Abstract
Cancer therapy-induced adverse effects on the brain are a major challenge in neuro-oncology. Brain tissue necrosis (treatment necrosis [TN]) as a consequence of brain directed cancer therapy remains an insufficiently characterized condition with diagnostic and therapeutic difficulties and is frequently associated with significant patient morbidity.
A better understanding of the underlying mechanisms, improvement of diagnostic tools, development of preventive strategies, and implementation of evidence-based therapeutic practices are pivotal to improve patient management. In this comprehensive review, we address existing challenges associated with current TN-related clinical and research practices and highlight unanswered questions and areas in need of further research with the ultimate goal to improve management of patients affected by this important neuro-oncological condition.
Keywords: complications, malignant glioma, radiation necrosis, treatment effects, treatment necrosis
Cancer treatment-related effects on the central nervous system remain a challenging issue in neuro-oncology.1,2 Specifically, treatment-induced brain tissue necrosis (treatment necrosis [TN]), perhaps inappropriately referred to as “radiation necrosis,” continues to be a challenge for clinical management and can be a significant cause of patient morbidity and even mortality.3–6 Radiographic and clinical presentation of TN is usually indistinguishable from those of residual/recurrent tumor (progressive disease [PD]), causing a major dilemma in patient management. Establishing a reliable diagnosis based on clinical assessment and conventional MRI is difficult, frequently necessitating a surgical tissue biopsy.1,2,5,7 The pathophysiology of TN is complex and incompletely understood.8,9 Depending on the location and extent of the necrotic lesion and the degree of associated mass effect, the condition’s clinical course may be heterogeneous and unpredictable.5 To date, no standard of care (SOC) for TN exists and treatment is mostly directed at controlling associated neurological symptoms.5 Experimental therapies have shown mixed efficacy and await robust evidence-based assessment5,10; a consensus regarding best practices for efficient preventative, diagnostic, and therapeutic measures to manage TN has not yet been established.5,11
This review discusses diagnostic and therapeutic strategies directed at management of patients with TN, focusing on clinical pitfalls and research barriers that have precluded advancement of this field. Of note, the term “treatment (-induced) necrosis (TN)”12–14 (unlike the conventional clinical term “radiation necrosis”) reflects emerging knowledge of the mechanisms driving this condition. Specifically, existing studies point to a contribution of chemotherapeutic agents such as temozolomide (TMZ)13 or tyrosine kinase inhibitors15 and preexisting comorbidities to the development of TN.
Treatment-Induced Necrosis: A Clinical Challenge
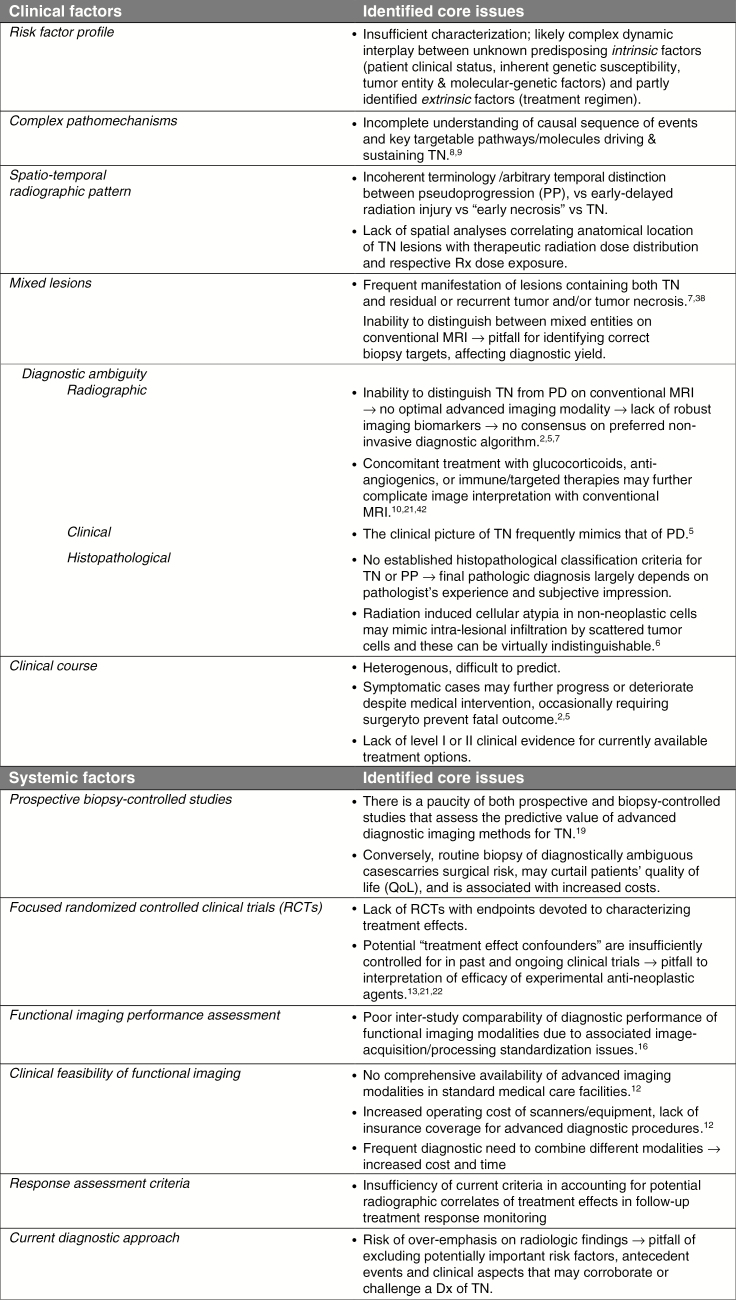
Our observations and those of others1,2,5,12–14,16–22 suggest that numerous clinical and systemic factors complicate the understanding and management of TN, as summarized in Fig. 1. Addressing these challenges is essential to define risk factors and preventative strategies, reliable diagnostic and monitoring algorithms, and effective patient management practices.
Fig. 1.
Overview of clinical and systemic factors challenging the study and better understanding of TN. Dx = diagnosis; QoL = quality of life; Rx = radiation.
