Abstract
Background
O6-methylguanine DNA-methyl transferase (MGMT) promoter methylation status is predictive for alkylating chemotherapy, but there are non-benefiting subgroups.
Methods
This is the long-term update of NOA-08 (NCT01502241), which compared efficacy and safety of radiotherapy (RT, n = 176) and temozolomide (TMZ, n = 193) at 7/14 days in patients >65 years old with anaplastic astrocytoma or glioblastoma. DNA methylation patterns and copy number variations were assessed in the biomarker cohort of 104 patients and in an independent cohort of 188 patients treated with RT+TMZ-containing regimens in Heidelberg.
Results
In the full NOA-08 cohort, median overall survival (OS) was 8.2 [7.0–10.0] months for TMZ treatment versus 9.4 [8.1–10.4] months for RT; hazard ratio (HR) = 0.93 (95% CI: 0.76–1.15) of TMZ versus RT. Median event-free survival (EFS) [3.4 (3.2–4.1) months vs 4.6 (4.2–5.0) months] did not differ, with HR = 1.02 (0.83–1.25). Patients with MGMT methylated tumors had markedly longer OS and EFS when treated with TMZ (18.4 [13.9–24.4] mo and 8.5 [6.9–13.3] mo) versus RT (9.6 [6.4–13.7] mo and 4.8 [4.3–6.2] mo, HR 0.44 [0.27–0.70], P < 0.001 for OS and 0.46 [0.29–0.73], P = 0.001 for EFS). Patients with glioblastomas of the methylation classes receptor tyrosine kinase I (RTK I) and mesenchymal subgroups lacked a prognostic impact of MGMT in both cohorts.
Conclusion
MGMT promoter methylation is a strong predictive biomarker for the choice between RT and TMZ. It indicates favorable long-term outcome with initial TMZ monotherapy in patients with MGMT promoter-methylated tumors primarily in the RTK II subgroup.
Keywords: glioma, radiotherapy, temozolomide, MGMT, methylation classifier
Key Points.
Single compound temozolomide holds activity in MGMT methylated glioblastoma.
Methylation profiles may be necessary to assess the value of MGMT.
The RTK II methylation class shows the biggest benefit from MGMT promotor methylation.
Importance of the Study.
There is a specific need for trials of elderly patients with malignant gliomas. NOA-08 provided interesting monotherapy data and thereby offered the opportunity to reassess the predictive impact of MGMT promoter methylation. Whereas comparative data between combined chemoradiation and TMZ alone are missing, this updated analysis of NOA-08 offers mature data for RT or chemotherapy alone and allows some hypothesis generation in comparison to the CCTG CE.6/EORTC 26062 trial. As methylation profiles become increasingly relevant for more precise glioma classification and subgrouping, the present data also offer a thought-provoking restriction of the impact of MGMT, dependent on methylation subclasses, with the most impact of MGMT promoter hypermethylation being detected in RTK II class malignant astrocytoma.
A major challenge in the care of patients with malignant astrocytomas includes improvement of outcome in the elderly and defining predictive biomarkers. Whereas patients <65–70 years of age most likely will be treated with concomitant chemoradiotherapy until 60 Gy (radiotherapy [RT]/temozolomide [TMZ]) and at least 6 cycles of maintenance TMZ,1 in the elderly there is evidence suggesting that a longer course of RT is not superior to a shorter course,2 and a shorter (hypofractionated) RT regimen is inferior to combined chemoradiotherapy with TMZ at 75 mg/m2 body surface area and RT until 40.05 Gy.3 In addition, there is evidence that TMZ is ineffective when the O6-methylguanine DNA-methyltransferase (MGMT) promoter in the tumor tissue is not hypermethylated.2,4 Yet, neither the clinically relevant extent and pattern of promoter methylation, nor the precise cutoff, nor the standard test method have been defined and generally accepted to date. It is noteworthy that trials with alkylating chemotherapy and data on MGMT status do not uniformly show benefit for patients with a hypermethylated MGMT status.5 The subgroup(s) that do not derive the same or any benefit is not yet defined.
The distinction between non-elderly and elderly6 remains a matter of controversy. Based on randomized data showing that RT/TMZ followed by 12 cycles of TMZ is superior to RT for all patients,3 RT/TMZ should be administered in centers that do not test for the MGMT promoter methylation status.
While the Neurooncology Working Group (NOA) of the German Cancer Society Study 8 (NOA-08) and the Nordic Elderly trial suggested an MGMT status–tailored use of RT and TMZ, recent data from the Canadian Clinical Trials Group (CCTG) CE.6/European Organisation for Research and Treatment of Cancer (EORTC) 26062 trials showed a hypofractionated RT plus TMZ to be superior to RT for unselected elderly patients, and revealed a less profound association of MGMT status with the effect of TMZ.
Demonstration of MGMT promoter methylation in the tumor tissue according to the available international guidelines of the European Association of Neuro Oncology1 and the National Comprehensive Cancer Network (NCCN) should trigger the use of TMZ in elderly glioblastoma patients. Whether or not these patients benefit from the early addition of RT has not been examined in randomized trials. When the primary analysis was published, NOA-08 did not report a mature median overall survival (OS) for patients with MGMT hypermethylated tumors and primary treatment with TMZ4 and, therefore, did not provide metrics for a comparison to the CCTG CE.6/EORTC 26062 data.
