdated concerns.82,83 An ongoing effort is needed to ensure that eventually glioma patients will routinely be included in phase I trials if there is biologic rationale and the drug has reasonable access across the blood–brain barrier.
Novel technology development
Comparative Studies.
In neuro-oncology as well as other cancer indications, trials assessing novel technology interventions pose further challenges for accrual associated with clinical equipoise. Do we have the clinical equipoise of “first do no harm” prior to embarking on randomized comparisons of technology? The example of proton versus photon radiation therapy underscores such a challenge. For this technical improvement comparison, the treating physician and the patient are both aware that one technology can reduce unwanted and unnecessary radiation dose to normal tissues. Patients and treating physicians may disregard such trials due to concern of randomizing to the “less desirable” arm. This lack of equipoise in technology testing serves as a major impediment to accrual and is underscored by several languishing proton versus photon therapy or intensity-modulated radiation therapy (IMRT) versus standard radiotherapy phase III trials (eg, NCT021790860). In this context, third party coverage decisions, resulting in inordinate delays in initiating treatment, also substantially impede trial accrual.84
Endpoint Selection.
Conventional trials incorporate traditional endpoints such as PFS, OS, and response rate. In contrast, technology trials may be best suited to provide results focused on “unconventional endpoints.” For example, the fluorescent dyes used to enhance surgical resection of gliomas categorically demonstrate the ability to produce a more complete tumor resection, which translates to short-term, but not long-term, survival benefit, and therefore FDA approval of such agents lagged Europe’s approval by more than a decade. Endpoints for technology trials need to be tailored to the expected outcome to be tested. For example, craniospinal irradiation with protons might permit a greater ability to provide dose-intense chemotherapy for medulloblastoma patients, or volume-sparing radiation techniques might cause less lymphopenia, or preoperative stereotactic radiosurgery (SRS) might reduce the local failure rate and pattern observed with postoperative SRS of brain metastases. Consideration of unconventional endpoints may be appropriate beyond technology trials, such as the use of seizure control, change in neurocognition, or preservation of neurologic function as endpoints for lower-grade glioma trials.85 However, these endpoints are “non-traditional,” and therefore often not considered germane, leading to incorporation of traditional yet less appropriate endpoints that may impact enthusiasm for accrual.
Site and Organizational Factors
Clinical research is distinct from routine clinical care. Centers with clinical research capabilities have specialized personnel, training, infrastructure, resources, dedicated research nurses, specialized institutional facilities for specimen collection and analysis, infusion centers, and imaging. Clinical trials for brain tumor patients have become increasingly complicated in recent years, having evolved into more subgroup-focused studies, and therefore enroll smaller numbers of patients per trial. Genomic profiling, patient screening, and added procedures require greater infrastructure support. At many larger centers, additional sources of research support are used, such as philanthropy and funding from not-for-profit foundations; but these are mostly available to research-focused clinical centers. The critical need for funding is highlighted by the fact that centers in the US with NCI- or industry-sponsored clinical trials have greater trial enrollment,86,87 whereas unfunded sites have found a lack of specialized resources to be a barrier to trial enrollment.88
It follows, therefore, that health care organizations are critical to the successful conduct of neuro-oncology clinical trials. Even if physicians are engaged in research and eligible patients are available, accrual to clinical trials is dependent upon the proper stage being set by the organization. If health care organizations focus on setting the stage with infrastructure, clinical practice models, and appropriate staff necessary for clinical trial conduct, then trial accrual to neuro-oncology studies will improve.
At present, many clinical centers do not view providing access of their patients to clinical trials as a goal of high or even modest priority. Internationally, this is also true for academic centers, since their reimbursement systems usually do not differ from those of non-academic centers and since patients enrolled onto clinical trials incur more administrative effort, but often less income. Thus, organizations are often faced with difficult decisions when funding different aspects of patient care. At a different level, publicity generated by the participation in clinical trials is usually limited and clinical centers tend to be sensitive to activities that raise national or local publicity. The major administrative and financial burdens may differ somewhat between countries and individual sites but commonly include the institutional review boards (IRBs) or ethics committees, challenges related to contracting between trial sponsors and the institutions, maintaining compliance with research billing, and financially supporting research support staff.
