Abstract
Glioblastomas are the most common form of malignant primary brain tumor and an important cause of morbidity and mortality. In recent years there have been important advances in understanding the molecular pathogenesis and biology of these tumors, but this has not translated into significantly improved outcomes for patients. In this consensus review from the Society for Neuro-Oncology (SNO) and the European Association of Neuro-Oncology (EANO), the current management of isocitrate dehydrogenase wildtype (IDHwt) glioblastomas will be discussed. In addition, novel therapies such as targeted molecular therapies, agents targeting DNA damage response and metabolism, immunotherapies, and viral therapies will be reviewed, as well as the current challenges and future directions for research.
Keywords: glioblastoma, diagnosis, therapy, clinical trials
Glioblastomas are the most common type of malignant primary brain tumor and account for the majority of deaths among patients with primary brain tumors.1 Although there has been progress in understanding the biology of these tumors, this has not translated into significant improvements in therapies or outcomes for patients. In this consensus review from SNO and EANO recent advances in the management of glioblastoma are discussed, as well as the current challenges and future directions for research. The focus will be on the 90–95% of glioblastomas that do not harbor IDH mutations (IDHwt) and have a worse prognosis.2,3 We concur with the current considerations to regroup IDH-mutant glioblastomas with other IDH-mutant gliomas in the framework of the revision of the World Health Organization (WHO) classification of brain tumors, and to restrict the term “glioblastoma” to tumors without IDH mutations.4
Epidemiology
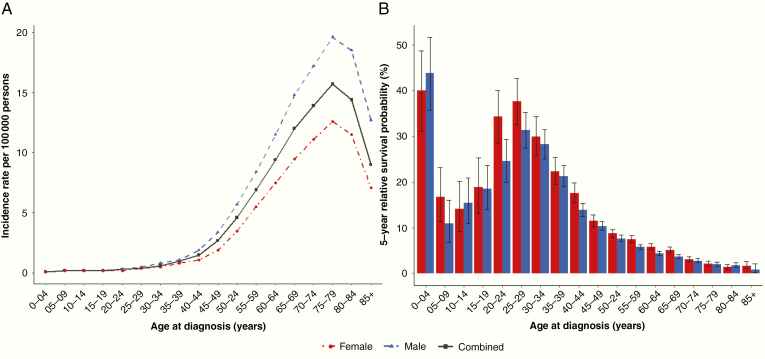
The overall age-adjusted incidence of glioblastoma in the United States is 3.22/100 000 persons, and increases with advanced age at diagnosis and male sex (Fig. 1A; Central Brain Tumor Registry of the United States, 2012–2016).1 Incidence also varies worldwide.5 Recent data show no trend toward increased incidence in the US or Canada,6 although data from England indicate that the incidence is increasing.7,8 These differences might reflect differing surveillance procedures, coding, and changes in classifications of glioblastoma over time.2 Glioblastomas contribute disproportionately to morbidity and mortality, with a 5-year overall relative survival of only 6.8%, which varies by age at diagnosis and by sex (Fig. 1B; National Program of Cancer Registries, 2012–2016).1 Known risk factors for glioblastoma account for only a small proportion of cases.9 In multiple independent studies, one risk factor, ionizing radiation exposure to the head and neck, and one protective factor, history of atopic diseases (including allergies, asthma, eczema, and hay fever), have been validated for all brain tumors (as reviewed by Ostrom et al9). While cell phone use (ie, non-ionizing radiation exposure) has been heavily studied as a potential risk factor for brain tumors, studies have shown no consistent evidence of any association.9,10 However, the latency period for disease after exposure to non-ionizing radiation is not known, hence continued careful monitoring of the incidence trend is advised.
Fig. 1.
Glioblastoma. (A) Incidence rate per 100 000 persons by age at diagnosis and sex, Central Brain Tumor Registry of the United States (CBTRUS) 2012–1016 (50 US states and Puerto Rico included) and (B) 5-year relative survival probability (with 95% confidence intervals) by age at diagnosis and sex, National Program of Cancer Registries (NPCR) 2012–2016 (43 US states included). **Glioblastoma defined by International Classification of Disease-Oncology (ICD-O) version 3 codes 9440/3, 9441/3, 9442/3.
The vast majority of glioblastoma patients do not have a family history of cancer. Approximately 5% of all gliomas are familial,11 and there are multiple rare Mendelian inherited syndromes that involve adult glioma and glioblastoma12 (Table 1 adapted from Ostrom et al9). The frequency of germline variants is higher than expected based on family history data with up to 13% of glioma patients harboring at least one deleterious or likely deleterious alteration in the germline.13 Genome-wide association studies of genetic risk factors have validated 25 single nucleotide polymorphisms associated with increased risk for glioma, where 11 are specific to glioblastoma.14 While the biological significance of these associations remains to be elucidated, this genome-wide approach identified loci containing critical glioma genes such as telomerase reverse transcriptase (TERT), RTEL1, epidermal growth factor receptor (EGFR), and cyclin-dependent kinase inhibitor 2B (CDKN2B).14 The majority of these loci are associated with molecularly defined glioma subtypes.15 Continued improvements in accurate measurement of potential risk factors and advances in technology allowing for discovery of additional germline and tumor molecular features will be critical to future understanding of causes and risk factors for glioblastoma.
Table 1.
Inherited syndromes associated with adult gliomas (and adult glioblastomas) (adapted from Ostrom et al9)
| Gene Symbol (Chromosome Location) | Disorder/Syndrome (OMIM ID) | Mode of Inheritance | Phenotypic Features | Associated Brain Tumors |
|---|---|---|---|---|
| APC, MMR (5q21) | Familial adenomatous polyposis (FAP, 175100), Turcots syndrome type 2 | Dominant | Development of multiple adenomatous colon polyps (>100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma |
| ATM (11q22.3) | Ataxia- telangiectasia (208900) | Autosomal recessive trait | Progressive cerebellar ataxia, susceptibility to infections, predisposition to lymphoma and lymphocytic leukemia. | Astrocytoma and medulloblastoma |
| CDKN2A (9p21.3) | Melanoma-neural system tumor syndrome (155755) | Dominant | Predisposition to malignant melanoma and malignant brain tumors | Glioma |
| IDH1/IDH2 (2q33.3/15q26.1) | Ollier disease | Acquired post-zygotic mosaicism, dominant with reduced penetrance | Development of intraosseous benign cartilaginous tumors, cancer predisposition | Glioma |
| MLH1, PMS2 | Turcots syndrome type 1 | Autosomal recessive trait | Development of multiple adenomatous colon polyps (<100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma, |
| MSH2,MLH1,MSH6,PMS2 | Lynch syndrome (120435), biallelic mismatch repair deficiency, constitutional MMR deficiency | Dominant | Predisposition to gastrointestinal, endometrial and other cancers | Glioblastoma, other gliomas |
| MSH2,MLH1,MSH6,PMS2 | Mismatch repair deficiency syndrome (276300) | Recessive | Pediatric cancer predisposition; café-au-lait spots; colon polyps | Glioma |
| NF1 (17q11.2) | Neurofibromatosis 1 (NF1) (162200) | Dominant | Neurofibromas, schwannomas, café-au-lait macules | Astrocytoma, schwannomas, optic nerve glioma |
| RB1 (13q14) | Retinoblastoma | Dominant | Development of multiple tumors of the eye, increased risk of some brain tumors | Retinoblastoma, pineoblastoma, malignant glioma |
| TP53 (17p13.1) | Li–Fraumeni syndrome (151623) | Dominant | Predisposition to numerous cancers, especially breast, brain, and soft-tissue sarcoma | Glioblastoma, other gliomas |
| TSC1,TSC2 (9q34.14,16p13.3) | Tuberous sclerosis (TSC) (191100, 613254) | Dominant | Development of multisystem nonmalignant tumors | Giant cell astrocytoma |
Abbreviations used: ATM, ataxia telangiectasia; APC, adenomatous polyposis coli; CDKN2A, cyclin-dependent kinase inhibitor 2A; MLH1, MutL homolog 1, colon cancer, nonpolyposis type 2; MSH2, MutS protein homolog 2; MSH6, MutS protein homolog 6; OMIM, Online Mendelian Inheritance in Man; PMS2, postmeiotic segregation increased homolog 2; RB1, retinoblastoma transcriptional corepressor 1; TP53, tumor protein p53.
Biology
Glioblastomas are thought to arise from neuroglial stem or progenitor cells and are characterized by molecular heterogeneity. Detailed discussion of glioblastoma biology is beyond the scope of this paper, but has recently been reviewed.16–23
Molecular Pathogenesis and Genomics
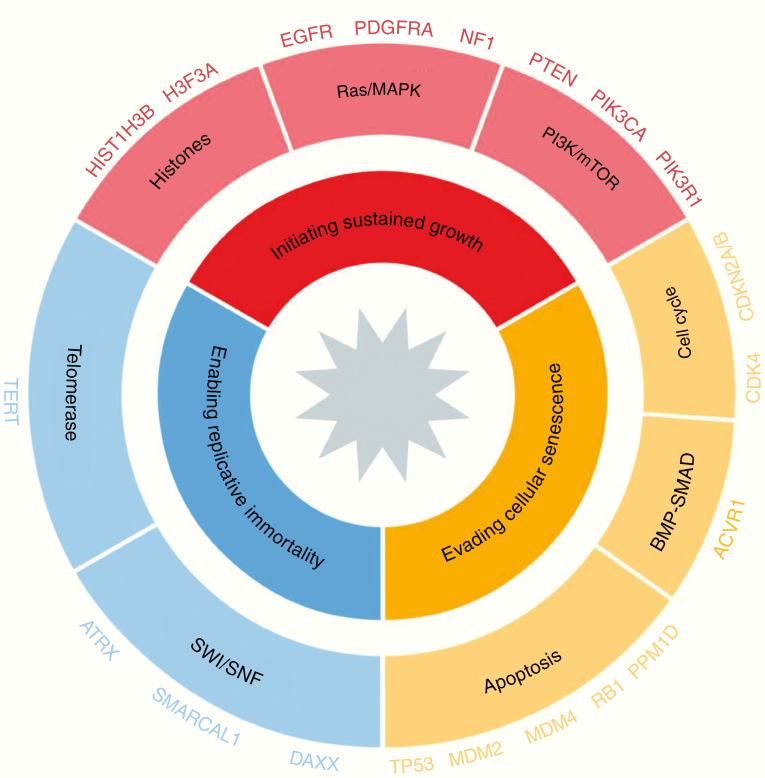
Molecular profiling has identified genes and core pathways that are commonly mutated in sporadic glioblastoma (Fig. 2).24,25,26 Extension of this work to more tumors and additional dimensions (gene expression, DNA methylation) identified 3 main glioblastoma subgroups, each enriched for specific somatic alterations. The proneural gene expression/receptor tyrosine kinase (RTK) I/LGm6 DNA methylation group is marked by cyclin-dependent kinase 4 (CDK4) and platelet derived growth factor alpha (PDGFRα) amplifications and is most common in relatively younger adults. The classical gene expression/classic-like/RTK II DNA methylation group shows a high frequency of EGFR amplifications and homozygous loss of CDKN2A/B. The mesenchymal/mesenchymal-like subtype is enriched for tumors with neurofibromatosis type 1 (NF1) loss and increased tumor infiltration with macrophages. These 3 groups, and mixed entities between them, account for the vast majority of glioblastomas, and are all associated with TERT promoter mutations.27–30 The molecular classification of glioblastoma into distinct subtypes provides a framework for research, but its clinical utility remains unclear. None of the glioblastoma subtypes are predictive for treatment response to current therapies, and assignment of glioblastoma subtype can be challenging in some tumors due to apparent coexistence of multiple subtypes within the same tumor and subtype “switching” through the course of the disease.
Fig. 2.
Glioblastomas are characterized by somatic molecular defects in 3 major processes: initiating tumor growth, evading senescence and enabling immortal growth. Genomic abnormalities in each of the 3 processes appear required for gliomagenesis. The 3 processes are shown here, as are some of the most frequently altered genes and pathways.
One important finding in more recent studies has been the identification of rare glioblastoma entities and their properties. For example, the alternative lengthening of telomeres phenotype, defined by alpha thalassemia/mental retardation syndrome X-linked (ATRX) mutation associated with TP53 mutation, is mostly found in glioblastomas with mutations in IDH1/2, H3K27M, or H3G34R. FGFR3-TACC3 fusion positive glioblastomas have been found to activate oxidative phosphorylation and appear to be metabolically distinct from the more common glycolytic glioblastomas.31 Epigenetic tumor profiles have been particularly informative in distinguishing tumor entities beyond glioma, as they contain information retained from the cell of origin and acquired tumor associated changes. Characteristic epigenetic patterns are associated with certain presumed driver mutations, including mutant IDH1 and IDH2, mutations in either H3F3A or HIST1H3B genes, specifically H3K27M in diffuse midline gliomas, and H3G34R/H3G34V mutations in younger patients with glioblastomas.32,33
After first-line therapy, which typically includes surgical resection, radiation, and chemotherapy, tumor cell subclones may emerge with distinct features—for example, deficiency in DNA mismatch repair (MMR).34,35 About 10% of recurrent, post-temozolomide (TMZ) glioblastomas show a markedly higher mutation rate.36 DNA “hypermutation” is associated with germline defects in MMR genes and can be acquired following therapy with DNA alkylating agents,37–39 the latter occurring more commonly in O6-methylguanine-DNA methyltransferase (MGMT) methylated gliomas, including those with IDH mutations. Oncogene amplification on extrachromosomal DNA, which is common in sporadic adult glioblastoma, likely represents another mechanism for tumor cells to overcome scarcity in resources within the tumor microenvironment.40,41 Comparison of tumor samples obtained at diagnosis and at recurrence show that 80% of mutations and copy-number variants remained unchanged between the primary and recurrent tumors.36,42 Mutations of PIK3CA, TERT, and EGFR amplification in the primary tumor were usually retained in the recurrent tumor, whereas amplifications of PDGFRA, mutations in EGFR, and presence of the variant III (EGFRvIII) rearrangement were the genetic events most likely to be lost. The most frequent genetic changes acquired in recurrent tumors included TP53, EGFR, and phosphatase and tensin homolog (PTEN) mutations. These molecular changes between initial and recurrent tumors may potentially affect the design of clinical trials for recurrent glioblastomas if the tumor genotype is based on analysis of the initial tumor. For trials targeting genetic changes that are frequently altered at recurrence, re-biopsy may be indicated.
Novel sequencing technologies add another layer of detail to our understanding of intratumoral heterogeneity and tumor evolution in glioblastoma. Single-cell transcriptomics show that glioblastomas are mixtures of cells from each of the 3 gene expression subtypes, not one single category,43 corroborating previous findings from multisector bulk gene expression profiling.44 Single-cell DNA profiling confirmed prior fluorescence in situ hybridization findings, showing that many glioblastomas contain admixtures of subclones,45 each of which has amplification of a different RTK (eg, EGFR, PDGFRA, MET).46,47 More recently, single-cell analyses of glioblastoma samples revealed 4 cellular states within individual tumor samples that demonstrate plasticity and are influenced by tumor genetics and the microenvironment.19 Lastly, sequencing of circulating tumor DNA (ctDNA) in cerebrospinal fluid (CSF) can yield a genetically faithful snapshot of the glioma genome in 50% of patients and may eventually obviate the need for tumor re-biopsy in certain instances.48 As technology improves, evaluation of plasma ctDNA may also be feasible in the future.
Genomic profiling has advanced our understanding of the molecular pathogenesis of glioblastoma and identified opportunities for the development of genotype-directed therapies for subsets of patients. Thus far, however, treatment outcomes for patients with glioblastoma have not improved despite this knowledge. Silencing of MGMT-mediated DNA repair, typically the result of MGMT promoter methylation and loss of the second allele of chromosome 10, currently remains the only predictive biomarker of treatment response to TMZ.49 It is thus critical to annotate the molecular data with relevant clinical information through cooperative data-sharing efforts such as the Glioma Longitudinal Analysis (GLASS) Consortium,42 and to incorporate prospective tumor profiling into hypothesis-driven, genotype-directed clinical trials.
Pathology and Classification of Glioblastoma
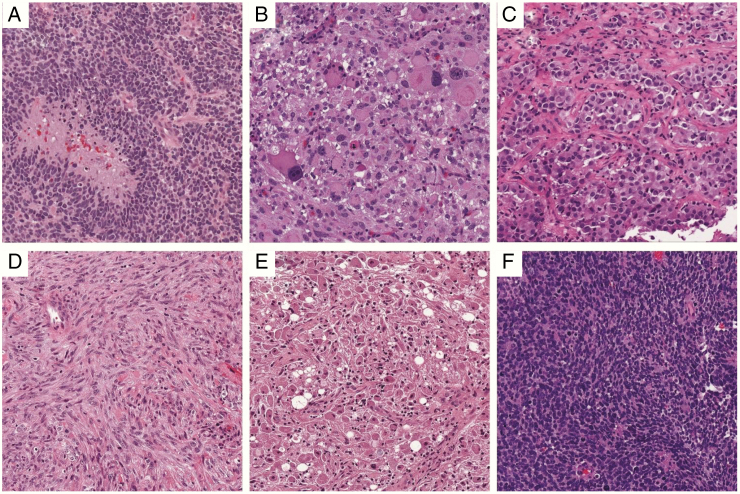
The pathologic hallmarks of glioblastoma are that of a diffusely infiltrative neoplasm with astroglial appearance (angulated nuclei and irregular chromatin), microvascular proliferation, and/or pseudopalisading necrosis (Fig. 3).50 Mitoses are usually easy to identify. Some variants include giant cell astrocytoma (which tends to have a high frequency of TP53 mutations but only rare EGFR amplifications) and gliosarcoma. Epithelioid glioblastoma resembles metastatic poorly differentiated carcinoma and is characterized by frequent BRAFV600E mutations,2 although many of these tumors may be difficult to distinguish from pleomorphic xanthoastrocytomas (Fig. 3).51
Fig. 3.
The many forms of GBM. (A) Classic GBM, with pseudopalisading necrosis and microvascular proliferation. (B) Giant cell GBM. (C) Epithelioid GBM with BRAF V600E. (D) Gliosarcoma. (E) Granular cell GBM. (F) Small cell GBM. All images are from the UPMC Neuropathology Virtual Slide Database, 200x magnification.
Occasionally, a tumor specimen does not show the classic histopathologic features of a glioblastoma. Before the era of integrated histopathology-molecular classification, such tumors would have been assigned a lower WHO grade. However, numerous studies have consistently shown that if such a tumor contains the molecular signature of a glioblastoma, it will act like one and should be treated as such. This was incorporated into the third update of cIMPACT-NOW (the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy), which recommended diagnostic criteria for “diffuse astrocytic gliomas, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” 52 In the absence of IDH mutations, either TERT promoter mutations, the combination of gain of chromosome 7 and loss of chromosome 10, or EGFR amplification are now considered sufficient molecular evidence of glioblastoma with similar clinical outcome, even when histologic examination meets only WHO grade II or III criteria.52,53 The recently described CNS tumor methylation classifier33 represents a major advance in the diagnostic armamentarium in the goal of diagnostic accuracy of brain tumors, and specific glioblastoma subclasses are defined. While the clinical utility of these glioblastoma subtypes is not yet shown, use of the classifier to confirm a glioblastoma diagnosis can be helpful in selected cases, especially in unusual clinical situations (for example, unusual histopathology or history of long-term patient survival).
Conversely, mutations in IDH1/2 in adult diffuse gliomas allow prediction of extended patient survival.3,54 A fast, inexpensive upfront screen for IDH mutation is mutation-specific immunohistochemistry for the most common variant, IDH1-R132H, which comprises well over 90% of all IDH mutations in glioblastoma.54,55 Reflex sequencing for non-canonical IDH mutations, such as IDH2 (codon 172), and non-R132H mutations in IDH1 (for example, R132C or R132S), is common practice at many institutions, especially when it is part of a larger next-generation sequencing (NGS) panel. However, targeted sequencing for “antibody-negative” glioblastoma (ie, tumors which are not positive on IDH1 R132H immunohistochemistry) is considered optional when patients are ≥55 years old, since IDH mutations overall, and especially those that are non-canonical, are very uncommon in older patients.56 On a practical level, it is also very unusual for a glioblastoma, upon initial diagnosis, to have an IDH mutation when it contains microthrombi and/or unequivocal pseudopalisading necrosis.57 Finally, the use of ATRX immunohistochemistry can be a useful screen, since most cases of IDH-mutant glioblastoma show concomitant loss of ATRX (although not all cases of histologically defined glioblastoma with ATRX loss are IDH-mutant). In keeping with the distinct biology and clinical behavior of grade IV gliomas as a function of IDH mutation status, the cIMPACT-NOW consensus group suggests that the term “glioblastoma” no longer apply to IDH-mutant tumors, and suggests instead the term “astrocytoma, IDH-mutant, WHO grade IV” for such tumors, to distinguish them from IDHwt glioblastoma.4
Predictive Biomarkers
Genomic profiling has advanced our understanding of the molecular pathogenesis of glioblastoma and identified opportunities for the development of genotype-directed therapies for subsets of patients. Thus far, however, treatment outcomes for glioblastoma patients have not improved despite this knowledge.