Incidence and Clinical Relevance
TN constitutes a serious and relatively common treatment-related adverse effect, particularly since combined chemotherapy and radiation therapy (RT) with concurrent and sequential TMZ23 was established as the SOC treatment for glioblastoma (GBM).13,14,17 The exact incidence and prevalence of TN remains unknown; depending on the type of neoplastic lesion, treatment regimen, and data acquisition parameters, TN incidence ranges from 3–24%9,24 or 5–50%.8,25 For high-grade glioma patients, Ruben et al reported a 4.9% incidence of TN following RT (± adjuvant chemotherapy).24 However, this study was not fully biopsy-controlled and patient data derived from an era before standard chemo-RT23 was implemented. Since then, Chamberlain et al13 found a 14% incidence of biopsy-confirmed TN in TMZ-based chemo-RT treated GBM patients, supporting the notion that the incidence with combined chemo-RT may be higher. Any improvement of patient overall survival (OS) with use of novel anti-neoplastic treatments will likely be associated with an increase in TN manifestation.4 Moreover, the incidence and severity of TN is influenced by the choice of treatment modality, including targeted therapies, immunotherapies, anti-angiogenic therapies, and concurrent steroid use. For instance, TN incidences may be higher in patients treated for brain metastasis with tyrosine kinase inhibitors15 and lower in those concurrently treated with corticosteroids26 and anti-angiogenic therapies.27,28 Whether immune checkpoint inhibitors may increase the risk of TN in patients with metastatic brain cancer has been discussed controversially.29,30
Risk Factors and Prevention
Prevention of TN is limited by an incomplete understanding of risk factors and a lack of efficacious neuroprotective strategies. Apart from anti-neoplastic treatment parameters, such as RT type (eg, brachytherapy, stereotactic radiosurgery) and radiation modality (proton vs photon radiation), radiation dose, -volume, -fraction size and/or hyperfractionation regimen, and use of concurrent and/or adjuvant chemotherapy, other potential risk factors for TN include patient age, survival time, and vascular comorbidities.7,10,14,24,31–34 However, poor predictability and heterogeneity of TN suggest that additional yet unidentified risk factors are implicated.35
Radiographic Appearance and Spatiotemporal Pattern
Lacking a distinctive radiographic signature, TN is mostly indistinguishable from PD on conventional structural MRI.2,7,14 As such, TN commonly occurs in close proximity to the original tumor location, usually appearing as a focal (or multiple) contrast enhancing nodule(s) with associated T2/fluid attenuated inversion recovery signal hyperintensity consistent with perilesional vasogenic edema1,2,7 (Fig. 2). While thought to occur most commonly at the site of maximum radiation exposure (ie, adjacent to the tumor or surgical resection cavity),7,14,17 a detailed correlative analysis of the spatial pattern of TN with the radiation field has, to our knowledge, not yet been carried out. Interestingly, solitary or multiple de novo necrotic lesions can also occur more remotely, on ipsilateral or even contralateral cerebral hemispheres.7
Fig. 2.
Progressive treatment necrosis (A–C; T1-weighted gadolinium-enhanced axial MRI sequences). (A) A 35-year-old male with right frontal low-grade astrocytoma (World Health Organization grade II) underwent surgical resection followed by TMZ-based chemo-RT treatment. Eight months post-RT completion he developed headaches of increased frequency and was found to have a new nodular focus of enhancement in the right frontal lobe subjacent to the resection cavity, with periventricular and corpus callosum involvement, a biopsy of which revealed TN. (B) Sequential TMZ was resumed and completed over the next 6 months; however, the patient experienced worsening of his symptoms as the region of enhancement continued to expand. (C) Despite initiation of corticosteroid and bevacizumab treatment, he developed progressive left-sided hemiparesis and cognitive decline over the following 2 years, prompting a second biopsy of the continually enhancing lesion, which again confirmed TN. Therapeutic management of symptomatic TN was continued; however, the patient deteriorated further, necessitating a transfer to hospice care, where he eventually passed away 2 years after the second biopsy.
The periventricular white matter is considered a predilection site for TN, likely due to its high susceptibility to radiation-induced microvascular injury.7,14,36 Some have observed a high frequency of corpus callosum involvement and subependymal expansion with TN as opposed to PD,16,37 although the opposite was observed by others.38 Further distinct MRI features of radionecrotic lesions, such as a “Swiss cheese” or “soap bubble”‒like interior enhancement,7 a “spreading wavefront” pattern of the lesion,38 or a radiographic lesion quotient,39 have been put forward. Despite these efforts, authoritative diagnosis of the condition based solely on conventional MRI has remained largely elusive.14 Lastly, the frequent presence of “mixed” brain lesions, consisting of both TN and residual and/or recurrent (necrotic) tumor,7,38,39 causes additional ambiguity on conventional MRI, making it a poor diagnostic tool for TN.
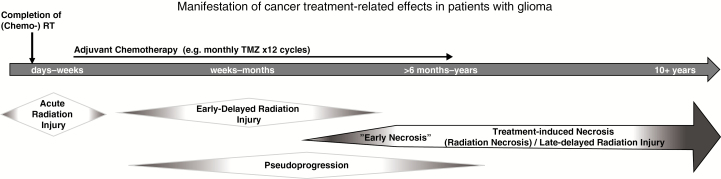
The temporal manifestation pattern of TN is highly variable.5 While late-delayed radiation injury—predominantly manifesting as TN—frequently occurs within 12 months post-RT,5,17,40 TN may develop months to many years after treatment, occasionally occurring up to a decade later.3,41 Recent findings point to an increasing appearance of “early necrosis” developing within the first 6 months post-RT in those patients with glioma who receive standard chemo-RT, suggesting that concurrent TMZ may act as a radiosensitizing agent.13 In this context, it has been hypothesized that (early) TN manifestation might serve as a predictive biomarker for a more durable treatment response.13,17 This assumption should be interpreted with caution, as survival analyses carried out in patients with treatment-related effects are inherently reflective of a selected patient population with an implicit time bias, which needs to be accounted for.36
Finally, the distinction between different types of treatment-related effects is the subject of active clinical debate.5 Apart from TN, other less severe and usually more transient types of treatment-related effects include acute and early-delayed radiation injury,3,8,41 as well as pseudoprogression (PP).1,14 While these entities are primarily distinguished by differences in temporal and clinical patterns, they are somewhat arbitrarily defined and may occasionally overlap, creating diagnostic ambiguities (Fig. 3).5 In particular, the delineation between PP and TN has been complicated by semantic inconsistencies regarding the meaning of the term “pseudoprogression.” PP likely represents a unique, transient scenario in patients with high-grade glioma within the first 3 months of combined TMZ-based chemo-RT.1 Recently, van West et al employed this term to describe late enhancing, treatment-related lesions (median onset 12 mo post-RT) they observed and characterized in patients with low-grade glioma.36 Concluding that the delayed onset for these lesions differed clearly from the earlier timeframe for PP in patients with high-grade gliomas, the authors suggest that these lesions “could be small areas of radiation necrosis.”36
Fig. 3.
Timeline schematic illustrating the temporal manifestation pattern and clinical course of cancer treatment–related effects. Acute and early-delayed types of radiation injury represent transient, reversible neurotoxic phenomena observed within days to weeks, and weeks to several months following chemo-RT.41 By contrast, TN typically constitutes a late-delayed type of radiation injury observed >6 months post-RT with a frequently irreversible and progressive course1; however, concurrent TMZ-based chemo-RT may contribute to increasing incidences of “early necrosis.”13 Pseudoprogression (PP) likely represents a unique, transient, predominantly radiographic phenomenon encountered in patients with high-grade glioma within the first 3 months of combined TMZ-based chemo-RT.1 Differentiation between these entities remains a clinical challenge.