Cancer care accreditation as an incentive for trial enrollment
Accreditation is a useful tool to recognize sites for clinical excellence. In the US, the Commission on Cancer (CoC) recognizes cancer care programs for their commitment to provide comprehensive, high-quality, and multidisciplinary patient-centered care. Research participation is an integral component of the CoC standards. To meet accreditation standards, cancer centers must therefore place an emphasis on research participation, which supports the financial investment cancer centers put toward research activities. The development of accreditation for neuro-oncology centers through the Society for Neuro-Oncology (SNO) would spur organizational and site investment in research infrastructure, similar to what is seen for the general oncology practice through CoC accreditation.
IRB issues
Centralizing operations may also relieve some of the administrative and financial burdens for individual sites. Moving toward a central IRB (eg, on a country or trial level) could be an important step that remains yet to be realized in most countries; moreover, issues remain on an international level, notably with European involvement, since processes of harmonization among different countries in Europe remain slow. However, even when a central IRB is available, not all institutions will recognize that approval in lieu of their own internal processes. In the US, a central IRB is now required for all multi-site, NIH-funded studies, which has relieved a significant burden from individual sites and has led to more rapid trial conduct.
Contracting issues
Contracting challenges more often come from the institution than from external trial sponsors. Both industry and cooperative study groups prefer to institute a single contract per site, which facilitates trial conduct and money flow, but this requires contract review, cooperation, and communication at the various levels within each institution. Supporting disciplines like pathology, radiology, radiation oncology, and pharmacy often request separate contracts, which drives administrative burden and cost. Contracting issues can be especially problematic for investigator-initiated multicenter trials and greater use of master contracts would reduce the timelines involved.
Another and often underrecognized challenge relates to the determination of which study-related events or procedures are considered to be “standard of care” versus “experimental.” It is generally recognized that SOC procedures should be covered by traditional means (eg, insurance or national health care plan) and that experimental procedures, including additional imaging, blood tests, etc, should be covered by the sponsor. Often there is some disagreement as to which medical tests, measures, and tasks should be considered SOC and which should be considered trial related. While some of these costs may be covered, at least in part, by the sponsors, there are certain costs (for example, the primary investigator’s professional time) that are not recognized. The level of necessary insurance coverage for clinical trial conduct may add complexity in some countries. In the US, these coverage determinations come in the form of a Medicare Coverage Analysis (MCA). It has been recognized that for each individual site to complete a separate MCA is unnecessary. The development of central MCAs which are provided for NCI cooperative group trials is ongoing.
Another significant expense relates to the processing of adverse event (AE) reports. These reports are as important to the evaluation of a new treatment as are the primary outcome measures, and the amount of time spent on recognizing and reporting AEs is typically not covered in clinical trial contracts. These reports require the input of the investigators, research nurses, protocol managers, and other administrative personnel; and they often require multiple revisions in collaboration with the study sponsor. The most significant cost of these activities is time, particularly that of the primary investigator. The primary investigator is also expected to attend steering committee meetings (in person or by telephone) and other internal research regulatory meetings, which also deplete the finite resource of time and are usually not reimbursed. Especially if an investigational agent is investigated in several parallel large trials, the number of serious AE reports may prevent adequate reading.
Clinical practice models
Organizational support of a clinical practice model that fosters research participation is also essential to improve neuro-oncology clinical trial accrual. Practice models may vary between academic and community centers. In academic institutions, there are centralized models (eg, central core units including phase I sections where cancer trials across tumor entities are conducted) as opposed to decentralized models where small dedicated teams take care of brain tumor patients, both outside and within clinical trials. The former units are commonly more sustainable, better capable of more complex trial supportive measures, and often preferred by institutions, whereas the latter are more likely to meet specific requirements addressing the specific needs of brain tumor patients, notably their impairment including aphasia, paresis, or seizures. Practice models in community centers vary widely and relate to various factors, including whether neuro-oncology patients are seen in a neurology clinic versus a general medical oncology practice, or whether they are seen in a rural or urban setting. In general, research subjects in community practices are not seen in a separate unit. Thus, any guidance toward developing criteria for a clinical practice supporting research activities must be broad to include multiple practice models. The value of a dedicated assessment tool to benchmark clinical trial infrastructure is currently being explored.89
For several reasons, enrolling patients into clinical trials becomes even more difficult if the respective disease is diagnosed and treated in a multidisciplinary fashion. Tailoring diagnosis and treatment strategies to individual patients with brain tumors is commonly believed to be done best in a multidisciplinary tumor board, which a priori requires additional resources from all departments involved. Tumor boards represent the ideal setting for research staff to gather feedback about upcoming trials and to educate all departments about open studies. Such boards also appear to be the best platform to ensure that eligible patients for each open trial are readily identified. Still, since disease experts cannot be expected to universally agree with all trials of the institution’s portfolio, it is important that once a decision to join a trial is made, the whole team of disciplines supports this trial, notwithstanding potential reservations of individual team members. This is particularly relevant for clinical trials which may be perceived to challenge one’s own discipline (eg, trials which examine withholding surgery or radiotherapy). Also, requirements for time-consuming molecular testing can imply that eligibility for a trial becomes confirmed shortly before treatment is to start, which requires additional efforts from departments that bring their services to the trial without being involved and without formal recognition. To overcome this threat requires strong leadership of the multidisciplinary team and a broad institutional mindset that considers a strong clinical trial portfolio an asset to an academic medical unit. Tumor boards, with involvement of outside experts or use of virtual tumor boards, are particularly useful for community practices as their input may represent the sole point in time when the full multidisciplinary team is together.