Multiple phase III trials have shown that the presence of MGMT promoter methylation results in approximately 50% longer median survival for glioblastoma patients treated with TMZ.49,58,59 In glioblastomas that lack MGMT promoter methylation, TMZ has little or no benefit.49,60 Whether TMZ may be withheld from these patients, especially in the context of clinical trials, remains controversial, although an increasing number of studies are doing so.61
There are multiple ways to test for MGMT promoter methylation, including methylation-specific polymerase chain reaction (MS-PCR), methylation-specific high-resolution melting, pyrosequencing, and MethyLight, as well as other methodologies.62 A recent method, STP-27,63 employing data obtained from the Illumina methylation array (the same methodology in use for the diagnostic brain tumor classifier33) has also shown promise. The method with the most prospective clinical trial validation is quantitative MS-PCR. However, one retrospective study employing various methods on the same set of TMZ-treated glioblastomas suggested that pyrosequencing might actually provide the best stratification in terms of outcomes, although this needs to be validated by other independent studies.62 Due to the large number of assays available and differences in cutoffs for calling methylation, there is a nontrivial amount of interlaboratory heterogeneity, and better harmonization of MGMT promoter methylation testing is critically needed. In addition, approximately 10% of patients fall into a “gray zone” with tumors that are neither truly methylated nor unmethylated but appear to derive some benefit from TMZ.64 Immunohistochemistry has been proven to be unreliable and should not be used.65
Diagnosis and Imaging
Most glioblastomas are diagnosed following symptomatic presentation due to their rapid expansion and displacement, or infiltrative destruction of brain structures. Suggestive symptoms may include new onset epilepsy, progressive headaches, focal neurologic signs, and mental status alterations in combination with signs of increased intracranial pressure.66 Contrast-enhanced MRI is the diagnostic tool of choice for glioblastoma. These tumors typically manifest as an enhancing, necrotic-appearing mass surrounded by non-enhancing signal abnormalities consisting of edema and infiltrative tumor (Fig. 4). Hemorrhage, cystic changes, or multicentric enhancement is also frequently present.67 When combined with the clinical history, radiological diagnosis of glioblastoma is often achieved with confidence, although challenges may arise as other intra-axial neoplasms, including metastasis, some lower-grade gliomas, and occasionally lymphoma can share similar imaging findings. Nonneoplastic neurologic conditions, such as abscess or demyelinating lesions, may also have a similar appearance. MRI also provides essential anatomic details of the tumor and its adjacent brain structures for surgical planning. For tumors located close to eloquent locations, functional MRI can help plan optimal surgical trajectory and achieve safe maximal resection of enhancing tumor with the goal to improve patient survival.68,69 For clinical trials, the standardized brain tumor imaging protocol is recommended to reduce variability and increase reliability.70 Ideally this protocol would also be incorporated into routine clinical imaging of glioblastoma patients.
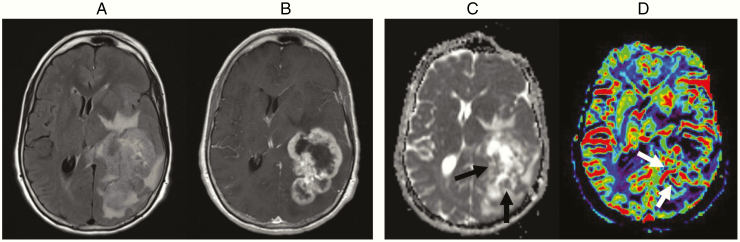
Fig. 4.
Sixty-four-year-old with a glioblastoma who presented with word finding difficulty. FLAIR (A) and contrast-enhanced T1W (B) images show a large, necrotic-appearing, enhancing mass with surrounding T2/FLAIR signal abnormality in the periventricular regions. There is evidence of hypercellularity on ADC map (black arrow in C) and elevated blood volume on CBV map (white arrow in D)
Advanced MRI techniques are increasingly available to assist in the diagnosis of glioblastomas by evaluating their physiological or metabolic properties. Perfusion-weighted imaging such as dynamic susceptibility contrast (DSC) MRI measures cerebral blood volume (CBV), an imaging marker that correlates with microvessel density and area (Fig. 4D).71,72 Since microvascular proliferation due to tumor-induced angiogenesis is a hallmark of glioblastoma,73 CBV may allow differentiation of glioblastoma from other tumor types74–76 or histological grades.77 DSC-MRI may also be useful for differentiation of pseudoprogression in response to radiotherapy (RT) and immunotherapies from true progression, although both false negative and false positive studies may occur.78,79 Apparent diffusion coefficient (ADC), derived from diffusion weighted MRI, inversely correlates with tumor cell density.80,81 ADC values for glioblastomas are lower than for lower grade glioma82 but higher than for lymphoma.83,84 (Fig. 4C) Magnetic resonance spectroscopy (MRS) can detect alterations of metabolite concentrations within the tumor85; glioblastomas typically show markedly elevated choline due to increased cell proliferation and reduced N-acetyl aspartate from neuronal loss. These changes are sensitive but not specific for the diagnosis of glioblastoma, since similar changes can also be observed with other neoplasms or inflammatory disease.79
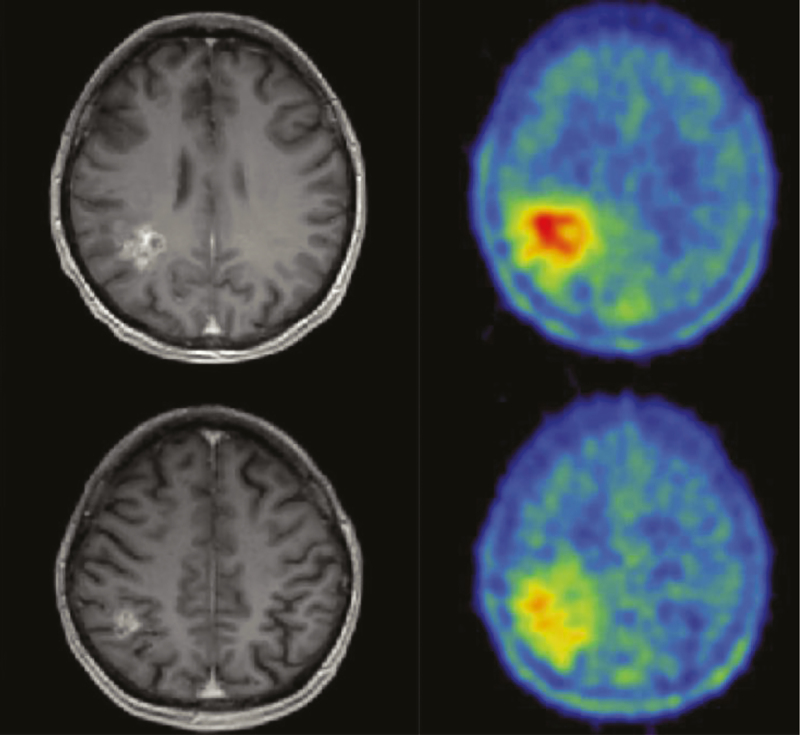
Positron emission tomography (PET) can also provide additional information about biology, differential diagnosis, delineation of tumor extent for surgical and RT planning, and posttreatment surveillance (progression vs pseudoprogression).86,87 Amino acids are the preferred PET tracer (11C-MET, 18F-FET, 18F-FDOPA) based on higher specificity and lower signal/noise ratio than glucose (18F-FDG) (Fig. 5).88 However, the lack of insurance coverage currently limits the widespread incorporation of these studies into standard clinical practice in the United States.
Fig. 5.
Contrast-enhanced MRI T1W (left) and 18FET-PET (right) of a 42-year-old patient showing much larger extent of a glioblastoma on the PET images compared with the enhancing tumor evident by MRI. The tumor extent on the PET image was confirmed by histology.
Accurate determination of response and progression remains a challenge. Currently, the Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas is the most widely used standard in clinical trials for glioblastoma.89,90 These criteria use 2D tumor measurements and provide guidance on evaluating pseudoresponse, non-enhancing progression, and pseudoprogression. More recently, modifications to the RANO criteria have been suggested using a post-RT baseline,91,92 and confirmation of progression on subsequent scans has been advised, especially for agents associated with pseudoprogression, to ensure that patients are not removed from therapies prematurely. This schema also lowers the possibility that patients with spontaneously improving pseudoprogression would be offered salvage options or placed inappropriately on clinical trials for presumed progressive disease.91,93 Additional work is needed to improve response assessment for glioblastomas, with first reports on automated volumetric measurements and deep learning algorithms showing that these may improve outcome assessment.94,95
Medical Management and Supportive Care
Corticosteroids, preferably dexamethasone (in conjunction with gastric protection if used at high doses), are given to reduce symptomatic peritumoral vasogenic edema.96 Dexamethasone alleviates neurologic deficits and signs of increased intracranial pressure such as headache and drowsiness. Low doses (eg, 4 mg/day given in 1–2 doses) are effective in most clinically symptomatic patients without signs of herniation.97,98 There is no need to give dexamethasone 4 times a day.98 Side effects of dexamethasone worsen with increased dose and duration of treatment.99,100 There is also growing evidence that corticosteroids may have an adverse effect on patient outcome, so they should be avoided if patients are not symptomatic.101 Patients on chronic corticosteroids (≥20 mg prednisone equivalents daily for ≥1 month) should be considered for prophylaxis for osteoporosis and pneumocystis jerovecii pneumonia.102
Seizures affect 23% of glioblastoma patients at presentation103 and an additional 20% later in the disease course. While patients with seizures require anti-epileptic drugs (AEDs), studies have not clearly shown a benefit of prolonged primary AED prophylaxis in patients who have never had a seizure.104,105 Current guidelines recommend tapering AEDs 1–2 weeks after surgery and avoiding long-term prophylaxis.106 There is no role for primary perioperative prophylaxis (ie, in patients who have never had a seizure). A meta-analysis of 6 studies,107 a Cochrane systematic review,108 and a subsequent randomized trial of phenytoin versus no prophylaxis109 have all shown no significant benefit from primary prophylaxis. When AEDs are used, newer agents including levetiracetam and lacosamide are preferred over older drugs because of generally more favorable side effect profiles, reduced laboratory monitoring requirements, and lack of drug-drug interactions.110 Emerging data suggesting that neurons and glioma cells form synapses via AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors raises the possibility that AEDs that inhibit these receptors, such as perampanel, may be beneficial not only in controlling seizures, but also through possible antiglioma activity.111,112 However, a prior trial with another glutamate inhibitor, talampanel, was ultimately interpreted to be negative.113
Venous thromboembolism (VTE) risk is high in the perioperative period and persists well beyond, with one-year incidence of approximately 20%,114,115 mandating a low threshold for pursuing diagnostic studies.115 Most,116,117 though not all,118 studies suggest that the risk of precipitating intratumoral hemorrhage with anticoagulants is acceptably low, even in patients receiving bevacizumab.119 The preferred anticoagulant is not well studied in brain tumors; in systemic cancer, low molecular weight heparin (LMWH) is preferred over warfarin.120 Direct oral anticoagulants (DOACs) (factor Xa and thrombin inhibitors) have been reported to be safe in patients with brain tumors.121 However, no randomized data are available for glioma patients and randomized trials on secondary prophylaxis of VTE with DOACs enrolling cancer patients have generally shown a similar or slightly higher efficacy than LMWH but with a slightly higher risk of bleeding.122,123
A high incidence of recurrent VTE with inferior vena cava (IVC) filters limits their use to patients with recent intracranial surgery, intratumoral hemorrhage, or absolute contraindications to anticoagulation.110 Prophylaxis with anticoagulation outside of the perioperative setting has not been definitively studied, as the only trial addressing this issue was prematurely terminated for slow accrual.124 A meta-analysis of pooled randomized clinical trial data indicated no survival benefit from anticoagulation in glioblastoma patients, but rather suggested that VTE should be treated more vigorously in this patient population.125
Cognitive deficits, personality changes, and mood disturbances are major comorbidities for glioblastoma patients.96 Before treatment, up to 91% of brain tumor patients have cognitive deficits, with only moderate correlation with cognitive complaints.126,127 The frequent presence of fatigue and sleep disturbance contributes to cognitive impairment.128,129 Medical treatments with acetylcholinesterase inhibitors (donepezil) or psychostimulants (methylphenidate, modafinil) to prevent cognitive decline and fatigue after RT in patients with brain tumors (<50% were glioblastoma) have been unsuccessful.130–133 Although the 6-month prevalence of clinical depression is about 20% in brain tumor patients,134 randomized studies on medical treatment are lacking.
Regular exercise,135 adoption of a healthy diet, avoidance of hyperglycemia,136 early discussion of goals of care, and involvement of palliative care should be considered. Despite extensive interest in ketogenic diets and cannabinoids, there are currently no clinical data supporting their routine use.
Standard Therapy
Despite recent advances in our understanding of glioma biology, the prognosis of patients with glioblastoma remains poor. With standard-of-care consisting of surgery, RT, and TMZ chemotherapy, median overall survival (OS) in well-selected patients in clinical trials is approximately 15–18 months,58,59,137 and 5-year survival is less than 10%.138 Once glioblastomas recur, median OS is estimated to be 24–44 weeks.139–141 Standard-of-care therapies for patients with newly diagnosed glioblastoma are summarized in Table 2, Fig. 6 and recurrent glioblastoma in Table 3, Fig. 7. Because none of these treatments are curative, the National Comprehensive Cancer Network (NCCN) recommends clinical trials as the preferred option for eligible patients.142 Treatment must also be tailored to the individual based on age, functional status, goals of care, etc. Integration of palliative care early in the course of the illness is important, and best supportive care may be the most appropriate course in some patients.66
Table 2.
Selected phase III clinical trials in patients with newly diagnosed glioblastoma
| Study Design | Study Population and Key Eligibility Criteria | Extent of Resection | Radiation Scheme | Systemic/Experimental Agent | Median OS, months | Median PFS/EFS, mo |
|---|---|---|---|---|---|---|
| Randomized phase III trial of RT ± TMZ (N = 573)58 | Newly diagnosed GBM Age 18–70 years WHO PS ≤ 2 | Biopsy: 16–17% Partial resection: 44–45% Complete resection: 39–40% | Fractionated focal irradiation in daily fractions of 2 Gy given 5 days per week for 6 weeks, for total of 60 Gy | TMZ 75mg/m2/day during radiation from the first to the last day of RT (up to 49 days) followed by 6 cycles of adjuvant TMZ 150–200 mg/m2 for 5 days during each 28-day cycle) | RT alone: 12.1 (95% CI, 11.2–13.0) RT + TMZ: 14.6 (95% CI, 13.2–16.8). Unadjusted HR for death 0.63; 95 % CI, 0.52–0.75; P < 0.001 | RT alone: 5.0 (95% CI, 4.2–5.5) RT + TMZ: 6.9 (95% CI, 5.8–8.2) |
| Randomized, open-label trial of adjuvant TMZ ± TTF (N = 695)143 | Newly diagnosed GBM who had completed concomitant RT + TMZ Age ≥ 18 years KPS ≥ 70 Supratentorial tumor | Biopsy: 13% Partial resection: 33–34% Gross total resection 53–54% | N/A | Adjuvant TMZ as per Stupp regimen above TTF initiated 4–7 weeks from last day of RT until second progression or for a maximum of 24 months | TMZ alone: 15.6 TMZ + TTF 20.5 HR 0.64; 99.4% CI, 0.42–0.98; P = 0.004 | TMZ alone: 4.0 TMZ + TTFields: 6.7 HR 0.63; 95% CI, 0.52–0.76; P < 0.001 |
| Studies in MGMT methylated GBM | ||||||
| Randomized, open-label, phase III trial of standard TMZ versus lomustine-TMZ 144 | Newly diagnosed GBM Age ≥ 18 years KPS ≥ 70 Centrally confirmed methylated MGMT promotor | Biopsy: 2–5% Partial resection: 35–36% Complete resection: 59–63% | Standard involved-field RT to total dose of 59–60 Gy in 30–33 single day fractions | Standard concurrent + adjuvant TMZ as per Stupp above Lomustine-TMZ: 6-week cycles of lomustine 100 mg/m2 on Day 1 and TMZ 100–200 mg/m2 on days 2–6 for up to 6 cycles, starting in the first week of RT | In modified intention to treat population. Standard TMZ: 31·4 (95% CI, 27·7–47·1) Lomustine-TMZ: 48·1 (95% CI, 32·6–not assessable) HR 0·60; 95% CI 0·35–1·03; P = 0.0492 | In modified intention to treat population. Standard TMZ: 16·7 (95% CI, 11·4–24·2) Lomustine-TMZ: 16·7 (95% CI, 12·0–32·0) HR 0·91; 95% CI 0·57–1·44; P = 0.4113 |
| Studies in elderly patients (age ≥65 years) | ||||||
| Randomized phase III trial of hypofractionated RT ± TMZ (N = 562) 145 | Newly diagnosed GBM Age ≥65 years ECOG PS ≤2 Deemed by their physicians not to be suitable to receive conventional RT | Biopsy: 31.7% Partial or complete resection: 68.3% | Fractionated focal irradiation administered in 15 daily fractions over a period of 3 weeks, for total of 40.05 Gy | TMZ 75 mg/m2/day during radiation from the first to the last day of RT (21 consecutive days) followed by adjuvant TMZ 150–200 mg/m2 for 5 days during each 28 day cycle) for up to 12 cycles | RT alone: 7.6 RT + TMZ: 9.3 HR 0.67 for death; 95% CI, 0.56 to 0.80; P < 0.001 | RT alone: 3.9 RT + TMZ: 5.3 HR 0.50 for disease progression or death; 95% CI, 0.41 to 0.60; P < 0.001 |
| NOA-08: Noninferiority, randomized phase III trial of TMZ vs RT (N = 373)146 | Newly diagnosed GBM or AA Age ≥ 65 years KPS ≥ 70 | Biopsy: 37–41% Partial resection: 31–35% Complete resection; 20–27% | Fractionated focal irradiation administered in 30 daily fractions over 6–7 weeks, total 60.0 Gy | TMZ 100 mg/m2 for 1 week on, 1 week off | TMZ: 8·6 (95% CI, 7·3–10·2) RT: 9·6 (95% CI, 8·2–10·8) HR 1·09, 95% CI 0·84–1·42, pnon-inferiority = 0·033 | TMZ: 3·3 (95% CI, 3·2–4·1) RT: 4·7 (p5% CI, 4·2–5·2) HR 1·15, 95% CI 0·92–1·43, pnon-inferiority = 0·043 |
| Nordic: Randomized, phase III trial of TMZ vs 6-week RT vs hypofractionated RT (N = 291)147 | Newly diagnosed GBM Age ≥60 y WHO PS ≤ 2 | Biopsy: 26–27% Partial or complete resection: 73–74% | Hypofractionated RT: 34·0 Gy administered in 3·4 Gy fractions over 2 weeks Standard RT: 60·0 Gy administered in 2·0 Gy fractions over 6 weeks | TMZ 200 mg/m2 for 5 days during each 28 day cycle for up to 6 cycles | In comparison with standard RT: 6·0 months (95% CI, 5·1–6·8) TMZ: 8·3; HR 0·70; 95% CI 0·52–0·93, P = 0.01 Hypofractionated RT: 7·5 (95% CI, 6·5–8·6), HR 0·85; 95% CI 0·64–1·12, P = 0.24 | Deliberately not collected |
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; HR, hazard ratio; KPS, Karnofsky performance status; OS, overall survival; PFS, progression-free survival; PS, performance status; RR, radiographic response rate; TTF, tumor-treating fields.