Assessment of DNA methylation patterns employing large-scale, microarray-based technology is a powerful tool for brain tumor classification.7 It allows the characterization of novel molecular subgroups such as the methylation classes (i) glioblastoma, isocitrate dehydrogenase (IDH) wildtype, subgroup receptor tyrosine kinase I (RTK I); (ii) glioblastoma, IDH wildtype, subgroup RTK II (RTK II); and (iii) glioblastoma, IDH wildtype, subgroup mesenchymal (MES).8
Here we report that the present long-term analysis of NOA-08 shows that elderly patients with RTK II glioblastomas demonstrate the most profound benefit from TMZ and strengthens the role of MGMT promoter methylation as a strong predictive biomarker for the choice between RT and TMZ offering unexpectedly favorable long-term outcome with initial TMZ monotherapy. In an independent dataset, the lack of impact of MGMT promoter methylation in RTK I subgroup patients is confirmed. The present data provide a confirmation of the prior hypothesis9 that RTK I subgroup glioblastoma fails to demonstrate the MGMT effect as well as that the MES subgroup does not uniformly benefit.
Methods
Patients, Trial Design, and Treatment
Included were patients with histologically confirmed de novo anaplastic astrocytoma or glioblastoma with central post hoc review at the Brain Tumor Reference Center of the German Society of Neuropathology and Neuroanatomy in Düsseldorf, who were >65 years of age, with Karnofsky performance score (KPS) ≥60, no prior systemic chemotherapy or RT to the brain, adequate bone marrow reserve, and liver and renal function. NOA-08 (German Cancer Trials Registry ID 386 and NCT01502241) was conducted from May 15, 2005 through November 2, 2010, with the last patient randomized on November 2, 2009. Consenting patients were centrally randomized 1:1 to receive RT or TMZ in a one-week-on/one-week-off schedule.4 At progression, which was diagnosed locally, or treatment delay related to toxicity (Common Terminology Criteria for Adverse Events v3.0) in the TMZ arm >4 weeks prior to 6 months of therapy, salvage treatments with RT in the TMZ arm and with TMZ in the RT arm were recommended per protocol. MGMT status was tested centrally in batches and not for entry into the trial using methylation-specific polymerase chain reaction (PCR).4 The potential impact of toxicity in this frailer patient cohort led to the decision to replace progression-free survival (PFS) by event-free survival (EFS), which generally is identical to PFS, but may in occasional patients define an event as inability of the patient to receive the recommended therapy rather than a tumor progression.
Both treatment arms in NOA-08 achieved similar results in the first analysis conducted after a median follow-up of 2.1 years.4 Median follow-up time for the present analysis is 7.5 years (95% CI: 8.6–10.2). All patients consented in writing to exploratory molecular analyses performed with study data and materials.
Biomarker Cohort
The NOA-08 biomarker cohort consists of 104 patients—28% of the total study cohort—with available formalin-fixed paraffin-embedded tissue or previously extracted DNA suitable for DNA methylation array analysis.10 Patient characteristics of the biomarker cohort are included in Supplementary Table 5. Treatment characteristics (likelihood to cross over or number of salvage treatments) do not differ from the overall cohort.4 Of note, in 221 patients of the whole study cohort, MGMT promoter methylation status was available from methylation-specific PCR (MSP). These patients are referred to as the MSP cohort.
Heidelberg Validation Cohort
The Heidelberg patients’ cohort consists of 188 patients from the Heidelberg Neuro-Oncology center diagnosed with glioblastoma between July 2014 and January 2018 based on histopathological and molecular characteristics and treated with chemoradiotherapy with TMZ in order to allow assessment of the value of MGMT in the context of methylation group and alkylator-containing treatment. Methylation array diagnostic was performed on the primary tumor tissue with either Illumina 450k or EPIC arrays. These 188 patient tumors had a molecular classifier assignment to one of the 3 most common glioblastoma IDH wildtype groups: glioblastoma MES, glioblastoma RTK I, or glioblastoma RTK II. These patients were included in the validation cohort. Post-surgical treatment was administered according to international standard guidelines and subject to the treating physician’s individualized decision.
PFS was calculated from diagnosis until progression according to Response Assessment in Neuro-Oncology (RANO) criteria based on local assessment.
Array-Based DNA Methylation Profiling
The Illumina Infinium HumanMethylation450 (450k) bead chip and MethylationEPIC (EPIC) kits were used at the Genomics and Proteomics Core Facility of the German Cancer Research Center in Heidelberg to obtain the DNA methylation status at approximately 450 000 and 850 000 CpG sites, respectively.7,11 A detailed description of the bioinformatic processing is provided in the Supplementary Methods.
Statistics
After collection on case report forms with onsite support until a cutoff at April 1, 2018, forms of all long-term data were fed into the database at the coordinating study center in Heidelberg. Kaplan–Meier estimator and Cox proportional hazards regression were performed to assess survival data (R package ‘survival,’ v2.42–6). To compare the performance of Cox regression models, prediction error curves were generated using the R package ‘pec,’ 12 and analysis of deviance tables were computed. Processing and analysis of Illumina HumanMethylation 450k and EPIC arrays as well as molecular subgrouping are described7,8,13 and specific analyses detailed in the Supplementary Methods. A P-value of <0.05 was considered statistically significant without adaptation for multiple testing. All tests were two-sided. All tests were explorative as the long-term analysis was not the primary endpoint of the study. Analyses were carried out using R v3.5.1 and Stata IC v12.1. All methylation raw and processed data are made accessible under the Gene Expression Omnibus accession numbers GSE122920 (NOA-08 biomarker cohort) and GSE122994 (Heidelberg validation cohort). The protocol had been approved by an institutional review board (266/2004).