Physicians and research staff
Specific challenges associated with physician engagement in clinical research were discussed previously in this article. Physician engagement with research varies within each clinical trial site, and thus accrual suffers if particular physicians are not engaged.67 This is particularly true in community settings as opposed to academic settings. Non-research-focused clinical centers, including community centers, may have fewer incentives to enroll patients in clinical trials. Physicians in larger academic centers are evaluated and incentivized with promotion, tenure, and academic credit for developing—and enrolling patients in—clinical trials. In the US, the same incentives are typically absent at smaller community hospitals and smaller academic centers, where success of the provider and center might be based on revenue-generating procedures and overall patient volume (eg, RVU-based incentives). Usually, due to pressures to increase patient volume activity, protected time is limited for community physicians to open clinical trials and spend the time necessary to explain the value of clinical research to patients. A greater collaboration between research-based centers and community partners in local or statewide networks as well as enhancing incentives for patient enrollment in clinical trials (discussed previously in the “Physician and Provider Factors” section) could greatly improve trial accrual and patient satisfaction.
In the US, up to 80% of cancer patients are treated in community settings, highlighting the importance of greater partnership between academic centers and community physicians. Educational efforts focused on community physicians to emphasize the importance of clinical research would be helpful. Although benefits from state-specific awareness campaigns have been short lived,90,91 it is imperative that the brain tumor community identifies optimal strategies to increase clinical trial awareness. Closer academic–community collaboration is essential. Realigning incentives within the community is important to facilitate patient referrals to centers of excellence for enrollment. Medicare, Medicaid, and third-party insurance reimbursements could be linked to patient trial enrollment. Additionally, opening more clinical trials in community centers may lead to greater patient participation.
Nonetheless, each health care organization maintains the ultimate responsibility to hire physicians engaged in research activities and to maintain engagement by developing the infrastructure and clinical practice model necessary to facilitate physician involvement in research. If sites place an emphasis on research, they will seek to hire new physicians who share similar goals. To maintain engagement, sites may develop strategies to recognize and financially reward physicians for research activities, such as a research RVU system.
In addition to physicians, research staff and support staff at an institution, including nurse practitioners, nurses, study coordinators, social workers, and translators, play a critical role in trial accrual. These personnel are crucial in establishing patient trust in otherwise complex and impersonal medical systems and provide a more compassionate and human touch to necessary medical encounters and decision making. At many research institutions, especially community sites, the research nurse is the primary point of contact for patients to learn details about clinical trial participation. This interaction plays a key role in whether a patient elects to participate in a particular trial. Development of education materials and accreditation specific for neuro-oncology will improve each site’s ability to hire and maintain support staff necessary for the conduct of trials and to enhance accrual.
Plans for the Future
More research is needed to better define the factors specific to neuro-oncology affecting accrual. To better understand this issue, clinical trial investigators should systemically document the reasons why patients do not enroll on trials. However, an analysis alone of the complex factors contributing to poor trial accrual to neuro-oncology trials is insufficient. The next step is to determine a roadmap to overcome these barriers (see Table 1 for summary). Borrowing from the field of behavioral economics, we must find “nudges” 92 that can effect significant, long-lasting change and promote clinical trial participation. Provider and patient educational campaigns as well as engagement with advocacy groups may help change the mindset of physicians, patients, and caregivers to one where enrollment in clinical trials is the best option for most patients. This is now reflected in the NCCN guidelines for central nervous system tumors, where clinical trials are now the preferred option for most tumors.60 We must also harness modern technology to spread information about clinical trials through social media campaigns, smartphone apps, or internet-based clinical trial matching services that simplify clinical trial searches for patients and caregivers, increasing the number of patient navigators to guide patients through the process, as well as creation of national brain tumor registries where patients can contribute tumor tissues for sequencing which may help pair them with clinical trial options. Until we can expand clinical trial opportunities to more community-based settings (perhaps through a more efficient national clinical trial network, allowance of SOC components of the trials to be performed locally, and use of telemedicine for virtual visits), any patient with a GBM or uncommon brain cancer should be offered a referral to a major neuro-oncology center(s) in their relative proximity for consideration of clinical trials.