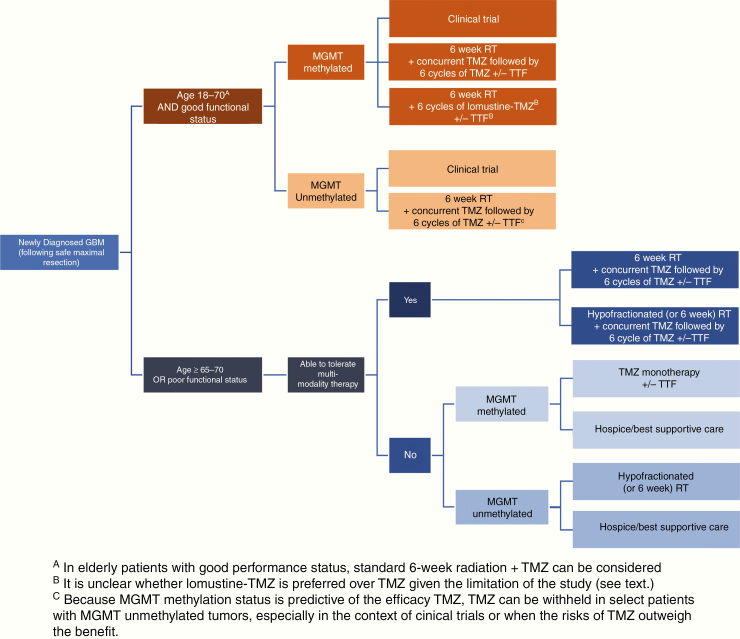
Fig. 6.
Standard of care treatment paradigm for newly diagnosed glioblastoma.
Table 3.
Selected clinical trials of systemic agents in patients with recurrent glioblastoma
| Systemic Agent(s) | Study Design | Study Population | Median OS, mo | Median PFS/TTF, mo | 6M-PFS | RR |
|---|---|---|---|---|---|---|
| EORTC 26101: Lomustine (nitrosurea) ± bevacizumab (VEGF inhibitor)148 | Randomized phase III trial of lomustine +/- bevacizumab in recurrent GBM | 437 with recurrent GBM at first progression | Combination: 9.1 Lomustine: 8.6 HR 0.95 [95% CI 0.74–1.21; P = 0.65) | Combination: 4.2 Lomustine: 1.5 HR 0.49 [95% CI, 0.39–0.61; P < 0.001). | Combination: 30.2% Lomustine: 16.9% | Combination: 41.5% Lomustine: 13.9% |
| RESCUE Study: TMZ rechallenge149 | Nonrandomized, phase II trial of continuous TMZ 50 mg/m2 daily for recurrent GBM | Recurrent GBM at first progression Group B1: 33 with early progression during the first 6 cycles of adjuvant TMZ Group B2: 27 with progression on adjuvant TMZ beyond standard 6 cycles but before completing of adjuvant TMZ Group B3: 28 who progressed after completing of upfront adjuvant TMZ (treatment free interval > 2 months) | NR | Group B1: 3.6 Group B2: 1.8 Group B3: 3.7 | Group B1: 27.3% Group B2 7.4% Group B3: 35.7% | Group B1:3% Group B2: 0% Group B3: 11.1% |
| Director Trial: Temozolomide rechallenge150 | Randomized, phase II trial of two different dose-intense TMZ regimens (note trial prematurely closed due to withdrawal of support) | Recurrent GBM at first progression randomized to Arm A: TMZ 120 mg/m2 one week on, one week off Arm B: TMZ 80 mg/m3 three weeks on, one week off | Arm A: 9.8 Arm B: 10.6 | Arm A: 1.8 Arm B: 2.0 | Arm A: 17.1% Arm B: 25.0% | Arm A: 8% Arm B: 16% |
Abbreviations: NR, not reported; PFS, progression-free survival; RR, radiographic response rate; VEGF, vascular endothelial growth factor.
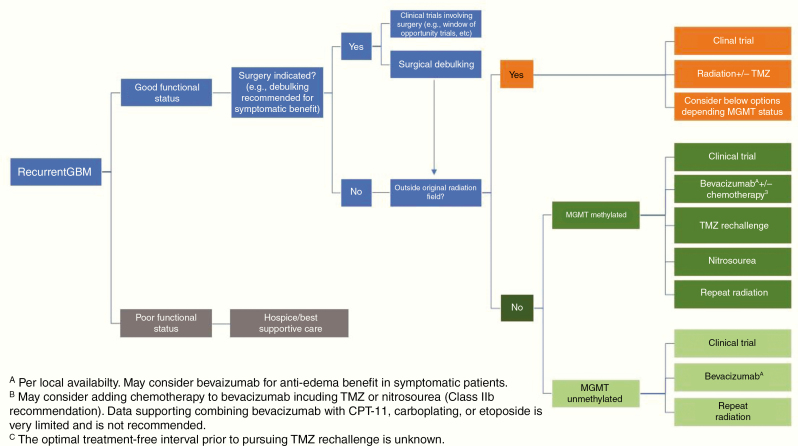
Fig. 7.
Standard of care treatment paradigm for recurrent glioblastoma.
Surgical Management
Surgical procedures should be tailored to individual patients, taking into consideration indications, risk-benefit ratio, and prognostic impact for each patient. In the past, tumor extent has been mostly defined on MRI by T1-weighted sequences with contrast enhancement; however, non-contrast enhancing tumor volume has to be incorporated as well into the target volume for resection.151 Whenever microsurgical resection is deemed to be high risk based on the patient’s medical condition and/or the functional topography or eloquence of the affected brain region, a stereotactic or open biopsy should be performed to obtain at least a histological and molecular diagnosis.66 In order to obtain sufficient material for histological diagnosis and grading, the surgeon aims to target and biopsy areas of solid tumor mass that contain viable tumor cells, preferably avoiding necrotic areas or adjacent nonneoplastic brain. The most frequently requested genetic markers (IDH1/2 mutation and MGMT promoter methylation) appear to be present homogeneously throughout the tumor, so the risk of a sampling error by obtaining a “false-negative” result or misclassification of the molecular profile is relatively low.152 However, since additional molecular markers may gain clinical relevance in the future, multiple (larger) samples should be considered for more advanced genomic analyses. Whenever possible, areas of enhancement must be included in the target for the biopsy to ensure accurate WHO grade classification of the tumor. Extent of resection should be verified by an early postoperative contrast enhanced MRI, preferably within 48 hours after surgery.151
Radical microsurgical resection of a glioblastoma is limited by the highly invasive nature of the tumor with infiltrating tumor cells typically extending significant distances from the main tumor mass.153 Nevertheless, the goal for glioblastoma surgery should be gross total resection of the enhancing solid tumor mass whenever feasible. While some studies report gradually improved outcome with increasing extent of resection above 78%, only gross total resection is likely to be associated with improved outcome in both newly diagnosed69,154–158 and recurrent glioblastoma.159,160 The goal is to leave the smallest amount of residual postoperative enhancing volume possible as this correlates with survival.161 Current standard surgical adjuncts include stereotactic navigation systems using anatomical and functional MRI datasets, intraoperative MRI, ultrasound, intraoperative functional monitoring, and the fluorescent dye 5-aminolevulinic acid (5-ALA) to visualize vital tumor tissue, all of which are increasingly used to improve and maximize the extent of resection while reducing the risk of new neurologic deficits (Fig. 8).155,162,163 As a general principle, preventing new permanent neurologic deficits is more important than maximizing the extent of resection, because glioblastomas are not cured by surgery alone, while recognizing and taking into consideration the benefits of maximal safe resection. Postoperative deficits due to emerging complications are a negative prognostic factor.164,165 This emphasizes the relevance of a risk-adapted concept which embeds surgery into a thorough prognostic evaluation. Given the complexities of surgery for glioblastoma, consideration should be given to referring patients to high-volume centers specializing in the care of brain tumor patients.
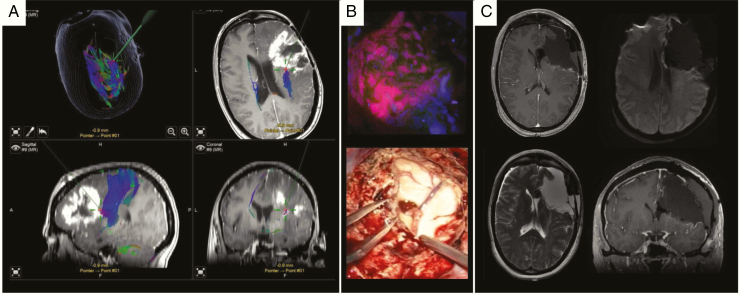
Fig. 8.
Microsurgical resection of a right-sided recurrent IDHwt glioblastoma WHO grade IV using intraoperative neuronavigation, neuromonitoring and 5-ALA fluorescence techniques. (A) T1 contrast enhanced axial, sagittal and coronal planes including DTI fiber tracking (blue fibers). The green trajectories/red points represent the pointer for intraoperative neuronavigation. (B) Upper image: corresponding intraoperative 5-ALA fluorescence image taken from the area as depicted by neuronavigation. Lower image: opening of the right ventricle due to critical involvement by tumor formation. (C) Postoperative MRI confirms gross total resection without residual contrast enhancement, no perilesional ischemia (diffusion-weighted image upper right).
Biodegradable polifeprosan 20 with carmustine wafers inserted at the time of surgery is approved by the US FDA and the European Medicines Agency for the treatment of both newly diagnosed high-grade glioma and recurrent glioblastoma.166,167 They were shown to produce a modest survival advantage of approximately 2 months but are used only sporadically, in part because the efficacy data stem from the pre-temozolomide era, carmustine from the wafers has limited brain penetration, safety and tolerability are an issue in low-volume centers, and this treatment may preclude patients from enrolling into clinical trials.
Postsurgical Management of Newly Diagnosed Glioblastoma
Following maximal safe resection, the generally accepted treatment for glioblastoma is radiotherapy (RT) with concurrent TMZ (75 mg/m2/day × 6 wk) and maintenance TMZ (150–200 mg/m2/day × 5 days for six 28-day cycles) (Figure 6).58,138 Because MGMT promoter methylation status is predictive of the efficacy of TMZ,49 TMZ can be withheld in select patients with MGMT unmethylated tumors where the benefit of TMZ is minimal, especially in the context of clinical trials,61 or when the risks of TMZ outweigh the benefit (ie, toxicity limits TMZ use). During adjuvant TMZ, the addition of tumor treating fields (TTF), which provide low intensity, intermediate frequency (200 kHZ), alternating electric fields to produce antimitotic effects selective for dividing tumor cells with limited toxicity, extended survival by a median of 4.9 months in one study.143 Neither dose-dense TMZ regimens,59 extending the length of adjuvant TMZ treatment beyond 6 cycles,168–170 nor the addition of bevacizumab137,171 yield additional survival benefit.
A recent small randomized phase III trial examined the benefit of an intensified lomustine-TMZ regimen for newly diagnosed MGMT promoter methylated glioblastoma. When combined with radiotherapy, median OS increased from 31.4 months with standard TMZ to 48.1 months with lomustine-TMZ.144 Since the sample size was small (~70 patients in each arm), the survival curves separated late (after 2–3 y), and in univariate analysis the effect was small, the role of this regimen remains unclear.172 Hematologic toxicity was greater in the lomustine-TMZ arm, and fewer patients were able to complete all 6 cycles of adjuvant treatment.144
Radiotherapy Considerations
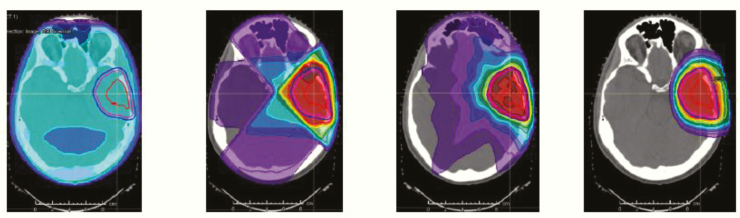
Most standard approaches recommend delivering RT in the range of 60 Gy in 30 fractions of 2 Gy, based on targets selected using the immediate postsurgical MRI. The recommendation of the European Organisation for Research and Treatment of Cancer (EORTC) is to perform RT in a single phase (60 Gy, 2 Gy per fraction) while the Radiation Therapy Oncology Group (RTOG) approach uses an initial larger volume defined by the fluid attenuated inversion recovery (FLAIR) abnormality plus a 2-cm margin, which receives 46 Gy, in 23 fractions of 2 Gy each, plus additional 14 Gy given to the resection cavity and residual enhancing tumor.173 Great attention is paid to limit exposure of structures that are at risk of radiotherapy-induced damage, including ophthalmic and optic structures, brainstem, cervical cord, cochlea, and, where feasible, temporal lobes and/or hippocampi.174 A number of institutions have published modifications of this approach, in an attempt to decrease the volume of normal brain irradiated.175 There remains considerable disagreement regarding the optimum RT volume and margin expansions, and advanced imaging has not yet helped resolve this issue.175,176 Several ongoing research efforts are focusing on better defining the volume that truly needs to be boosted to a higher dose by incorporating advanced imaging such as perfusion/diffusion MR, MRS, and amino-acid PET, but these remain investigational. Prior dose-escalation efforts have largely failed, but these were conducted in the pre-temozolomide era, and current trials are investigating whether RT dose-escalation might be beneficial, at least in some patients, when combined with TMZ (eg, NCT02179086). It is also unclear whether modern RT techniques will yield superior outcomes (Fig. 9). For example, emerging data suggest that the reduction in the low dose volume to normal brain decreases therapy-associated lymphopenia,177 and this has been suggested to be indirectly associated with improved survival.178
Fig. 9.
This figure shows, from left to right, how the transition from 2D RT to 3D RT to intensity modulated radiotherapy to intensity modulated proton therapy harnesses the potential for sparing normal, uninvolved brain substructures from unnecessary RT dose; whether this produces meaningful patient clinical benefit is a subject of current clinical trial testing.
Pseudoprogression
Radiochemotherapy can produce transient worsening of contrast enhancement on MRI for several months in approximately 10–30% of patients, sometimes associated with symptoms of intracranial mass effect.179,180 A similar problem may occur with immunotherapies. The diagnosis of pseudoprogression can be problematic; DSC-MRI78,79 and amino acid PET imaging, as described above, may be helpful.86 Because of the difficulty in differentiating pseudoprogression from progression, the RANO working group has recommended avoiding enrolling patients within 3 months of completion of radiochemotherapy into clinical trials for recurrent disease, unless the recurrence is mainly outside the RT field or there is tissue confirmation of progression.89 However, histopathological distinction of ”residual tumor” (apparently dormant and damaged) versus truly “recurrent tumor” (healthier and actively proliferating) can be challenging. 181
Elderly Patients
Since the median age of glioblastoma is 65 years, a significant number of patients are considered “elderly.” 1 Their treatment represents a particular challenge, as they generally have a worse prognosis and are less tolerant of toxicities.182 There is evidence that hypofractionated RT (40 Gy/15 fractions of 2.67 Gy over 3 weeks) is as effective as the standard 60 Gy over 6 weeks.183 An international phase III trial of newly diagnosed glioblastoma patients age 65 and older demonstrated an OS advantage with hypofractionated RT (40 Gy/15 fractions of 2.67 Gy) with TMZ compared with RT alone (9.3 vs 7.6 mo), with clinical benefit predominantly in patients with methylated MGMT promoter.145 However, there has never been a direct comparison of hypofractionated RT with TMZ compared with the standard 6 weeks of RT with TMZ. For patients with poor functional status, single modality therapy may be better tolerated, but the recommendation varies depending on the MGMT promoter methylation status. In both the NOA-08146 and the Nordic Clinical Brain Tumor Study Group trials147 which compared RT versus TMZ, RT was more effective than TMZ for MGMT promoter-unmethylated tumors, whereas TMZ was more effective than RT for MGMT promoter-methylated tumors. Radiotherapy schedules used in the elderly population include 40 Gy delivered in 15 fractions,183 34 Gy in 10 fractions,147 or 25 Gy in 5 fractions,184 although the role of the latter regimen is more controversial.
Recurrent Glioblastoma
Glioblastoma patients invariably recur after a median interval of less than 7 months,58 and there is no clear standard-of-care salvage therapy (Fig. 7). NCCN guidelines list clinical trials as the preferred option for eligible patients.142 Surgery may have a role for symptomatic and/or large lesions. However, only patients who undergo complete resections have any survival benefit.160 Other options include systemic therapy such as TMZ rechallenge, nitrosoureas, bevacizumab, re-irradiation, and TTF (in the US),185 none of which have been shown to prolong survival in randomized trials in this setting, or palliative care for patients with poor performance status.
Bevacizumab
Multiple studies of the humanized vascular endothelial growth factor (VEGF) antibody bevacizumab for glioblastoma have failed to demonstrate a survival benefit.148 However, bevacizumab is often effective in reducing peritumoral edema and related clinical symptoms and signs.186 It is approved in the United States and some other countries, but not in the European Union, for use in recurrent glioblastoma due to improvement in progression-free survival (PFS) and reduction in corticosteroid use.148 Continuation of bevacizumab post progression did not improve outcome in a small study.187 Patients with recurrent glioblastoma should ideally be considered for clinical trials before receiving bevacizumab, as most trials exclude prior use of bevacizumab. Bevacizumab has also been proven to be effective in radiation-induced necrosis, although the doses used are lower than standard dosing for recurrent glioblastoma (typically 7.5 mg/kg every 3 wk for a maximum of 4 treatments).188
Temozolomide Rechallenge
Rechallenge with TMZ may be reasonable, especially in patients with MGMT promoter methylated glioblastoma that relapses more than a few months after completion of maintenance TMZ in the first-line setting.149,150 The uncontrolled RESCUE study observed that patients who lived longest with dose-dense TMZ were those who progressed after a treatment-free interval.149 While MGMT status was not predictive of outcome in the RESCUE study, the DIRECTOR trial did demonstrate increased time to treatment failure with TMZ rechallenge in patients with MGMT promoter methylated versus unmethylated tumors.150 However, there is no evidence to suggest that TMZ rechallenge is superior to nitrosoureas in any patient population.
Nitrosoureas
Nitrosoureas, including lomustine, carmustine, and fotemustine, have good blood–brain barrier (BBB) penetration.189 Fotemustine is available in some European countries, but has not been approved for use in the United States. Lomustine is generally preferred over carmustine given its oral formulation, schedule of administration, and better safety profile. In several phase III randomized trials, the lomustine monotherapy arm (dosed as 6 wk cycles of 100–130 mg/m2 for up to 6 cycles) was associated with median OS of 7.1–8.6 months and PFS of 1.5–3 months.148,190 Data from these trials also suggest that patients with MGMT-methylated tumors are more likely to benefit from nitrosoureas than those with unmethylated MGMT.148,191,192
Other Therapies
Although other chemotherapeutic agents such as irinotecan, carboplatin, procarbazine, and etoposide are sometimes used for patients with recurrent glioblastomas, there are no data suggesting that they are beneficial.142 A recent randomized phase II trial suggested that regorafenib, a VEGF receptor 2 and multikinase inhibitor, increased survival in patients with recurrent glioblastoma compared with lomustine.193
Re-Irradiation
Repeat RT in the form of radiosurgery or hypofractionated radiotherapy (30–35 Gy in 5–15 fractions) is increasingly used for recurrent glioblastoma, although there is currently no definitive data regarding benefit.194,195 A secondary analysis of the NRG Oncology/RTOG 0525 trial showed no significant survival benefit of re-irradiation over systemic therapy after tumor progression.196 Preliminary results of the NRG phase II trial comparing bevacizumab alone versus bevacizumab with re-irradiation in patients with recurrent glioblastomas showed that the addition of re-irradiation improved PFS (7.1 mo with the combination vs 3.8 mo with bevacizumab alone; P = 0.05) but not OS.197
Novel Therapies
Given the poor outcomes with current therapies, there is great interest in various experimental approaches under investigation.198 These will be discussed in the following sections.