Results
Patient Characteristics
Between May 15, 2005 and November 2, 2009, three hundred seventy-three patients >65 years of age and with KPS ≥60 with centrally reviewed anaplastic astrocytoma (11%) or glioblastoma (89%) were randomized and received at least one dose of dose-dense TMZ or one fraction of RT.
The mean age was 71 (RT group) and 72 (TMZ group) years, and clinical risk factors, such as clinical features, histology, and corticosteroid use (with a tendency for higher use in the RT group), as well as the frequency of salvage therapies, were balanced within the groups when stratified according to MGMT promoter methylation status.
Since the initial report in 20124 of molecular analyses, 104 samples using 450k or EPIC Illumina DNA methylation arrays have been performed. There was no difference in the baseline characteristics between the full study cohort and the biomarker subset, with a numerically better outcome in the biomarker cohort (Supplementary Table 1). Characteristic copy-number variations (CNVs) and the associated methylation classes are depicted in Supplementary Figure 1.
Clinical Efficacy
At a median follow-up of 7.5 years (95% CI: 8.6–10.2), 369 deaths have been observed in the intention-to-treat population (N = 373, RT: 176, TMZ: 193). Data are thus much more mature, since the initial publication had a median follow-up of 2.1 years and an OS event had been documented for only 61.1% of patients.
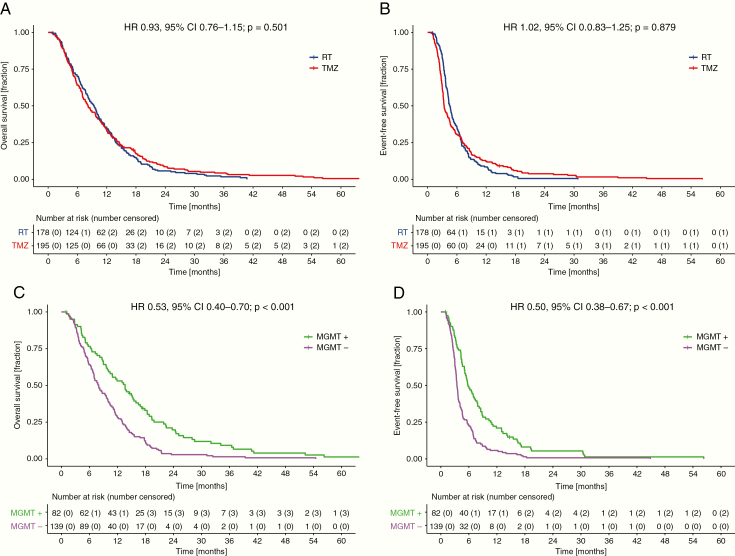
At the data cutoff of April 1, 2018, an OS event was documented for 98.6% of patients. The median OS was 8.2 [7.0–10.0] months for TMZ treatment and 9.4 [8.1–10.4] months for RT; the hazard ratio (HR) 0.93 (95% CI: 0.76–1.15) of TMZ versus RT did not differ between both groups (Fig. 1A, Table 1). Also, median EFS [3.4 (3.2–4.1) months for TMZ treatment and 4.6 (4.2–5.0) months for primary RT] did not differ, with HR = 1.02 (0.83–1.25) (Fig. 1B, Supplementary Table 2).
Fig. 1.
Kaplan–Meier survival estimates of the long-term analysis of NOA-08. (A) OS and (B) EFS with comparison of RT vs TMZ. (C) OS and (D) EFS according to MGMT promoter methylation status. MGMT+ = MGMT promoter methylated; MGMT− = MGMT promoter unmethylated.
Table 1.