We must also critically evaluate clinical trial design that fosters accrual and removes excessive hindrances for participants. Continuing the momentum of work by ASCO/FOCR in collaboration with NCI, we will plan to work with the neuro-oncology community and clinical trial consortia to modernize eligibility criteria for brain tumor trials.
However, these efforts to increase clinical trial accrual are likely to have limited impact if there are insufficient good quality trials. It will be critical that parallel efforts be undertaken in neuro-oncology to address this issue as well. How this can be accomplished is beyond the scope of this paper, although the neuro-oncology community must work together to increase the number of high quality clinical trials, especially in the NCI National Clinical Trials Network in the US and the EORTC in Europe, and to develop more trials utilizing efficient clinical trial designs (ie, adaptively randomized trials such as AGILE93 and INSIGhT94).
Funding
This work was supported by Adult Brain Tumor Consortium (ABTC) NIH/NCI UM1 CA137443.
Conflict of interest statement. Brian Alexander: Personal fees from Abbvie, Schlesinger Associates, Precision Health Economics, BMS. Research support from Celgene, Eli Lilly, Puma. Employment by Foundation Medicine, Inc.
John de Groot: Grant or Research Support from Sanofi-Aventis, Astrazeneca, EMD-Serono; Eli Lilly, Novartis, Deciphera Pharmaceuticals, Mundipharma. Paid Consultant: Celldex; Deciphera Pharmaceuticals, AbbVie, FivePrime Therapeutics, Inc., GW Pharma, Carthera, Eli Lilly, Kadmon, Boston Biomedical Inc.,Taiho Pharmaceuticals, Kairos Venture Investments, Syneos Health, Monteris, Agios, Mundipharma Research, GenomiCare, Blue Earth Diagnostics. Advisory Boards: Genentech, Celldex, Foundation Medicine, Inc., Novogen, Deciphera, Astrazeneca, Insys Therapeutics, Merck, Eli Lilly, Novella Clinical, Kiyatec, Blue Earth Diagnostics. Other Relevant Financial or Material Interests: DSMB: VBL Therapeutics; DSMB: Novella; VBI Vaccines, Inc. Stock Ownership: Ziopharm Oncology, Gilead. Company Employment (Spouse): Ziopharm Oncology.
Maryam Fouladi: Grant or research funding from Pfizer, PTC Therapeutics, Merck, and BMS
Eudocia Q. Lee: Consulting for Eli Lilly. Contributor to Up to Date, Inc. and Medlink.
Jose Pablo Leone: Research support (paid to institution) from Merck
Nancy Lin: Research funding: from Pfizer, Genentech, Novartis, Seattle Genetics. Consulting for Genentech, Seattle Genetics, Puma, Daichii.
Minesh Mehta: Consulting relationships with honoraria with Varian, Agenus, Insys, Remedy, IBA, Astra-Zeneca, Celgene, Tocagen, Abbvie; Board of Directors position with stock options with Oncoceutics; and DSMB for Monteris.
Kathy Oliver: Mission support funding and grants (paid to the International Brain Tumour Alliance/IBTA from 2005 to date) from AbbVie, Accuray, Antisense Pharma, Apogenix, Archimedes, Ark Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Brain Tumor Network (USA), Brain Tumor Resource and Information Network (USA), Bristol-Myers Squibb Celldex Therapeutics, Crusade, Dijon Designs (UK), Elekta, Eli Lilly, Gerry & Nancy Pencer Brain Trust (Canada), Gosling Foundation (UK), GlaxoSmithKline (GSK), Ivy Foundation (USA), Lilly, Link Pharmaceuticals, MagForce, Medac, Merck Serono, Merck, MGI Pharma, MSD Oncology, NeoPharm, Neuroendoscopy (Australia), Northwest Biotherapeutics, Novartis, Novocure, Pediatric Brain Tumor Foundation (USA), Pfizer, Photonamic, Roche, Schering-Plough (Global), Sontag Foundation (USA), Spink (UK), to-BBB, Vane Percy (UK), VBL Therapeutics and the Wallerstein Foundation (USA). Honoraria for advisory board work and conference speaking from AbbVie, Bayer, Bristol-Myers Squibb, GSK, Lilly, Novartis, Sarcoma Patients EuroNet
David Reardon: Research support (paid to institution): Acerta Phamaceuticals; Agenus; Celldex; EMD Serono; Incyte; Inovio; Midatech; Omniox; Tragara. Advisory/consultation (paid to self): Abbvie; Advantagene; Agenus; Bristol-Myers Squibb; Celldex; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc. Honoraria (paid to self): Abbvie; Advantagene; Agenus; Bristol-Myers Squibb; Celldex; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc.
