Abstract
Background:
Patients diagnosed with metastatic melanoma have varied clinical courses, even in patients with similar disease characteristics. We examine the impact of initial stage of melanoma diagnosis, BRAF status of primary melanoma, and receiving adjuvant therapy on post metastatic survival.
Methods:
We studied melanoma patients presenting to Perlmutter Cancer Center at New York University (NYU) and prospectively enrolled in NYU’s melanoma biospecimen database and followed up on protocol-driven schedule. Patients were stratified by stage at initial melanoma diagnosis as per AJCC 7th edition guidelines. Post-metastatic survival was determined using the Kaplan-Meier method, and Cox proportional hazards models were used to assess hazard ratios
Results:
Three hundred and four out of 3204 patients developed metastatic disease over the time of follow up (median follow up 2.2 years, range 0.08–35.2 years). Patients diagnosed with stage I (n=96) melanoma had longer pmOS (29.5 months) than those diagnosed with stage II (n=99, pmOS 14.9 months) or stage III (n=109, pmOS 15.1 months) melanoma (p=0.036). Initial stage of diagnosis remained significant in multivariate analysis when controlling for LDH and site of metastases (primary diagnosis stage II (HR 1.44, p=0.046), stage III (HR 1.5, p=0.019)). Adjuvant treatment was associated with better survival but BRAF mutation status did not show an association.
Conclusion:
Our data challenge the general assumption that primary melanomas converge upon diagnosis of metastatic disease and behave uniformly. Primary stage of melanoma at the time of diagnosis may be prognostic of outcome, similar to LDH and metastatic disease sites.
Keywords: metastatic melanoma, initial stage, prognosis, overall survival, clinical course
Introduction
Despite recent advances in treatment options, over 10,000 patients died of metastatic melanoma in 2016 in the US alone 1. Systemic PD-1 inhibitors, combination CTLA-4/PD-1 immunotherapy, and combination BRAF/MEK dual targeted therapy offer the best survival benefit to patients, with clinical trials showing one-year survival rates of 60–75%, 85%, and 50–60%, respectively 2–6. Predictive factors are needed to better identify patient outcomes.
According to 2009 AJCC 7th edition cancer staging guidelines, the only independent prognostic factors for stage IV melanoma are location of metastases and serum LDH levels.7 The presence of visceral metastases (excluding lung metastases) has repeatedly been identified as a negative prognostic factor in studies of stage IV melanoma patients.8–11 Presence of metastases in more than one visceral site as opposed to a single location has also been shown to be predictive of poor survival, however this distinction is not included in AJCC staging guidelines.7–11
Most metastatic melanoma patients present with disease history that predates stage IV diagnosis. The impact of initial cancer stage at the time of diagnosis has been studied in other tumor types but not adequately studied in melanoma.12–15 It is unknown what impact disease history, including stage at initial diagnosis, and primary tumor characteristics have on prognosis following onset of metastatic disease. Observations regarding the relationship between stage at diagnosis and survival following stage IV diagnosis may be clinically relevant for predicting patient outcomes, and may in turn suggest that stage-dependent biological characteristics of disease at first intervention are persistent following recurrence at a later stage.
Here, we examine the association between stage of initial melanoma diagnosis and survival following the diagnosis of metastatic melanoma using our database of NYU patients enrolled within the last 14 years. We compared tumor and patient characteristics at time of initial diagnosis and at time of diagnosis of metastatic disease, in addition to association with survival. We assessed if presence of a BRAF mutation affected outcomes, as well as the effect of adjuvant therapy on survival outcomes.
Methods
Study Population
We studied melanoma presenting to Perlmutter Cancer Center, NYU Langone Medical Center from August 2002 to December 2015 and prospectively enrolled in the Interdisciplinary Melanoma Cooperative Group (IMCG) database16. Some patients were diagnosed with melanoma prior to their presentation to NYU. The study was approved by the Institutional Research Board at NYU and all patients provided informed written consent at the time of enrollment. All patients underwent surgical treatment of the initial primary tumor. BRAF and NRAS mutational status was determined directly, either for clinical purposes, including clinical trial enrollment, or research, by qPCR, sequencing, or targeted next generation sequencing, from tumor specimens, when available. Other clinical characteristics were extracted from patient charts. Date of diagnosis was defined as the biopsy date of the primary tumor and was used to calculate age at diagnosis, follow-up time, and time to recurrence. Recurrence and survival information were collected by active prospective follow-up every six months for all patients enrolled in the database.