Long-term analysis of OS in the NOA-08 study
| Population | N | Median | 6 Months | 12 Months | 18 Months | 24 Months |
|---|---|---|---|---|---|---|
| Overall Survival | Months (95% CI) | Percent (95% CI) | ||||
| All patients | ||||||
| RT | 178 | 9.4 (8.1–10.4) | 70.1 (63.6–77.1) | 34.4 (28.0–42.2) | 14.9 (10.5–21.2) | 5.7 (3.1–10.5) |
| TMZ | 195 | 8.2 (7.0–10.0) | 64.1 (57.7–71.2) | 32.8 (26.8–40.1) | 17.8 (13.1–24.1) | 8.6 (5.4–13.7) |
| All patients | ||||||
| MGMT− | 139 | 8.0 (6.9–9.9) | 64.0 (56.5–72.5) | 28.1 (21.5–36.6) | 12.2 (7.8–19.1) | 2.9 (1.1–7.6) |
| MGMT+ | 82 | 13.6 (10.1–16.5) | 76.5 (67.9–86.4) | 53.1 (43.3–65.2) | 33.0 (24.1–45.1) | 19.8 (12.6–30.9) |
| Patients with MGMT− | ||||||
| RT | 60 | 10.2 (8.0–12.0) | 76.7 (66.7–88.2) | 35.0 (24.8–49.4) | 16.7 (9.5–29.3) | 3.3 (0.9–13.0) |
| TMZ | 79 | 6.7 (5.6–8.2) | 54.4 (44.5–66.6) | 22.8 (15.2–34.2) | 8.9 (4.4–18.0) | 2.5 (0.6–10.0) |
| Patients with MGMT+ | ||||||
| RT | 43 | 9.6 (6.4–13.7) | 66.7 (53.8–82.6) | 40.5 (28.0–58.4) | 16.7 (8.5–32.8) | 9.5 (3.8–24.2) |
| TMZ | 39 | 18.4 (13.9–24.4) | 87.2 (77.3–98.3) | 66.7 (53.4–83.2) | 50.7 (37.1–69.3) | 31.0 (19.1–50.2) |
Prognostic and Predictive Factors
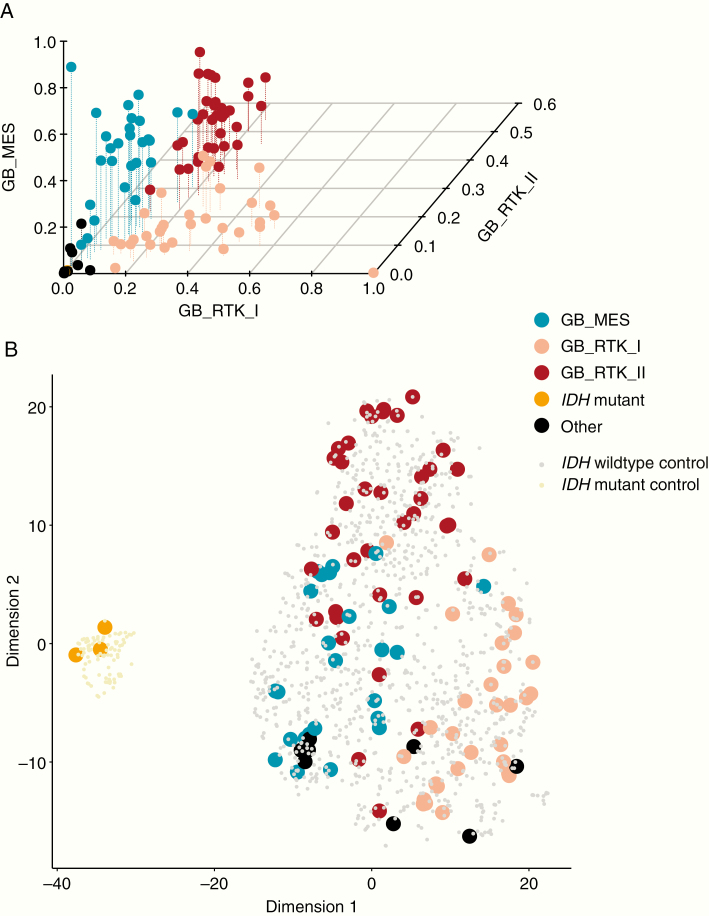
The biomarker cohort resembles the overall study cohort with respect to risk factors4 and outcome (Supplementary Table 1) and consists of previously established molecular glioblastoma subgroups, with the vast majority of tumors corresponding to the MES, RTK I, or RTK II methylation subgroups7 (Fig. 2A). The t-distributed stochastic neighbor embedding analysis of the DNA methylation data matching NOA-08 samples with a group of independent glioblastoma samples demonstrates that the NOA-08 cohort is representative for adult-type molecular subgroups of glioblastoma (Fig. 2B). Chromosome 7 gain and 10 loss were less frequent in the mesenchymal group, whereas chromosome 19 gain differentiates RTK II from RTK I tumors (Supplementary Figure 2). Further, MGMT promoter analysis as performed by MSP4 was largely in accordance with the data calculated from the global methylation arrays, and discrepant patients showed an intermediate survival (Supplementary Figure 3, Supplementary Table 3).
Fig. 2.
Standard glioblastoma methylation classes in the NOA-08 study. Methylation array data from the biomarker cohort (n = 104) were grouped according to the proposed subgroups (Capper et al. 2018) (A). t-Distributed stochastic neighbor embedding blot of these groups matched on an independent cohort of glioblastoma samples demonstrate a regular distribution of NOA-08 samples including the 3 IDH mutated outliers also grouped with IDH mutated glioblastoma (B).