Solmaz Sahebjam: Research support (paid to institution) from BMS and Merck.
Martin van den Bent: Consultanting for Cellgene, BMS, Agios, Boehringer, Abbvie.
Michael Vogelbaum: Indirect equity and patient royalty interests from Infuseon Therapeutics. Honoraria from Celgene, Tocagen, and Blue Earth Diagnostics.
Michael Weller: Research grants from Abbvie, Bayer, Merck, Sharp & Dohme (MSD), Dracen, Merck (EMD), Novocure, OGD2, Piqur, Roche and Adastra. Honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, Bristol Meyer Squibb, Celgene, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Orbus, Roche, Teva and Tocagen.
Patrick Wen: Research support from Agios, Astra Zeneca, Beigene, Eli Lily, Genentech/Roche, Karyopharm, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Sanofi-Aventis, VBI Vaccines. Participated on advisory boards for Abbvie, Agios, Astra Zeneca, Blue Earth Diagnostics, Eli Lilly, Genentech/Roche, Karyopharm, Kiyatec, Puma, Vascular Biogenics, Taiho, Deciphera, VBI Vaccines, Tocagen. Speaker for Merck, Prime Oncology.
No other authors report conflicts of interest.
Acknowledgments
We would like to thank patients (who wish to remain anonymous) as well as Joohee Sul from the US Food and Drug Administration (FDA) for their invaluable input on this manuscript.
References
- 1. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. St Germain D, Denicoff AM, Dimond EP, et al. Use of the National Cancer Institute Community Cancer Centers Program screening and accrual log to address cancer clinical trial accrual. J Oncol Pract. 2014;10(2):e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mannel RS, Walker JL, Gould N, et al. Impact of individual physicians on enrollment of patients into clinical trials. Am J Clin Oncol. 2003;26(2):171–173. [DOI] [PubMed] [Google Scholar]
- 4. Albrecht TL, Eggly SS, Gleason ME, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26(16):2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wujcik D, Wolff SN. Recruitment of African Americans to National Oncology Clinical Trials through a clinical trial shared resource. J Health Care Poor Underserved. 2010;21(1 Suppl):38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang SM, Barker FG 2nd, Schmidt MH, et al. Clinical trial participation among patients enrolled in the Glioma Outcomes Project. Cancer. 2002;94(10):2681–2687. [DOI] [PubMed] [Google Scholar]
- 7. Bates AJ, Couillard SA, Arons DF, et al. HOUT-15. Brain tumor patient and caregiver survey on clinical trials: identifying attitudes and barriers to patient participation. Neuro Oncol. 2017;19(Suppl 6):vi109. [Google Scholar]
- 8. Vanderbeek AM, Rahman R, Fell G, et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol. 2018;20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers J, Acquaye A, Vera E, Bates A, Wen PY, Armstrong T. Provider reported challenges and barriers to referring patients to Neuro-Oncology clinical trials: a report from the society for Neuro-Oncology member survey. Accepted for publication by Neuro-Oncology Practice. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 11. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nass SJ, Balogh E, Mendelsohn J. A National Cancer Clinical Trials Network: recommendations from the Institute of Medicine. Am J Ther. 2011;18(5):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paskett ED, Reeves KW, McLaughlin JM, et al. Recruitment of minority and underserved populations in the United States: the Centers for Population Health and Health Disparities experience. Contemp Clin Trials. 2008;29(6):847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc. 1998;90(3):141–145. [PMC free article] [PubMed] [Google Scholar]
- 16. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Comis RL, Miller JD, Aldigé CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21(5):830–835. [DOI] [PubMed] [Google Scholar]
- 18. Dillard JP, Meyer BJ, Solomon DH, Manni A. Factors associated with participation in a prevention trial aimed at reducing biomarkers of breast cancer risk. Patient Educ Couns. 2015;98(5):640–644. [DOI] [PubMed] [Google Scholar]
- 19. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. [DOI] [PubMed] [Google Scholar]
- 20. Marson DC, Martin RC, Triebel KL, Nabors LB. Capacity to consent to research participation in adults with malignant glioma. J Clin Oncol. 2010;28(24):3844–3850. [DOI] [PubMed] [Google Scholar]