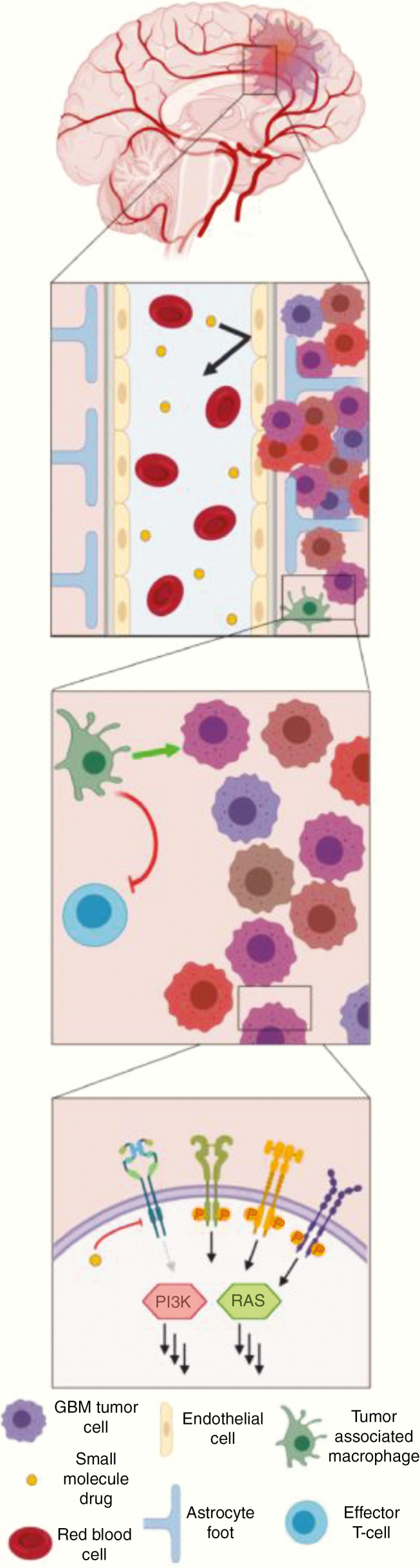
Targeted Molecular (Precision) Therapies
Despite advances in understanding the molecular pathogenesis of glioblastoma, there has been only modest progress in developing effective targeted molecular therapies.199 Challenges include the paucity of agents that effectively cross the BBB,22 the relative lack of “easy” targets such as BRAFV600E mutations, redundant signaling pathways,28 and tumor heterogeneity.19,36,42
The 2016 update of the WHO classification incorporated molecular parameters into the definition of certain brain tumors.50 Several of these markers are easily assessed by immunohistochemistry, including IDH1-R132H and histone H3 K27M, while other point mutations can be determined by sequencing. BRAFV600E mutation status, while challenging by immunohistochemistry is easily assessed by sequencing and has therapeutic implications for a subset of glioblastoma patients. For more comprehensive profiling, targeted NGS panels have proven to be useful, while some centers also have the capacity to perform whole exome sequencing or whole genome sequencing. Microsatellite instability can be readily assessed by either genome-wide or medium-sized panel approaches, and is relevant given the tumor-agnostic approval by the FDA for pembrolizumab for cancers with high microsatellite instability. Copy number variations—for example, the aforementioned chromosomal +7/−10 pattern—are relevant for glioma diagnosis and possibly treatment. Fusion detection, to identify a potentially relevant and druggable group of alterations (for example, fusions of neurotrophic-tropomyosin RTK [NTRK fusions]), requires specific coverage by either DNA-based approaches or alternatively mRNA-based analyses. Routine examination of these (and potentially additional) alterations will be critical if we are to make substantial steps forward for precision glioblastoma therapies.200
Examples of putative treatment-predictive biomarkers exist. The most often investigated biomarkers, high level EGFR amplification and EGFRvIII mutation, have been targeted with and without tumor pretesting, with the aim of suppressing pathway activation with EGFR inhibitors such as erlotinib,201 targeting the heterogeneously expressed EGFRvIII neoantigen by vaccination with a peptide vaccine, rindopepimut,202 or using the conformational change for specific binding of an antibody-drug conjugate, depatuxizumab mafodotin (ABT414)203–207 without clinical activity.207 Targeting BRAFV600E mutations showed responses to monotherapy with Raf inhibitors such as vemurafenib,208 or dual therapy with combined BRAF/MEK inhibition with trametinib and dabrafenib,209 but these mutations are rare in glioblastoma except for epithelioid glioblastoma,210 a somewhat controversial entity likely to be often confused with pleomorphic xanthoastrocytoma. Other potentially targetable mutations, such as NTRK fusions,211H3K27M mutations,212,213 and FGFR mutations and FGFR3-TACC3 fusions,214 are all uncommon in glioblastoma. Of note, mutations in the telomerase reverse transcriptase (TERT) promoter are found in up to 85% of glioblastomas,215 although to date this mutation has been challenging to target.
The lack of success in targeted therapy trials in glioblastoma is likely due to tumor heterogeneity, lack of knowledge of the contribution of genetic alterations to tumor maintenance, targeting subclonal or unstable genetic alterations instead of stable and clonal oncogenic drivers, redundant signaling pathways, use of archival instead of freshly obtained recurrent tumor tissue for biomarker testing, insufficient assessment of drug brain tumor concentrations, failure of target inhibition, and development of rapid secondary resistance and clonal selection.
Currently, most therapeutic strategies and biomarkers are focused on single or multiple biological features that are differentially detected in patient groups responding to a given therapy. In several studies post-hoc exploratory analyses suggested subsets of patients that may have benefited from experimental treatments, but in the absence of validation, these remain only hypothesis generating. For example, the proneural subtype of glioblastoma defined by expression analyses216,217 or MRI features218 may derive benefit from the addition of bevacizumab to standard treatment. Lower levels of carboxypeptidase G2 promoter methylation of cluster of differentiation (CD)95 ligand (CD95L) were correlated with improved Overall survival with the CD95 inhibitory treatment asunercept (APG101) in combination with re-irradiation compared with re-irradiation alone.219 Also, based on a retrospective analysis, mammalian target of rapamycin (mTOR) Ser2448 phosphorylation may be a putative predictive biomarker of response to the mTOR inhibitor temsirolimus plus radiation in patients with newly diagnosed glioblastoma lacking MGMT promoter methylation.220 Others have suggested PTEN loss predicts benefit from mTOR inhibitors.221 Without preselection, mTOR inhibition is not only ineffective but may even confer a survival disadvantage compared with the standard of care. For example, the addition of a different mTOR inhibitor, everolimus, resulted in worse outcome in an unselected group of patients with newly diagnosed glioblastoma irrespective of MGMT status (Table 4).222
Table 4.
Selected completed trials with targeted molecular therapies
| Molecular Target | Signaling Pathway | Therapy | Trial | Trial Concept (examples) | Trial Result |
|---|---|---|---|---|---|
| BRAFV600 mutation | Vemurafenib208 | NCT01524978 | Basket trial with recurrent glioma arm | ORR 25% overall 3/6 GBM had SD as best response | |
| BRAFV600E mutation | Dabrafenib + Trematenib210 | NCT02034110 | Phase II basket trial using novel Bayesian hierarchical statistical design | ORR for GBM 29%; 62% for low grade gliomas | |
| EGFR amplification | Depatuxizumab mafodotin (DM) (ABT414)205 | NCT02573324 (Intellance 1) | Randomized phase III trial in newly diagnosed GBM with EGFR amplification comparing RT + TMZ ± DM | 639 patients randomized Ocular toxicity common DM MS 18.9 (17.4, 20.8) Placebo: 18.7 (17.0, 20.3) HR 1.02 (0.82, 1.26); P = 0.63 | |
| EGFR amplification | Depatuxizumab mafodotin (DM) (ABT414)206 | NCT02343406 (Intellance 2) | Randomized phase II in recurrent GBM comparing DM, DM + TMZ, or TMZ alone | 260 patients 25–30% grade 3 or 4 ocular toxicity Hazard ratio (HR) for the combination arm DM+TMZ compared with the TMZ was 0.71, 95% CI [0.50, 1.02]; P = 0.062 at initial analysis. On long-term follow-up, HR for the comparison of the DM+TMZ compared with control was 0.66 (95% CI = 0.48, 0.93), P = 0.017. Efficacy of DM monotherapy was comparable to that of TMZ (HR = 1.04, 95% CI [0.73, 1.48]; P = 0.83) | |
| Exportin 1 | Important for transport of tumor suppressor proteins and oncoprotein mRNA from nucleus to cytoplasm | Selinexor | NCT01986348 | Multi-arm phase II trial in recurrent GBM | ORR 10% PFS6 19% 6 cycle PFS (24 weeks) 30% |
| FGFR mutations and FGFR-TACC gene fusions | Highly oncogenic FGFR mutations and FGFR-TACC gene fusion that confers sensitivity to FGFR inhibitors | AZD4547 | NCT02824133 | Phase I/II study in patients recurrent glioma positive for FGFR fusion | Not available |
| FGFR mutations and FGFR-TACC gene fusions | Highly oncogenic FGFR mutations and FGFR-TACC gene fusion that confers sensitivity to FGFR inhibitors | Infigratinib (BGJ398)223 | NCT01975701 | Phase II study in recurrent GBM with FGFR1-TACC1, FGFR3-TACC3 fusion and/or activating mutation in FGFR1, 2 or 3 | 26 patients ORR 7.7% 4 patients disease control > 1 year (2 FGFR1 mutations, 1 FGFR3 mutation, 1 FGFR3-TACC3 fusion) PFS6 16% |
| mTOR | Everolimus222 | NCT01062399 | Randomized phase II trial of RT+TMZ ± everolimus in newly diagnosed GBM | 171 patients No difference in PFS (median PFS 8.2 m for everolimus vs 10.2 m for control; P = 0.79) OS for everolimus was inferior to that for control patients (median OS: 16.5 vs 21.2 m, respectively; P = 0.008) | |
| mTOR | Temsirolimus | NCT01019434 | Randomized phase II of RT+TMZ versus RT + temsirolimus in newly diagnosed unmethylated GBM | 111 patients randomized Not difference in 1year survival (72.2% in TMZ arm; 69.6% in the temsirolimus arm. (HR 1.16; P = 0.47]. Phosphorylation of mTORSer2448 in tumor (HR 0.13; P = 0.002), detected in 37.6%, associated with benefit from temsirolimus | |
| Phosphatidylinositol 3-kinase (PI3K) | PIK3CA or PIK3R1 mutation, loss of PTEN activity through PTEN mutation, homozygous deletion or negative PTEN expression (<10% of tumor cells that stained positive), or positive phosphorylated AKTS473 (pAKTS473) | Buparlisib224 | NCT01339052 | Multicenter, open-label, multi-arm, phase II trial in patients with PI3K pathway-activated glioblastoma at first or second recurrence | ORR = 0 PFS6 8% Median PFS 1.7 m |
| VEGF | Bevacizumab171 | NCT0094382 (AVAGlio) | Phase III placebo-controlled trial comparing RT + TMZ ± bevacizumab | 921 patients randomized Median PFS longer in the bevacizumab group than in the placebo group (10.6 months vs 6.2 months;HR 0.64; P < 0.001). OS did not differ between groups (HR, 0.88; P = 0.10). | |
| VEGF | Bevacizumab137 | NCT00884741 (RTOG 0825) | Phase III placebo-controlled trial comparing RT + TMZ ± bevacizumab | 637 patients randomized No difference in OS (bevacizumab median, 15.7 m, control 16.1 m (HR 1.13) PFS was longer in the bevacizumab group (10.7 months vs 7.3 months;HR, 0.79) | |
| VEGF | Bevacizumab148 | NCT01290939 (EORTC 26101) | Phase III trial comparing lomustine to lomustine + bevacizumab in recurrent GBM | 437 patients randomized No survival advantage with addition of bevacizumab Median OS 9.1 m with lomustine compared with 8.6 m in combination group (HR 0.95) PFS 4.2 m with bevacizumab + lomustine compared with 1.5 m with lomustine alone (HR 0.49; P < 0.001) | |
| VEGF receptors 1, 2, and 3 and PDGF receptors | No test yet required | Regorafenib193 | NCT02926222 | Randomized phase II comparing regorafenib with lomustine in patients with relapsed glioblastoma (REGOMA): | 7·4 months (95% CI 5·8–12·0) in the regorafenib group and 5·6 months (4·7-7·3) in the lomustine group (hazard ratio 0·50, 95% CI 0·33-0·75; log-rank P = 0.0009) |
| VEGFR2, cMET, AXL, RET | No testing required | Cabozantinib225,226 | NCT00704288 | Single arm phase II in recurrent GBM | 220 patients Bevacizumab naïve 14.5–17.6% ORR; PFS6 22.3 to 27.6% Bevacizumab failure 4.3% ORR. |
| CD95/CD95ligand | Lower levels of methylation of the CpG2 in the promoter of the CD95 ligand | Asunercept219 | NCT01071837 NCT03152708 | Re-irradiation ± asunercept in progressive GBM CAN008 biomarker CD95 Ligand and CpG2 methylation in Chinese patients with GBM | PFS6 rates were 3.8% [95% CI, 0.1–19.6] for rRT and 20.7% (95% CI, 11.2–33.4) for rRT+APG101 (P = 0.048). Ongoing |
Abbreviations: CI, Confidence intervals; GBM, glioblastoma; HR, hazard ratio; mTOR; mammalian target of rapamycin; m, months; MS, Median survival; ORR, objective response rate; PFS, progression-free survival; PFS6, Progression-free survival at 6 months; rRT, reirradiation; SD, stable disease; VEGF, vascular endothelial growth factor.
Several clinical trials are based on well-defined molecular characteristics of the tumor, confirmation of adequate drug penetration and biological efficacy (eg, target engagement and modulation in neoadjuvant, “window-of-opportunity” surgery-based trials),221,224 as well as necessary retrospective validation of potential biomarkers (Table 5). Several large clinical trials are underway where prospectively assigned biomarkers will enrich predefined patient cohorts for potentially benefiting patients. The National Center for Tumor Diseases‒Heidelberg Neuro Master Match (N2M2) (NCT03158389), a trial of molecularly matched targeted therapies plus RT in patients with newly diagnosed glioblastoma without MGMT promoter methylation is currently ongoing.227 Similarly, the National Cancer Institute MATCH trial, while designed mainly for extracranial solid tumors, does allow patients with glioblastoma if they meet the eligibility criteria. The Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT) trial evaluating EGFR, mTOR/DNA-PK, and CDK4/6 inhibitors,228 and the GBM Adaptive, Global, Innovative Learning Environment (AGILE) consortium229 are taking a different approach by enrolling patients into unselected cohorts with given therapies first, assessing potential biomarkers as the trial accrues and integrating this information via adaptive randomization processes to enrich specific arms that may be showing benefit with particular biomarkers (Table 5).229
Table 5.
Selected ongoing trials with molecularly targeted treatments
| Molecular Target | Therapy | Phase | Design | Tumor Type | Trial |
|---|---|---|---|---|---|
| MDM2 | AMG-232 | Phase 0/I | • P53 wildtype status • Phase 0/I to measure concentrations in tumor in patients with recurrent GBM and of AMG 232 in combination with RT in patients with newly diagnosed GBM and unmethylated MGMT promoter |
Recurrent and newly diagnosed GBM | NCT03107780 |
| mTORC1/2 | Sapanisertib (MLN0128) | Phase 0 | • No selection • Phase 0 to evaluate tumor PK and PD effects |
Recurrent GBM | NCT02133183 |
| CDK4/6 | Abemaciclib | 0/II | • Patients with activation of CDK4/6 pathway and intact RB | Recurrent GBM | NCT02981940 |
| CDKs 1, 2, 7, and 9, JAK2 and FLT3 | Zotiraciclib (TG02) with metronomic TMZ | I/randomized phase II | • Phase I with metronomic TMZ followed by randomized phase II comparing zoltiraciclib = TMZ versus TMZ | Recurrent grade III glioma and GBM | NCT02942264 |
| CDKs 1, 2, 7 and 9, JAK2 and FLT3 | Zotiraciclib (TG02) with metronomic TMZ | I | • Zotiraciclib + RT for unmethylated MGMT patients • Zotiraciclib with TMZ for methylated MGMT patients • Zotiraciclib alone for recurrent patients |
Newly diagnosed and recurrent grade III glioma and GBM in elderly population | NCT03224104 |
| H3K27M mutation | ONC201 (dopamine receptor D2 inhibitor and ClpP agonist)212,213 | II | • H3K27M mutated gliomas • Non H3K27M mutated midline GBM |
Recurrent H3K27M mutated gliomas and other GBM | NCT02525692 NCT03295396 |
| Interleukin-4 (IL-4) receptor | MDNA55 | II | • Convection enhanced delivery of genetically engineered IL-4 linked to a modified version of the Pseudomonas aeruginosa exotoxin A | Recurrent GBM | NCT02858895 |
| HIF2α | PTC2977 | II | • No selection | Recurrent GBM | NCT02974738 |
| Proteasome | Marizomib | III | • No selection | Newly diagnosed GBM | NCT03345095 |
| Platform Trials | |||||
| Alk MDM2 SHH CDK4/6 mTOR | Alectinib Idasanutlin Vismodegib Palbociclib Temsirolimus | Phase II | • Umbrella trial N2M2/NOA-20 • Alk expression • P53wild-type/MDM2 high • SHH activation • CDK4/6 high or codeletion of CDKN2A/B • Phospho mTOR Ser 24448 |
Newly diagnosed GBM without MGMT hypermethylation, targeted treatment according to molecular profile | NCT03158389 |
| EGFR mTOR/DNA PK CDK4/6 | Neratinib CC115 Abemaciclib | Bayesian adaptive randomized phase II platform trial | INSIGhT Agnostic (assessment post hoc) | Newly diagnosed unmethylated GBM | NCT02977780 |
| Agnostic (assessment post hoc) | Multiple regimens (regorafenib) | Bayesian adaptive randomized phase II/III platform trial | GBM AGILE Multiple | Newly diagnosed and recurrent GBM | NCT03970447 |
Abbreviations: GBM, glioblastoma; HIF2α, hypoxia-inducible factor alpha; MDM2, mouse double minute 2; m, months; ORR, objective response rate; PD, pharmacodynamics; PK, pharmacokinetics; RB, retinoblastoma; SD, stable disease; SHH, sonic hedgehog.
The extensive tumor heterogeneity in glioblastoma suggests that combination therapy may be more effective than treatment with single agents. However, combination studies to date have been associated with little activity and often significant toxicity, and increase the need for assessment of the targets in the tumor.199,230 Potentially, combinations of more potent selective agents with less off-target effects may be better tolerated. To address the issues of heterogeneity and redundant signaling pathways, there is significant interest in exploiting synthetic lethality (targeting tumor stem cells231) or common downstream pathways with agents such as marizomib, a proteasome inhibitor (NCT03345095), and selinexor, an exportin 1 inhibitor.232
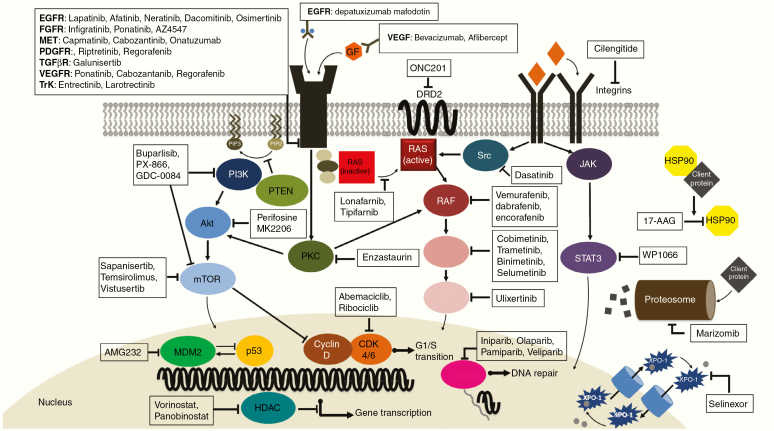
Fig. 10 shows selected targeted molecular therapies evaluated in recently completed or ongoing trials.
Fig. 10.
Selected recently completed or ongoing trials with targeted molecular therapies. CDK = cyclin-dependent kinase; EGF = epidermal growth factor; EGFR = epidermal growth factor receptor; FGFR = fibroblast growth factor receptor; GF = growth factor; HDAC = histone deacetylase; HSP = heat shock protein; MDM2 = murine double minute 2; mTOR = mammalian target of rapamycin; PARP = poly(ADP-ribose) polymerase; PDGFR = platelet derived growth factor receptor; PKC = protein kinase C; RTK = receptor tyrosine kinase; TGF-β = transforming growth factor beta; TGFβR = transforming growth factor beta receptor; TrK = tropomyosin receptor kinase; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor; XPO1 = exportin 1.
Targeting DNA Damage Response Pathways
The most effective nonsurgical treatments for glioma are DNA-damaging agents, including RT and cytotoxic chemotherapy.58 Enhancing their effect in tumor while sparing normal tissue is an appealing strategy that is particularly relevant in tumors such as glioblastoma. One emerging approach is to target tumor-specific DNA repair vulnerabilities in glioblastoma, which appears to have a significant stem cell compartment in which DNA repair is upregulated and contributes to treatment resistance.233,234
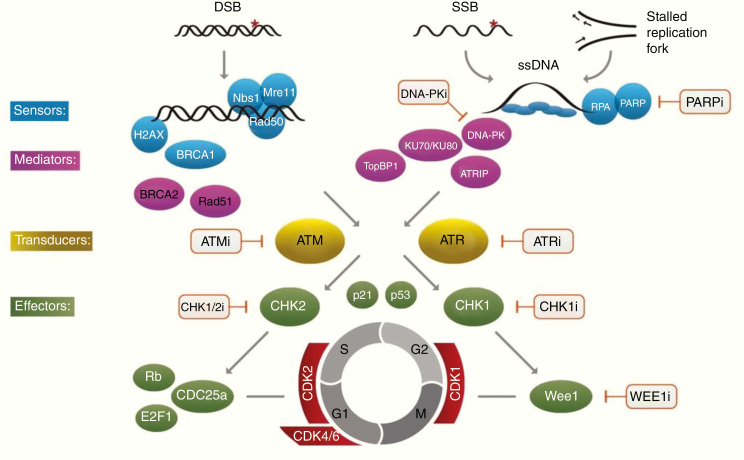
The complex signaling and effector events following DNA damage, often referred to as the DNA damage response (DDR), are summarized in Fig. 11 and have been reviewed recently.236–238 DNA double-strand breaks (DSBs) are the main toxic lesion induced by DNA damaging agents, but single strand breaks (SSBs) are also now recognized as important lesions for lethality. Unrepaired SSBs are thought to stall replication forks, which may indirectly contribute to the DSB load, particularly in the context of replication stress. Combining DNA damaging agents with DDR inhibitors will increase the levels of unrepaired DSBs and SSBs in cells, and thus has the potential for significant chemo- and radiosensitization. However, specific DDR inhibitors, such as poly(ADP-ribose) polymerase (PARP) inhibitors, induce myelosuppression when given with DNA damaging agents, potentially limiting their use in combination with TMZ. As such, it will be important to identify glioma-associated molecular biomarkers (eg, tumor mutations not found in normal tissue), which could allow the administration of active but safe drug combinations.