Primary melanoma diagnosis refers to the time at which the patient was first diagnosed with melanoma—either Stage I, II, or III, as defined by AJCC cancer staging 7th edition. Metastatic disease refers to the time at which the patient was diagnosed with metastatic melanoma, stage IV disease. Characteristics recorded for each patient included age, gender, date of primary melanoma diagnosis, primary tumor thickness, ulceration, anatomic site, clinical stage at primary melanoma diagnosis, adjuvant therapy received, date of metastatic diagnosis, site of distant metastases, LDH level, BRAF and NRAS mutational status, systemic treatment received, and ECOG performance status. Adjuvant therapy was defined as treatment given after the initial surgery and before the date of metastatic diagnosis. Therapy included interferon, GM-CSF (Leukine®), and vaccine clinical trials.
Statistical Analysis
Patients were categorized based on clinical stage at primary melanoma diagnosis, as defined by AJCC cancer staging 7th edition criteria. Protocol based follow-up of disease status was used to identify melanoma patients who developed metastatic disease and assess disease course and response to treatment. Time to metastatic recurrence was defined as the time from primary melanoma diagnosis until diagnosis of metastatic disease. Post metastatic OS (pmOS) was defined as the time from diagnosis of metastatic disease until death or date of last follow up. The Kaplan-Meier method was used to calculate survival curves based on clinical stage at primary melanoma diagnosis, adjuvant therapy, systemic treatment types, and BRAF mutational status. The p-values for the difference of survival curves are calculated based on log-rank test. Cox Proportional hazards models were used to assess hazard ratios, 95% confidence intervals, and p-values for pmOS. The univariate and multivariate Cox model was used to assess the associations of clinical stage at primary melanoma diagnosis, primary tumor characteristics, adjuvant therapy, other clinical characteristics of metastatic disease, and systemic treatment types with survival outcomes.
Results
Primary Diagnosis of Stage I Melanoma Associated with Improved Post-Metastatic Survival
In our cohort of 3204 melanoma patients presenting to NYU between 2002 and 2015, 304 patients (11%) developed metastatic disease during follow up. The median time to diagnosis of metastatic disease from time of initial diagnosis was 2.2 years (range 0.08 to 35.2 years). Median follow-up was 25 months post diagnosis of metastatic disease (range 0.5 to 167.8 months) for surviving patients. Patient characteristics at the time of primary melanoma diagnosis are summarized in Table 1.
Table 1.
Melanoma patient characteristics stratified by stage at diagnosis.
| Initial stage at
diagnosis |
||||||
|---|---|---|---|---|---|---|
| Patient/tumor characteristics | All (N=304) |
Stage I (N=96) |
Stage II (N=99) |
Stage III (N=109) |
P-value |
|
