Abstract
Background
Nonfunctioning pituitary adenoma (NFPA) and growth hormone pituitary adenoma (GHPA) are major subtypes of pituitary adenomas (PAs). The primary treatment is surgical resection. However, radical excision remains challenging, and few effective medical therapies are available. It is urgent to find novel targets for the treatment. Bromodomain-containing protein 4 (BRD4) is an epigenetic regulator that leads to aberrant transcriptional activation of oncogenes. Herein, we investigated the pathological role of BRD4 and evaluated the effectiveness of BRD4 inhibitors in the treatment of NFPA and GHPA.
Methods
The expression of BRD4 was detected in NFPA, GHPA, and normal pituitary tissues. The efficacies of BRD4 inhibitors were evaluated in GH3 and MMQ cell lines, patient-derived tumor cells, and in vivo mouse xenograft models of PA. Standard western blots, real-time PCR, and flow cytometry experiments were performed to investigate the effect of BRD4 inhibitors on cell cycle progression, apoptosis, and the expression patterns of downstream genes.
Results
Immunohistochemistry studies demonstrated the overexpression of BRD4 in NFPA and GHPA. In vitro and in vivo studies showed that treatment with the BRD4 inhibitor ZBC-260 significantly inhibited the proliferation of PA cells. Further mechanistic studies revealed that ZBC-260 could downregulate the expression of c-Myc, B-cell lymphoma 2 (Bcl2), and related genes, which are vital factors in pituitary tumorigenesis.
Conclusion
In this study, we determined the overexpression of BRD4 in NFPA and GHPA and assessed the effects of BRD4 inhibitors on PA cells in vitro and in vivo. Our findings suggest that BRD4 is a promising therapeutic target for NFPA and GHPA.
Keywords: BRD4, GHPA, NFPA, target, ZBC-260
Key Points.
BRD4 is overexpressed in NFPA and GHPA.
BRD4 inhibitors reduced PA cell proliferation in vitro and in vivo.
Importance of the Study.
This is the first study to explore the therapeutic potential of targeting BRD4 in PAs. Both genetic knockdown and pharmacological inhibition of BRD4 with the BRD4 inhibitor ZBC-260 significantly reduced the proliferation of NFPA and GHPA cells, which provides a strong rationale for the development of BRD4 inhibitors for translation in the future.
Pituitary adenoma (PA) is one of the most common intracranial neuroendocrine tumors. As previous epidemiology studies have suggested, the prevalence of PA ranges from 1 in 865 persons to 1 in 2688 persons and has increased in recent years.1 PA can result in significant morbidity and mortality caused by mass effects and excessive pituitary hormone secretion.2 Depending on their hormone-secreting capabilities, PAs are classified as clinically nonfunctioning PAs (NFPAs) or functioning PAs (FPAs). FPAs may secrete one or more of the following hormones: prolactin (PRL), growth hormone (GH), adrenocorticotropic hormone, thyroid-stimulating hormone, follicle-stimulating hormone, and luteinizing hormone. PRLPA is the most common subtype, accounting for 32‒51% of PA, and medical therapy with dopamine agonist is the preferred initial treatment for these patients.2 NFPA and GHPA are the two most common types in surgical series, comprising approximately 14‒54% and 8‒16% of all adenomas, respectively.1 Patients with NFPA can range from being asymptomatic to experiencing significant headaches, hypopituitarism, and visual field defects.1 Prolonged exposure to excess GH can lead to a wide range of systemic manifestations, including extremity and facial feature enlargement and cardiovascular, metabolic, endocrine, gastrointestinal, respiratory, and skeletal system complications, which are associated with increased mortality.3
Transsphenoidal resection has been adopted as the primary therapy for the treatment of NFPA and GHPA. However, radical excision can be difficult, since NFPA and GHPA are usually macroadenomas and sometimes feature suprasellar or parasellar extensions.4 Furthermore, NFPA and GHPA may also exhibit aggressive behavior characterized by high proliferation potential, which can lead to early recurrence after surgical resection.5,6 Therefore, to achieve long-term remission, an optimal treatment strategy combining surgery, medication, and radiation therapy is recommended. Unfortunately, for GHPA, the reported biochemical response rate of the currently available first-line medical therapies, somatostatin analogues (SSAs), ranges from only 17% to 37%.7 For NFPA, no effective medical therapy has yet been approved. Although the expression of dopamine receptors and somatostatin receptors has been confirmed in NFPA by ex vivo study, only a minority of NFPA patients show benefits from dopamine agonists and SSAs.8 The current situation highlights the urgent need to find additional effective drugs for the treatment of NFPA and GHPA.
As emerging evidence in recent years has indicated that epigenetic alterations contribute to PA formation, we tend to investigate the epigenetic modulators as new antitumor drugs for the treatment of PA.9,10 Bromodomain-containing protein 4 (BRD4) belongs to the bromodomain and extraterminal domain (BET) family.11 Functionally, it acts as a chromatin reader that specifically recognizes the acetylated lysine in histone tails and facilitates the localization of chromatin regulatory cofactors, thereby regulating transcription initiation and elongation.12,13 BRD4 also serves as a mitotic bookmark and plays a key role in cell cycle progression.14 Ectopic expression of BRD4 in cultured cells results in G1/S arrest, while inhibition of BRD4 function by injection of anti-BRD4 antibodies arrests cells in G2/M phase.15 Emerging evidence has confirmed that dysfunction of BRD4 could lead to aberrant transcriptional activation of oncogenes, notably c-Myc and B-cell lymphoma 2 (Bcl2), in hematological carcinoma and a wide range of solid tumors (melanoma, neuroblastoma, breast cancer, glioblastoma, etc).16–19 BRD4 inhibitors, which are now in active clinical development, can effectively downregulate the expression of c-Myc and Bcl2, induce cell cycle arrest, and suppress tumor growth and metastasis both in vitro and in vivo.20–22 In addition, combinations of BET inhibitors and other targeted small molecules have shown significant cytotoxic effects in human cancers and become a promising strategy to overcome drug resistance.23–25 According to the literature, abnormal expression of c-Myc and Bcl2 has long been indicated in PA, which suggests that c-Myc and Bcl2 interactions are vital factors in pituitary tumorigenesis and progression.26–29 However, a role for BRD4 in PA remains elusive and needs further investigation.