Fig. 11.
A simplified overview of signaling from common types of DNA damage to the DDR and cell cycle checkpoint pathways. Initial damage is sensed by proteins including the histone γ -H2AX, which is rapidly phosphorylated by ATM at a specific serine residue in response to chromatin structure alteration at DBS sites, activating recruitment of repair proteins including BRCA1 and the MRN complex (MRE11, Rad51, NBS1). DSB repair is undertaken by the end-joining pathway involving the kinase DNA-PK and Ku protein binding partners and the homologous recombination pathway involving Rad51 and associated proteins. Single strand breaks (SSB) and replication stress leading to stalled replication forks activate PARP which in turn recruits repair factors including XRCC1 and promotes chromatin remodeling at the break site and base excision repair. ATR and ATM function both in the initial signaling cascade and as transducers to downstream activation of the cell cycle checkpoints inhibitors, Chk1 and Chk2 producing cell cycle delay to facilitate repair. Points in the pathway at which specific inhibitors are available are indicated. As predicted from their roles in the DDR pathway, ATM and ATR inhibitors sensitize to a broad range of DNA damaging agents causing single or double strand breaks. PARPi and cell cycle checkpoint inhibitors including Wee1 inhibitors are specifically effective in cells undergoing rapid replication. DSB = Double Strand Break; SSB = Single Strand Break.
Multiple DDR inhibitors are now being tested in clinical trials for glioblastoma (summarized in Table 6). Recent studies have elucidated important links between intrinsic DNA repair defects and sensitivity to specific DDR inhibitors in glioblastoma, which likely will serve as key molecular biomarkers for patient selection in these trials. Loss of MGMT protein expression is a possible predictor for TMZ sensitivity, and emerging data suggest that it may also be an important biomarker for TMZ-based combinations with inhibitors of PARP, ataxia telangiectasia, and Rad3-related protein.239–242
Table 6.
Current Clinical Trials Testing DDR Inhibitors in Glioma
| Target | Agent | Trial Name | Phase | Regimen | Tumor Type(s)/ Patient Populations | Status | PI/Co-PI | Trial ID(s) |
|---|---|---|---|---|---|---|---|---|
| PARP | Veliparib | A071102 (Alliance) | 2/3 | TMZ-/+Veliparib | Newly diagnosed GBM, Adults | Active, not recruiting | Sarkaria | NCT02152982 |
| Olaparib | OPARATIC | 1 | Olaparib and TMZ | Recurrent GBM, adults | Completed | Chalmers | - | |
| PARADIGM | 1/2 | Olaparib and RT | Newly diagnosed GBM, 65+ y | Recruiting (Phase II) | Chalmers | - | ||
| PARADIGM-2 | 1 | Olaparib and RT | Newly diagnosed GBM, Adults | Recruiting (MGMT- cohort) | Chalmers | - | ||
| ETCTN 10129 | 2 | Monotherapy | Recurrent IDH1/2-mutant glioma, adults | Recruiting | LoRusso/Bindra | NCT03212274 | ||
| BGB290 | Study 104 | 1/2 | BGB290 with RT and/or TMZ | Recurrent GBM, adults | Recruiting | Brachman | NCT03150862 | |
| ABTC-1801 | 1/2 | BGB290 with TMZ | Recurrent IDH1/2-mutant glioma, adults | Recruiting | Bindra/Schiff | NCT03914742 | ||
| PNOC017 | 1 | BGB290 with TMZ | Recurrent IDH1/2-mutant glioma, ages 13–25 y | Recruiting | Marks/Bindra | NCT03749187 | ||
| ATM | AZD1390 | AstraZeneca | 1 | AZD1390 and RT | Newly diagnosed and recurrent GBM, adults | Recruiting | Wen | NCT03423628 |
| DNA-PK | CC115 | INSIGhT | GBM | CC115 and GBM | Newly diagnosed GBM, adults | Completed | Wen/Alexander | NCT02977780 |
| Wee1 | AZD1775 | ABTC-1202 | GBM | AZD1775, TMZ and RT | Newly diagnosed and recurrent GBM, adults | Completed | Lee | NCT01849146 |
| AZD1775 | - | 0 | Monotherapy | Recurrent GBM, adults | Completed | Sanai | NCT02207010 |
Abbreviations: RT, Radiotherapy; TMZ, TMZ; GBM, Glioblastoma.
Targeting Tumor Metabolism
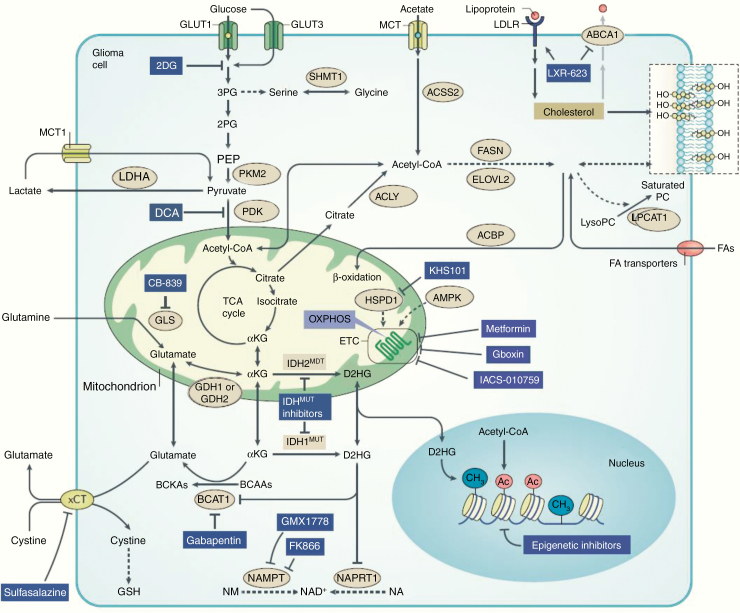
In the past decade there have been converging data to support tumor metabolism as a key determinant of glioma progression. Oncogenic mutations modulate glioblastoma metabolism to promote survival, proliferation, and evasion of therapy, in addition to tumor microenvironmental factors influencing glioblastoma metabolism.20,242 Data suggest that regulators of glioblastoma metabolism can be used as prognostic, diagnostic, and therapeutic tools that can advance management of glioblastoma.20 There is also growing evidence that tumor genotype and the brain’s biochemical and cellular microenvironment shape the metabolic reprogramming of glioblastoma cells, generating vulnerabilities that could be exploited therapeutically (Fig. 12).20
Fig. 12.
An expanded pharmacopoeia of metabolic drug targets in glioblastoma (Adapted with permission from Bi et al. Nature Rev Cancer 2020;20:57–70.) The extensive focus on altered glioma metabolism has led to a considerable expansion in the list of potential drug targets. Receptor tyrosine kinase (RTK)-driven metabolic dependencies have also been identified. For example, epidermal growth factor receptor (EGFR) amplification produces major changes in metabolic enzyme dependencies, including in glucose uptake, glycolysis, fatty acid (FA) synthesis, membrane lipid remodeling, cholesterol uptake, NAD+ production and epigenetic remodeling. Targeting lysophosphatidylcholine (LysoPC) acyltransferase 1 (LPCAT1) decreases the level of saturated phosphatidylcholines (PCs) and disrupts plasma membrane localization of EGFR variant III (EGFRvIII), which blocks EGFRvIII- driven oncogenic signaling and suppresses glioblastoma (GBM) tumor growth. LXR-623, a brain- penetrant liver X receptor (LXR) agonist, targets the cholesterol homeostasis of GBM cells by promoting ATP- binding cassette subfamily A member 1 (ABCA1)-mediated cholesterol efflux and inhibiting low- density lipoprotein receptor (LDLR)-mediated cholesterol uptake. Isocitrate dehydrogenase (IDH) mutants in glioma cells generate the oncometabolite d-2-hydroxyglutarate (D2HG), which defines the dependencies of NAD+ and glutathione (GSH) production and impacts epigenetic events in glioma cells. The oxidative phosphorylation (OXPHOS) inhibitors, including metformin, Gboxin and IACS-010759, target glioma cells by inhibiting transmembrane protein complexes in the mitochondrial inner membrane, known as the electron transport chain (ETC). 2DG, 2-deoxy-d-glucose; 2PG, 2-phosphoglyceric acid; αKG, α-ketoglutarate; ACBP, acyl-CoA-binding protein; ACLY, ATP citrate lyase; ACSS2, acetyl-CoA synthetase; AMPK, AMP-activated protein kinase; BCAA, branched-chain amino acid; BCAT1, branched- chain amino acid transaminase 1; BCKA, branched-chain keto acid; BRD4, bromodomain containing 4; DCA, dichloroacetate; ELOVL2, ELOVL FA elongase 2; FASN, FA synthase; GDH1, glutamate dehydrogenase 1; GLS, glutaminase; GLUT1, glucose transporter 1; HSPD1, heat shock protein family D (Hsp60) member 1; IDH1, isocitrate dehydrogenase 1; LDHA, lactate dehydrogenase A; MCT1, monocarboxylate transporter 1; NA, nicotinic acid; NAMPT, nicotinamide phosphoribosyl-transferase; NAPRT1, nicotinate phosphoribosyltransferase domain containing 1; NM, nicotinamide; PDK, pyruvate dehydrogenase kinase; PEP, 2-phosphoenolpyruvate; PKM2, pyruvate kinase muscle isozyme M2; SHMT1, serine hydroxymethyltransferase 1; TCA, tricarboxylic acid; xCT, cystine/glutamate transporter. Reproduced with permission from Bi J, et al. 20
A classic and recognized biochemical adaptation in glioblastomas, as with other solid cancers, is the metabolic shift to aerobic glycolysis rather than mitochondrial oxidative phosphorylation, regardless of oxygen availability, a phenomenon referred to as the Warburg effect.20 Targeting genes that regulate tumor metabolism can be an ideal candidate for rational drug design. Some of the regulators involved in glioblastoma have been shown to be PTEN induced kinase 1 (PINK1)243 and hexokinase 2 (HK2),244 where inhibition of HK2 and activation of PINK1 in preclinical models have shown therapeutic benefit in glioblastoma. Similarly, cholesterol metabolism may be a therapeutic target in certain glioblastomas. In EGFR driven tumors, there is high dependency on cholesterol uptake, rendering the glioblastoma cells vulnerable to liver X receptor agonists, which reduce cholesterol uptake.20,245
Immunotherapies
Unlike several other solid tumors, no breakthrough has been achieved with current immunotherapy strategies for glioblastoma.21,246 Although the concept that relieving glioma-associated immunosuppression to allow immune-mediated antitumor responses is attractive and has received preclinical experimental support,247–252 clinical trials testing this hypothesis using targeted therapies such as transforming growth factor beta (TGF-β)253 or colony-stimulating factor receptor inhibitors,254 vaccines,202 or more recently, immune checkpoint blockade with the anti–programmed cell death protein 1 (PD1) antibody nivolumab in recurrent and newly diagnosed MGMT unmethylated glioblastoma, as well as other agents were unsuccessful (Table 7).21,246,255 However, that does not necessarily imply that attempts to improve outcome with immunotherapy approaches may not be effective, as appropriate target inhibition in the glioma microenvironment or even immunogenicity assessments were largely lacking, and read-out was mainly limited to classical efficacy endpoints. As more is learned about the role of the tumor microenvironment in immunotherapy responses, it may be possible to enrich for select glioblastoma patient subsets that respond to specific immunotherapy regimens.
Table 7.
Selected completed trials with immunotherapies (including viral therapies)
| Vaccines | Type | Phase | Trial | Trial Result |
|---|---|---|---|---|
| ICT107 (dendritic cell vaccine against MAGE-1, HER-2, AIM-2, TRP-2, gp100, and IL13Rα2)273 | Newly diagnosed GBM | Randomized placebo controlled phase II | NCT01280552 | • 124 patients randomized • PFS increased by 2.2 months (P = 0.011) • OS increased by 2.0 months (NS) • HLA-A2 subgroup showed increased clinical benefit and immune response |
| Rindopepimut (EGFRvIII peptide vaccine) ACT IV202 | Newly diagnosed GBM with EGFRvIII mutation | Double blind phase III | NCT01480479 | 745 patients randomized • No difference in outcome with addition of rindopepipimut • Median overall survival was 20·1 months (95% CI 18·5–22·1) in the rindopepimut group versus 20·0 months (18·1–21·9) in the control group (HR 1·01, 95% CI 0·79-1·30; P = 0.93) |
| DC Vax (dendritic cell vaccine)256 | Newly diagnosed GBM | Phase III | NCT00045968 | • 331 patients treated • Primary endpoint of PFS not reported • 90% crossover at progression • Median OS was 23.1 months from surgery |
| Checkpoint Inhibitors | Type | Phase | Trial | Trial result |
| Nivolumab versus bevacizumab (CheckMate 143)255 | Recurrent GBM | III | NCT02017717 | • 369 patients randomized • No difference in outcome between the nivolumab or bevacizumab arm • Median OS was 9.8 months with nivolumab and 10.0 months with bevacizumab (NS), and the 12-mo OS rate was 42% in both arms. • Median PFS was 1.5 months for nivuolumab and 3.5 months for bevacizumab ORRs were 8% for nivolumab and 23% for bevacizumab • No steroid use and MGMT promoter methylation were associated with longer OS in the nivolumab arm versus the bevacizumab arm |
| Viral Therapies | Type | Phase | Trial | Trial Result |
| DNX-2401 (Delta-24-RGD) Oncolytic adenovirus257 | Recurrent GBM | I | NCT02197169 | • 37 patients • Fairly well-tolerated • 20% of patients survived > 3 years • 12% response • Evidence of virus replication in tumor • Cases of pseudoprogression |
| Polio virus (PVSRIPO)258 | Recurrent GBM | 1 (convection enhanced delivery) | NCT01491893 | • 61 patients enrolled • Dose level −1 (5.0×107 TCID50) was identified as the phase 2 dose • 19% of the patients had a PVSRIPO-related adverse event of grade 3 or higher • OS reached a plateau of 21% (95% confidence interval, 11 to 33) at 24 months that was sustained at 36 months |
| Ad-RTS-hIL12 (adenovirus producing IL-12) + velidimex259 | Recurrent GBM | 1 | NCT03679754 | • 31 patients • Fairly well tolerated but cytokine syndrome observed in some • Median OS = 12.7 months • Inflammatory responses seen in recurrent tumors • Concurrent corticosteroids negatively affected survival: Patients cumulatively receiving >20 mg versus ≤20 mg of dexamethasone (days 0 to 14), median OS was 6.4 and 16.7 months, respectively |
| Gene mediated cytotoxic immunotherapy (GMCI; AdV-Tk) + valacyclovir + RT and TMZ260 | Newly diagnosed GBM | II | NCT00589875 | • 48 patients • No dose-limiting toxicities • Median OS was 17.1 months for GMCI + SOC versus 13.5 months for SOC alone (P = 0.0417) • Greatest benefit was observed in gross total resection patients: median OS of 25 versus 16.9 months (P = 0.0492) |
| TOCA 511 (replication competent retrovirus which transduces tumor cells with the cytosine deaminase gene) in combination with TOCA FC (5-flucytosine) versus SOC (TOCA 5 Study)261 | Recurrent GBM | II/IIII | NCT02414165 | • 403 patients treated • Fairly well tolerated • No difference in primary endpoint of OS between TOC 511 and lomustine (HR = 1.06 (95% CI: 0.83, 1.35; P-value = 0.6154) • Median OS: Toca 511: 11.07 months • SOC 12.22 months • Durable response rate 2.5% with TOCA 511; 4.5% with SOC (NS) |
GBM; glioblastoma; NS, not significant; SOC, standard of care; Tk, thymidine kinase.
It has become evident that glioblastoma is an immunologically “cold” tumor characterized by a paucity of tumor infiltrating effector lymphocytes.18,21 In understanding mechanisms of immune resistance, we have evolved from the 3 “E” hypothesis of elimination, equilibrium, and escape.262 Hence to reconcile this concept of “hot” and “cold” tumors with the 3 “E” hypothesis, we must also consider the magnitude of a tumor’s adaptive and intrinsic resistance.18 Factors that drive intrinsic resistance for glioblastoma include a paucity of neoantigens (most glioblastomas have low mutational burden relative to other cancers) and active inhibition including the release of soluble immunosuppressive mediators such as TGF-β, interleukin (IL)-10, and prostaglandin E2, and production of tryptophan and indolamine 2,3 dioxygenases and arginase, which deplete tryptophan and arginine and result in the accumulation of metabolites such as kynurenine, leading to suppression of T-cell activity.18,21,263 Furthermore, there are data to suggest that location (ie, having a tumor in the brain) negatively influences the immune system globally by actively deleting antigen specific T cells264 and potentially sequestering them in the bone marrow.265 Glioblastoma induces adaptive resistance by promoting exhaustion of infiltrating T cells266 and recruiting suppressive myeloid cells and regulatory T cells.18,246 In addition, the corticosteroids frequently used in these patients also contribute to immunosuppression and impair the efficacy of immunotherapies.259,267
The immunologically “cold” microenvironment of glioblastoma tumors likely contributed to the negative phase III studies of the PD1 antibody nivolumab in patients with recurrent (CheckMate-143)21,255 and MGMT unmethylated, newly diagnosed (CheckMate-498) glioblastoma. Hence current strategies are focusing on overcoming both intrinsic and/or adaptive resistance. Efforts to enhance effector immune infiltrate into the microenvironment such as cellular therapies including chimeric antigen receptor (CAR) T cells,269,270 oncolytic viruses (OVs),259,271,272 and vaccines267,273,274 are being developed to meet this challenge (Table 8).
Table 8.