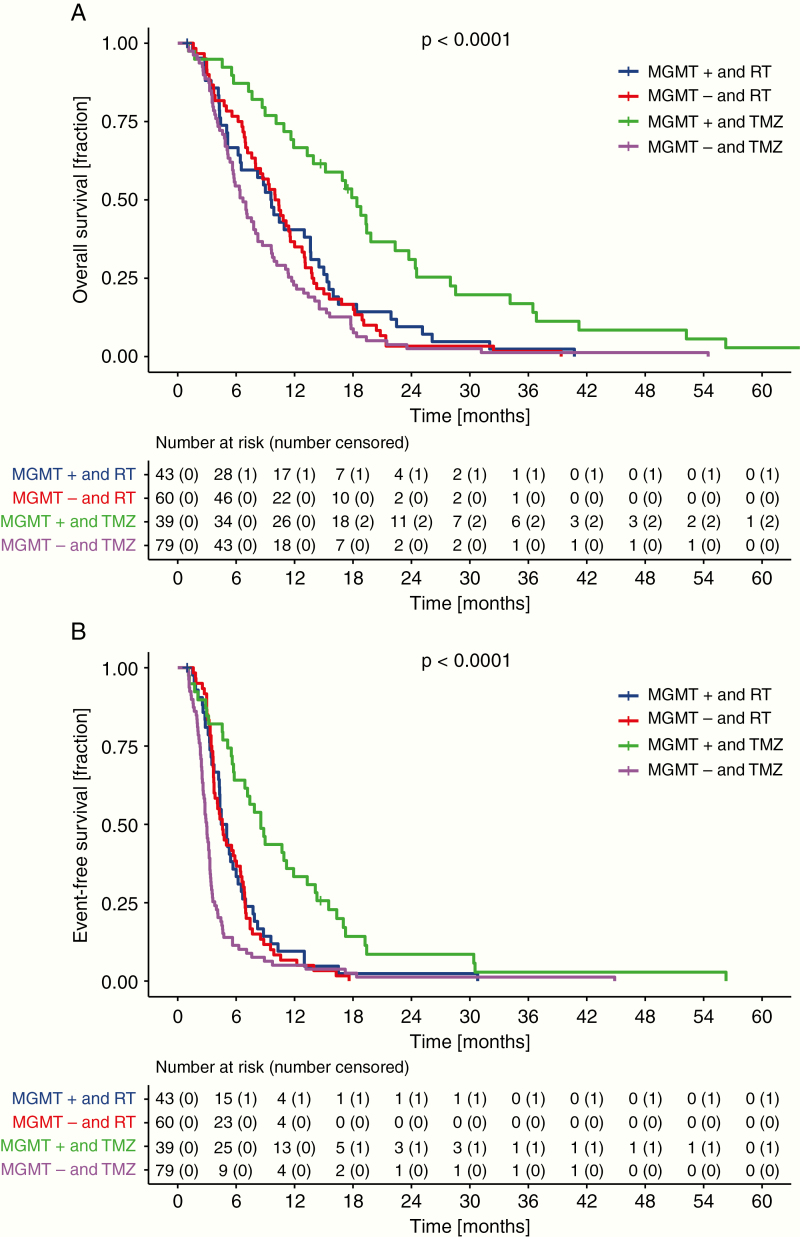
Median OS was longer in patients with MGMT promoter hypermethylated tumors (82/221 patients, MSP cohort, 37.1%), to 13.6 [10.1–16.5] versus 8.0 [6.9–9.9] months, resulting in an HR of 0.53 (0.40–0.70), P < 0.0001 (Fig. 1C, Table 1). Similarly, median EFS was longer in patients with MGMT promoter hypermethylated tumors, to 5.8 [5.1–7.9] versus 3.4 (3.2–3.7) months (Fig. 1D, Supplementary Table 2). The effect on median but also landmark OS and EFS was pronounced when patients with MGMT promoter hypermethylated tumors were treated with TMZ (OS: 18.4 [13.9–24.4] mo and EFS: 8.5 [6.9–13.3] mo) versus RT (OS: 9.6 [6.4–13.7] mo and EFS: 4.8 [4.3–6.2] mo), resulting in an HR of 0.44 [0.27–0.70], P < 0.001 for OS and 0.46 [0.29–0.73], P = 0.001 for EFS. Patients whose tumors lacked MGMT promoter methylation had shorter EFS, with TMZ at 3.0 [2.6–3.3] months versus 4.6 [3.7–6.4] months with RT and a numerically shorter OS, however, with the statistical testing being not significant when treated with TMZ (6.7 [5.6–8.2] mo) versus RT (10.2 [8.0–12.0] mo), resulting in HRs of 1.86 [1.32–2.62], P < 0.001 for EFS and 1.33 [0.95–1.87], P = 0.099 for OS (Fig. 3, Table 1, Supplementary Table 4). Matching the high concordance of MGMT assessment by either methylation-specific PCR or DNA methylation array analysis, the survival impact per treatment group was likewise retained in the biomarker cohort stratified for the MGMT status obtained by 450k or EPIC array analysis and consecutive prediction according to Bady et al (Supplementary Table 3, Supplementary Figure 4).14
Fig. 3.
Patients with MGMT promoter methylated tumors show strong survival benefit with TMZ treatment. OS (A) and EFS (B) were analyzed according to treatment and MGMT promoter methylation status defined by methylation-specific PCR.