Diagnostic Considerations
Defining a reliable diagnostic algorithm for accurate detection of TN has been hampered by its radiographic similarity to PD on conventional MRI2,7 and frequent manifestation as a mixed pathology with recurrent or residual tumor.7,39 Moreover, complex radiographic findings seen after combinatorial anti-angiogenic, cytotoxic, and immunotherapy regimens21,42,43 compromise adequate MRI-based follow-up monitoring and characterization of treatment response with Macdonald and revised Response Assessment in Neuro-Oncology (RANO) criteria.44–46 While existing RANO criteria limit clinical trial enrollment to patients with radiographic PD in whom contrast enhancing lesions appear at or beyond 12 weeks post-RT,46 treatment-related effects (especially TN) frequently manifest beyond this cutoff point (Fig. 3). Misdiagnosis of tumor progression could result in premature first-line treatment discontinuation and administration of a salvage treatment (which should have been withheld until true PD) or may delay a necessary treatment change in cases where treatment effects, such as PP or TN, are mistakenly assumed.20,22,44 Furthermore, erroneous inclusion of misdiagnosed patients into clinical trials condones misinterpretation of the efficacy of any investigational agent.13,21,22
Beyond efforts to revise currently employed radiographic treatment response assessment criteria,18,21 attempts to identify more accurate, clinically feasible diagnostic imaging biomarkers and, ultimately, enable a “virtual biopsy” of TN1,12,17,40 have included the assessment of diffusion weighted47 and diffusion tensor48 MRI, MRI perfusion studies,49–51 CT perfusion (CTP) studies,52 MR spectroscopy (MRS),53–55 positron emission tomography (PET),56–59 single photon emission computed tomography (SPECT),60 or combinations thereof.55,61,62 Notwithstanding, histopathological evaluation remains the diagnostic gold standard,5,11 albeit many of the aforementioned non-invasive technologies hold substantial additive value in complementing conventional MRI findings and improving diagnostic certainty in cases of suspected TN and when a surgical tissue biopsy is too risky or otherwise not feasible.1,12,17,19,20,40 Further advantages include guidance for stereotactic biopsy procedures and more tailored, less neurotoxic radiation field mapping for radiotherapeutic interventions16 (eg, via quantitative TN versus PD distinction within mixed lesions), identification of tumor “hot spots,” and characterization of the degree of tumor infiltration into perilesional brain parenchyma. Techniques such as MRI-localized biopsies and radiographic-histopathological correlations (eg, via MR signal intensity to cell density correlation maps)63 have addressed the challenges of tumor sampling resulting from the high degree of intratumoral heterogeneity and frequent presence of mixed pathology following anti-neoplastic treatment.
Several reviews have evaluated the growing body of literature on the role of advanced imaging in TN diagnosis.12,16,19,20,40,64 Concluding that a preferred non-invasive diagnostic gold standard for TN is still lacking, several reports identify distinct strengths and weaknesses of various imaging modalities, and provide valuable recommendations for clinical practice and research design (Table 1). Methodological problems involve the lack of randomized controlled clinical trials, absence of histopathological verification of lesions identified by imaging, poorly matched patient groups, high variability in clinical practices at time of radiographic disease progression, and potential operator dependency in radiographic assessment.12,19,20,64 Moreover, most studies investigate a single imaging modality, whereas combined use of multiple functional imaging modalities has become a common clinical reality with improved diagnostic accuracy.12,20,55,62 Other difficulties relate to producing methodologically accurate meta-analyses of published data due to inconsistencies in defining TN40 and unresolved standardization in image acquisition and processing.16
Table 1.
Comprehensive overview of existing reviews assessing the diagnostic performance of different advanced imaging modalities for TN vs PD
| Study/Type | No./Types of Studies Reviewed | Selected Notable Findings | Key Issues Identified | Overall Recommendations |
|---|---|---|---|---|
|
Alexiou et al, (2009)
19
- Systematic Review - Focus on value of MRI techniques, SPECT, PET to differentiate TN from glioma recurrence. |
46 clinical studies - 3 Class I, - 9 Class III, - and 34 Class IV evidence level studies |
DWI / MRS: several Class III & IV studies. - 1 biopsy-controlled Class I study (Rock et al, 2004) showing MRS ratios (Cho/NAA, NAA/normal Cr and NAA/Cho) can reliably differentiate TN from PD. ADC values improved differentiation, but not in mixed lesions. PET: majority Class III & IV studies. - Accuracy of 18F-FDG-PET hampered by high background signal; ranges of 62–100% sensitivity and 40–100% specificity in evaluated studies. - Novel PET tracers (11C-MET,18F-FDOPA,18 F-FET) with different advantage/ disadvantage profiles, but potentially improved diagnostic sensitivity (75–100%) and specificity (75–100%). - 1 prospective biopsy-controlled study (Mehrkens et al, 2008) showed 84% pos. predictive value of 18F-FET PET for detecting glioma recurrence. |
- Majority of studies had ↓evidence levels - Many studies not biopsy-controlled - Mostly retrospective design - Unclear methodology in some studies |
- Tentative recommendation to use multivoxel MRS and/or PET with newer radiotracers to detect true tumor recurrence - Recommendation to carry out prospective, biopsy-confirmed studies with higher evidence levels. |
|
Jain et al, (2010)
16
- Comprehensive Review - Discusses individual advantages, limitations, and clinical utility of functional neuro-imaging modalities in distinguishing between TN and PD. |
Unspecified number of key studies discussed: - Perfusion imaging studies - MRS studies - DWI/DTI studies - PET/SPECT studies |
Perfusion imaging: limited performance in mixed lesions and in pat. receiving anti-angiogenic treatments. - Potential advantage of CTP over MR perfusion, due to relative ease to generate quantitative perfusion parametric maps through defined arterial input & venous output function. - CTP clinical utility limited by Rx exposure + iodinated contrast agent; MR perfusion easily obtainable as additional sequence to conventional Gd-MRI. - 1 biopsy-controlled CTP study showed 83.3% sensitivity / 100% specificity for TN vs PD detection (Jain et al, 2007) MRS: most studies lack biopsy-controls. - MRS metabolic ratios can reliably differentiate pure, but not mixed lesions with tissue heterogeneities below current spatial resolution (~1 cc). - Multivoxel > single voxel MRS for diagnostic performance (Chernov et al, 2005) |
- Most techniques lack standardization of image acquisition & post-processing parameters → 1) difficulty to use as treatment response monitoring tool. 2) difficulty to conduct multicenter studies or compare different studies. - Most techniques have ↓resolution → difficulty for in vivo quantification of (particularly mixed) lesions. |
- Advanced imaging can facilitate TN/PD distinction; however, clinical feasibility is still limited by several remaining issues. - Critical need for further development and greater clinical use of functional imaging biomarkers → conventional imaging is insufficient for radiographic characterization of effects produced by new and combinatorial treatment regimens |
|
- Longer scan times required to obtain reproducible data. DWI: Unresolved ongoing discussion on which lesion type (TN or PD) has higher ADC values. PET / SPECT: Overall more limited availability and ↓spatial resolution - 18F-FDG-PET downsides: ↑background signal, potential false-negatives (LGG appear hypometabolic) or false-positives (abscess or reactively inflamed TN lesions can appear hypermetabolic). - These challenges might be improved by employing novel amino acid tracers or combinations thereof with FDG, as well as co-registration of PET with structural MRI. |
||||
|
Caroline & Rosenthal (2012)