Examples of ongoing trials with immunotherapies
| Vaccine | Tumor Type | Phase | Trial |
|---|---|---|---|
| Personalized neoantigen cancer vaccine with RT and pembrolizumab | Newly diagnosed unmethylated GBM | I | NCT02287428 |
| Personalized neoantigen DNA vaccine in combination with immune checkpoint blockade therapy | Newly diagnosed unmethylated GBM | I | NCT03068832 |
| Personalized neoantigen- based vaccine in combination with nivolumab and ipilimumab | Newly diagnosed unmethylated GBM | I | NCT03422094 |
| Personalized peptide vaccine in combination with standard therapy and TTFields | Newly diagnosed GBM | I | NCT03223103 |
| pp65 CMV RNA-Pulsed Dendritic Cells With Tetanus-Diphtheria Toxoid Vaccine | Newly diagnosed | II | NCT02465268 |
| SurVaxM (peptide vaccine against survivin) with TMZ following RT and TMZ | Newly diagnosed GBM | II | NCT02455557 |
| INO-5401 (DNA plasmids targeting Wilms tumor gene-1 (WT1) antigen, prostate-specific membrane antigen (PSMA) and human telomerase reverse transcriptase (hTERT) genes) and INO-9012 (DNA plasmid expressing IL-12) in combination with cemiplimab (REGN2810), with radiation and chemotherapy | Newly diagnosed GBM | I/II | NCT03491683 |
| VXMO1 (Attenuated Salmonella typhi Ty21a carrying a plasmid encoding for vascular endothelial growth factor receptor (VEGFR)-2) + avelumab | Recurrent GBM | I | NCT03750071 |
| CMV vaccine VBI-1901 | Recurrent GBM | I | NCT03382977 |
| CMV pp65 peptide DC vaccine in combination with nivolumab | Recurrent GBM | I | NCT02529072 |
| Pembrolizumab and ATL-DC (dendritic cell tumor lysate) vaccine | Recurrent GBM | I | NCT04201873 |
| EO2401, Multipeptide Vaccine, With and Without Check Point Inhibitor | Recurrent GBM | I/II | NCT04116658 |
| IMA950/Poly-ICLC + pembrolizumab | Recurrent GBM | I/II | NCT03665545 |
| CMV RNA- loaded DC vaccine +/– anti- CD27 therapy varlilumab (to deplete Treg cells) | Recurrent GBM | II | NCT03688178 |
| WT1 peptide vaccine (DSP-7888) in combination with bevacizumab | Recurrent GBM | II | NCT03149003 |
| SurVaxM + pembrolizumab | Recurrent GBM | II | NCT04013672 |
| Checkpoint Inhibitors and combinations | Tumor Type | Phase | Trial |
| Nivolumab + Gene Mediated Cytotoxic Immunotherapy (GMCI; Ad-Tk) + valacyclovir + RT + TMZ | Newly diagnosed GBM | I | NCT03576612 |
| Nivolumab + ipilimumab + short course RT | Newly diagnosed unmethylated GBM | I | NCT03367715 |
| Nivolumab, IDO inhibitor (BMS-986205), and RT With or Without TMZ | Newly diagnosed GBM | I | NCT04047706 |
| Pembrolizumab + vorinostat (HDAC inhibitor) with RT and TMZ | Newly diagnosed GBM | I | NCT03426891 |
| Atezolizumab + RT and TMZ | Newly diagnosed GBM | I/II | NCT03174197 |
| Nivolumab + TMZ versus TMZ (NUTMEG) | Newly diagnosed elderly GBM | II | NCT03367715 |
| Nivolumab + Gliadel wafers | Newly diagnosed GBM | II | |
| RT +/- Nivolumab (CheckMate 498) * | Newly diagnosed unmethylated GBM | III | NCT02617589 |
| RT + TMZ +/- Nivolumab (CheckMate 548)# | Newly diagnosed methylated GBM | III | NCT02667587 |
| Nivolumab +anti-LAG3 or anti-CD137 | Recurrent GBM | I | NCT02658981 |
| Avelumab + laser interstitial therapy | Recurrent GBM | I | NCT03341806 |
| Nivolumab + Ad-RTS-hIL12 (adenovirus producing IL-12) + velidimex | Recurrent GBM | I | NCT03636477 |
| Cemiplimab + Ad-RTS-hIL12 + velidimex | Recurrent GBM | I | NCT04006119 |
| Biomarker driven therapy using immune activators with nivolumab (Nivolumab + anti-GITR (MK4166) or Nivolumab + IDO1 inhibitor (INCB024360) or Nivolumab + Ipilimumab | Recurrent GBM | I | NCT03707457 |
| Anti-TIM3 monoclonal (TSR-022) +/– nivolumab | Advanced solid tumors | I | NCT02817633 |
| Indoximod (IDO inhibitor) and TMZ | Recurrent GBM | I | NCT02052648 |
| Neoadjuvant pembrolizumab | Recurrent GBM undergoing surgery | II | NCT02852655 |
| Pembrolizumab | Hypermutated recurrent GBM | II | NCT02658279 |
| Nivolumab +ipilimumab | Hypermutated recurrent GBM | II | |
| Nivolumab | Recurrent IDH mutated glioma | II | NCT03718767 |
| Pembrolizumab and re-irradiation | Recurrent GBM (bevacizumab naïve or refractory) | II | NCT03661723 |
| Nivolumab + standard or low dose bevacizumab | Recurrent GBM | II | NCT03452579 |
| Nivolumab + ipilimumab + TTF | Recurrent GBM | II | NCT03430791 |
| Pembrolizumab + DNX-2401 (CAPTIVE) | Recurrent GBM | II | NCT02798406 |
| Pembrolizumab + abemaciclib (CDK4/6 inhibitor) | Recurrent GBM | II | NCT04118036 |
| Pembrolizumab + levantinib (VEGFR and multikinase inhibitor) | Recurrent GBM | II | NCT03797326 |
| Intratumor INT230-6 (amphiphilic cell penetration enhancer molecule combined with cisplatin and vinblastine) + nivolumab and ipilimumab | Recurrent GBM + other cancers | I/II | NCT03058289 |
| Targeted Therapies and Other Agents | Tumor Type | Phase | Trial |
| WP1066 (STAT 3 inhibitor) | Recurrent GBM and melanoma | I | NCT01904123 |
| Cellular Therapies Including CAR T cells | Tumor Type | Phase | Trial |
| EGFRvIII CAR T cells + pembrolizumab | Newly diagnosed EGFRvIII mutated unmethylated GBM | I | NCT03726515 |
| Intracerebral EGFRvIII CAR T cells (INTERCEPT) | Recurrent GBM | I | NCT03283631 |
| IL13Ralpha2-Targeted CAR T cells with or without nivolumab and ipilimumab | Recurrent GBM | I | NCT04003649 |
| Memory-Enriched T Cells Transduced to Express a HER2-Specific, Hinge-Optimized, 41BB-Costimulatory Chimeric Receptor and a Truncated CD19 | Recurrent GBM | I | NCT03389230 |
GBM, glioblastoma; HDAC, histone deacetylase; VEGFR2, RT, radiotherapy; TMZ, termozolomide; vascular endothelial growth factor receptor 2; TTF, tumor treating fields.
*Reported to be negative.
# PFS reported to be negative; OS results pending.
Sporadic partial and complete responses with immune checkpoint blockade among patients with hypermutated tumors due to germline DNA repair deficits suggest that these tumors likely exhibit low innate resistance and possess immunologically relevant mutations, including tumor-specific neoantigens or tumor-associated antigens that the immune system can recognize and attack.275,276 However, the numbers of mutations alone may not be sufficient to generate an immune response. Roughly 10% of patients may develop hypermutated tumors at recurrence after TMZ chemoradiotherapy,36,277 and an 8% response rate was seen in the CheckMate-143 study with nivolumab in recurrent glioblastoma.255 Yet, importantly, tumor mutational burden at recurrence was not captured in this study, precluding firm assumptions that hypermutation was indeed associated with response; in fact, the immunogenicity and clonality of mutations, not just their quantity, may determine responsiveness to immunotherapy.278 Furthermore, the negative phase III study of rindopepimut,202 an EGFRvIII peptide vaccine, argues that targets must be present and stably expressed in all tumor cells or that targeting multiple tumor antigens may be important. Current approaches to overcome intrinsic resistance have revolved around novel antigen identification strategies by targeting multiple overexpressed and private mutations derived from NGS267,274,279 and mass spectrometry analysis of the human leukocyte antigen (HLA) ligandome. Whereas peptide-based approaches targeting these antigens have been successful in eliciting systemic as well as local antigen-specific CD8 responses, the limited magnitude of the response may benefit from other potentially augmentative strategies such as transgenic T-cell receptors, combinations with appropriate checkpoint inhibitors, or other measures targeting the suppressive myeloid compartment.21,246
Approaches to overcome adaptive resistance for glioblastoma initially focused on checkpoint molecules.21,246 This approach has not been successful for at least two key reasons: first, intratumoral T cells are severely exhausted with loss of effector function and hence these cells appear to not be rescuable with immune checkpoint inhibitor therapy; and second, myeloid cells including macrophages and/or microglia are programmed by glioblastomas to be highly suppressive in the tumor microenvironment.18,251 Therapies such as OVs that can activate macrophages from an M2 to an M1 phenotype, induce antigen presentation, and promote migration of antigen presenting cells to regional lymph nodes may overcome some of these obstacles.272 However, it should be noted that since macrophages exist in complex activation continuums the situation is likely to be more complicated.280 Combination approaches with local therapies such as stereotactic radiosurgery,248,249 laser ablation (NCT03277638), and local chemotherapy281 are also potential means to overcome the adaptive resistance of glioblastomas. Another interesting approach is the concept of neoadjuvant anti-PD1 treatment administered prior to planned debulking surgery. Two recent studies demonstrated favorable modulation of local immune reactivity in recurrent glioblastoma patients using such an approach.282,283 An improved outcome in one of these studies,282 as well as encouraging benefit in other solid tumors, including significant rates of pathologic response,284,285 suggest that further evaluation of neoadjuvant checkpoint administration in glioblastoma is warranted.
There is also growing interest in cellular therapies, especially CAR T cells, and more recently, CAR-transduced natural killer cells. CAR T cells have been engineered to express CAR molecules on their surface, which allow them to recognize and bind to specific antigens on tumor cells, leading to target cell killing in an HLA-independent manner.286 Although one patient with leptomeningeal spread of glioblastoma responded to treatment with CAR T cells against IL-13 receptor subunit alpha-2,269 the experience with CAR T cells to date for glioblastoma has been generally disappointing.270,286 Current efforts are focused on developing next generation CAR T cells directed against multiple antigens, designing them to induce epitope spreading, combining them with checkpoint inhibitors to help overcome immunosuppression or with conventional therapies such as radiotherapy, and delivering them directly into the tumor.21,287,288
Viral Therapies
There has been resurgent interest in OVs and gene therapy (GT) in clinical trials for glioblastoma.272 Oncolytic viruses are either natural viral strains or genetically engineered viruses designed to infect and/or replicate selectively in tumor cells.289,290 Gene therapy instead utilizes viruses that have been rendered replication incompetent but deliver anticancer cDNAs. With either therapy there is an initial phase of direct cytotoxic activity caused by OV replication or the GT-delivered anticancer cDNA. This cytotoxicity may then induce a second phase of innate and adaptive antitumor immunity caused by released tumor antigens.291 There is one OV (talimogene laherparepvec) that has been FDA approved for melanoma,292 and several GTs have been approved since 2017, such as voretigene neparvovec (Luxturna) for blindness and onasemnogene abeparvovec (Zolgensma) for spinal muscular atrophy.
For glioblastoma, several phase I clinical trials of both OVs and GTs are in progress or have been completed, usually in the recurrent setting.293,294 In most, the treatment is delivered by intratumoral injection at the time of surgery. Currently there are several open GT trials (see Clinicaltrials.gov) that include: (i) injection in the resected recurrent glioblastoma cavity of an adenoviral GT vector that delivers an IL-12 cDNA whose transcription is activated by an oral agent, veledimex (NCT03636477)259; and (ii) injection into the resected newly diagnosed glioblastoma cavity of an adenoviral vector that delivers a thymidine kinase cDNA that leads to cytotoxicity when subjects take the oral drug, valacyclovir, combined with chemoradiation (NCT03576612).260 Both trials also entail neoadjuvant or adjuvant immune checkpoint inhibition to counteract T-cell dysfunction. The latter trial is also being tested in pediatric brain tumors. Currently open OV trials in patients with recurrent glioblastoma include: (i) stereotactic injection of an oncolytic herpes simplex virus type 1 (oHSV) that delivers an IL-12 cDNA (NCT02062827); (ii) stereotactic injection of an oHSV that has been engineered to replicate better in glioblastoma cells that express the stem cell marker, nestin (NCT03152318); and (iii) monthly injections of oHSV G47∆.295 There are also trials delivering OV with stem cells: (i) intra-arterial delivery of allogeneic bone marrow‒derived human mesenchymal stem cells loaded with the oncolytic adenovirus DNX-2401 (BM-hMSCs-DNX2401) (NCT03896568); and (ii) injection of neural stem cells that deliver an oncolytic adenovirus into newly diagnosed glioblastoma (NCT03072134). A general conclusion is that both OV and GT treatments have been well tolerated. When post-tissue treatment is available, there has been evidence of increased infiltration of immune cells, including cytotoxic T cells that also may have upregulated inhibitory immune checkpoint signaling.296
More advanced trials (phase II and beyond) are also ongoing mostly for OVs in the recurrent glioblastoma setting. These include: (i) convection-enhanced delivery of an engineered poliovirus (PVSRIPO; NCT02986178); 258 (ii) stereotactic injection of an oncolytic adenovirus with selectivity for glioblastoma cells driven by the p16/retinoblastoma (RB) pathway and integrin expression (tasadenoturev; DNX-2401) in combination with pembrolizumab (NCT02798406); 257 and (iii) intracavitary injection after glioblastoma resection of a retrovirus that delivers a cytosine deaminase cDNA that provides chemosensitivity to 5-fluorocytosine (Toca 511; NCT02414165).271,297 However, the phase III trial of Toca 511 was recently reported to show no survival benefit compared with standard of care in patients with recurrent glioblastoma.261 A phase III trial of another viral therapy, ofranergene obadenovec (VB-111), which targets tumor endothelium, also failed to show a survival advantage in combination with bevacizumab compared with bevacizumab alone,298 although it is possible that the simultaneous administration of bevacizumab may have impeded the effects of the virus.298,299 Therefore, while there is currently optimism in the pursuit of novel concepts, this optimism must also be coupled with ongoing efforts to find molecular and immunologic variables and targets that may be associated with benefit.
Other Therapies
Overall, almost 100 therapies are under evaluation for glioblastomas. In addition to the ones listed above, other treatments include cytotoxic agents such as Val 083 (NCT02717962), BAL101553 (NCT03250299), and agents that may augment the activity of TMZ, such as ibudilast (NCT03782415).
Improving Clinical Trial Design
An important factor limiting the development of more effective therapies for glioblastoma is the slow and inefficient clinical trial process. Frequently glioblastoma patients are excluded from phase I oncology trials evaluating novel agents without sound rationale.300 As glioblastoma patients are generally healthy,301 greater inclusion of these patients in phase I oncology trials will facilitate the identification of novel agents for further testing at an earlier stage.
Since the ability of many agents to cross the BBB to achieve therapeutic tumor concentrations and inhibit the appropriate molecular pathways adequately is either unknown or inadequate, there is a need for more “window-of-opportunity” “phase 0” surgical trials early during drug development. In these studies patients receive a therapeutic agent for 1–2 weeks prior to surgery, and tumor from both enhancing and non-enhancing areas obtained at surgery are analyzed for drug concentrations and pharmacodynamic effects. There is a need to develop more efficient clinical trial networks focused on these studies to identify agents worthy of further development.
Most treatments in glioblastoma have been initially assessed in uncontrolled single-arm studies using PFS or OS compared with contemporary or historical controls as primary endpoints. Limitations of these approaches have been the inadequacy of historical controls compared with external control data from prior trials302 and, commonly, the failure to develop a biomarker to enrich patient populations in parallel or to inform on likelihood of success or failure of a new treatment. These shortcomings led to several inadequate phase II to III transitions, including the development of cilengitide,303 enzastaurin,304 bevacizumab,148,186 cediranib,190 rindopepimut,202 and nivolumab.255 Moreover, the frequent failure to understand why these trials were unsuccessful prevented lessons that could be learned to inform the design of future trials. No single therapeutic biomarker, not even MGMT promoter methylation, has been uniformly applied despite evidence that even TMZ only benefited approximately a third of patients with glioblastoma.49 Beyond that, it seems unlikely today that there are new treatment options that would be active in an all comer trial, reinforcing the need for more elaborate research efforts prior to embarking on large clinical trials.
Steps to increase the likelihood of a drug to be successful in glioblastoma include preclinical modeling and window-of-opportunity (phase 0) surgical assessment, the parallel (and mandatory) assessment of tissue, CSF or blood for biomarkers and molecular imaging that may aid in enriching for benefiting versus failing patients, and the use of active, randomized control groups in earlier stages.302 Innovative clinical trial concepts are based on the idea that phase II clinical evaluation must have a control arm, but could add several experimental arms. The endpoints of such trials might be pharmacodynamic-biomarker based or mainly imaging based—relying on more advanced MRI (or PET) techniques, including artificial intelligence algorithms94,95—and allow termination or expansion of cohorts in a dynamic fashion based on their likelihood of success. If biomarker based, adaptations may take place on the basis of quick, prospectively assessed biomarkers that are correlated to patients’ performance with a given treatment and allow enrichment for subsequent biomarker-positive patients with the same therapy. Examples of this approach are GBM AGILE (NCT03970447)229 and INSIGhT (NCT02977780).228 Another opportunity is the assignment of patients to a specific therapy based on real-time assessment of a panel of biomarkers to be tested or even on high-throughput molecular tumor characterization, to allow (theoretically) treatment in the group of greatest likelihood of success, as done in the N2M2 trial.227 These trials are most efficiently performed with multiple experimental groups and one standard arm. The basic requirements are a recent, non-historical tissue sample, adequate tests and assignment algorithms, and a broader tumor board, including biomarker/bioinformatics specialists.
The only randomized effort so far to evaluate the value of treatment allocation based on molecular testing over standard of care, the French SHIVA trial,305 comes from the non–neuro-oncology area and failed to demonstrate overall benefit using historical tissue information guiding treatment decisions at progression. Nonetheless, there are many options to improve on this important first effort. One example is the WINTHER trial, which tested the role of the tumor transcriptome in identifying tumor vulnerabilities that may be treated with targeted therapies, an approach that may expand data-driven therapeutic options for patients.306 While such modern clinical trial concepts are innovative and promising, the risk is that most glioblastomas are not a single pathway-driven disease and therefore will not be amenable to single agent targeted therapy selected based on molecular profiling.
As the design of clinical trials improves, it will also be important to consider incorporation of patient-reported outcomes and the input of patient advocates.
Challenges and Future Directions
Despite decades of research into the biology and treatment of glioblastoma, many challenges remain to treat this universally lethal cancer (Fig. 13). Glioblastomas are particularly aggressive and treatment refractory, resulting in disproportionate mortality, as reflected in the fact that they account for only 1.4% of cancers but 2.9% of cancer-related deaths.307
Figure 13.
Challenges to effectively treat GBM include (top) the presence of the blood–brain barrier that precludes the delivery of many drugs into the brain coupled to (middle) an immunosuppressive microenvironment and (bottom) compensatory signaling networks that can render GBM therapies ineffective.
A major impediment to improving outcome is the fact that currently only approximately 11% of newly diagnosed glioblastoma patients enroll in clinical trials.308 Reasons for this deficiency were recently reviewed and include many factors, including lack of knowledge regarding availability of trials309 and the fact that physical or cognitive symptoms may reduce ability or willingness to travel for patients in the community who are seeking clinical trials at academic centers. Indeed, a survey of 57 patients demonstrated that travel time below 1 hour was significantly (4x) associated with increased willingness to consider clinical trial participation.310 Developing strategies to improve clinical trial accrual will be critical.309 Overly strict eligibility criteria are another barrier to accrual, and efforts are under way to address this.311
There are many other challenges that need to be addressed to improve therapy for patients with glioblastoma. A key consideration is the CNS location of these tumors and the need to consider treatment-related neurologic toxicities (eg, RT-induced neurocognitive injury, accelerated atherosclerotic disease),312 which may profoundly impact quality of life. Another critical consideration is the BBB. Glioblastomas reside in and intertwine with the brain, where these tumors exploit the brain’s natural defense mechanism against toxins via the BBB.22,313 The BBB is composed of endothelial cells linked by tight junctions against a basement membrane that are surrounded by pericytes and astrocyte foot processes.22 This barrier limits the diffusion of compounds to small, uncharged, lipid-soluble molecules. The vast majority of drugs do not possess these properties and therefore do not cross the BBB to a significant degree.314 In addition to the physical barrier, the BBB is also reinforced with ATP-binding cassette transporter family proteins—drug efflux transporters on the luminal side of the BBB that remove toxic metabolites, xenobiotics, and drugs from the brain.22,314 Together, these components prevent 98% of all small molecules from crossing the BBB.314 Although it is well recognized that portions of glioblastoma tumors can have a leaky, compromised BBB, significant regions of the tumor (often the infiltrative tumor edge left behind in the patients after resection) still have an intact BBB and impede effective drug delivery.22,313 With many recent major clinical trials failing to improve survival due to the compounds not achieving therapeutic concentrations at the target site,315 the issue of brain penetration remains a major challenge to the treatment of glioblastoma. Strategies to overcome this issue include the development of significantly more agents with good BBB penetration,316 hijacking endogenous influx transporters such as low-density lipoprotein receptor-related protein 1,317 inhibiting efflux pumps, cell mediated drug delivery, convection-enhanced delivery, and focused ultrasound and microbubbles to transiently disrupt the BBB.22,318
Another critical therapeutic challenge for glioblastoma is the high degree of inter- and intratumoral heterogeneity. As the first cancer to be characterized by The Cancer Genome Atlas, glioblastoma has been shown to have multiple different genetic drivers.28 The differences among glioblastomas are further complicated by the existence of intratumoral heterogeneity at both molecular and functional levels. For example, different regions within the same tumor may contain cells having distinct genetic compositions,44,45 transcriptional subtypes,19,43 and/or proliferation kinetics.319,320 While the impact of this intratumor heterogeneity on therapeutic outcome remains poorly characterized, preclinical evidence suggests that functionally distinct glioma cells (eg, putative glioma stemlike cells compared with more differentiated counterparts) within a tumor can have differential responses to TMZ319,320 or ionizing radiation,234 which may underlie resistance to these conventional treatments. Additional work is necessary to examine the impact of glioblastoma heterogeneity on more contemporary therapies, including molecularly targeted therapies and immunotherapies. For example, comparisons of tumor specimens obtained at diagnosis and at recurrence suggest that temporal heterogeneity may occur with certain genetic alterations, such as EGFRvIII mutations, which are lost in 30–60% of recurrent tumors.36,42,202 This raises the possibility that re-biopsy and genotyping, and/or improvements in minimally invasive liquid biopsy technology, may be necessary for therapies directed at targets that change over time.