In this study, we detected overexpression of BRD4 in NFPA and GHPA and assessed the effect of a BRD4 inhibitor on PA cells in vitro and in vivo. Our findings suggest that BRD4 is a promising therapeutic target for NFPA and GHPA.
Materials and Methods
Cell Culture
The GH3 cell line (cat. no. CCL-82.1) and MMQ cell line (cat. no. CRL-10609) were purchased from the American Type Culture Collection and cultured in Ham’s F-12K medium (Shanghai BasalMedia Technologies) supplemented with 2.5% fetal bovine serum, 15% horse serum, and 1% penicillin/streptomycin (all Gibco). Before experiments, all cell cultures were maintained in an incubator at 37°C supplemented with 5% CO2.
Microarray Data
The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE51618 as detailed in the Supplementary Material.
Immunohistochemistry
Paraffin sections of human normal pituitary, NFPA, GFPA, and xenograft tumors were immunostained with anti-BRD4 antibody and anti-hormones antibodies as detailed in the Supplementary Material. The immunoreactivity was measured and quantified with Image-Pro Plus 6.0.
Cell Viability Assay
Cells were seeded in 96-well plates at a density of 5000 cells per well and then incubated with dimethyl sulfoxide (DMSO; control) or a series of 2-fold-diluted concentrations of JQ-1 (cat. no. HY-13030, MedChemExpress), OTX-015 (cat. no. HY-15743, MedChemExpress), and ZBC-260 for 4 days. Three replicates were made for each concentration of the drugs. After incubation, Cell Titer-Glo luminescent assays (cat. no. G7572, Promega) were performed to measure cell viability following the manufacturer’s protocol.
RNA Interference Study
The small interfering (si)RNAs for BRD4 and negative control siRNA (siCtrl) were synthesized by Shanghai Genomeditech, and the transfection was performed following the instructions of Lipofectamine RNAiMAX Transfection Reagent (cat. no. 13778075, Invitrogen). These experiments were performed in triplicate and are detailed in the Supplementary Material.
Western Blot Analysis
Western blot was performed as previously described.30 The blots were blocked in 5% nonfat milk and incubated with primary antibody overnight at 4°C, followed by incubation with horseradish peroxidase–conjugated second antibody at room temperature for 1 hour and the bands were detected in a ChemiScope 3400 imaging system using electrochemiluminescence substrate (cat. no. 32106, Thermo Scientific). All experiments, as outlined in the Supplementary Material, were repeated 3 times.
Real-Time Polymerase Chain Reaction
Total RNA from cells was extracted using TRIzol reagent (cat. no. R401-01, Vazyme Biotech). Reverse transcription was carried out to obtain cDNA using HiScript II qRT SuperMix (cat. no. R222-01, Vazyme Biotech). Real-time PCR was performed using AceQ qPCR SYBR Green Master Mix (cat. no. Q141-02, Vazyme Biotech), and amplification was detected with a Quant Studio 6 Flex Real-Time PCR System (ABI). Every sample was tested in triplicate. The primer sequences are listed in Supplementary Table 1.
Clonogenic Assay
Clonogenic assay was performed as previously described.31 For the isRNA and inhibitor-treated GH3 cells, the colonies were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet as detailed in the Supplementary Material. Three replicates were made in clonogenic assay. The colonies were counted with Image-Pro Plus 6.0.
Flow Cytometry Assay
GH3 cells were plated in 6-well plates at a concentration of 1 ×106 cells/well and treated with different concentrations of ZBC-260 for 48 hours. For cell cycle analysis, samples were stained with propidium iodide (cat. no. A211-01, Vazyme Biotech) for 30 minutes at room temperature. For apoptosis assay, cells were incubated with a fluorescein isothiocyanate–labeled annexin V antibody and propidium iodide (cat. no. A211-01, Vazyme Biotech) for 10 min. The cell cycle phase distribution and apoptosis were detected with FACSCalibur (BD Pharmingen) as previously reported.30 Data were presented as the mean ± SD of triplicate samples.
Tissue Samples and Primary Cell Culture
Two normal rat pituitary samples were derived from 3-month-old Fischer-344 male rats. For immunohistochemistry (IHC) study, a total of 62 samples were collected, comprising 5 with normal pituitary tissues, 41 with NFPAs, and 16 with GHPAs (Supplementary Table 2). Normal pituitary samples were obtained from cadaveric organ donations with no evidence of any endocrine disease (Fudan University). For primary cell culture, 7 tumor samples (Supplementary Table 2) were processed into single-cell suspensions immediately after surgical detachment and were cultured with 10% fetal bovine serum containing DMEM as previously reported.32 PA tissues were surgically removed at the Neurosurgery Department, Huashan Hospital, Fudan University, between 2018 and 2019. Histological diagnoses were performed at the Pathology Department, Huashan Hospital, Fudan University, according to the 2017 World Health Organization classification. The clinical diagnosis of adenomas was based on the hormone levels measured in the serum of the patients and on histological diagnosis including hormone expression. NFPA was further classified pathologically into gonadotroph adenomas (GPAs), null cell adenomas (NCAs), and silent adenomas (SPAs). Silent adenomas were tumors for which patients present without endocrine hypersecretion syndrome or laboratory repercussion but had histological and IHC features consistent with a well-differentiated lineage-specific adenoma. The study was approved by the ethics committee at Huashan Hospital, and the samples were obtained after acquiring written informed consent from all patients.
Xenograft Experiment
BALB/c nude mice were used for generation of the GH3 xenograft model.33 Six- to 8-week-old male mice were subcutaneously injected with GH3 cells (2.5 × 106) mixed with Matrigel Matrix (cat. no. 354262, Corning) in one flank. Tumor size was measured every 3 days, and tumor volume was calculated as width2 × length × 0.5. ZBC-260 was intragastrically administered at a dose of 2.5 mg/kg once daily when the tumor volumes approached approximately 100 mm3. All mice were sacrificed upon completion of the 26-day experiment, and the tumors were excised, weighed, and frozen at −80°C for protein studies. A portion of each tumor was embedded in paraffin for IHC studies. All animal experiments were approved by the Institutional Animal Care and Use Committee, Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 software. The data in this study are shown as the mean ± SD. Mann–Whitney tests and two-tailed Student’s t-tests were used for comparisons between 2 groups. P-values <0.05 were considered to indicate significance.