- 21. Harrison RA, Anderson MD, Cachia D, et al. Clinical trial participation of patients with glioblastoma at The University of Texas MD Anderson Cancer Center. Eur J Cancer. 2019;112:83–93. [DOI] [PubMed] [Google Scholar]
- 22. Ellis PM, Butow PN, Tattersall MH, Dunn SM, Houssami N. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. J Clin Oncol. 2001;19(15):3554–3561. [DOI] [PubMed] [Google Scholar]
- 23. Ramers-Verhoeven CW, Perrone F, Oliver K. Exploratory research into cancer patients’ attitudes to clinical trials. Ecancermedicalscience. 2014;8:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Godskesen T, Hansson MG, Nygren P, Nordin K, Kihlbom U. Hope for a cure and altruism are the main motives behind participation in phase 3 clinical cancer trials. Eur J Cancer Care (Engl). 2015;24(1):133–141. [DOI] [PubMed] [Google Scholar]
- 25.Tufts Center for the Study of Drug Development. Poor Physician and Nurse Engagement Driving Low Patient Recruitment. Impact Report. 2017;19(1). http://csdd.tufts.edu/files/uploads/Summary-JanFebIR1207.pdf . Accessed January 1, 2019. [Google Scholar]
- 26. Haddad AQ, Singla N, Gupta N, et al. Association of distance to treatment facility on quality and survival outcomes after radical cystectomy for bladder cancer. Urology. 2015;85(4):876–882. [DOI] [PubMed] [Google Scholar]
- 27. Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol. 2015;137(3):497–502. [DOI] [PubMed] [Google Scholar]
- 28. Wu TC, Sarraj A, Jacobs A, et al. Telemedicine-guided remote enrollment of patients into an acute stroke trial. Ann Clin Transl Neurol. 2015;2(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304(2):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. FDA. FDA’s MyStudies Application (App). 2018. https://www.fda.gov/Drugs/ScienceResearch/ucm624785.htm. Accessed April 8, 2019. [Google Scholar]
- 31. FDA. Payment and Reimbursement to Research Subjects - Information Sheet. 2018. https://www.fda.gov/RegulatoryInformation/Guidances/ucm126429.htm. Accessed January 21, 2019. [Google Scholar]
- 32. Hale C. FDA Clarifies Stance on Clinical Trial Reimbursements for Patient Travel, Lodging Center Watch Weekly. 2018. https://www.centerwatch.com/cwweekly/2018/02/05/fda-clarifies-stance-clinical-trial-reimbursements-patient-travel-lodging/. Accessed January 21, 2019. [Google Scholar]
- 33. European Organisation for Research and Treatment of Cancer. SPECTA | EORTC: EORTC. 2016. http://www.eortc.org/specta. Accessed April 10, 2019. [Google Scholar]
- 34. Dizon DS, Graham D, Thompson MA, et al. Practical guidance: the use of social media in oncology practice. J Oncol Pract. 2012;8(5):e114–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sedrak MS, Attai DJ, George K, Katz MS, Markham MJ. Integrating social media in modern oncology practice and research. Am Soc Clin Oncol Educ Book. 2018;38:894–902. [DOI] [PubMed] [Google Scholar]
- 36. Pew Internet & Techology Center. Social Media Use in 2018. Washington, DC: Pew Research Center; 2018. https://www.pewinternet.org/2018/03/01/social-media-use-in-2018/. Accessed January 15, 2019. [Google Scholar]
- 37. Gorman JR, Roberts SC, Dominick SA, Malcarne VL, Dietz AC, Su HI. A diversified recruitment approach incorporating social media leads to research participation among young adult-aged female cancer survivors. J Adolesc Young Adult Oncol. 2014;3(2):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Cancer Institute. At the Crossroads of Social Media and Clinical Trials Executive Summary. 2018. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/patient-engagement/social-media-clinical-trials-workshop-executive-summary.pdf. Accessed June 1, 2018. [Google Scholar]
- 39. National Cancer Institute. At the Crossroads of Social Media and Clinical Trials: A Workshop Summary. 2018. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/patient-engagement/social-media-clinical-trials-workshop-workshop-summary.pdf. Accessed June 1, 2018. [Google Scholar]
- 40. Comerci GD, Macdonald DI. Prevention of substance abuse in children and adolescents. Adolesc Med. 1990;1(1):127–144. [PubMed] [Google Scholar]