64
- Systematic Review - Assesses efficacy of imaging modalities to distinguish between PP, TN, and PD (HGGs). |
26 clinical studies - 4 main groups of imaging modalities: MRI, PET, SPECT, and combinations thereof. |
MRI-based techniques:
- Conventional Gd-MRI and MRS appear to be more sensitive than specific. - MR perfusion using rCBF appears to be more specific than sensitive. - DWI and DTI appear to have similar accuracy (86.7% and 85.7%, respectively) in detecting PD PET / SPECT: - 201Tl-SPECT may be more specific (100% specificity / 84–100% sensitivity range) than FDG or amino acid based PET tracers. - Combined MRI w/ 201Tl-SPECT may have ↑sensitivity than combined MRI w/ 18 F-FDG-PET; using combinations of PET tracers may exceed the level of diagnostic accuracy reached by single tracers alone. |
- Many included studies had small sample sizes or were not biopsy-controlled - Overall lack of prospective biopsy-controlled studies in the field |
- No specific recommendations on preferred imaging techniques given - Advocated need for large, prospective, biopsy-controlled studies. |
|
Shah et al, (2013)
40
- Systematic Review - Assesses case reports/case series/prospective studies for efficacy of imaging modalities to distinguish TN from recurrent glioma. |
17 clinical studies - All selected studies included at least 1 case of histological confirmation. |
- SPECT had the highest combined mean specificity (97.8%) out of the reviewed studies. Its mean sensitivity (87.6%) was comparable to that of conventional MRI, the most sensitive modality (88.9%) - MET-PET has ↑mean sensitivity and specificity (84.2% and 82.4%, respectively) than FDG-PET (70.1 and 64.8%, respectively). - CTP combined with a permeability surface air product (PS) yielded 100% sensitivity, 89% specificity in a biopsy-controlled cohort of 38 pat. (Jain et al, 2011) |
Limitations noted in own review:
- No differentiation between TN and PP made in analysis - Predominance of PD cases over TN cases → potential bias in sensitivity/ specificity values Other identified issues: - Potential operator dependency/ subjective bias in studies - Clinicians must ensure that technology is available and that neuroradiologists are familiar with it. |
- SPECT, in particular Tc-99 SPECT, may be the modality of choice for diagnostic purposes. - CTP is recommended if maximal sensitivity for detection of PD is clinically desired. - MRI alone and18F-FDG-PET have low specificity and should be avoided. |
|
Verma et al, (2013)
12
- Comprehensive Review - Discusses efficacy and limitations of structural & functional imaging modalities in distinguishing TN from PD. |
Tabular analysis of: - 8 DWI /DTI studies (ADC and FA values) - 10 perfusion studies (MR or CT-based) - 14 MRS studies (MRS ratios) - 16 PET studies - 14 SPECT studies |
DWI/DTI: Remains largely at exploratory stage, awaits thorough evaluation. - Measurements (esp. ADC values) affected by scanner type, magnetic field strength → difficult to establish standardized parameters and universal threshold values to differentiate TN from PD. - Effects of necrosis, gliosis, fibrous scar tissue, tissue granulation on ADC and FA values not well understood - Mean ADC and FA values easily skewed by mixed lesions. Perfusion imaging: Variable Pro/Con profile for each technique. DSC MR imaging potentially most clinically feasible. - DSC MRI: Pros- better SNR, shorter scan times, ease of use, better availability. Cons- prone to susceptibility artifacts → limited application in pat. w/ hemorrhages, calcifications, surgical clips. - DCE MRI: Pros- robust against susceptibility artefacts, ↑spatial resolution that better characterizes mixed lesions. Cons- complex/error prone hemodynamic parameter quantification → no FDA-approved standardized software exists. |
Majority of studies focus on single imaging modalities only, have small sample sizes, lack biopsy-control Limited clinical utility: - ↓scanner availability - lack of insurance coverage - ↑operation costs - frequent diagnostic need for multiple combined imaging techniques further limits clinical feasibility |
- Multiple combined imaging techniques should be used in case of mixed lesions to yield a) better physiological characterization of lesions and b) reduce misinterpretation of lesions. - Results from multimodal diagnostic imaging should be contextualized with info on patient demographics, therapeutic history, and primary tumor type. - Quantitative approaches using morphometric image feature analysis to detect fine-grained differences between TN and PD warrant further investigation. |
|
- CTP: Pros- technology widely available, no magnetic susceptibility artefacts, parameter quantification linear and less error prone. Cons- ↓clinical feasibility than MRI → toxicity (ionizing radiation, iodinated contrast agents), ↓resolution, image acquisition and processing less flexible. MRS: Multivoxel MR measuring abnormal spectra beyond the contrast-enhanced area could help detect extent of perilesional tumor infiltration → potential for improved radiation field mapping/ reduction of TN risk. - Frequent tissue necrosis in PD may metabolically mimic TN (↑lipid and ↑lactate levels) - Prone to ↑variability (low SNR, acquisition- and biological variability, inaccurate voxel relocalization during spectrum averaging) → ↓reproducibility of measurements - Limited clinical feasibility → long scan times, high cost, no insurance coverage, lack of universal consensus (↑metabolite ratio variability across studies) |
||||
|
Multimodal imaging: - In a prospective, biopsy-controlled study, structural MRI when used in conjunction with FET-PET and MRS could boost accuracy of PD detection from 68% to 97% (Floeth et al, 2005) |
||||
|
Ryken et al, (2014)
20
-Systematic Review -Focus on which imaging techniques best differentiate PD from TN and PP in patients with previously diagnosed GBM. |
57 clinical studies, 46 focused on advanced imaging techniques -8 MRI perfusion studies -5 MRI diffusion studies -13 MRS studies -10 PET studies -10 SPECT studies |
See detailed imaging recommendations with corresponding levels of evidence (Class I–III)a. Multimodal imaging: - Combined use of multiple imaging techniques and multi-parametric analyses are classified as class 3 data (lacking independent validation), but may offer greatly improved diagnostic accuracy - A 55 pat. cohort study (36 pat. w/ biopsy-confirmed diagnosis) showed a 96% diagnostic accuracy of MRS combined with DWI in detecting TN vs PD (Zeng 2007) |
- Reviewed studies lack high levels of evidence due to: -poor study design -heterogeneity of pat. population -variability in practices at time of progression - Paucity of prospectively collected data with well-matched pat. groups |
- MRI (w/ or w/o Gd.) as imaging surveillance method to detect progression of GBM (Level II evidence) - MRS (Level II) or SPECT (Level III) as diagnostic methods for PD vs TN / PP differentiation. - Routine use of PET to identify PD is not recommended (Level III) |
Abbreviations: 11C-MET = (11)c-methionine;18F-FDG-PET = fluorodeoxyglucose;18F-FDOPA = fluorodopa;18F-FET = fluoro-ethyl-tyrosine; 201Tl = (201)thallium; ADC = apparent diffusion coefficient; Cho = choline; Cr = creatine; CTP = computed tomography perfusion imaging; DCE = dynamic contrast-enhanced; DSC = dynamic susceptibility contrast; DTI = diffusion tensor imaging; DWI = diffusion weighted imaging; FA = fractional anisotropy; GBM = glioblastoma multiforme; Gd = gadolinium; HGG = high-grade glioma; LGG = low-grade glioma; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; NAA = N-acetylaspartate; pat. = patients; PD = progressive disease; PET = positron emission tomography; PP = pseudoprogression; rCBF = regional cerebral blood flow; Rx = radiation; SNR = signal-to-noise ratio; SPECT = single-photon emission computed tomography; Tc-99 = technetium-99; TN = treatment necrosis; w/ = with; w/o = without
a Grading of evidence levels in this study was carried out according to “a three-tiered system for assessing studies addressing diagnostic testing as approved by the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Committee on Guidelines criteria.”