Results
BRD4 Is Overexpressed in NFPA and GHPA
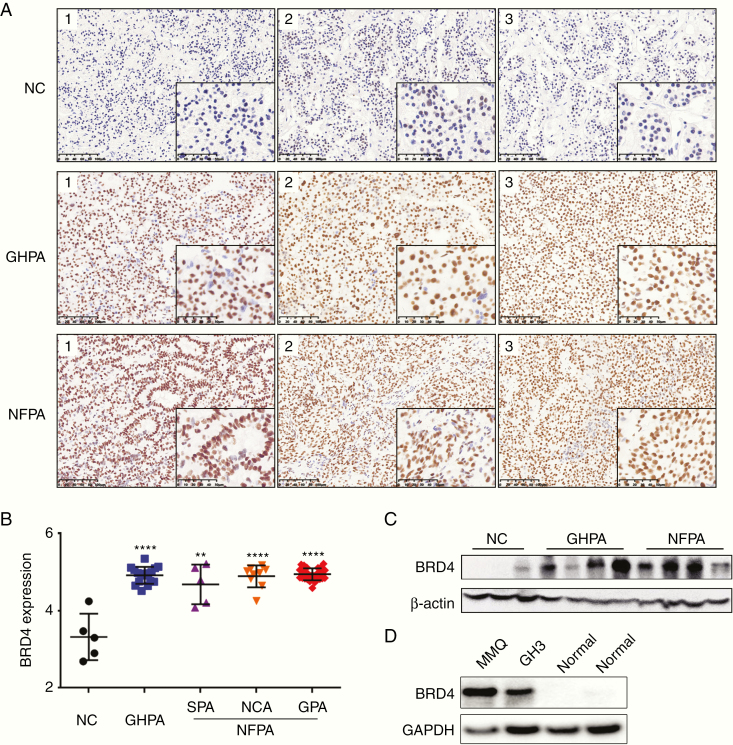
Analysis of publicly available gene expression data from the Gene Expression Omnibus repository datasets (GSE51618) revealed a strong overexpression of BRD4 in NFPA compared with normal pituitary tissue (Supplementary Figure 1). For identifying the expression profile of BRD4 in NFPA and GHPA, archived paraffin-embedded specimens including 5 normal adenohypophysis, 41 NFPA, and 16 GHPA samples were selected for IHC (Fig. 1A). There were 27 GPAs, 9 NCAs, and 5 SPAs contained in the NFPA group. The immunoreactivity of BRD4 protein in each sample was identified and quantified by using integrated optical density (IOD). Comparison of the IOD values between normal and tumor tissues revealed significant increases in BRD4 expression in NFPA (P < 0.0001) and GHPA (P < 0.0001) tissues, but there was no significant difference in BRD4 expression between the NFPA subtypes (Fig. 1B). Furthermore, in the sequential IHC sections of normal pituitary tissue, it was shown that all neuroendocrine hormone-positive cell clusters in normal pituitary tissue were negative for BRD4 (Supplementary Figure 2). To verify the IHC findings, we compared the BRD4 protein levels in normal adenohypophysis tissue with those in NFPA and GHPA tissues by western blot analysis. We also performed western blot analysis on normal rat pituitary, rat GH3, and rat MMQ cell lines. The results indicated that BRD4 was significantly overexpressed in PA tissues and GH3 and MMQ cell lines, which was consistent with the IHC results (Fig. 1C, D). The upregulation of BRD4 in NFPA and GHPA suggests a potential role for BRD4 in promoting PA transformation and growth.
Fig. 1.
BRD4 is overexpressed in NFPA and GHPA. (A) IHC image of BRD4 expression in normal pituitary (NC), NFPA, and GHPA. Each column represents a different sample. Scale bars: 100 μm left, 50 μm right. (B) Logarithmized IOD value of BRD4 expression in IHC images of NC, GHPA, and NFPA (silent adenomas, SPAs; null cell adenomas, NCAs; gonadotroph adenomas, GPAs). (C) Immunoblot analysis of BRD4 expression level in NC, NFPA, and GHPA tissues. (D) Immunoblot analysis of BRD4 expression level in normal rat pituitary, rat GH3, and rat MMQ pituitary adenoma cell lines. **P < 0.01; ****P < 0.0001. Bar represents mean ± SD.
BRD4 Knockdown Displayed Anti-Proliferative Effects in GH3 Cells
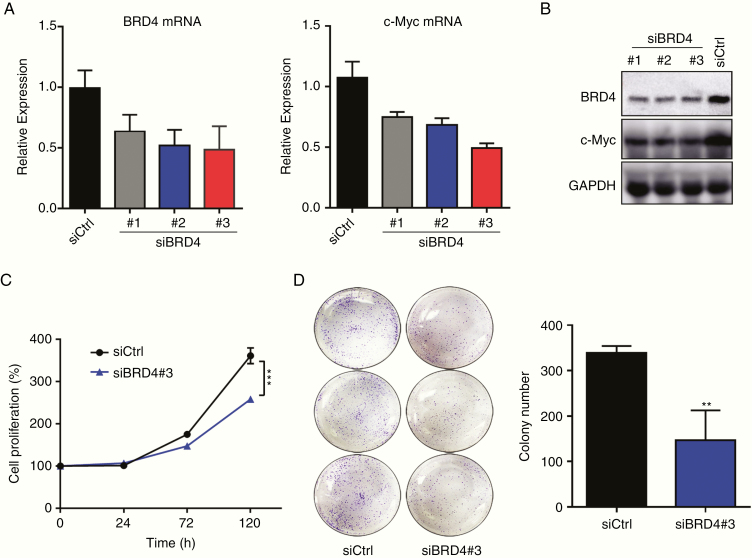
To determine the function of BRD4 in PA, 3 BRD4-specific siRNAs were constructed and delivered to GH3 cells. The efficiency of siRNA-mediated knockdown was confirmed by real-time PCR and western blot analysis at 24 hours post transfection (Fig. 2A, B). SiRNA#3 was chosen for further studies because it had the greatest BRD4 knockdown. Notably, genetic knockdown of BRD4 significantly reduced GH3 cell proliferation (P < 0.001) and impaired GH3 cell colony formation (P < 0.01) (Fig. 2C, D). Taken together, our data suggest that BRD4 is indispensable for the maintenance of GH3 cell proliferation and may be a therapeutic target for PA.