- 41. Institute of Medicine (US) committee on understanding and eliminating racial and ethnic disparities in health care. In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 42. Dean LT, Gehlert S, Neuhouser ML, et al. Social factors matter in cancer risk and survivorship. Cancer Causes Control. 2018;29(7):611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nahleh Z, Otoukesh S, Mirshahidi HR, et al. Disparities in breast cancer: a multi-institutional comparative analysis focusing on American Hispanics. Cancer Med. 2018;7(6):2710–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pratte MA, Griffin A, Ogazi C, et al. Racial/ethnic disparities in cervical cancer screening services among contractors of the connecticut breast and cervical cancer early detection program. Health Equity. 2018;2(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DeRouen MC, Schupp CW, Yang J, et al. Impact of individual and neighborhood factors on socioeconomic disparities in localized and advanced prostate cancer risk. Cancer Causes Control. 2018;29(10):951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campbell JD, Lathan C, Sholl L, et al. Comparison of prevalence and types of mutations in lung cancers among black and white populations. JAMA Oncol. 2017;3(6):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langford AT, Resnicow K, Dimond EP, et al. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer. 2014;120(6):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colon-Otero G, Smallridge RC, Solberg LA Jr, et al. Disparities in participation in cancer clinical trials in the United States: a symptom of a healthcare system in crisis. Cancer. 2008;112(3):447–454. [DOI] [PubMed] [Google Scholar]
- 49. Ibraheem A, Polite B. Improving the accrual of racial and ethnic minority patients in clinical trials: time to raise the stakes. Cancer. 2017;123(24):4752–4756. [DOI] [PubMed] [Google Scholar]
- 50. Ford JG, Howerton MW, Bolen S, et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evid Rep Technol Assess (Summ). 2005;( 122):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. [DOI] [PubMed] [Google Scholar]
- 52. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 53. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shin JY, Yoon JK, Diaz AZ. Racial disparities in anaplastic oligodendroglioma: an analysis on 1643 patients. J Clin Neurosci. 2017;37:34–39. [DOI] [PubMed] [Google Scholar]
- 55. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith MM, Rose SH, Schroeder DR, Long TR. Diversity of United States medical students by region compared to US census data. Adv Med Educ Pract. 2015;6:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lathan CS. Lung cancer care: the impact of facilities and area measures. Transl Lung Cancer Res. 2015;4(4):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652–658. https://www.ncbi.nlm.nih.gov/pubmed/29509930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 60. National Comprehensive Cancer Network. Central Nervous System Cancers. Version 1. 2019. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf Accessed April 22, 2019. [DOI] [PubMed] [Google Scholar]
- 61. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Penner LA, Albrecht TL, Coleman DK, Norton WE. Interpersonal perspectives on black–white health disparities: social policy implications. Soc Issues Policy Rev. 2007;1(1):63–98. [Google Scholar]
- 63. Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67(3):478–486. [DOI] [PubMed] [Google Scholar]
- 64. Jernigan VB, Hearod JB, Tran K, Norris KC, Buchwald D. An examination of cultural competence training in US medical education guided by the tool for assessing cultural competence training. J Health Dispar Res Pract. 2016;9(3):150–167. [PMC free article] [PubMed] [Google Scholar]
- 65. Winkfield KM, Flowers CR, Patel JD, et al. American Society of Clinical Oncology strategic plan for increasing racial and ethnic diversity in the oncology workforce. J Clin Oncol. 2017;35(22):2576–2579. [DOI] [PubMed] [Google Scholar]
- 66. Cohen JJ. The consequences of premature abandonment of affirmative action in medical school admissions. JAMA. 2003;289(9):1143–1149. [DOI] [PubMed] [Google Scholar]
- 67. Jacobs SR, Weiner BJ, Reeve BB, Weinberger M, Minasian LM, Good MJ. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin Trials. 2014;11(5):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Langley GR, Sutherland HJ, Wong S, Minkin S, Llewellyn-Thomas HA, Till JE. Why are (or are not) patients given the option to enter clinical trials? Control Clin Trials. 1987;8(1):49–59. [DOI] [PubMed] [Google Scholar]