| Age (y) at primary diagnosis, median (range) | 60 (18–93) | 55 (18–82) | 67 (31–93) | 57 (20–84) | <0.001 | |
| Sex, n (%) | Female | 102 (33.6) | 32 (33.3) | 30 (30.3) | 40 (36.7) | 0.81 |
| Male | 202 (66.4) | 64 (66.7) | 69 (69.7) | 69 (63.3) | ||
| Primary tumor thickness (mm), n (%) |
≤1.0 | 50 (17.2) | 45 (47.9) | 0 (0.0) | 5 (5.1) | <0.001 |
| 1.01–2.0 | 78 (26.9) | 49 (52.1) | 14 (14.3) | 15 (15.3) | ||
| 2.01–4.0 | 81 (27.9) | 0 (0.0) | 47 (48.0) | 34 (34.7) | ||
| >4.0 | 81 (27.9) | 0 (0.0) | 37 (37.8) | 44 (44.9) | ||
| Primary tumor ulceration, n (%) | Absent | 134 (49.8) | 70 (90.9) | 25 (26.0) | 39 (40.6) | <0.001 |
| Present | 135 (50.2) | 7 (9.1) | 71 (74.0) | 57 (59.4) | ||
| Primary tumor site, n (%) | Extremity | 120 (40.3) | 38 (39.6) | 40 (40.4) | 42 (40.8) | 0.06 |
| Head/neck | 76 (25.5) | 22 (22.9) | 37 (37.4) | 17 (16.5) | ||
| Trunk | 98 (32.9) | 34 (35.4) | 22 (22.2) | 42 (40.8) | ||
| Many | 4 (1.3) | 2 (2.1) | 0 (0.0) | 2 (1.9) | ||
| Clinical stage at
primary diagnosis, n (%) |
IA | 25 (8.2) | 25 (26.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| IB | 71 (23.4) | 71 (74.0) | 0 (0.0) | 0 (0.0) | ||
| IIA | 30 (9.9) | 0 (0.0) | 30 (30.3) | 0 (0.0) | ||
| IIB | 41 (13.5) | 0 (0.0) | 41 (41.4) | 0 (0.0) | ||
| IIC | 28 (9.2) | 0 (0.0) | 28 (28.3) | 0 (0.0) | ||
| IIIA | 29 (9.5) | 0 (0.0) | 0 (0.0) | 29 (26.6) | ||
| IIIB | 46 (15.1) | 0 (0.0) | 0 (0.0) | 46 (42.2) | ||
| IIIC | 34 (11.2) | 0 (0.0) | 0 (0.0) | 34 (31.2) | ||
| Primary tumor
histologic subtype, n (%) |
Acral lentiginous | 19 (8.1) | 5 (7.8) | 4 (4.5) | 10 (12.3) | <0.001 |
| Nodular | 123 (52.6) | 16 (25.0) | 57 (64.0) | 50 (61.7) | ||
| Superficial spreading |
69 (29.5) | 38 (59.4) | 13 (14.6) | 18 (22.2) | ||
| Other | 23 (9.8) | 5 (7.8) | 15 (16.9) | 3 (3.7) | ||
| Adjuvant therapy received
at primary diagnosis, n (%) |
No | 213 (70.1) | 74 (77.1) | 70 (70.7) | 69 (63.3) | 0.20 |
| Yes | 91 (29.9) | 22 (22.9) | 29 (29.3) | 40 (36.7) | ||
| Time to metastatic recurrence (months), median (range) | 26.7 (0.0–422.8) | 65.1 (0.5–350.1) | 22.6 (0.4–422.8) | 16.2 (0.0–117.6) | <0.001 | |
| Clinical substage at
metastatic recurrence, n (%) |
M1a | 25 (8.2) | 9 (9.4) | 6 (6.1) | 10 (9.2) | 0.78 |
| M1b | 44 (14.5) | 18 (18.8) | 13 (13.1) | 13 (11.9) | ||
| M1c | 235 (77.3) | 69 (71.9) | 80 (80.8) | 86 (78.9) | ||
| Site of distant metastases, n (%) | Brain | 32 (10.5) | 12 (12.5) | 8 (8.1) | 12 (11.0) | 0.97 |
| Visceral | 176 (57.9) | 56 (58.3) | 58 (58.6) | 62 (56.9) | ||
| Brain & visceral | 96 (31.6) | 28 (29.2) | 33 (33.3) | 35 (32.1) | ||
| LDH, n (%) | High | 52 (30.4) | 21 (35.6) | 15 (28.8) | 16 (26.7) | 0.75 |
| Normal | 119 (69.6) | 38 (64.4) | 37 (71.2) | 44 (73.3) | ||
| BRAF status, n (%) | Mutant | 115 (47.9) | 48 (56.5) | 30 (41.1) | 37 (45.1) | 0.25 |
| Wild type | 125 (52.1) | 37 (43.5) | 43 (58.9) | 45 (54.9) | ||
| NRAS status, n (%) | Mutant | 32 (19.8) | 8 (15.4) | 13 (23.6) | 11 (20.0) | 0.76 |