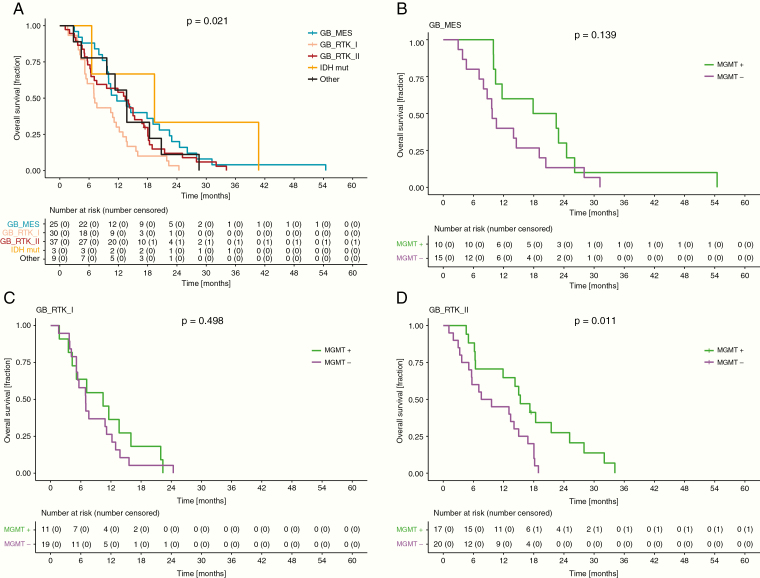
As a potential to embed MGMT data into the global methylation patterns, array-based DNA methylation analysis confirmed evidence for a differential prognosis within the 3 molecular subgroups of IDH-wildtype glioblastoma,9 with the RTK I class being associated with the worst outcome and an absence of a prognostic impact of the MGMT status and the MES subgroup also not harboring a significant MGMT effect, as opposed to the RTK II subgroups (Fig. 4). To assess the stability of this important finding, the analyses were repeated for an independent patient cohort (Supplementary Table 5) that also demonstrated that unlike those in RTK II, patients in the RTK I and MES subgroups had an overall worse OS and PFS, with MGMT promoter methylated glioblastoma resulting in the same outcome as MGMT unmethylated tumors (Supplementary Figures 5 and 6, Supplementary Table 6). These results were retained in a multivariate Cox proportional hazards model assessing MGMT promoter methylation and glioblastoma subgroup in the NOA-08 and validation cohort (Supplementary Table 9), despite some differences in these cohorts, with resection being a significant risk factor in the Heidelberg cohort as it was in the full NOA-08 dataset, but not in the smaller biomarker cohort; MGMT status is not predictive in the Heidelberg cohort and the prognostic differences of the methylation subclasses are likewise not significant.
Fig. 4.
RTK I tumors show worse survival and similar to MES tumors lack of benefit from MGMT promoter methylation in NOA-08 patients. (A) OS of patients in the biomarker cohort according to classifier assignment. OS of patients according to MGMT promoter methylation status with (B) mesenchymal, (C) RTK I, and (D) RTK II classifier assignment.
Discussion
The NCCN guidelines allow withholding TMZ from patients with glioblastoma harboring an unmethylated MGMT promoter in the elderly age group >70 years, TMZ alone is an option for patients with MGMT hypermethylated glioblastoma, whereas the CCTG CE.6/EORTC 26062 trial3 defined the currently widest accepted option, which is hypofractionated RT with concurrent and maintenance TMZ. At >98% of patients having reached the primary endpoint, NOA-08 confirms that elderly patients with glioblastoma may be treated with RT or TMZ alone when information on the MGMT promoter methylation status is available. There is a strong predictive impact of MGMT status for the choice between RT and TMZ, with a survival benefit for patients with MGMT promoter methylated tumors when treated with primary TMZ as opposed to primary RT. Notably, this survival advantage cannot be compensated with salvage TMZ. Although the inverse comparison fails to reach significance, it is intriguing to recognize the numeric superiority for primary RT in patients with MGMT promoter unmethylated tumors (Table 1). As MGMT seems powerful, but not perfect, to stratify between TMZ-benefiting and TMZ-failing patients and global methylation information on the biomarker subset of the NOA-08 trial got available, it was intriguing to postulate that the impact of MGMT was not the same in all methylation subclasses. In the NOA-08 patients, for whom tumor methylation profiles were available and confirmed in an independent dataset with clinical and methylation information available, RTK I and MES glioblastoma had an increased risk for short PFS and OS as proposed before9 and did not show the predictive impact of MGMT status (Fig. 4), implying an integrated analysis of MGMT in the context of global DNA methylation profiles.
These data are not impacted by the choice between 2 accepted methods, quantitative PCR and CpG island quantification according to array data (Supplementary Figure 3), although the few discordant samples further may mandate careful evaluation of the assays used.13 The confirmed DNA methylation subgroups of IDH-wildtype glioblastoma, RTK I and MES, do not show the prognostic effect of MGMT status, and in contrast the strong effect of MGMT in the RTK II subgroup9 will aid the further development of MGMT promoter methylation as a predictive biomarker in glioblastoma. These data provide another argument to use DNA methylation arrays7 and impact the selection of patients who definitely benefit from MGMT testing.
As a limitation, biomarker data except MGMT had not been prespecified in the protocol of the trial and could be generated only for a subset, for which sufficient material had been available. As observed in other trials, there was a trend toward superior outcome for biomarker cohorts, which are less protected from randomization. Despite the lack of a clear biasing factor for differential benefit from any treatment of a patient qualifying for the biomarker cohort, it is possible that the most likely confounding factor—extent of resection and therefore tissue availability—is differentially impacting treatments. However, glioblastomas in elderly patients correspond to unselected adult-type glioblastomas according to DNA methylation profiles (Fig. 2) and associated CNV (Supplementary Figure 1). We challenged the finding of a negative prognostic impact of RTK I methylation subgroup and the absence of the predictive impact of MGMT in the RTK I and MES subgroups in an independent cohort that was specifically looking at the impact of the global methylation status on the prognostic role of MGMT, but not aiming at adding to the elderly data and confirmed the data from the NOA-08 biomarker subgroup that patients with RTK I tumors do worse (Supplementary Figure 5). Further, the positive impact of a hypermethylated MGMT promoter is not visible in RTK I and MES tumors, but the MGMT methylated tumors confer the same prognosis as a lack of MGMT methylation in the other subgroups (Supplementary Figure 4a, b). The reason for this effect is not clear, but differential copy number profiles between RTK I and RTK II tumors include chromosome 19, which had been associated with better prognosis in glioblastoma.15 However, it cannot simply be explained by other major differences in copy numbers of the MGMT gene (Fig. 4c). We hope that existing trials3,16 enhance their biomarker efforts to glioblastoma methylation profiles and further support or challenge the present finding.