Fig. 2.
BRD4 knockdown inhibits GH3 cells proliferation in vitro. (A, B) Knockdown efficiency of the BRD4-specific siRNAs and their impact on BRD4 relevant oncogene c-Myc expression in GH3 cells, tested by real-time PCR and western blotting. (C) Normalized proliferation curve of siCtrl and siBRD4#3-treated GH3 cells. (D) Macroscopic images and quantified colonies formed by GH3 cells. All the experiments were repeated 3 times with consistent results. **P < 0.01; ***P < 0.001. Bar represents mean ± SD.
BRD4 Inhibitors Attenuated the Viability of GH3 and MMQ Cell Lines and Primary PA Cells In Vitro
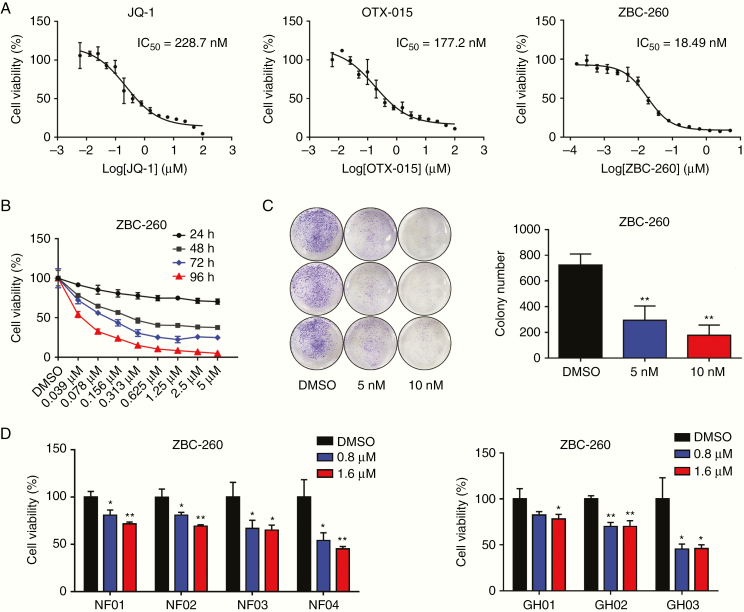
To further test the sensitivity of PA to treatment with BRD4 inhibitors, we tested the anti-proliferative effects of 3 well-studied BRD4 inhibitors (JQ-1, OTX-015, and ZBC-260) in the GH3 cell line. After 4 days of treatment, the BRD4 inhibitors exhibited promising anti-proliferative effects in GH3 cells, among which ZBC-260 was the most potent compound, with a half-maximal inhibitory concentration value of 18 nM (Fig. 3A). Subsequently, we exposed GH3 cells to ZBC-260 at concentrations varying from 5 μM to 39 nM for 24, 48, 72, and 96 hours. The cytostatic effect of ZBC-260 was time and dose dependent (Fig. 3B). Moreover, to further evaluate the long-term inhibitory effect of ZBC-260 on GH3 cells, a clonogenic assay was performed. As demonstrated in Fig. 3C, prolonged exposure to ZBC-260 at concentrations of 5 nM (P < 0.01) and 10 nM (P = 0.001) for 3 weeks also significantly inhibited the colonization of GH3 cells. The anti-proliferative effects of JQ1, OTX015, and ZBC-260 were also potent in the MMQ cell line (Supplementary Figure 3A). The positive cell line–based results impelled us to further investigate the impact of the compound on primary pituitary adenoma cells. Patient-derived PA cells were then incubated with ZBC-260 at concentrations of 0, 0.8, and 1.6 µM for 4 days. The results showed that ZBC-260 also significantly suppressed the viability of primary NFPA and GHPA cells (Fig. 3D). In summary, all of these data suggest that BRD4 inhibitors may be plausible agents for the treatment of PA.
Fig. 3.
BRD4 inhibitors attenuated the viability of GH3 cells and primary PA cells in vitro. (A) Cell viability assays with the treatment of indicated concentrations of JQ-1, OTX-015, and ZBC-260 for 4 days. Each condition was performed in triplicate. (B) Cell viability assay upon the treatment of ZBC-260 for 24, 48, 72, or 96 hours. (C) Macroscopic images and quantified colonies formed by GH3 cells after the incubation with ZBC-260 or DMSO control for 3 weeks. (D) Cell viability of primary NFPA and GHPA cells treated with ZBC-260 or DMSO for 4 days. Three independent experiments were repeated for each result. *P < 0.05; **P < 0.01. Bar represents mean ± SD.