In addition to the differential intrinsic drug sensitivity across distinct glioblastoma cell subpopulations, glioblastoma cells also display remarkable plasticity as a means to circumvent the toxic effects of cancer therapy. In response to targeted tyrosine kinase inhibitors, glioblastoma has been shown to adapt and survive through a wide variety of mechanisms, including the dynamic regulation of extrachromosomal DNA, chromatin remodeling to a slow-cycling/drug-tolerant persistent state, suppression of PTEN tumor suppressor, and reactivation in oncogenic signaling pathways (eg, phosphatidylinositol-3 kinase, Ras‒mitogen-activated protein kinase signaling).221,321,322 This redundancy in restoring oncogenic signaling flux can manifest via RTK switching323 or the coactivation of multiple RTKs,325 both of which can maintain persistent oncogenic signaling to promote tumor viability. Although there have been some examples of benefit with targeted molecular therapies (dabrafenib and trametinib for glioma with BRAFV600E mutations,209 and entrectinib and larotrectinib for NTRK fusions,211 and possibly ONC201 for H3K27M mutations),212,213 most targeted therapies have failed because of low BBB penetration of the drugs employed, redundant signaling pathways, molecular heterogeneity, as well as the enhanced toxicity of the drug combinations, thus requiring suboptimal dosing. Alternative combination approaches, such as those that target orthogonal signaling/functional networks to induce synthetic lethality in glioblastoma,322,324 are potential options to augment drug responses to therapies against the primary genetic driver. A likely corollary to these advanced approaches is that an approach based on a single mutation matched with a putative targeted therapy is unlikely to work in most glioblastomas, and efforts to integrate multiplatform molecular analyses (gene expression, copy number changes, immune cell/pathway profiling) are going to be required in future clinical trial designs to utilize advanced enriching strategies and maximize what we can learn even when specific therapeutic agents are not efficacious in a glioblastoma patient population
Although immunotherapy holds great promise as a new treatment option for glioblastoma, the negative results in large randomized studies to date21,202,255 indicate the increased complexity and difficulty in achieving a clinically meaningful immune therapy effect in glioblastoma. In fact, it appears that glioblastoma provides challenges in almost every area of the cancer immunity cycle, including limited antigenicity, impaired antigen presentation, intrinsic and therapy-induced systemic immune suppression, and a unique immune suppressive microenvironment.18 A functional and mechanistic understanding of these immune deficits will be required in order to construct effective immune therapies for glioblastoma.
Summary
Although there has been important progress in understanding the molecular pathogenesis and biology of glioblastoma, this has not translated into significantly improved outcomes for patients. While much remains to be learned, important therapeutic strategies have been identified that are being translated clinically. In addition to developing novel therapies based on strong scientific rationale, there is a need to increase the efficiency with which they are evaluated in clinical trials. This includes greater inclusion of glioblastoma patients in phase I oncology trials, an expanded network for conducting “window-of-opportunity” “phase 0” surgical studies to assess BBB penetration and pharmacodynamic effects, greater incorporation of molecular imaging and blood and CSF biomarkers, integration of a broad range of molecular biomarkers into clinical trial schema, more efficient design of clinical trials, and significantly increased trial accrual. These changes will hopefully lead to the identification of more effective therapies for patients with glioblastoma.
Acknowledgments
We are grateful to Mary Jane Lim-Fat, MD for her helpful comments and to Debbie Ferreira for editorial assistance.
Conflicts of Interest
Brian M. Alexander: Employment—Foundation Medicine, Inc.; Equity—Hoffman-La Roche; Research support—Puma, Eli Lilly, Celgene.
Tracy Batchelor: Advisory Board Genomicare.
Susan M. Chang: Research support: Agios.
E. Antonio Chiocca: Advisor to Advantagene Inc., Alcyone Biosciences, Insightec, Inc., DNAtrix Inc, Immunomic Therapeutics, Sangamo Therapeutics, Seneca Therapeutics; equity interest in DNAtrix, Immunomic Therapeutics, Seneca Therapeutics; Advisory board: Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx, Ziopharm Oncology, Cerebral Therapeutics, Genenta. Merck, Janssen, Karcinolysis, Shanghai Biotech. Research support from NIH, US Department of Defense, American Brain Tumor Association, National Brain Tumor Society, Alliance for Cancer Gene Therapy, Neurosurgical Research Education Foundation, Advantagene, NewLink Genetics and Amgen. Named inventor on patents related to oncolytic HSV1 and noncoding RNAs.
Timothy Cloughesy: Consulting services: Roche, Trizel, Medscape, Bayer, Amgen, Odonate Therapeutics, Pascal Biosciences, Bayer, Del Mar Pharmaceuticals, Tocagen, Karyopharm, GW Pharma, Kiyatec, Abbvie, Boehinger Ingelheim, VBI, Deciphera, VBL, Agios, Merck, Roche, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, Medqia. Stock options: Notable Labs. Member of the board for the 501c3 Global Coalition for Adaptive Research. U.S. Provisional Application No.: 62/819,322.
Evanthia Galanis: General Consulting from MedImmune, Inc.; F. Hoffman La Roche, Ltd (compensation to Mayo), Tactical Therapeutics, Inc.; Oncorus (personal compensation). Advisory Board on Vyriad (compensation to Mayo), Celgene Corporation; KIYATEC (personal compensation). Clinical Trial Funding from MedImmune, Inc; Tracon; Genentech; Bristol-Myers Squibb (Mayo).
John DeGroot: Grant or Research Support: Sanofi-Aventis, AstraZeneca, EMD-Serono; Eli Lilly, Novartis, Deciphera Pharmaceuticals, Mundipharma. Paid Consultant: Celldex; Deciphera Pharmaceuticals, AbbVie, FivePrime Therapeutics, Inc, GW Pharma, Carthera, Eli Lilly, Kadmon, Boston Biomedical, Inc,Taiho Pharmaceuticals, Kairos Venture Investments, Syneos Health, Monteris, Agios, Mundipharma Research, GenomiCare, Blue Earth Diagnostics, Del Mar Pharmaceuticals, Insightec, Voyager Therapeutics, Inc, Merck & Co, Tocagen, Bioasis Technologies, Inc, ResTORbio, Inc Advisory Boards: Genentech, Celldex, Foundation Medicine, Inc, Novogen, Deciphera, Astrazeneca, Insys Therapeutics, Merck, Eli Lilly, Novella Clinical, Karyopharm Therapeutics, Blue Earth Diagnostics, Kiyatec, Vanquish Oncology, Orsenix, Insightec, Prelude Therapeutics, Debiopharm Therapeutics, Inc Other Relevant Financial or Material Interests: DSMB: VBL Therapeutics [Glioblastoma (VB111)]; DSMB: Novella [Glioblastoma (ICT-107)]; VBI Vaccines, Inc [Glioblastoma (VBI-1901)].
Stock Ownership: Ziopharm Oncology, Gilead. Company Employment (spouse): Ziopharm Oncology.
Andrew B. Lassman: Research support (to the institution) from: NCI grants UM1 CA189960 and P30CA013696, the Rhodes Center for Glioblastoma at NewYork-Presbyterian Hospital, Voices Against Brain Cancer, Genentech/Roche, Amgen, AbbVie, Millenium, Celldex, Novartis, Pfizer, Aeterna Zenaris, Karyopharm, RTOG-Foundation, Kadmon, VBI Vaccines, Beigene, Oncoceutics, Bayer, Agios, Orbus. ABCC; Honoraria/travel support in the last (or expected in the next) 12 months from Orbus, Bioclinica as an expert blinded independent reviewer of clinical and imaging data for a BMS-sponsored trial, Sapience, Novocure, Karyopharm, Abbott, QED, Forma, Bayer, and AbbVie.
Eudocia Q. Lee: Consulting for Eli Lilly. Royalties from Up to Date, Inc Honoraria from Prime Oncology.
Emilie LeRhun: Honoraria for advisory boards from Tocagen, Abbvie, Daiichy Sankyo.
Michael Lim: Research Support from Arbor, BMS, Accuray, DNAtrix, Tocagen, Biohaven, Kyrin-Kyowa; Consultant for Tocagen, SQZ Technologies, VBI; Patent: Focused radiation + checkpoint inhibitors; Non-research consultant—Stryker.
Minesh Mehta: Consulting relationships with honoraria with Varian, Agenus, Insys, Remedy, IBA, Astra-Zeneca, Celgene, Tocagen, Abbvie; Board of Directors position with stock options with Oncoceutics; and DSMB for Monteris.
Ingo K. Mellinghoff: Research funding from General Electric, Agios, Amgen, and Lilly; advisory roles with Agios, Debiopharm Group, Puma Biotechnology, and Voyager Therapeutics; and honoraria from Roche for a presentation.
Michael Platten: Research support (paid to institution) from Pfizer, Genentech/Roche, and honoraria for consulting from Affiris, Bayer (paid to self) and Apogenix, Novartis, VAXIMM (paid to institution).
Matthias Preusser: Research support from: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. Honoraria for lectures, consultation or advisory board participation from: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen.
David Reardon: Research support (paid to institution): Acerta Phamaceuticals; Agenus; Celldex; EMD Serono; Incyte; Inovio; Midatech; Omniox; Tragara. Advisory/consultation (paid to self): Abbvie; Advantagene; Agenus; Amgen, Bayer, Bristol-Myers Squibb; Celldex; Delmar, EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc.
Patrick Roth: Research grants from MSD and Novocure. Honoraria for advisory board participation or lectures from Bristol-Myers Squibb, Covagen, Debiopharm, Medac, Merck, MSD, Novartis, Novocure, QED, Roche and Virometix.
Marc Sanson: Research support (paid to institution) from Astra-Zeneca; Travel grant from Abbvie; Consulting from Genenta and Abbvie; Speaker honoraria from Abbvie and Astra-Zeneca.
Susan Short: Advisory board: Abbvie, HOX Therapeutics and Tocagen.
Jonathan Tsang: U.S. Provisional Application No.: 62/819,322 Title: Compositions and Methods for Treating Cancer.
Joerg Christian Tonn: Speakers honoraria: BrainLab, CarThera; Royalties: Springer Publishing
Martin van den Bent: Consulting for Celgene, BMS, Agios, Boehringer, Abbvie, Bayer, Carthera, Nerviano, Genenta.
Roel Verhaak: Stock options Boundless Bio.
Michael Weller: Research grants from Abbvie, Adastra, Bristol Meyer Squibb (BMS), Dracen, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Piqur and Roche, and honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, Bristol Meyer Squibb (BMS), Celgene, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Orbus, Roche and Tocagen.
Patrick Wen: Research Support from Agios, AstraZeneca, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics, VBI Vaccines. Advisory Board for Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Deciphera, Elevate Bio, Immunomic Therapeutics, Imvax, Karyopharm, Kiyatec, Puma, QED, Taiho, Vascular Biogenics, VBI Vaccines, Voyager, Tocagen. Speaker for Merck, Prime Oncology. Consultant: Integral Health.
No conflicts reported for Kenneth Aldape Jill Barnholtz-Sloan, Floris Barthel, Mark R. Gilbert, Monika Hegi, Craig Horbinski, and Martin Taphoorn.
No part of this manuscript has been previously published or submitted concurrently to any other journal.
All co-authors have read and approved of its submission to this journal.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. 2017;19(11):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis FG, Smith T, Gittleman H, Ostrom Q, Kruchko C, Barnholtz-Sloan JS. Glioblastoma multiforme incidence in Canada and the US in comparison with England 1995–2015. Neuro Oncol. 2020;22(2):301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Philips A, Henshaw DL, Lamburn G, O’Carroll MJ. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995–2015 Suggests an Adverse Environmental or Lifestyle Factor. J Environ Public Health. 2018:7910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Philips A, Henshaw DL, Lamburn G, O’Carroll MJ. Authors’ comment on “Brain tumours: rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor.” J Environ Public Health. 2018:2170208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostrom QT, Adel Fahmideh M, Cote DJ, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019;21(11):1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostrom QT, Gittleman H, Stetson L, Virk S, Barnholtz-Sloan JS. Epidemiology of Intracranial Gliomas. Prog Neurol Surg. 2018;30:1–11. [DOI] [PubMed] [Google Scholar]
- 11. Ranger AM, Patel YK, Chaudhary N, Anantha RV. Familial syndromes associated with intracranial tumours: a review. Childs Nerv Syst. 2014;30(1):47–64. [DOI] [PubMed] [Google Scholar]
- 12. Vijapura C, Saad Aldin E, Capizzano AA, Policeni B, Sato Y, Moritani T. Genetic syndromes associated with central nervous system tumors. Radiographics. 2017;37(1):258–280. [DOI] [PubMed] [Google Scholar]
- 13. Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019;25(18):5537–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. ; GliomaScan Consortium Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Labreche K, Kinnersley B, Berzero G, et al. Diffuse gliomas classified by 1p/19q co-deletion, TERT promoter and IDH mutation status are associated with specific genetic risk loci. Acta Neuropathol. 2018;135(5):743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung E, Alfonso J, Osswald M, Monyer H, Wick W, Winkler F. Emerging intersections between neuroscience and glioma biology. Nat Neurosci. 2019;22(12):1951–1960. [DOI] [PubMed] [Google Scholar]
- 17. Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019;33(11-12):591–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–1109. [DOI] [PubMed] [Google Scholar]
- 19. Neftel C, Laffy J, Filbin MG, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849 e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bi J, Chowdhry S, Wu S, Zhang W, Masui K, Mischel PS. Altered cellular metabolism in gliomas - an emerging landscape of actionable co-dependency targets. Nat Rev Cancer. 2020;20(1):57–70. [DOI] [PubMed] [Google Scholar]
- 21. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee JH, Lee JE, Kahng JY, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. [DOI] [PubMed] [Google Scholar]
- 24. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barthel FP, Wesseling P, Verhaak RGW. Reconstructing the molecular life history of gliomas. Acta Neuropathol. 2018;135(5):649–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 28. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frattini V, Pagnotta SM, Tala, et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature. 2018;553(7687):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14(2):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Körber V, Yang J, Barah P, et al. Evolutionary trajectories of IDHWT glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell. 2019;35(4):692–704.e12. [DOI] [PubMed] [Google Scholar]
- 36. Draaisma K, Chatzipli A, Taphoorn M, et al. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J Clin Oncol. 2020;38(1):81–99. [DOI] [PubMed] [Google Scholar]
- 37. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turner KM, Deshpande V, Beyter D, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543(7643):122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. deCarvalho AC, Kim H, Poisson LM, et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat Genet. 2018;50(5):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barthel FP, Johnson KC, Varn FS, et al. ; GLASS Consortium Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Francis JM, Zhang CZ, Maire CL, et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 2014;4(8):956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. [DOI] [PubMed] [Google Scholar]
- 47. Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization. Histological Classification of Tumors of The Central Nervous System. Lyon, France: IARC; 2016. [Google Scholar]
- 51. Korshunov A, Chavez L, Sharma T, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28(5):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2-wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125(5):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DeWitt JC, Jordan JT, Frosch MP, et al. Cost-effectiveness of IDH testing in diffuse gliomas according to the 2016 WHO classification of tumors of the central nervous system recommendations. Neuro Oncol. 2017;19(12):1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 59. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weller M. Where does O6 -methylguanine DNA methyltransferase promoter methylation assessment place temozolomide in the future standards of care for glioblastoma? Cancer. 2018;124(7): 1316–1318. [DOI] [PubMed] [Google Scholar]
- 61. Hegi ME, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter—still a dilemma? Neuro Oncol. 2015;17(11):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quillien V, Lavenu A, Karayan-Tapon L, et al. Comparative assessment of 5 methods (methylation-specific polymerase chain reaction, MethyLight, pyrosequencing, methylation-sensitive high-resolution melting, and immunohistochemistry) to analyze O6-methylguanine-DNA-methyltranferase in a series of 100 glioblastoma patients. Cancer. 2012;118(17):4201–4211. [DOI] [PubMed] [Google Scholar]
- 63. Bady P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012;124(4):547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hegi ME, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of 4 clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 66. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 67. Ly KI, Wen PY, Huang RY. Imaging of central nervous system tumors based on the 2016 World Health Organization Classification. Neurol Clin. 2020;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 68. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 70. Ellingson BM, Bendszus M, Boxerman J, et al. ; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hu LS, Eschbacher JM, Dueck AC, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sadeghi N, D’Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol. 2008;29(3):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wesseling P, van der Laak JA, de Leeuw H, Ruiter DJ, Burger PC. Quantitative immunohistological analysis of the microvasculature in untreated human glioblastoma multiforme. Computer-assisted image analysis of whole-tumor sections. J Neurosurg. 1994;81(6):902–909. [DOI] [PubMed] [Google Scholar]
- 74. Kickingereder P, Wiestler B, Sahm F, et al. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;272(3):843–850. [DOI] [PubMed] [Google Scholar]
- 75. Lee B, Park JE, Bjørnerud A, Kim JH, Lee JY, Kim HS. Clinical value of vascular permeability estimates using dynamic susceptibility contrast MRI: improved diagnostic performance in distinguishing hypervascular primary CNS lymphoma from glioblastoma. AJNR Am J Neuroradiol. 2018;39(8):1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Suh CH, Kim HS, Jung SC, Park JE, Choi CG, Kim SJ. MRI as a diagnostic biomarker for differentiating primary central nervous system lymphoma from glioblastoma: a systematic review and meta-analysis. J Magn Reson Imaging. 2019;50(2):560–572. [DOI] [PubMed] [Google Scholar]
- 77. Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25(5):746–755. [PMC free article] [PubMed] [Google Scholar]
- 78. Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors. J Magn Reson Imaging. 2018;48(3):571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gharzeddine K, Hatzoglou V, Holodny AI, Young RJ. MR perfusion and MR spectroscopy of brain neoplasms. Radiol Clin North Am. 2019;57(6):1177–1188. [DOI] [PubMed] [Google Scholar]
- 80. Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. [DOI] [PubMed] [Google Scholar]
- 81. Hayashida Y, Hirai T, Morishita S, et al. Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol. 2006;27(7):1419–1425. [PMC free article] [PubMed] [Google Scholar]
- 82. Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–846. [DOI] [PubMed] [Google Scholar]
- 83. Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–183. [DOI] [PubMed] [Google Scholar]
- 84. Lu X, Xu W, Wei Y, et al. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: a systematic review and meta-analysis. Neurol Sci. 2019;40(5):947–956. [DOI] [PubMed] [Google Scholar]
- 85. Oz G, Alger JR, Barker PB, et al. ; MRS Consensus Group Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Suchorska B, Albert NL, Bauer EK, Tonn JC, Galldiks N. The role of amino-acid PET in the light of the new WHO classification 2016 for brain tumors. Q J Nucl Med Mol Imaging. 2018;62(3):267–271. [DOI] [PubMed] [Google Scholar]
- 88. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 90. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wick W, Chinot OL, Bendszus M, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18(10):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kickingereder P, Isensee F, Tursunova I, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 2019;20(5):728–740. [DOI] [PubMed] [Google Scholar]
- 95. Chang K, Beers AL, Bai HX, et al. Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019;21(11):1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology palliative care task force European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 97. Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44(4):675–680. [DOI] [PubMed] [Google Scholar]
- 98. Lim-Fat MJ, Bi WL, Lo J, et al. Letter: when less is more: dexamethasone dosing for brain tumors. Neurosurgery. 2019;85(3):E607–E608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee EQ, Wen PY. Corticosteroids for peritumoral edema: time to overcome our addiction? Neuro Oncol. 2016;18(9):1191–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ly KI, Wen PY. clinical relevance of steroid use in Neuro-Oncology. Curr Neurol Neurosci Rep. 2017;17(1):5. [DOI] [PubMed] [Google Scholar]
- 101. Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36(30):3043–3054. [DOI] [PubMed] [Google Scholar]
- 103. Chang SM, Parney IF, Huang W, et al. ; Glioma Outcomes Project Investigators Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 104. Forsyth PA, Weaver S, Fulton D, et al. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003;30(2):106–112. [DOI] [PubMed] [Google Scholar]
- 105. Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46(4):985–991. [DOI] [PubMed] [Google Scholar]
- 106. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 107. Kuijlen JM, Teernstra OP, Kessels AG, Herpers MJ, Beuls EA. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta-analysis. Seizure. 1996;5(4):291–298. [DOI] [PubMed] [Google Scholar]
- 108. Pulman J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post-craniotomy seizures. Cochrane Database Syst Rev. 2013;2:CD007286. [DOI] [PubMed] [Google Scholar]
- 109. Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013;118(4):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. 2015;17(4):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumor progression. Nature. 2019;573(7775):532–538. [DOI] [PubMed] [Google Scholar]
- 113. Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27(25):4155–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Laws ER, Parney IF, Huang W, et al. ; Glioma Outcomes Investigators Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 115. Czap AL, Becker A, Wen PY. Thrombotic complications in gliomas. Semin Thromb Hemost. 2019;45(4):326–333. [DOI] [PubMed] [Google Scholar]
- 116. Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993;43(6):1111–1114. [DOI] [PubMed] [Google Scholar]
- 117. Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006;24(8):1310–1318. [DOI] [PubMed] [Google Scholar]
- 118. Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129(25):3379–3385. [DOI] [PubMed] [Google Scholar]
- 119. Norden AD, Bartolomeo J, Tanaka S, et al. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012;106(1):121–125. [DOI] [PubMed] [Google Scholar]
- 120. Lee AY, Levine MN, Baker RI, et al. ; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 121. Carney BJ, Uhlmann EJ, Puligandla M, et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019;17(1):72–76. [DOI] [PubMed] [Google Scholar]