NOA-08 aimed at overcoming MGMT-conferred TMZ resistance by a weekly alternating TMZ schedule, which is not of relevance anymore.17 The Nordic trial asked the same RT versus TMZ question as NOA-08 and drew the same conclusions but substructured RT between a conventionally fractionated schedule and a hypofractionated regimen.2 The outcome of this second comparison supported earlier phase II data18 and in conjunction with the CCTG CE.6/EORTC 26062 trial3 paved the way for hypofractionated RT being accepted as a standard for RT of elderly patients with or without reduced clinical performance.1 CCTG CE.6/EORTC 26062 complemented these two trials with the adapted combined RT/TMZ schedule that had proven superiority to mere RT in patients ≤70 years of age with newly diagnosed glioblastoma.19 It provided a remarkable benefit from the combination treatment. The predictive impact of MGMT was rather small3 and future molecular analysis may aid to overcome these data which contrast several other datasets.2,4,19 The initially intended 3-armed trial (RT or TMZ or RT/TMZ) was deferred as a consequence of the parallel European studies. Therefore, currently only scientifically strongly limited cross trial comparisons are possible to get an impression of the missing comparisons. Assuming similar basic risk factors, but a largely different number of patients, between CCTG CE.6/EORTC 26062 and NOA-08 (Supplementary Table 7) and a numerically better outcome with the regular fractionated RT in NOA-08 compared with the hypofractionated regimen in the CCTG trial for OS and PFS (Table 2, Supplementary Table 8), efficacy seems to be superior in the CCTG trial starting with combined RT/TMZ versus the monotherapy with TMZ in the NOA-08 trial. Agnostic to the MGMT status, the combined RT/TMZ may therefore provide a benefit not only over RT as shown in the CCTG trial, but also over TMZ alone (Table 2, Supplementary Table 8). The picture changes with focus on the biomarker cohorts of both trials. Whereas patients with MGMT promoter unmethylated glioblastoma show no difference between the RT group of NOA-08 and the RT/TMZ group of the CCTG/EORTC trial, outcome in patients with MGMT promoter methylated glioblastoma treated with TMZ alone in NOA-08 seems numerically superior to RT/TMZ in the CCTG CE.6/EORTC 26062. Whether or not this is due to the dose-dense regimen of TMZ in NOA-08, earlier treatment interruptions in the CCTG trial due to potentially higher treatment toxicity or to the prolonged treatment of benefiting patients in NOA-08 (until progression) compared with the fixed 14 months therapy of the CCTG CE.6/EORTC 26062 trial is speculative. OS was measured from diagnosis in the CCTG trials and from randomization in the NOA-08 study. There is no reason to believe that this rather small difference, which is equal for all groups in the trial, accounts for overall efficacy differences or the discrepancy in the impact of MGMT. PFS in the NOA-08 trial could have been a toxicity event triggering premature cessation of the treatment, which is termed EFS. However, there was no difference between the 2 concepts in NOA-08 already in the primary publication.4
Table 2.
Overall survival comparison between the NOA-08 study and CCTG CE.6/EORTC 26062
| Population | NOA-08 | CCTG CE.6/EORTC 26062* | ||
|---|---|---|---|---|
| Overall Survival | N | Months (95% CI) | N | Months (95% CI) |
| All patients | ||||
| TMZ (+RT*) | 195 | 8.2 (7.0–10.0) | 281 | 9.3 (8.3–10.3) |
| RT | 178 | 9.4 (8.1–10.4) | 281 | 7.6 (7.0–8.4) |
| HR | 0.93 (0.76–1.15) | 0.67 (0.56–0.80) | ||
| Patients with MGMT− | ||||
| TMZ (+RT*) | 79 | 6.7 (5.6–8.2) | 93 | 10.0 (8.3–10.7) |
| RT | 60 | 10.2 (8.0–12.0) | 96 | 7.9 (6.9–10.0) |
| HR | 1.33 (0.95–1.87) | 0.75 (0.56–1.01) | ||
| Patients with MGMT+ | ||||
| TMZ (+RT*) | 39 | 18.4 (13.9–24.4) | 88 | 13.5 (10.2–15.3) |
| RT | 43 | 9.6 (6.4–13.7) | 77 | 7.7 (5.8–10.7) |
| HR | 0.44 (0.27–0.70) | 0.53 (0.38–0.73) | ||
*For NOA-08 TMZ alone, for CCTG CE.6/EORTC 26062 TMZ + RT is shown.