| Wild type | 130 (80.2) | 44 (84.6) | 42 (76.4) | 44 (80.0) | ||
| Chemotherapy
received at metastatic recurrence, n (%) |
No | 163 (53.6) | 53 (55.2) | 56 (56.6) | 54 (49.5) | 0.76 |
| Yes | 141 (46.4) | 43 (44.8) | 43 (43.4) | 55 (50.5) | ||
| Immunotherapy received at metastatic recurrence, n (%) |
No | 160 (52.6) | 42 (43.8) | 58 (58.6) | 60 (55.0) | 0.20 |
| Yes | 144 (47.4) | 54 (56.2) | 41 (41.4) | 49 (45.0) | ||
| Targeted
therapy received at metastatic recurrence, n (%) |
No | 228 (75.0) | 65 (67.7) | 79 (79.8) | 84 (77.1) | 0.24 |
| Yes | 76 (25.0) | 31 (32.3) | 20 (20.2) | 25 (22.9) | ||
| ECOG performance
status, n (%) |
0 | 204 (77.9) | 67 (80.7) | 72 (79.1) | 65 (73.9) | 0.73 |
| >0 | 58 (22.1) | 16 (19.3) | 19 (20.9) | 23 (26.1) | ||
| Survival outcome, n (%) | Alive | 75 (24.7) | 29 (30.2) | 23 (23.2) | 23 (21.1) | 0.76 |
| Deceased | 217 (71.4) | 62 (64.6) | 73 (73.7) | 82 (75.2) | ||
| Unknown | 12 (3.9) | 5 (5.2) | 3 (3.0) | 4 (3.7) | ||
| Follow up time (months), median (range) | 25 (0.5–167.8) | 22 (5.1–155.8) | 37.5 (5.5–141.3) | 27.7 (0.5–167.8) | 0.84 | |
The majority of patients were male (66.4%, 202/304) and the median age at the time of primary melanoma diagnosis was 59.5 years (range 17.7 to 93.6 years), reflective of the general worldwide melanoma distribution. Fifty patients (17.2%) presented with primary tumors ≤1.0 mm in thickness, 78 (26.9%) with tumors between 1.01 and 2.0 mm, 81 (27.9%) between 2.01 and 4.0 mm, and 81 (27.9%) greater than 4 mm. Primary tumor ulceration was assessed in 88.5% (269/304) of the cohort, of which 50.2% (135/269) were ulcerated. The median time to diagnosis of metastatic disease from time of initial diagnosis was 26.6 months (range 0 to 422.8 months).
When categorized by the initial stage of primary melanoma diagnosis, 96 patients (31.6%) presented with stage I disease, 99 (32.6%) with stage II disease, and 109 (35.8%) with stage III disease. Survival analysis of patients stratified by clinical stage at the time of primary melanoma diagnosis demonstrated a significant difference in pmOS among the groups (p=0.036; Figure 1). Patients diagnosed with stage I melanoma had better pmOS (29.5 months) than patients diagnosed with stage II or III disease (pmOS 14.9 and 15.1 months, respectively). No significant differences in post metastatic OS were found when comparing substages of each clinical stage (e.g. stage IA vs. IB) at the time of primary melanoma diagnosis (Supplemental Figure 1). Univariate analysis revealed that primary melanoma diagnosis of stage II (HR 1.42, p=0.041) or stage III (HR 1.51, p=0.015) were associated with worse post metastatic OS than those initially diagnosed with stage I disease (Table 2). The association between initial stage II and stage III diagnosis and pmOS (adjusted HR 1.42, p=0.046 and adjusted HR 1.56, p=0.010, respectively) remained statistically significant in multivariate analysis adjusting for LDH, age, gender, and presence of brain metastases.
Figure 1.