Most reviews emphasize a critical necessity for prospective, biopsy-controlled studies to improve the current body of evidence.12,19,20,64 Moreover, widespread adoption of advanced imaging is difficult to achieve in clinical practice due to limited availability, high operational costs, and common lack of insurance coverage for such procedures.12 Low spatial resolution of most techniques and limited utility for accurate longitudinal monitoring (due to standardization issues) are additional concerns.16
Recommendations on diagnostic imaging for TN versus PD distinction vary. Several groups endorse multivoxel MRS,19,20,65 PET with novel amino acid based radiotracers,19 (technetium-99) SPECT,20,40 and CTP.16,40 Conversely, routine diagnostic use of fluorodeoxyglucose (18F-FDG) PET is discouraged due to its low specificity and poor signal-to-noise ratio.20,40 Nevertheless, virtually all neuroimaging techniques were found to bear some specific disadvantages (see Table 1). Others have therefore advocated a multimodal diagnostic approach through the combined use of several techniques,12 such as MRS with diffusion-weighted imaging (DWI),55 or MRI combined with fluoro-ethyl-tyrosine (FET) PET and MRS.62 The advent of hybrid PET-MRI56 may facilitate such combinatorial approaches in becoming more clinically feasible and less time-consuming.18 An interesting novel approach includes the use of delayed-contrast MRI to construct treatment response assessment maps (TRAMs) for differentiation of PD from treatment effects based on delayed contrast accumulation (nontumor tissues) versus contrast clearance (representing active tumor).66 Histological validation demonstrated 100% sensitivity and 92% positive predictive value to active tumor of this approach, including adequate representation of tumor burden by TRAMs.
Blood-based biomarkers are increasingly explored for diagnosis and treatment response in neuro-oncology, including efforts to achieve liquid biopsy-based differentiation of treatment effects from PD, with technical limitations mainly pertaining to sensitivity issues.67 One recent study investigated expression profile differences of myeloid-derived suppressor cells (MDSCs) as a potential biomarker for predicting recurrent GBM and differentiating it from TN.68 While early results of this approach have been encouraging, potential diagnostic feasibility of the MDSC biomarker for lower-grade gliomas—where TN would be expected to occur even more frequently—remains to be established. The predictive value of this approach in the setting of “mixed lesions” remains unclear, as only TN lesions with <5% of active tumor were included.68 Other previous efforts have investigated blood-derived microvesicles as a potential diagnostic biomarker for PD versus TN/PP differentiation in chemo-RT treated GBM patients with equivocal imaging findings.69
Finally, histopathological diagnosis and classification of biopsied lesions raises several challenges. Currently, no specific guidelines for histopathological characterization of treatment-induced brain tissue necrosis or other treatment-related effects exist; the final pathological diagnosis depends largely on the pathologist’s professional experience and personal judgment. As the histopathological distinction between TN and PP remains challenging, findings are often summarized under the umbrella term “treatment effect.” Moreover, analyzed lesions frequently reveal “mixed results,” consisting of necrosis with differing quantities of scattered atypical tumor cells and/or foci of solid tumor (representing PD), thus making re-initiation of anti-neoplastic treatment a judgment call. Occasionally, lesions may contain inflammatory components, such as lymphocytic infiltrates, rather than plain necrosis. While rare atypical cells are found in most TN specimens, radiation-induced cellular atypia in non-neoplastic cells is a known phenomenon that may cause further diagnostic ambiguity.6
Establishing treatment effect–specific quantitative and qualitative measures for (i) more accurate histopathological differentiation between distinct types of TN or other treatment-induced phenomena like PP, and (ii) precise determination of the amount of tumor versus treatment-related pathology within the specimen would improve diagnostic accuracy and aid further patient management decisions and prognostication. Such measures may be more conceivable for specimens resected in toto, as tissue samples obtained by stereotactic needle biopsy—depending on the amount of available tissue—carry a higher risk of sampling error and non-diagnostic yield.70
Therapeutic Considerations
The clinical course of patients diagnosed with TN is highly variable. Necrotic lesions may develop entirely without symptoms (identified by neuroimaging only), but approximately 42%34 to 54%15 of patients will demonstrate progressive cognitive decline, diffuse and/or focal neurological deficits, signs of increased intracranial pressure, and/or seizures71 (ie, frequently mimicking the clinical picture of PD) (Fig. 1). While clinical symptoms may resolve gradually, some patients will get progressively worse, requiring medical and/or surgical therapeutic intervention to halt further neurological decline or, rarely, to prevent a fatal outcome.72 The rather ill-defined heterogeneous clinical picture of TN along with aforementioned radiological difficulties pose a management challenge,1 as therapeutic strategies for TN differ sharply from those for PD.73
No SOC treatment protocol for TN presently exists and the pathophysiology of the condition remains poorly understood. Histopathological correlates of TN commonly include thrombosis, hemorrhage, parenchymal necrosis, histiocytic infiltrates, gliosis, fibrinous exudates, and vascular abnormalities.6 While thought to be driven by a combination of treatment-induced vascular endothelial injury, glial cell injury, hypoxic injury/vascular endothelial growth factor (VEGF) overexpression and (auto)immune-mediated responses,6,8,9,17 the exact sequence of pathomechanisms and key targetable molecular drivers of TN remain uncertain.