The BRD4 Inhibitor Arrested the Cell Cycle at G2/M Phase and Induced Apoptosis in GH3 Cells
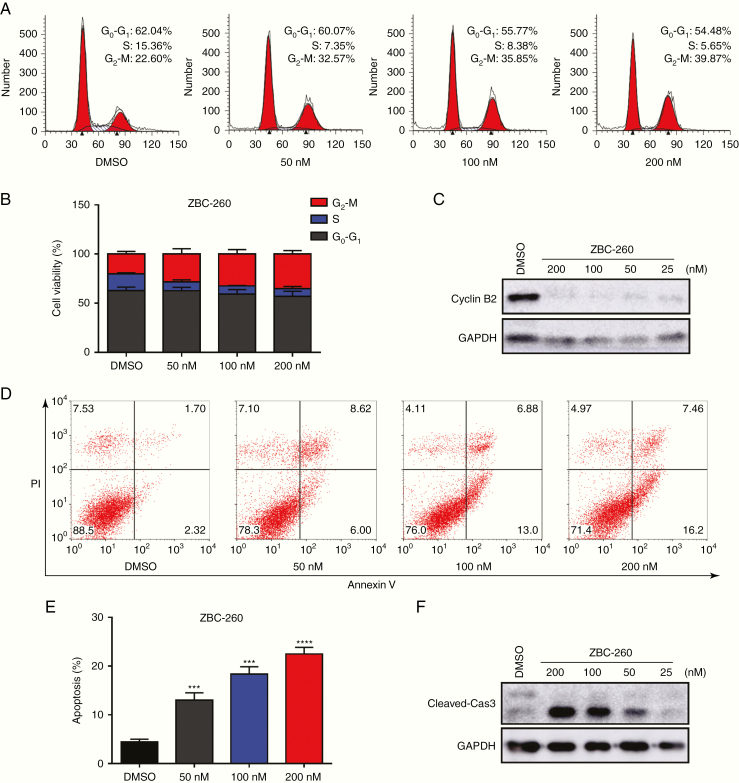
To preliminarily investigate the mechanism of the anti-proliferative effect of the BRD4 inhibitor in GH3 cells, cell cycle progression and apoptosis were analyzed after treating GH3 cells with ZBC-260 at concentrations of 200, 100, 50, and 0 nM for 48 hours. According to the flow cytometry results, BRD4 inhibition altered the cell cycle distribution, increasing the proportion of cells in G2/M phase (Fig. 4A, B). This finding was then reinforced by the decreased expression of the G2/M-specific protein cyclin B2 (Fig. 4C). Additionally, annexin V staining assays revealed that treatment with ZBC-260 dose-dependently increased the proportion of apoptotic cells followed by the activation of cleaved caspase-3, which was detected by western blotting (Fig. 4D–F). Taken together, these results reveal the molecular mechanism that accounts for the anti-proliferative effect of BRD4 inhibition.
Fig. 4.
The BRD4 inhibitor arrested the cell cycle at G2/M phase and induced apoptosis in GH3 cells. (A, B) Representative plots and statistical graph of the cell cycle phases of GH3 cells treated with ZBC-260 (50 nM, 100 nM, and 200 nM) or DMSO for 48 hours. (C) Immunoblot analysis of G2/M-phase specific protein cyclin B2. (D, E) Induction of apoptosis in GH3 cells treated with ZBC-260. (F) Immunoblot analysis of the apoptosis relative protein cleaved caspase-3. Each condition was tested in triplicate. ***P < 0.001; ****P < 0.0001. Bar represents mean ± SD.
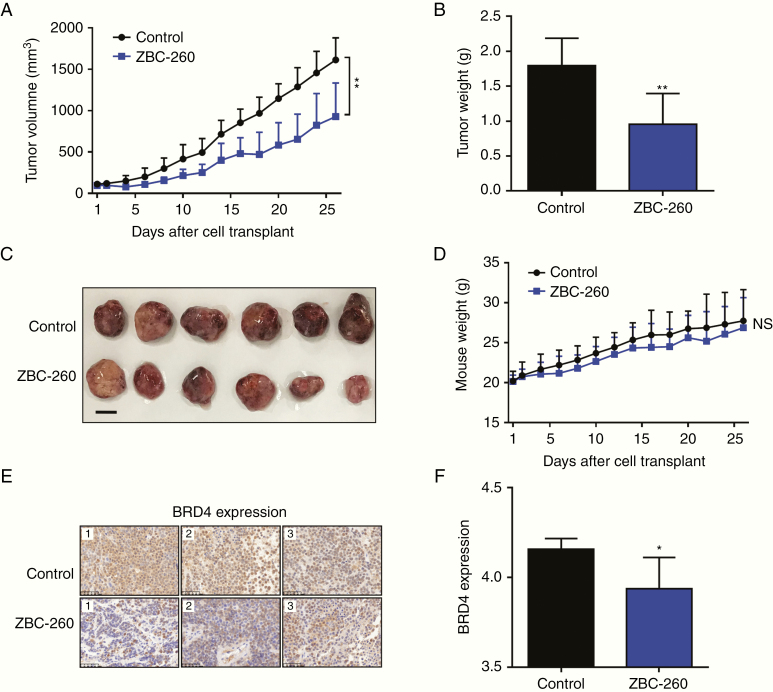
The BRD4 Inhibitor Impeded Pituitary Tumor Growth In Vivo
To evaluate the impact of the BRD4 inhibitor on GH3 cells in vivo, we generated a xenograft PA model by transplanting GH3 cells subcutaneously into the flanks of BALB/c nude mice. Once the tumor volume approached approximately 100 mm3, treatment was started with intragastric administration of 2.5 mg/kg ZBC-260 or vehicle control once daily for 26 days. All mice were sacrificed after completion of the 26-day experiment. Treatment with ZBC-260 significantly inhibited tumor growth with regard to tumor volume (43% inhibition, P < 0.01) and tumor weight (47% inhibition, P < 0.01) in comparison with the control regimen (Fig. 5A–C). Additionally, treatment with ZBC-260 showed minimal effects on mouse body weight, demonstrating its safety (Fig. 5D). Since ZBC-260 was designed as a highly efficacious BRD4 degrader, we further analyzed the expression profile of BRD4 in xenograft tumor tissues.34 As expected, significant decreases in BRD4 were observed in the ZBC-260–treated mice (P < 0.05) (Fig. 5E, F).
Fig. 5.
The BRD4 inhibitor suppressed tumor growth in subcutaneous PA models. (A) Tumor volume of mice treated with ZBC-260 (2.5 mg/kg) or vehicle control once daily, measured for 26 days. (B) Histogram of the tumor weight. (C) Macroscopic image of the resected tumor. Scale bar: 1 cm. (D) Mice weight measured for 26 days. (E) Representative IHC images of BRD4 expression in resected tumor tissues. Scale bars: 50 μm. (F) Logarithmized IOD value of BRD4 expression in IHC images. Each column represents a different sample. *P < 0.05; **P < 0.01. Bar represents mean ± SD.