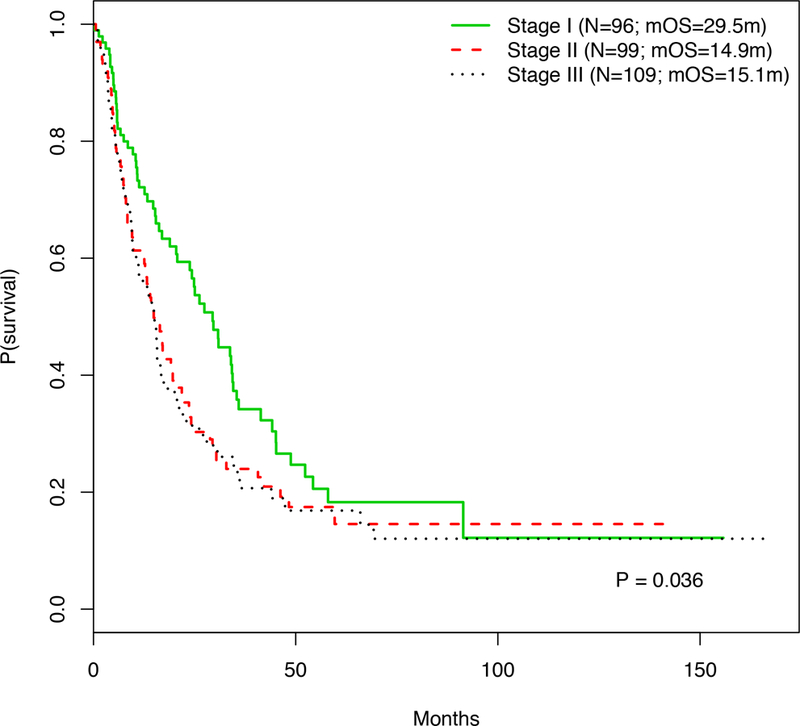
Kaplan-Meier curve displaying the post metastatic OS of patients stratified by their original clinical stage of their primary melanoma.
Table 2.
Univariate analysis and multivariate analysis of post-metastatic overall survival (pmOS) based on clinical stage of initial melanoma diagnosis and other clinicopathological characteristics.
| Patient/tumor characteristics | HR (95% CI) | P-value |
|---|---|---|
| Univariate analysis | ||
| Clinical stage at initial diagnosis (vs. stage I) | ||
| Stage II | 1.42 (1.15–2.00) | 0.041 |
| Stage III | 1.51 (1.08–2.10) | 0.015 |
| Multivariate analysis | ||
| Clinical stage at initial diagnosis (vs. stage I) | ||
| Stage II | 1.42 (1.01–2.01) | 0.046 |
| Stage III | 1.56 (1.11–2.18) | 0.010 |
| LDH (high vs. normal) | 1.65 (1.25–2.20) | 0.001 |
| Age at initial diagnosis (per 10 year increase) | 1.16 (1.06–1.27) | 0.001 |
| Sex (male vs. female) | 1.20 (0.89–1.60) | 0.225 |
| Site of metastasis (brain metastasis vs no
brain metastasis) |
1.61 (1.23–2.11) | 0.001 |
Elevated LDH and Stage M1c Disease at Metastatic Recurrence Associated with Worse Survival
Patient characteristics at the time of diagnosis of metastatic disease were also analyzed (Table 1). LDH levels were available for 171 patients (56.3%, 171/304), and 52 (30.4%, 52/171) of these patients had elevated LDH levels. Approximately 77 percent of patients (235/304) were classified as having substage M1c disease at the time of metastatic disease (Table 1). Two hundred and seventeen (71.4%) patients were confirmed deceased at the time of publication. The pmOS of all patients was 14.4 months (range 0 to 167.8 months). The median age of patients at the time of diagnosis of metastatic disease was 64 years (range 22.0 to 97.1 years). We next investigated the association between known prognostic factors and initial stage of melanoma diagnosis. In multivariate analysis, male gender is associated with worse prognosis, but is not statistically significant (adjusted HR 1.20, p=0.225, Table 2).. Increasing age at primary diagnosis, however, was prognostic of pmOS (adjusted HR 1.16 per 10 year increase in age, p=0.001, Table 2). High LDH level was associated with significantly worse OS in multivariate analysis (adjusted HR 1.65, p=0.001).
BRAF Mutation Status is not associated with Survival Outcomes
Genomic characterization of melanoma tumors has become standard practice, used to as characterize melanoma tumors, as well as identify suitable treatments with targeted therapies. We next assessed the association between BRAF mutation status and prognosis in this cohort. BRAF mutation status was available for 240 patients (79.0%, 240/304). Of the patients for whom data was available, 115 out of 240 (47.9%) had BRAF mutations. Analysis of time to metastatic disease based on BRAF status revealed no significant difference in progression free survival between patients with BRAF mutations and those who were wild-type (median time to recurrence 31.3 vs. 24.9 months, p=0.51; Figure 2A). Survival analysis based on BRAF mutation status showed no significant difference in pmOS between the two groups (pmOS 16.8 months vs. 18.9 months, BRAF mutant vs wild-type, respectively, p=0.81; Figure 2B).