Among numerous therapeutic strategies put forward for TN (see Supplementary Table 1 for a comprehensive overview of relevant published studies), no causal therapy is presently available as existing interventions are mostly limited to management of TN-associated symptoms.5 As such, vasogenic edema and associated mass effect, thought to be caused by radiation-induced blood–brain barrier disruption and inflammatory cytokine release,9,74 are commonly managed with corticosteroids.75 More recently, the VEGF-A monoclonal antibody bevacizumab (Avastin) has shown some promise in reversing neurological symptoms and radiographic changes in patients with TN.27,76–80 However, the long-term therapeutic feasibility of both medications is limited by their side effect profiles81 as well as treatment costs (in the case of bevacizumab).79 Single case reports of patients with TN experiencing paradoxical neurological worsening under bevacizumab treatment82 or developing acquired resistance to the drug83 have been documented. Anti-coagulant/anti-platelet drugs with vitamin E,84–86 hyperbaric oxygen therapy (HBOT),87–89 intramuscular nerve growth factor,90 and antibiotic applications91 constitute other experimental strategies, although response rates have been mixed and associated studies were generally of insufficient levels of clinical evidence.5,10 Minimally invasive techniques, such as laser interstitial thermal therapy (LITT),92–94 are being increasingly explored to treat TN or PD lesions that are surgically inaccessible94,95 and/or located in eloquent brain regions,96 or when open surgical procedures are contraindicated. Evidence from 2 biopsy-controlled retrospective studies95,97 and 1 multicenter prospective study has suggested clinical and radiographic improvement from LITT with minimal morbidity in patients with previously symptomatic TN lesions.98 Finally, surgical resection carries an implicit advantage of yielding diagnostic histopathological information that may guide future patient management. While potentially a life-saving intervention in the management of acutely symptomatic, mass-effect producing TN lesions, surgical intervention may bear the risk of procedure-related complications and worse neurological outcome.72 Delayed timing of surgery (usually after all conservative therapy has failed) may propel surgical risk, whereas more aggressive, early surgical intervention could potentially improve clinical outcome.72
Taken together, existing therapeutic options for patients with TN are limited. Most available treatment strategies lack sufficient clinical evidence to draw dependable conclusions on their possible therapeutic efficacy. Bevacizumab appears to have the most evidence to suggest favorable effects on both clinical and radiographic improvement as well as reducing steroid dependency, although the side effect profile and high treatment cost may preclude its long-term therapeutic feasibility.27,77,79,80 Intra-arterial anti-VEGF therapy might potentially reduce bevacizumab-associated side effects99,100; however, its efficacy remains to be shown in glioma patients affected by TN. Intramuscular nerve growth factor treatment has shown some early promise in reversing cognitive deficits and radiographic findings without significant adverse effects in patients with temporal lobe necrosis, warranting further investigation.90 Finally, the use of LITT to treat surgically inaccessible symptomatic TN lesions bears promise in alleviating neurological symptoms and reducing the need for steroids without the risk of conventional surgical approaches.95,97,98
Future Perspectives: Mapping the Field
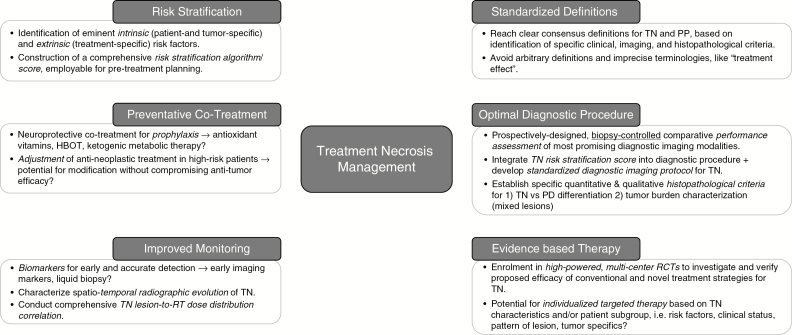
Improvement in the management of TN faces a number of clinical and systemic challenges (Fig. 1 and Fig. 2). While an array of advanced diagnostic imaging modalities and therapeutic strategies have been developed (Table 1 and Supplementary Table 1), no diagnostic or therapeutic consensus for TN presently exists. High-powered, prospective, and biopsy-controlled clinical studies may help to improve performance assessment of diagnostic neuroimaging and provide the basis to establish dependable, treatment-effect specific imaging criteria to supplement existing modified RANO criteria.45 Moreover, sufficient availability of biopsy material would facilitate research to advance histopathological characterization for different types of treatment effects (Fig. 3).
In addition to defining an evidence-based diagnostic and therapeutic SOC, future work should address prevention strategies and improved patient monitoring (Fig. 4). The former will necessitate assessment of putative risk factors for TN and, optimally, the construction of a clinically employable risk stratification tool to identify “high risk patients.” Adjustment of cancer therapy regimens and use of potential neuroprotective strategies, such as ketogenic metabolic therapy,101 high-dose antioxidants,86 or HBOT,88 during and after chemo-RT treatment are possible areas of investigation. Here, clinical evaluation should ideally include a non-inferiority design, to ensure that tumor response is not adversely affected. Additional challenges to clinical trial design relate to patient selection criteria, that is, whether stratification of patients with TN based on the underlying condition (malignant glioma, brain metastases, or nasopharyngeal carcinoma) would be reasonable. Finally, greater emphasis on comprehensive evaluation of treatment-related effects across the entire neuro-oncological care trajectory would permit more integrated analysis of collected clinical data.
Fig. 4.
Schematic illustrating 6 eminent, interdependent research pillars paramount to mapping the field of treatment necrosis management in neuro-oncology. Key research topics and unanswered questions are highlighted.
Conclusion
Progress in this complex field of TN is limited by several clinical and systemic factors. Critical questions pertaining to the true incidence and presentation of TN, risk factors, histopathological correlates, radiographic patterns, and the role of advanced functional imaging modalities remain to be addressed. Deriving conclusive answers from the current body of literature is chiefly precluded by the paucity of biopsy-controlled studies. A greater research focus on treatment-related effects through rigorous collection of clinical data and inclusion of relevant parameters as primary or secondary endpoints in multicenter randomized controlled trials would be of tremendous benefit to improve prevention, diagnosis, treatment response assessment, and therapeutic management of affected patients.