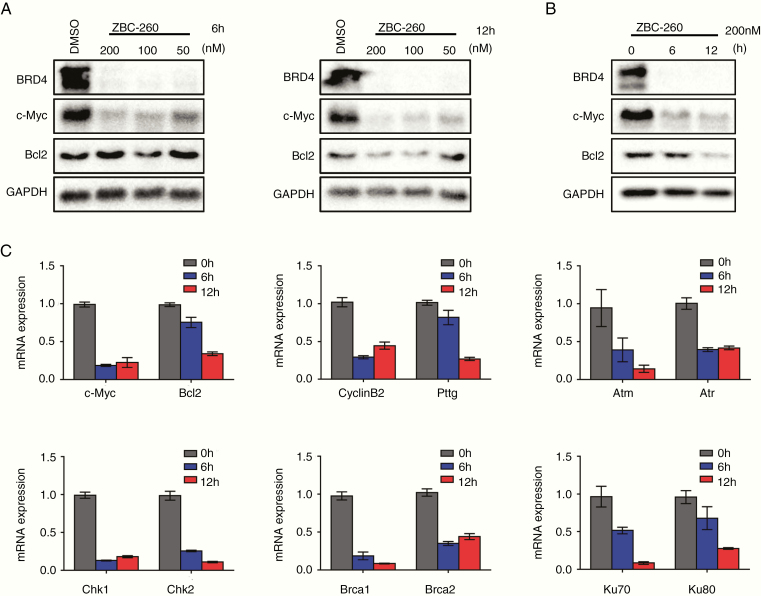
BRD4 Inhibitor Decreased the Expression of c-Myc and Bcl2 in GH3 and MMQ Cell Lines
Since BRD4 inhibition has been proven to be efficient in downregulating c-Myc and Bcl2 in many tumors, the influence of the BRD4 inhibitor on the expression of these 2 target genes in GH3 cells was then investigated to gain further mechanistic insight. Treatment with ZBC-260 for 6 hours and 12 hours notably decreased the expression of BRD4, Bcl2, and c-Myc (Fig. 6A, B). The effects of the BRD4 inhibitor ZBC-260 on the expression of BRD4 and c-Myc in MMQ cells were also tested (Supplementary Figure 3B). This finding was further confirmed by real-time PCR at the mRNA level (Fig. 6CSupplementary Figure 3C). Since previous studies have revealed that c-Myc dysregulation in tumor cells can promote proliferation by positively regulating cell cycle–relevant gene expression and can induce replication stress and the DNA damage/repair response,35–37 and since BRD4 has been proven to be essential in DNA non-homologous end joining (NHEJ) and is responsible for the c-Myc–driven pathway,20,38 we further investigated the expression of genes related to DNA damage (Atm, Atr, Chk1, and Chk2) and repair (Brca1, Brca2, Ku70, and Ku80). As expected, the transcription of all these genes was decreased upon BRD4 inhibition (Fig. 6C). In addition, BRD4 inhibition caused a reduction in the pituitary tumor-transforming gene (Pttg), which is an oncogene overexpressed in pituitary adenoma that plays a key role in the maintenance of genomic stability after DNA damage (Fig. 6C).39–41 Collectively, these results demonstrate that ZBC-260 could induce BRD4 degradation and inhibit the expression of downstream c-Myc‒related genes.
Fig. 6.
ZBC-260 altered the expression pattern of BRD4 downstream genes in GH3 cells. (A) Immunoblot analysis of BRD4, c-Myc, and Bcl2 after incubated with ZBC-260 (200 nM, 100 nM, and 50 nM) or DMSO for 6 hours and 12 hours. (B) The expression of BRD4, c-Myc, and Bcl2 after treating GH3 cells with 200 nM ZBC-260 for 0 hour, 6 hours, and 12 hours. (C) Real-time PCR analysis of the expression of c-Myc, Bcl2, cyclin B2, Pttg, and DNA damage/repair signal relevant genes upon treatment with ZBC-260 for 0 hour, 6 hours, and 12 hours in GH3 cells. The results were normalized with Gapdh. Each condition was tested in triplicate.
Discussion
Pituitary adenomas are the most common neuroendocrine adenomas and now occur with 3–4 times higher prevalence than previously reported.42 Although they are commonly regarded as benign tumors, 25–55% of PAs are infiltrative and exhibit aggressive behaviors.43 NFPA and GHFPA are the 2 major subtypes of PAs in surgical series and are usually large at diagnosis, presenting with invasion of surrounding vital structures.4,44 Surgical resection is the currently preferred first-line therapy for NFPA and GHPA. However, the cure rate with surgery alone for macroadenomas and for adenomas with supra- or parasellar infiltration is unfavorable.43 In addition, drug resistance remains a major hurdle for GHPA, and successful chemical intervention for NFPA is lacking.8 Thus, there is an urgent need to identify novel therapeutic targets for the treatment of NFPA and GHPA in this era of targeted drug therapy.
BRD4 is a chromatin reader that can specifically recognize the acetylated lysine residues in histone tails and play pivotal roles in cell proliferation, cell cycle progression, DNA damage repair, and downstream gene expression, including the expression of c-Myc and Bcl2.12–14,45 Emerging evidence has demonstrated the pathological function of BRD4 in the occurrence and progression of many malignancies, making it a promising target for drug intervention. Currently, many BRD4 inhibitors (OTX-015, ABBV-075, I-BET-762, etc) have entered clinical trials for the treatment of cancers, including glioblastoma, leukemia, and breast cancer, and these trials have demonstrated safety and efficacy.17,46 In PAs, c-Myc and Bcl2 are significantly upregulated, contributing to the development and progression of the tumor.26–29 However, the role of BRD4 in PAs remains elusive, and little is known about the therapeutic potential of BRD4 for the treatment of PAs.