Figure 2.
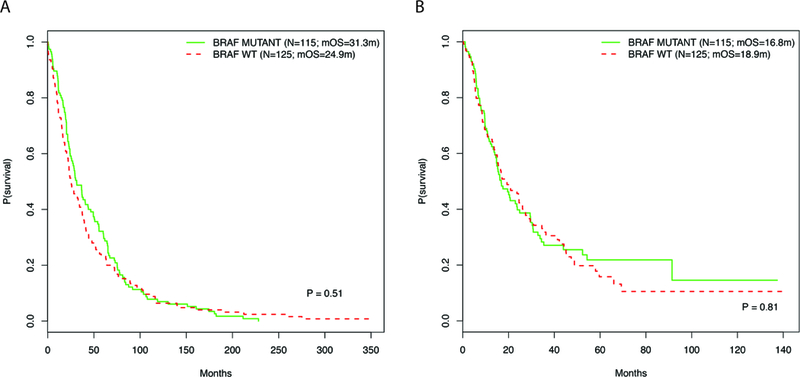
Kaplan-Meier curves displaying the (A) time to metastatic recurrence from primary melanoma diagnosis and (B) post-metastatic OS and for patients stratified by BRAF mutation status.
Adjuvant Therapy associated with longer Post-Metastatic Survival
We investigated the impact of additional interventions at the time of primary melanoma diagnosis on post metastatic survival outcomes. One hundred seventeen patients were eligible for adjuvant therapy prior to diagnosis of metastatic disease, and 91 patients (29.9%, 91/304) received adjuvant therapy. Twenty-five patients (8.2%, 25/304) received interferon, 21 (6.9%, 21/304) received GM-CSF (Leukine®), and 41 (13.4%, 41/304) were treated with a vaccine clinical trial; one patient received an unclassified adjuvant therapy; ten patients received more than one adjuvant treatment. Fifteen patients received combination adjuvant and systemic therapy and were not included for analysis. Survival analysis of patients stratified by receipt of adjuvant therapy demonstrated that those who had received specified adjuvant treatment had significantly better post metastatic OS than those who did not (median pmOS 24.3 vs. 15.7 months, p<0.01; Figure 3). Receipt of adjuvant therapy was also associated with significantly better post metastatic OS in multivariate analyses (adjusted HR 0.66, p=0.012; Table 2).When stratified by the specific type of adjuvant therapy received, there was no significant difference in post metastatic OS among the treatments (Interferon median OS 2.6 years vs. GM-CSF median OS 2.1 years vs. vaccine median OS 2.3 years, p=0.43; Supplemental Figure 2).
Figure 3.
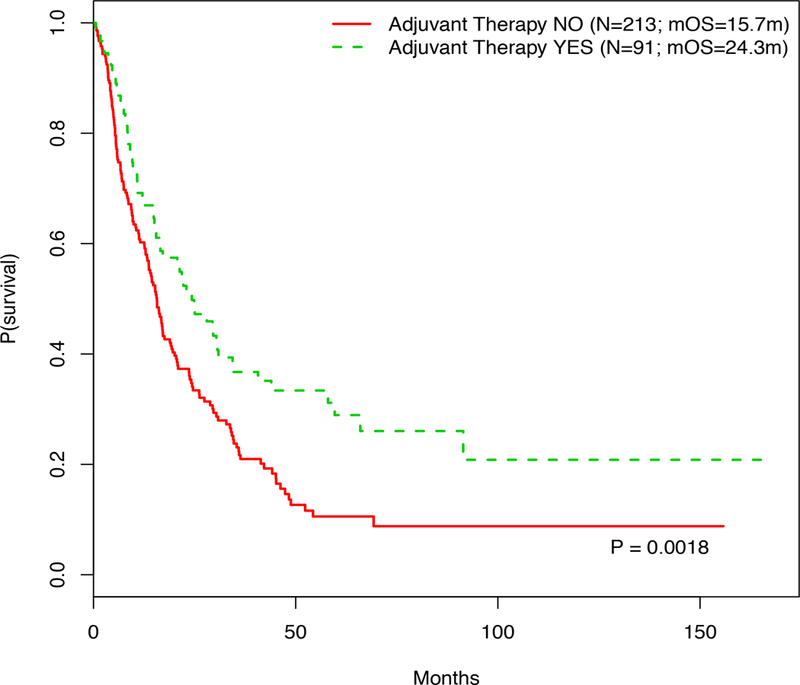
Kaplan Meier curve showing the post metastatic OS of patients based on receipt of adjuvant therapy at the time of primary melanoma diagnosis.