A new trial may focus on patients with MGMT promoter hypermethylated RTK II glioblastoma, an improved definition of an elderly or frail patient group, and assess the deferral of RT in these patients. Another option would be to specifically focus on the intensification of alkylating chemotherapy (with or without RT) in these patients building on the initial data of the CCNU/TMZ in glioblastoma (CETEG)/NOA-09 trial data.16 Until those results become available, we regard RT alone or TMZ alone as valid options for the respective MGMT promoter methylation–based subgroups with hypofractionated RT with concurrent and maintenance TMZ potentially being most attractive for the good performing, non-frail elderly patients.6
The intriguing cross trial comparisons do by no means replace future randomized efforts. The formal comparisons between hypofractionated and regular fractionated RT regimens have been done in small groups18 or in a difficult to assess 3-group trial (Nordic) with likewise small sample sizes and numerically inferior results due to a lack of second-line options.2 The question on the optimal RT regimen may therefore also not be answered to full satisfaction. Similarly, NOA-08 has used a non-standard dosing and scheduling for TMZ, aiming at more drug per 4-weekly cycle and dose escalation only limited by toxicity. Later trials, foremost Radiation Therapy Oncology Group (RTOG) 0525,20 have not confirmed any superiority of a dose-dense approach, although the RTOG trial used yet another 21/28 days instead of the 7/14 days regime from NOA-08.
In summary, the elderly trials support MGMT promoter methylation as a strong predictive biomarker in distinct glioblastoma methylation classes that guides clinicians to opt for or against the use of TMZ. RTK I and MES glioblastoma patients may benefit from alternative treatment to TMZ. NOA-08 long-term data conjoined with the results of CCTG CE-6/EORTC 26062 provoke the question whether TMZ alone with deferred RT may be a sufficient treatment in patients with MGMT promoter methylated tumors and hopefully help to kickstart a new trial.
Supplementary Material
Acknowledgments
We are indebted to the patients and their families for agreeing to participate in this trial, as well as to the nurses and data managers for their collaboration. The molecular analyses have been supported by the German Cancer Research Center and the NCT Heidelberg.
Conflict of interest statement. UH reports grants and personal fees from Roche, personal fees and non-financial support from Medac, Bristol-Myers Squibb, personal fees from Novocure, Novartis, Daichii-Sankyo, Rimester, Noxxon. MP reports non-financial support from Pfizer, Merck, and Bayer, honoraria from Bayer and Affiris and honoraria to the benefit of the institution from Novartis, Medac, and Roche. MW has received research grants from Abbvie, Acceleron, Actelion, Bayer, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, OGD2, Piqur, Roche, and Tragara, and honoraria for lectures or advisory board participation or consulting from Abbvie, BMS, Celgene, Celldex, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Orbus, Pfizer, Progenics, Roche, Teva, and Tocagen. WW reports non-financial support from Apogenix, Pfizer, and Roche and honoraria to the benefit of the institution from Abbvie, Bayer, BMS, and Roche.
Authorship statement: Conceptualization: WW, AW, TK, MW. Manuscript writing: WW, AW, TK. Trial conduct: AW, TK, MP, CM, MB, UH, JF, AW, KP, JPS, MS, JV, JD, JM, RK, CH, RM-S, SW, HB, DR, GR, FS, AvD, MW, WW. Final editing and approval of the manuscript: AW, TK, MP, CM, MB, UH, JF, AW, KP, JPS, MS, JV, JD, JM, RK, CH, RM-S, SW, HB, DR, GR, FS, AvD, MW, WW. Access to the raw data was limited to W.W., T.K., A.W., A.v.D., G.R., and M.W. The corresponding author had full access to all of the data and the final responsibility to submit the publication.
Data of this manuscript have been presented at the EANO Meeting 2018, Oct 14–16 and the SNO Meeting 2018, November 14–16.
Funding
The molecular analyses have been supported by the German Cancer Research Center, the NCT Heidelberg, and SFB 1389 (TP A03 to TK and WW). The original trial was supported by Merck, Sharp & Dohme (MSD). The funding source, former Schering Plough, now Merck, Sharp & Dohme, for the original trial and the Neurooncology Program at the National Center for Tumor Diseases Heidelberg (NCT) or the Deutsche Forschungsgemeinschaft (DFG) had no role in the study design, collection, analysis, or interpretation of the data, in writing the initial study report and the manuscript or this long-term outcome analyses.
References
- 1. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 2. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 3. Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 4. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 5. Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 6. Wick A, Kessler T, Elia AEH, et al. Glioblastoma in elderly patients: solid conclusions built on shifting sand? Neuro Oncol. 2018;20(2):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 8. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of human central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kessler T, Sahm F, Sadik A, et al. Molecular differences in IDH wildtype glioblastoma according to MGMT promoter methylation. Neuro Oncol. 2018;20(3):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiestler B, Claus R, Hartlieb SA, et al. ; Neuro-oncology Working Group (NOA) of the German Cancer Society Malignant astrocytomas of elderly patients lack favorable molecular markers: an analysis of the NOA-08 study collective. Neuro Oncol. 2013;15(8):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfaff E, Kessler T, Balasubramanian GP, et al. Feasibility of real-time molecular profiling for patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation-the NCT Neuro Master Match (N2M2) pilot study. Neuro Oncol. 2018;20(6):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50(11):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiestler B, Capper D, Hovestadt V, et al. Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro Oncol. 2014;16(12):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bady P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012;124(4):547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geisenberger C, Mock A, Warta R, et al. Molecular profiling of long-term survivors identifies a subgroup of glioblastoma characterized by chromosome 19/20 co-gain. Acta Neuropathol. 2015;130(3):419–434. [DOI] [PubMed] [Google Scholar]
- 16. Herrlinger U, Tzaridis T, Mack F, et al. ; Neurooncology Working Group of the German Cancer Society Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 17. Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357–3361. [DOI] [PubMed] [Google Scholar]
- 18. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 19. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 20. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.