- 122. Raskob GE, van Es N, Verhamme P, et al. ; Hokusai VTE Cancer Investigators Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624. [DOI] [PubMed] [Google Scholar]
- 123. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023. [DOI] [PubMed] [Google Scholar]
- 124. Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8(9):1959–1965. [DOI] [PubMed] [Google Scholar]
- 125. Le Rhun E, Genbrugge E, Stupp R, et al. Associations of anticoagulant use with outcome in newly diagnosed glioblastoma. Eur J Cancer. 2018;101:95–104. [DOI] [PubMed] [Google Scholar]
- 126. Gehring K, Taphoorn MJ, Sitskoorn MM, Aaronson NK. Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neurooncol Pract. 2015;2(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333; discussion 333. [DOI] [PubMed] [Google Scholar]
- 128. Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14(Suppl 4):iv65–iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Armstrong TS, Shade MY, Breton G, et al. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017;19(3):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1496–1501. [DOI] [PubMed] [Google Scholar]
- 132. Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee EQ, Muzikansky A, Drappatz J, et al. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016;18(6):849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rooney AG, McNamara S, Mackinnon M, et al. Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. J Clin Oncol. 2011;29(32):4307–4312. [DOI] [PubMed] [Google Scholar]
- 135. Ruden E, Reardon DA, Coan AD, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29(21):2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 139. Lamborn KR, Yung WK, Chang SM, et al. ; North American Brain Tumor Consortium Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12(2):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Clarke JL, Ennis MM, Yung WK, et al. ; North American Brain Tumor Consortium Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13(10):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Central Nervus System Cancers. Version 3.2019-October 18, 2019. NCCN; 2019. NCCN.org. [Google Scholar]
- 143. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Herrlinger U, Tzaridis T, Mack F, et al. ; Neurooncology Working Group of the German Cancer Society Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 145. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 146. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 147. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 148. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 149. Perry JR, Bélanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. [DOI] [PubMed] [Google Scholar]
- 150. Weller M, Tabatabai G, Kästner B, et al. ; DIRECTOR Study Group MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 151. Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70(1):234–243; discussion 243–244. [DOI] [PubMed] [Google Scholar]
- 152. Eigenbrod S, Trabold R, Brucker D, et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir (Wien). 2014;156(8):1427–1440. [DOI] [PubMed] [Google Scholar]
- 153. Sahm F, Capper D, Jeibmann A, et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol. 2012;69(4):523–526. [DOI] [PubMed] [Google Scholar]
- 154. Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 155. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ; ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 156. Kreth FW, Thon N, Simon M, et al. ; German Glioma Network Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117–3123. [DOI] [PubMed] [Google Scholar]
- 157. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ringel F, Pape H, Sabel M, et al. ; SN1 study group Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ellingson BM, Abrey LE, Nelson SJ, et al. Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018;20(9):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 163. Jenkinson MD, Barone DG, Bryant A, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev. 2018;1:CD012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Stummer W, Tonn JC, Mehdorn HM, et al. ; ALA-Glioma Study Group Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg. 2011;114(3):613–623. [DOI] [PubMed] [Google Scholar]
- 165. Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76(6):572–579. [DOI] [PubMed] [Google Scholar]
- 166. Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. [DOI] [PubMed] [Google Scholar]
- 167. Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Balana C, Vaz MA, Sepulveda JM, et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond six cycles in patients with glioblastoma (GEINO 14-01). Neuro-Oncol. 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Blumenthal DT, Gorlia T, Gilbert MR, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19(8):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gramatzki D, Kickingereder P, Hentschel B, et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology. 2017;88(15):1422–1430. [DOI] [PubMed] [Google Scholar]
- 171. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 172. Stupp R, Lukas RV, Hegi ME. Improving survival in molecularly selected glioblastoma. Lancet. 2019;393(10172):615–617. [DOI] [PubMed] [Google Scholar]
- 173. Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncoly 2016;118(1):35–42. [DOI] [PubMed] [Google Scholar]
- 174. Chang SM, Mehta MP, Vogelbaum MA, Taylor MD, Ahluwalia MS. Neoplasms of the central nervous system. In: Devita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 11th ed Wolters Kluwer Health; 2019:1568–1616. [Google Scholar]
- 175. Kruser TJ, Bosch WR, Badiyan SN, et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J Neurooncol. 2019;143(1):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wernicke AG, Smith AW, Taube S, Mehta MP. Glioblastoma: Radiation treatment margins, how small is large enough? Pract Radiat Oncol. 2016;6(5):298–305. [DOI] [PubMed] [Google Scholar]
- 177. Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3(4):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Grossman SA, Ye X, Lesser G, et al. ; NABTT CNS Consortium Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Strauss SB, Meng A, Ebani EJ, Chiang GC. Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Radiol Clin North Am. 2019;57(6):1199–1216. [DOI] [PubMed] [Google Scholar]
- 181. Haider AS, van den Bent M, Wen PY, et al. Towards a standard pathological and molecular characterization of recurrent glioma in adults: a RANO effort. Neuro Oncol. 2020;22(4):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wick A, Kessler T, Elia AEH, et al. Glioblastoma in elderly patients: solid conclusions built on shifting sand? Neuro Oncol. 2018;20(2):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 184. Roa W, Kepka L, Kumar N, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 185. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 186. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 187. Hovey EJ, Field KM, Rosenthal MA, et al. ; CABARET/COGNO investigators Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: an exploratory randomized phase II trial. Neurooncol Pract. 2017;4(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Brandes AA, Bartolotti M, Tosoni A, Franceschi E. Nitrosoureas in the management of malignant gliomas. Curr Neurol Neurosci Rep. 2016;16(2):13. [DOI] [PubMed] [Google Scholar]
- 190. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 192. Brandes AA, Finocchiaro G, Zagonel V, et al. AVAREG: a phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol. 2016;18(9):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–119. [DOI] [PubMed] [Google Scholar]
- 194. Straube C, Kessel KA, Zimmer C, et al. A second course of radiotherapy in patients with recurrent malignant gliomas: clinical data on re-irradiation, prognostic factors, and usefulness of digital biomarkers. Curr Treat Options Oncol. 2019;20(9):71. [DOI] [PubMed] [Google Scholar]
- 195. Scoccianti S, Francolini G, Carta GA, et al. Re-irradiation as salvage treatment in recurrent glioblastoma: a comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018;126:80–91. [DOI] [PubMed] [Google Scholar]
- 196. Shi W, Scannell Bryan M, Gilbert MR, et al. Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys. 2018;100(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tsien C, Pugh S, Dicker A, et al. ACTR-232. NRG Oncology RTOG 1205: randomized phase II trial of concurrent bevacizumab and re-irradiation versus bevacizumab alone as treatment for recurrent glioblastoma. Neuro Oncol. 2019;21:vi 20. [Google Scholar]
- 198. Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. [DOI] [PubMed] [Google Scholar]
- 199. Le Rhun E, Preusser M, Roth P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. [DOI] [PubMed] [Google Scholar]
- 200. Horbinski C, Ligon KL, Brastianos P, et al. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019;21(12):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 203. Reardon DA, Lassman AB, van den Bent M, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017;19(7):965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. van den Bent M, Gan HK, Lassman AB, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol. 2017;80(6):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lassman A, Pugh S, Wang A, et al. A randomized, double-blind, placebo-controlled phase 3 trial of depatuxizumab mafodotin (ABT-414) in Epidermal Growth Factor Receptor (EGFR) Amplified (Amp) Newly Diagnosed Glioblastoma (nGBM). Neuro Oncol. 2019;21:vi17. [Google Scholar]
- 206. van den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFRamplified glioblastoma. Neuro Oncol. 2019. doi: 10.1093/neuonc/noz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Reardon DA, Wen PY, Mellinghoff IK. Targeted molecular therapies against epidermal growth factor receptor: past experiences and challenges. Neuro Oncol. 2014;16(Suppl 8):viii7–viii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018:JCO2018789990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wen PY, Stein A, Van Den Bent M, et al. Updated efficacy and safety of dabrafenib plus trametinib in patients with recurrent/refractory BRAF V600E–mutated high-grade glioma (HGG) and low-grade glioma (LGG). Neuro Oncol. 2019;21(suppl 6;abstr ACTR-30). [Google Scholar]
- 210. Behling F, Barrantes-Freer A, Skardelly M, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30(Supplement_8):viii23–viii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Arrillaga-Romany I, Kurz S, Sumrall A, et al. Single agent ONC201 in previously-treated, progressive adult H3 K27M-mutant glioma. Neuro Oncol. 2019;21(Suppl 6):vi20–vi21. (Abstr.) [Google Scholar]
- 213. Chi AS, Tarapore RS, Hall MD, et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019;145(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lasorella A, Sanson M, Iavarone A. FGFR-TACC gene fusions in human glioma. Neuro Oncol. 2017;19(4):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sandmann T, Bourgon R, Garcia J, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wirsching HG, Tabatabai G, Roelcke U, et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open-label, phase II ARTE trial. Ann Oncol. 2018;29(6):1423–1430. [DOI] [PubMed] [Google Scholar]
- 218. Ellingson BM, Gerstner ER, Smits M, et al. Diffusion MRI phenotypes predict overall survival benefit from anti-VEGF monotherapy in recurrent glioblastoma: converging evidence from phase II trials. Clin Cancer Res. 2017;23(19):5745–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wick W, Fricke H, Junge K, et al. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20(24):6304–6313. [DOI] [PubMed] [Google Scholar]
- 220. Wick W, Gorlia T, Bady P, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 221. Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Chinnaiyan P, Won M, Wen PY, et al. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913. Neuro Oncol. 2018;20(5):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lassman AB, Sepúlveda-Sánchez JM, Cloughesy T, et al. Infigratinib (BGJ398) in FGFR altered recurrent malignant glioma: a multicenter phase II study. Neuro Oncology. 2019;21:vi20. [Google Scholar]
- 224. Wen PY, Touat M, Alexander BM, et al. Buparlisib in patients with recurrent glioblastoma harboring phosphatidylinositol 3-kinase pathway activation: an open-label, multicenter, multi-arm, phase II trial. J Clin Oncol. 2019;37(9):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Cloughesy TF, Drappatz J, de Groot J, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018;20(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Wen PY, Drappatz J, de Groot J, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018;20(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Wick W, Dettmer S, Berberich A, et al. N2M2 (NOA-20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro Oncol. 2019;21(1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Alexander BM, Trippa L, Gaffey S, et al. Individualized screening trial of innovative glioblastoma therapy (INSIGhT): a Bayesian adaptive platform trial to develop precision medicines for patients with glioblastoma. J Clin Oncol Precis Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Alexander BM, Ba S, Berger MS, et al. ; GBM AGILE Network Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–743. [DOI] [PubMed] [Google Scholar]
- 230. Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol. 2014;16(4):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127(2):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Lassman AB, Wen PY, van den Bent M, et al. Efficacy and safety of selinexor in recurrent glioblastoma. Neuro Oncol. 2019;21:iii3–iii4. [Google Scholar]
- 233. Carruthers RD, Ahmed SU, Ramachandran S, et al. Replication stress drives constitutive activation of the DNA damage response and radioresistance in glioblastoma stem-like cells. Cancer Res. 2018;78(17):5060–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 235. Yap TA, Plummer R, Azad NS, Helleday T. The DNA damaging revolution: PARP inhibitors and beyond. Am Soc Clin Oncol Educ Book. 2019;39:185–195. [DOI] [PubMed] [Google Scholar]
- 236. Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16(2):81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Beckta JM, Bindra RS, Chalmers AJ. Targeting DNA repair in gliomas. Curr Opin Neurol. 2019;32(6):878–885. [DOI] [PubMed] [Google Scholar]
- 238. Gupta SK, Kizilbash SH, Carlson BL, et al. Delineation of MGMT hypermethylation as a biomarker for veliparib-mediated temozolomide-sensitizing therapy of glioblastoma. J Natl Cancer Inst. 2016;108(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Gupta SK, Mladek AC, Carlson BL, et al. Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomide-sensitive versus -resistant glioblastoma multiforme xenografts. Clin Cancer Res. 2014;20(14):3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Gupta SK, Smith EJ, Mladek AC, et al. PARP inhibitors for sensitization of alkylation chemotherapy in glioblastoma: impact of blood–brain barrier and molecular heterogeneity. Front Oncol. 2018;8:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Jackson CB, Noorbakhsh SI, Sundaram RK, et al. Temozolomide sensitizes MGMT-deficient tumor cells to ATR inhibitors. Cancer Res. 2019;79(17):4331–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Venneti S, Thompson CB. Metabolic reprogramming in brain tumors. Annu Rev Pathol. 2017;12:515–545. [DOI] [PubMed] [Google Scholar]
- 243. Agnihotri S, Golbourn B, Huang X, et al. PINK1 is a negative regulator of growth and the warburg effect in glioblastoma. Cancer Res. 2016;76(16):4708–4719. [DOI] [PubMed] [Google Scholar]
- 244. Agnihotri S, Mansouri S, Burrell K, et al. Ketoconazole and posaconazole selectively target HK2-expressing glioblastoma cells. Clin Cancer Res. 2019;25(2):844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Villa GR, Hulce JJ, Zanca C, et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell. 2016;30(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. [DOI] [PubMed] [Google Scholar]
- 247. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 248. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9(7):e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. [DOI] [PubMed] [Google Scholar]
- 251. Wei J, Marisetty A, Schrand B, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Brandes AA, Carpentier AF, Kesari S, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18(8):1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020. (Epub 5/21/20) [DOI] [PMC free article] [PubMed]
- 256. Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Desjardins A, Gromeier M, Herndon JE 2nd, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Wheeler LA, Manzanera AG, Bell SD, et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Cloughesy T, Petrecca K, Walbert T, et al. TOCA 5: Toca 511 & Toca FC versus standard of care in patients with recurrent high grade glioma Late Breaking Abstract Society For Neuro-Oncology 2019. meeting. [Google Scholar]
- 262. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. [DOI] [PubMed] [Google Scholar]
- 263. Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401. [DOI] [PubMed] [Google Scholar]
- 264. Jackson CM, Kochel CM, Nirschl CJ, et al. Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin Cancer Res. 2016;22(5):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Weller M, Reardon DA, Brandes AA, et al. Nivolumab versus bevacizumab in patients with recurrent glioblastoima: exploratory analysis of MGMT mathylation status and baseline corticosteroid use. Neuro Oncol. 2019;21:vi12. [Google Scholar]
- 269. Brown CE, Alizadeh D, Starr R, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Cloughesy TF, Landolfi J, Vogelbaum MA, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20(10):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–849. [DOI] [PubMed] [Google Scholar]
- 273. Wen PY, Reardon DA, Armstrong TS, et al. Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients With Glioblastoma. Clin Cancer Res. 2019;25(19):5799–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 275. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 276. Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med. 2019;25(3):389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Danilova L, Anagnostou V, Caushi JX, et al. The mutation-associated neoantigen functional expansion of specific T cells (MANAFEST) assay: a sensitive platform for monitoring antitumor immunity. Cancer Immunol Res. 2018;6(8):888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370):370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 284. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther. 2020;205:107419. [DOI] [PubMed] [Google Scholar]
- 288. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. [DOI] [PubMed] [Google Scholar]
- 289. Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014;16(3):334–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Kwiatkowska A, Nandhu MS, Behera P, Chiocca EA, Viapiano MS. Strategies in gene therapy for glioblastoma. Cancers. 2013;5(4):1271–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 293. Chiocca EA, Nassiri F, Wang J, Peruzzi P, Zadeh G. Viral and other therapies for recurrent glioblastoma: is a 24-month durable response unusual? Neuro Oncol. 2019;21(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Iorgulescu JB, Reardon DA, Chiocca EA, Wu CJ. Immunotherapy for glioblastoma: going viral. Nat Med. 2018;24(8):1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Todo T. Results of phase II clinical trial of oncolytic Herpes virus G47Δ In patients with glioblastoma. Neuro Oncol. 2019;21:vi4 (Abstr.). [Google Scholar]
- 296. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Cloughesy TF, Brenner A, de Groot JF, et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2020;22(5):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Brenner AJ, Peters KB, Vredenburgh J, et al. Safety and efficacy of VB-111, an anti-cancer gene-therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro Oncol. 2020;22(5):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Wen PY, Schiff D, Cloughesy TF, et al. It is time to include patients with brain tumors in phase I trials in oncology. J Clin Oncol. 2011;29(24):3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Gounder MM, Nayak L, Sahebjam S, et al. Evaluation of the safety and benefit of phase I oncology trials for patients with primary CNS tumors. J Clin Oncol. 2015;33(28):3186–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Ventz S, Lai A, Cloughesy TF, Wen PY, Trippa L, Alexander BM. Design and evaluation of an external control arm using prior clinical trials and real-world data. Clin Cancer Res. 2019;25(16):4993–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 304. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Le Tourneau C, Delord JP, Gonçalves A, et al. ; SHIVA investigators Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–1334. [DOI] [PubMed] [Google Scholar]
- 306. Rodon J, Soria JC, Berger R, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25(5):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Surveillance, Epidemiology, and End Results: Cancer Stat Facts: Brain and Other Nervous System Cancer 2019. https://seer.cancer.gov/statfacts/html/brain.html. Accessed July 12, 2019.
- 308. Vanderbeek AM, Rahman R, Fell G, et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol. 2018;20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. Barriers to accrual and enrollment in brain tumor trials [published online ahead of print June 7, 2019]. Neuro Oncol. 2019:noz104. doi: 10.1093/neuonc/noz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Welch M, Segall L, Hillyer G, et al. EPID-11. identifying barriers to clinical research: a pilot study to improve access and enrollment to neuro-oncology trials at Columbia University Center Medical Center (CUMC). Neuro Oncol. 2017;19(suppl_6):vi71–vi71. [Google Scholar]
- 311. Lee EQ, Weller M, Sul J, et al. Optimizing eligibility criteria and clinical trial conduct to enhance clinical trial participation for primary brain tumor patients. Neuro Oncol. 2020;22(5):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122(3):421–429. [DOI] [PubMed] [Google Scholar]
- 313. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Heffron TP. Challenges of developing small-molecule kinase inhibitors for brain tumors and the need for emphasis on free drug levels. Neuro Oncol. 2018;20(3):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Vivanco I, Robins HI, Rohle D, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2(5):458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Wen PY, Cloughesy TF, Olivero AG, et al. First-in-human phase I study to evaluate the brain-penetrant PI3K/mTOR inhibitor GDC-0084 in patients with progressive or recurrent high-grade glioma. Clin Cancer Res. 2020;26(8):1820–1828. [DOI] [PubMed] [Google Scholar]
- 317. Drappatz J, Brenner A, Wong ET, et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19(6):1567–1576. [DOI] [PubMed] [Google Scholar]
- 318. Idbaih A, Canney M, Belin L, et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25(13):3793–3801. [DOI] [PubMed] [Google Scholar]
- 319. Lan X, Jörg DJ, Cavalli FMG, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017;549(7671):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Nathanson DA, Gini B, Mottahedeh J, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343(6166):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Liau BB, Sievers C, Donohue LK, et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell. 2017;20(2):233–246 e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013;3(5):534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Mai WX, Gosa L, Daniels VW, et al. Cytoplasmic p53 couples oncogene-driven glucose metabolism to apoptosis and is a therapeutic target in glioblastoma. Nat Med. 2017;23(11):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. [DOI] [PubMed] [Google Scholar]