Funding
This work was supported by the Charité Berlin/MDC joint Berlin Institute of Health (BIH) (MD Stipend Grant [S.F.W.]); the Rolf W. Günther Foundation of Radiological Sciences (R.W.G. Stiftung für Radiologische Wissenschaften) (S.F.W.); the German National Academic Foundation (Studienstiftung des deutschen Volkes) (S.F.W.); the American Brain Foundation (J.D.); the American Cancer Society (J.D.); and the Amy Gallagher Foundation (J.D.).
Conflict of interest statement. The authors declare no conflicts of interest.
Supplementary Material
References
- 1. Dietrich J, Winter SF, Klein JP. Neuroimaging of brain tumors: pseudoprogression, pseudoresponse, and delayed effects of chemotherapy and radiation. Semin Neurol. 2017;37(5):589–596. [DOI] [PubMed] [Google Scholar]
- 2. Dietrich J, Klein JP. Imaging of cancer therapy-induced central nervous system toxicity. Neurol Clin. 2014;32(1):147–157. [DOI] [PubMed] [Google Scholar]
- 3. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180–188. [DOI] [PubMed] [Google Scholar]
- 4. Na A, Haghigi N, Drummond KJ. Cerebral radiation necrosis. Asia Pac J Clin Oncol. 2014;10(1):11–21. [DOI] [PubMed] [Google Scholar]
- 5. Eisele SC, Dietrich J. Cerebral radiation necrosis: diagnostic challenge and clinical management. Rev Neurol. 2015;61(5):225–232. [PubMed] [Google Scholar]
- 6. Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. [DOI] [PubMed] [Google Scholar]
- 7. Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. [DOI] [PubMed] [Google Scholar]
- 8. Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502. [DOI] [PubMed] [Google Scholar]
- 9. Furuse M, Nonoguchi N, Kawabata S, Miyatake S, Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol. 2015;48(4):183–190. [DOI] [PubMed] [Google Scholar]
- 10. Fink J, Born D, Chamberlain MC. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12(3):276–285. [DOI] [PubMed] [Google Scholar]
- 11. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–83. [DOI] [PubMed] [Google Scholar]
- 14. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 15. Kim JM, Miller JA, Kotecha R, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133(2):357–368. [DOI] [PubMed] [Google Scholar]
- 16. Jain R, Narang J, Sundgren PM, et al. Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol. 2010;100(1):17–29. [DOI] [PubMed] [Google Scholar]
- 17. Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648–657. [DOI] [PubMed] [Google Scholar]
- 18. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 19. Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neurooncol. 2009;95(1):1–11. [DOI] [PubMed] [Google Scholar]
- 20. Ryken TC, Aygun N, Morris J, et al. The role of imaging in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3). [DOI] [PubMed] [Google Scholar]
- 21. Huang RY, Neagu MR, Reardon DA, Wen PY. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy—detecting illusive disease, defining response. Front Neurol. 2015;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanghera P, Rampling R, Haylock B, et al. The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol. 2012;24(3):216–227. [DOI] [PubMed] [Google Scholar]
- 23. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 24. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. [DOI] [PubMed] [Google Scholar]
- 25. Mikhael M. Dosimetric considerations in the diagnosis of radiation necrosis of the brain. In: Gilbert H, Kagan A, eds. Radiation Damage to the Nervous System. New York: Raven Press; 1980:59–91. [Google Scholar]
- 26. Lam TC, Wong FC, Leung TW, Ng SH, Tung SY. Clinical outcomes of 174 nasopharyngeal carcinoma patients with radiation-induced temporal lobe necrosis. Int J Radiat Oncol Biol Phys. 2012;82(1):e57–e65. [DOI] [PubMed] [Google Scholar]
- 27. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clarke J, Neil E, Terziev R, et al. Multicenter, phase 1, dose escalation study of hypofractionated stereotactic radiation therapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 2017;99(4):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du Four S, Janssen Y, Michotte A, et al. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med. 2018;7(10):4870–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol. 2017;133(3):595–602. [DOI] [PubMed] [Google Scholar]
- 31. Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–252. [DOI] [PubMed] [Google Scholar]
- 32. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 33. Lee AW, Foo W, Chappell R, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;40(1):35–42. [DOI] [PubMed] [Google Scholar]
- 34. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van West SE, de Bruin HG, van de Langerijt B, Swaak-Kragten AT, van den Bent MJ, Taal W. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro Oncol. 2017;19(5):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rogers L, Scarpace L, Gutierrez J, Ryu S. Magnetic resonance imaging features of histologically documented cerebral radiation necrosis. Neurology. 2006;66(5):A398–A399. [Google Scholar]
- 38. Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26(8):1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 39. Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63(5):898–903; discussion 904. [DOI] [PubMed] [Google Scholar]
- 40. Shah AH, Snelling B, Bregy A, et al. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol. 2013;112(2):141–152. [DOI] [PubMed] [Google Scholar]
- 41. Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153(4):357–370. [DOI] [PubMed] [Google Scholar]
- 42. Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol. 2013;10(1):14–26. [DOI] [PubMed] [Google Scholar]
- 44. Eisele SC, Wen PY, Lee EQ. Assessment of brain tumor response: RANO and Its offspring. Curr Treat Options Oncol. 2016;17(7):35. [DOI] [PubMed] [Google Scholar]
- 45. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25(2):201–209. [PMC free article] [PubMed] [Google Scholar]
- 48. Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24(9):1131–1142. [DOI] [PubMed] [Google Scholar]
- 49. Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 2013;55(3):361–369. [DOI] [PubMed] [Google Scholar]
- 50. Barajas RF Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99(1):81–88. [DOI] [PubMed] [Google Scholar]
- 52. Jain R, Narang J, Schultz L, et al. Permeability estimates in histopathology-proved treatment-induced necrosis using perfusion CT: can these add to other perfusion parameters in differentiating from recurrent/progressive tumors? AJNR Am J Neuroradiol. 2011;32(4):658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Plotkin M, Eisenacher J, Bruhn H, et al. 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol. 2004;70(1):49–58. [DOI] [PubMed] [Google Scholar]
- 54. Rock JP, Scarpace L, Hearshen D, et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery. 2004;54(5):1111–7; discussion 1117. [DOI] [PubMed] [Google Scholar]
- 55. Zeng QS, Li CF, Liu H, Zhen JH, Feng DC. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68(1):151–158. [DOI] [PubMed] [Google Scholar]