- 69. Fallowfield L, Ratcliffe D, Souhami R. Clinicians’ attitudes to clinical trials of cancer therapy. Eur J Cancer. 1997;33(13):2221–2229. [DOI] [PubMed] [Google Scholar]
- 70. Ramirez AG, Chalela P, Suarez L, et al. Early phase clinical trials: referral barriers and promoters among physicians. J Community Med Health Educ. 2012;2(8):1000173. [PMC free article] [PubMed] [Google Scholar]
- 71. Finn R. Surveys identify barriers to participation in clinical trials. J Natl Cancer Inst. 2000;92(19):1556–1558. [DOI] [PubMed] [Google Scholar]
- 72. Comis RL, Miller JD, Colaizzi DD, Kimmel LG. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Oncol Pract. 2009;5(2):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112–3124. [DOI] [PubMed] [Google Scholar]
- 74. Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21(12):2268–2275. [DOI] [PubMed] [Google Scholar]
- 75. Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. [DOI] [PubMed] [Google Scholar]
- 76. Severance HW. The Research RVU (rRVU): in search of a methodology to incentivize and compensate clinicians for participation in clinical research activities. Acad Med. 2016;91(1):9–10. [DOI] [PubMed] [Google Scholar]
- 77. Embi PJ, Tsevat J. Commentary: the relative research unit: providing incentives for clinician participation in research activities. Acad Med. 2012;87(1):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amato AA, Baskin PK, Gross RA. Updating Neurology® authorship criteria: ensuring inclusion of those making valuable intellectual contributions. Neurology. 2018;90(19):865–866. [DOI] [PubMed] [Google Scholar]
- 79. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(2):195–205. [DOI] [PubMed] [Google Scholar]
- 80. Beaver JA, Ison G, Pazdur R. Reevaluating eligibility criteria—balancing patient protection and participation in oncology trials. N Engl J Med. 2017;376(16):1504–1505. [DOI] [PubMed] [Google Scholar]
- 81. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 82. Wen PY, Schiff D, Cloughesy TF, et al. It is time to include patients with brain tumors in phase I trials in oncology. J Clin Oncol. 2011;29(24):3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gounder MM, Nayak L, Sahebjam S, et al. Evaluation of the safety and benefit of phase I oncology trials for patients with primary CNS tumors. J Clin Oncol. 2015;33(28):3186–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cox JD. Impediments to comparative clinical trials with proton therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):4–8. [DOI] [PubMed] [Google Scholar]
- 85. van den Bent MJ, Wefel JS, Schiff D, et al. Response Assessment in Neuro-Oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 86. Porter M, Ramaswamy B, Beisler K, et al. A comprehensive program for the enhancement of accrual to clinical trials. Ann Surg Oncol. 2016;23(7):2146–2152. [DOI] [PubMed] [Google Scholar]
- 87. Copur MS, Ramaekers R, Gonen M, et al. Impact of the National Cancer Institute community cancer centers program on clinical trial and related activities at a community cancer center in rural nebraska. J Oncol Pract. 2016;12(1):67–68, e44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw. 2007;5(8):655–664. [DOI] [PubMed] [Google Scholar]
- 89. Dimond EP, Zon RT, Weiner BJ, et al. Clinical trial assessment of infrastructure matrix tool to improve the quality of research conduct in the community. J Oncol Pract. 2016;12(1):63–64, e23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moffitt K, Brogan F, Brown C, et al. Statewide cancer clinical trial navigation service. J Oncol Pract. 2010;6(3):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Umutyan A, Chiechi C, Beckett LA, et al. Overcoming barriers to cancer clinical trial accrual: impact of a mass media campaign. Cancer. 2008;112(1):212–219. [DOI] [PubMed] [Google Scholar]
- 92. Sustein CR. Nudging: A Very Short Guide. 37 J. Consumer Pol’y 583. 2014. http://nrs.harvard.edu/urn-3:HUL.InstRepos:16205305. Accessed December 12, 2018. [Google Scholar]
- 93. Alexander BM, Ba S, Berger MS, et al. ; GBM AGILE Network Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–743. [DOI] [PubMed] [Google Scholar]
- 94. Alexander BM, Trippa L, Gaffey SC, et al. Individualized screening trial of innovative glioblastoma therapy (INSIGhT). J Clin Oncol. 2017;35(15_suppl):TPS2079. [DOI] [PMC free article] [PubMed] [Google Scholar]