Herein, based on IHC analysis of clinical samples, we have shown that BRD4 is significantly overexpressed in NFPA and GHPA tissues compared with normal tissues, and there is no selective expression of BRD4 in the normal gland. In the cell lines, both genetic knockdown using siRNA and pharmacological inhibition of BRD4 with the BRD4 inhibitor ZBC-260 significantly reduced the proliferation and survival of PA. Additionally, treatment with ZBC-260 led to cell cycle arrest at G2/M phase and induced apoptosis, which partially accounted for its anti-proliferative effect. In the primary pituitary adenoma cultures, as the cells grow slowly, ZBC-260 might not decrease the cell proliferation obviously. Instead, the cell viability assay was performed in patient-derived tumors, which could also demonstrate the efficiency of the BRD4 inhibitor. Further mechanistic studies showed that ZBC-260 decreased the expression of c-Myc and Bcl2. In addition, it led to the downregulation of c-Myc–related genes such as Atm, Atr, Chks, Brcas, Ku70, and Ku80, which play pivotal roles in DNA damage and repair. Notably, the expression of the oncogene Pttg, which activates angiogenesis and causes PA formation, was also decreased following BRD4 inhibition.47,48
Since the pituitary gland sites outside the blood–brain barrier, the inhibitor can freely access pituitary adenomas via blood.49 Thus, a murine xenograft flank model was applied to explore the efficacy of ZBC-260 on pituitary adenoma in vivo. ZBC-260 significantly inhibited GH tumorigenesis but did not show toxicity to mice when orally administered for 26 days. These data suggest that ZBC-260 is a potential candidate drug for PAs.
Besides NFPA and GHPA, the BRD4 inhibitors also presented great therapeutic potential on prolactin-secreting adenoma. Although the existing drugs such as bromocriptine and cabergoline have achieved good therapeutic effects on prolactin-secreting adenoma, the problem of drug resistance is emerging.50 Hence, it is urgent to discover new therapeutic methods. Based on our findings, the BRD4 inhibitors showed favorable effects on the proliferation of the MMQ cell line, which suggested a potential therapeutic approach to prolactin-secreting adenoma in the future.
In summary, our findings demonstrate the potential of BRD4 as a valuable target for PA treatment and provide deep insight into the pathological role of BRD4 in the development and progression of NFPA and GHPA. Our findings provide strong rationale for novel therapies targeting BRD4 in the future. BRD4 inhibitors could provide additional therapeutic options for optimal management of intractable pituitary adenomas.
Supplementary Material
Acknowledgments
We gratefully acknowledge Mrs Yun Zhang, Mrs Ye Wang, Mrs Qiuyue Wu and Mrs Lan Lai for sample collection. We thank Mrs Jingjing Zhu, Mr Chao Li, and Mrs. Liyan Yue for technical support. We thank Prof Ying Mao for technical consulting, who is supported by Natural Science Foundation and Major Basic Research Program of Shanghai (16JC1420100), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX03) and ZJ Lab.
Conflict of interest statement. No conflict of interest.
Funding
This study was supported by the Ministry of Science and Technology of China (National Key R&D Program of China, 2017YFB0202600), the “Personalized Medicines Molecular Signature-based Drug Discovery and Development”(Strategic Priority Research Program of the Chinese Academy of Sciences, XDA12020368) to Hong Ding; the China Pituitary Adenoma Specialist Council (CPASC), the National High Technology Research and Development Program of China (863 program, 2014AA020611), the Chang Jiang Scholars Program, the National Program for Support of Top-Notch Young Professionals, the National Science Fund for Distinguished Young Scholars (81725011), the Shanghai Rising-Star Tracking Program (12QH1400400) to Yao Zhao; the National Natural Science Foundation of China (81802496), the Shanghai Sailing Program (18YF1402700) to Zhao Ye; the National Natural Science Foundation of China (81802495), the Shanghai Sailing Program (18YF1403400) to Qilin Zhang; the National Science and Technology Major Project (2018ZX09711002-008-005), the Major Research Plan of the National Nature Science Foundation of China (grant no. 91753207), National Key R&D program of China (2018YFA0508200), K.C.Wong Education Foundation to Bing Zhou.
Authorship statement
Conception or design of the work: Hong Ding and Yao Zhao. Financial support: Hong Ding, Yao Zhao, Zhao Ye, Qilin Zhang and Bing Zhou. Subject recruitment: Yao Zhao, Yongfei Wang, Xiaoyun Cao, Xuefei Shou, Xiang Zhou, Zhao Ye, Zhaoyun Zhang, Yiming Li, Hongying Ye, Min He, Wenqiang He, Zhengyuan Chen, Jun Sun and Jianyong Cai. Pathological diagnosis: Hong Chen and Haixia Cheng. Inhibitor synthesis: Zizhou Li and Bing Zhou. Laboratory practice: Chengzhang Shi, Zhao Ye, Jie Han and Xiaoqing Ye. Xenograft experiment: Zengyi Ma, Qilin Zhang and Yichao Zhang. Data analysis and interpretation: Chengzhang Shi, Zhao Ye, Hong Ding, Yao Zhao. Drafting the article: Chengzhang Shi, Zhao Ye, Wenchao Lu and Chenxing Ji. Critical revision of the article: Chuanxin Huang, Fei Ye and Cheng Luo. All authors critically reviewed the article and approved the final manuscript.