- 56. Jena A, Taneja S, Jha A, et al. Multiparametric evaluation in differentiating glioma recurrence from treatment-induced necrosis using simultaneous 18F-FDG-PET/MRI: a single-institution retrospective study. AJNR Am J Neuroradiol. 2017;38(5):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 58. Takenaka S, Asano Y, Shinoda J, et al. Comparison of (11)C-methionine, (11)C-choline, and (18)F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir (Tokyo). 2014;54(4):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112(9):758–765. [DOI] [PubMed] [Google Scholar]
- 60. Zhang H, Ma L, Wu C, Xu BN. Performance of SPECT in the differential diagnosis of glioma recurrence from radiation necrosis. J Clin Neurosci. 2015;22(2):229–237. [DOI] [PubMed] [Google Scholar]
- 61. Hatzoglou V, Yang TJ, Omuro A, et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016;18(6):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Floeth FW, Pauleit D, Wittsack HJ, et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005;102(2):318–327. [DOI] [PubMed] [Google Scholar]
- 63. Chang PD, Malone HR, Bowden SG, et al. A multiparametric model for mapping cellularity in glioblastoma using radiographically localized biopsies. AJNR Am J Neuroradiol. 2017;38(5):890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caroline I, Rosenthal MA. Imaging modalities in high-grade gliomas: pseudoprogression, recurrence, or necrosis? J Clin Neurosci. 2012;19(5):633–637. [DOI] [PubMed] [Google Scholar]
- 65. Chernov M, Hayashi M, Izawa M, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg. 2005;48(4):228–234. [DOI] [PubMed] [Google Scholar]
- 66. Zach L, Guez D, Last D, et al. Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro Oncol. 2015;17(3):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zachariah MA, Oliveira-Costa JP, Carter BS, Stott SL, Nahed BV. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro Oncol. 2018;20(9):1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Soler DC, Young AB, Cooper KD, et al. The ratio of HLA-DR and VNN2+ expression on CD14+ myeloid derived suppressor cells can distinguish glioblastoma from radiation necrosis patients. J Neurooncol. 2017;134(1):189–196. [DOI] [PubMed] [Google Scholar]
- 69. Koch CJ, Lustig RA, Yang XY, et al. Microvesicles as a biomarker for tumor progression versus treatment effect in radiation/temozolomide-treated glioblastoma patients. Transl Oncol. 2014;7(6):752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23(2):71–75. [DOI] [PubMed] [Google Scholar]
- 71. Chi D, Behin A, Delattre J-Y.. Neurologic complications of radiation therapy. In: Schiff D, Kesari S, Wen P, eds. Cancer Neurology in Clinical Practice. Totowa, NJ: Humana Press; 2008:259–286. [Google Scholar]
- 72. McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol. 2004;68(1):41–47. [DOI] [PubMed] [Google Scholar]
- 73. Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–1228. [DOI] [PubMed] [Google Scholar]
- 74. Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164(5):662–668. [DOI] [PubMed] [Google Scholar]
- 75. Shaw PJ, Bates D. Conservative treatment of delayed cerebral radiation necrosis. J Neurol Neurosurg Psychiatry. 1984;47(12):1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–68. [DOI] [PubMed] [Google Scholar]
- 77. Furuse M, Nonoguchi N, Kuroiwa T, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis†. Neurooncol Pract. 2016;3(4):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sadraei NH, Dahiya S, Chao ST, et al. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. 2015;38(3):304–310. [DOI] [PubMed] [Google Scholar]
- 79. Lubelski D, Abdullah KG, Weil RJ, Marko NF. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol. 2013;115(3):317–322. [DOI] [PubMed] [Google Scholar]
- 80. Tye K, Engelhard HH, Slavin KV, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol. 2014;117(2):321–327. [DOI] [PubMed] [Google Scholar]
- 81. Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jeyaretna DS, Curry WT Jr, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. 2011;29(7):e159–e162. [DOI] [PubMed] [Google Scholar]
- 83. Zhuang H, Yuan X, Sun D, et al. Acquired-resistance of bevacizumab treatment for radiation brain necrosis: a case report. Oncotarget. 2016;7(11):13265–13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–366. [DOI] [PubMed] [Google Scholar]
- 85. Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–2027. [DOI] [PubMed] [Google Scholar]
- 86. ClinicalTrials.gov [Internet]. Identifyer NCT01508221. Evaluation of the Use of Trental and Vitamin E for Prophylaxis of Radiation Necrosis. Bethesda, MD: National Library of Medicine (US); 2000. [Google Scholar]
- 87. Chuba PJ, Aronin P, Bhambhani K, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80(10):2005–2012. [DOI] [PubMed] [Google Scholar]
- 88. Ohguri T, Imada H, Kohshi K, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(1):248–255. [DOI] [PubMed] [Google Scholar]
- 89. Valadão J, Pearl J, Verma S, Helms A, Whelan H. Hyperbaric oxygen treatment for post-radiation central nervous system injury: a retrospective case series. Undersea Hyperb Med. 2014;41(2):87–96. [PubMed] [Google Scholar]
- 90. Wang XS, Ying HM, He XY, Zhou ZR, Wu YR, Hu CS. Treatment of cerebral radiation necrosis with nerve growth factor: a prospective, randomized, controlled phase II study. Radiother Oncol. 2016;120(1):69–75. [DOI] [PubMed] [Google Scholar]
- 91. Feng Y, Chen X, Guo N, Gao X. Effects of antibiotics on CNS radiation necrosis. J Clin Oncol. 2015;33(15 SUPPL. 1). doi:10.1200/jco.2015.33.15_suppl.e17008 [Google Scholar]
- 92. Murovic JA, Chang SD. The pathophysiology of cerebral radiation necrosis and the role of laser interstitial thermal therapy. World Neurosurg. 2015;83(1):23–26. [DOI] [PubMed] [Google Scholar]
- 93. Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: an overview. Expert Rev Neurother. 2016;16(2):223–232. [DOI] [PubMed] [Google Scholar]
- 94. Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200. [DOI] [PubMed] [Google Scholar]
- 95. Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113(3):495–503. [DOI] [PubMed] [Google Scholar]
- 96. Iyer A, Halpern CH, Grant GA, Deb S, Li GH. Magnetic resonance-guided laser-induced thermal therapy for recurrent brain metastases in the motor strip after stereotactic radiosurgery. Cureus. 2016;8(12):e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Smith CJ, Myers CS, Chapple KM, Smith KA. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(Suppl 1):S59–S72. [DOI] [PubMed] [Google Scholar]
- 98. Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–811. [DOI] [PubMed] [Google Scholar]
- 99. Dashti SR, Spalding A, Kadner RJ, et al. Targeted intraarterial anti-VEGF therapy for medically refractory radiation necrosis in the brain. J Neurosurg Pediatr. 2015;15(1):20–25. [DOI] [PubMed] [Google Scholar]
- 100. ClinicalTrials.gov [Internet]. Low-dose Intra-arterial Bevacizumab for Edema and Radiation Necrosis Therapeutic Intervention (LIBERTI). Bethesda, MD: National Library of Medicine (US); 2000. [Google Scholar]
- 101. Winter SF, Loebel F, Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev Oncol Hematol. 2017;112:41–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.