References
- 1. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516–524. [DOI] [PubMed] [Google Scholar]
- 2. Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017;19(6):762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colao A, Grasso LFS, Giustina A, et al. . Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. [DOI] [PubMed] [Google Scholar]
- 4. Osamura RY, Kajiya H, Takei M, et al. . Pathology of the human pituitary adenomas. Histochem Cell Biol. 2008;130(3):495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raverot G, Vasiljevic A, Jouanneau E. Prognostic factors of regrowth in nonfunctioning pituitary tumors. Pituitary. 2018;21(2):176–182. [DOI] [PubMed] [Google Scholar]
- 6. Donoho DA, Bose N, Zada G, Carmichael JD. Management of aggressive growth hormone secreting pituitary adenomas. Pituitary. 2017;20(1):169–178. [DOI] [PubMed] [Google Scholar]
- 7. Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23(12):R551–R566. [DOI] [PubMed] [Google Scholar]
- 8. Tampourlou M, Karapanou O, Vassiliadi DA, Tsagarakis S. Medical therapy for non-functioning pituitary tumors-a critical approach. Hormones (Athens). 2019;18(2):117–126. [DOI] [PubMed] [Google Scholar]
- 9. Salomon MP, Wang X, Marzese DM, et al. . The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, Cushing’s disease and endocrine-inactive subtypes. Clin Cancer Res. 2018;24(17):4126–4136. [DOI] [PubMed] [Google Scholar]
- 10. Bi WL, Horowitz P, Greenwald NF, et al. . Landscape of genomic alterations in pituitary adenomas. Clin Cancer Res. 2017;23(7):1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filippakopoulos P, Picaud S, Mangos M, et al. . Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuang M, Zhou J, Wang L, Liu Z, Guo J, Wu R. Binding kinetics versus affinities in BRD4 inhibition. J Chem Inf Model. 2015;55(9):1926–1935. [DOI] [PubMed] [Google Scholar]
- 13. Yang Z, Yik JH, Chen R, et al. . Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19(4):535–545. [DOI] [PubMed] [Google Scholar]
- 14. Devaiah BN, Singer DS. Two faces of BRD4: mitotic bookmark and transcriptional lynchpin. Transcription. 2013;4(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282(18):13141–13145. [DOI] [PubMed] [Google Scholar]
- 16. Wadhwa E, Nicolaides T. Bromodomain inhibitor review: bromodomain and extra-terminal family protein inhibitors as a potential new therapy in central nervous system tumors. Cureus. 2016;8(5):e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng Z, Gong Y, Ma Y, et al. . Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res. 2013;19(7): 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuber J, Shi J, Wang E, et al. . RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segura MF, Fontanals-Cirera B, Gaziel-Sovran A, et al. . BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 2013;73(20):6264–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delmore JE, Issa GC, Lemieux ME, et al. . BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiskus W, Sharma S, Qi J, et al. . Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol Cancer Ther. 2014;13(5): 1142–1154. [DOI] [PubMed] [Google Scholar]
- 22. Filippakopoulos P, Qi J, Picaud S, et al. . Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramadoss M, Mahadevan V. Targeting the cancer epigenome: synergistic therapy with bromodomain inhibitors. Drug Discov Today. 2018;23(1):76–89. [DOI] [PubMed] [Google Scholar]
- 24. Adeegbe DO, Liu S, Hattersley MM, et al. . BET bromodomain inhibition cooperates with PD-1 blockade to facilitate antitumor response in Kras-mutant non-small cell lung cancer. Cancer Immunol Res. 2018;6(10):1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stratikopoulos EE, Dendy M, Szabolcs M, et al. . Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell. 2015;27(6):837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang DG, Johnston CF, Atkinson AB, Heaney AP, Mirakhur M, Buchanan KD. Expression of bcl-2 oncoprotein in pituitary tumours: comparison with c-myc. J Clin Pathol. 1996;49(10):795–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woloschak M, Roberts JL, Post K. c-Myc, c-fos, and c-myb gene expression in human pituitary adenomas. J Clin Endocrinol Metab. 1994;79(1):253–257. [DOI] [PubMed] [Google Scholar]
- 28. Turner HE, Nagy Z, Gatter KC, Esiri MM, Wass JA, Harris AL. Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumour behaviour. Br J Cancer. 2000;82(8):1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Formosa R, Gruppetta M, Falzon S, et al. . Expression and clinical significance of Wnt players and survivin in pituitary tumours. Endocr Pathol. 2012;23(2):123–131. [DOI] [PubMed] [Google Scholar]
- 30. Chen Z, Zhang H, Liu S, et al. . Discovery of novel trimethoxy-ring BRD4 bromodomain inhibitors: alphaScreen assay, crystallography and cell-based assay. Medchemcomm. 2017;8(6):1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. [DOI] [PubMed] [Google Scholar]
- 32. Ma ZY, Song ZJ, Chen JH, et al. . Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zubeldía-Brenner L, De Winne C, Perrone S, et al. . Inhibition of Notch signaling attenuates pituitary adenoma growth in nude mice. Endocr Relat Cancer. 2019;26(1):13–29. [DOI] [PubMed] [Google Scholar]
- 34. Zhou B, Hu J, Xu F, et al. . Discovery of a small-molecule degrader of bromodomain and extra-terminal (BET) proteins with picomolar cellular potencies and capable of achieving tumor regression. J Med Chem. 2018;61(2):462–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luoto KR, Meng AX, Wasylishen AR, et al. . Tumor cell kill by c-MYC depletion: role of MYC-regulated genes that control DNA double-strand break repair. Cancer Res. 2010;70(21):8748–8759. [DOI] [PubMed] [Google Scholar]
- 36. Wang WJ, Wu SP, Liu JB, et al. . MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73(3):1219–1231. [DOI] [PubMed] [Google Scholar]
- 37. Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–264. [DOI] [PubMed] [Google Scholar]
- 38. Li X, Baek G, Ramanand SG, et al. . BRD4 promotes DNA repair and mediates the formation of TMPRSS2-ERG gene rearrangements in prostate cancer. Cell Rep. 2018;22(3):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernal JA, Roche M, Méndez-Vidal C, Espina A, Tortolero M, Pintor-Toro JA. Proliferative potential after DNA damage and non-homologous end joining are affected by loss of securin. Cell Death Differ. 2008;15(1):202–212. [DOI] [PubMed] [Google Scholar]
- 40. Chesnokova V, Zonis S, Rubinek T, et al. . Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007;67(21):10564–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu R, Melmed S. Oncogene activation in pituitary tumors. Brain Pathol. 2001;11(3):328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. 2014;117(3):379–394. [DOI] [PubMed] [Google Scholar]
- 43. Di Ieva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K. Aggressive pituitary adenomas–diagnosis and emerging treatments. Nat Rev Endocrinol. 2014;10(7):423–435. [DOI] [PubMed] [Google Scholar]
- 44. Iglesias P, Rodríguez Berrocal V, Díez JJ. Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine. 2018;61(3):407–421. [DOI] [PubMed] [Google Scholar]
- 45. Donati B, Lorenzini E, Ciarrocchi A. BRD4 and cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrieu G, Belkina AC, Denis GV. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. 2016;19:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang X, Horwitz GA, Prezant TR, et al. . Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1999;13(1):156–166. [DOI] [PubMed] [Google Scholar]
- 48. Chengxian Y, Xinjie B, Renzhi W. Role of matrix metalloproteinases in pituitary adenoma invasion. Chinese Neurosurg J. 2018;4(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schankin CJ, Maniyar FH, Seo Y, et al. . Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain. 2016;139(Pt 7):1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Molitch ME. Management of medically refractory prolactinoma. J Neurooncol. 2014;117(3):421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.