Immunotherapy Improves Survival of Patients with Metastatic Disease
At the time of diagnosis of metastatic disease in the 304 patients, 144 patients (47.4%) received immunotherapy, 76 (25%) received targeted therapy, and 141 (46.4%) received chemotherapy as systemic treatment for their metastatic disease. Ninety six patients (31.2%) received more than one type of systemic therapy. Survival analysis of patients stratified by treatment demonstrated that patients who were treated with immunotherapy had significantly better pmOS than those who were not treated with immunotherapy (median OS 24.6 months vs. 10.8 months, respectively; p<0.01; Figure 4A). There was no significant difference in OS between patients treated with targeted therapy and those who were not treated with targeted therapy (median OS 17.6 months vs. 15.7 months, respectively; p=0.51; Figure 4B). Similarly, no difference was observed between those who received chemotherapy and those who did not receive chemotherapy (median OS 16.4 months vs. 19.6 months, respectively; p=0.85; Figures 4C). Treatment with immunotherapy was also associated with significantly better OS multivariate analysis (adjusted HR 0.57, p<0.001; Table 2). Treatment with either targeted therapy or chemotherapy was not associated with significant differences in pmOS (Table 2).
Figure 4.
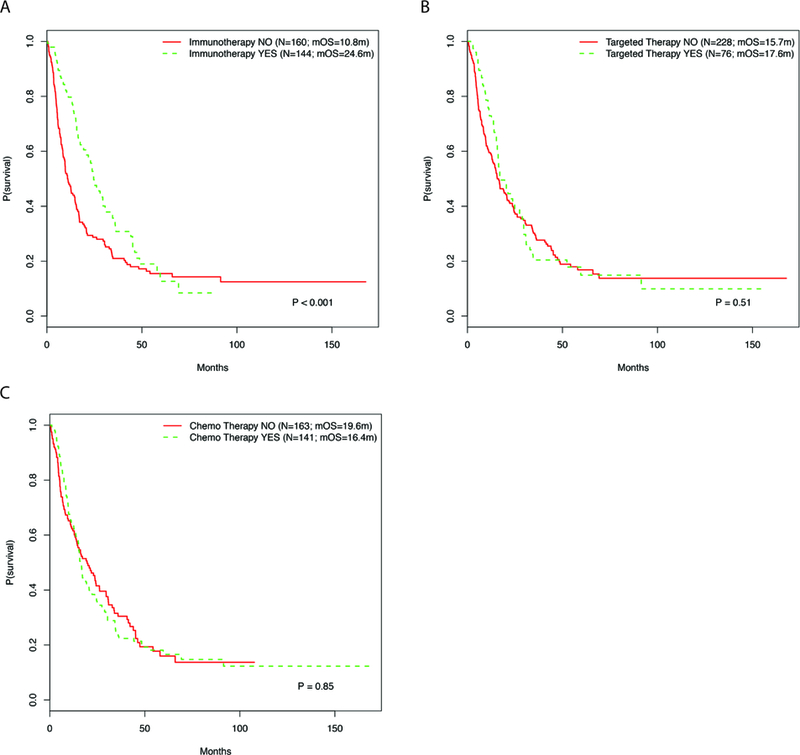
Kaplan Meier curves showing the post-metastatic OS of patients based on receipt of (A) immunotherapy, (B) targeted therapy, and/or (C) chemotherapy for treatment of their metastatic melanoma.
Discussion
Our investigation suggests that initial stage at primary melanoma diagnosis impacts survival after developing metastatic disease. Initial diagnosis of stage II and Stage III melanoma was associated with significantly worse pmOS compared to initial diagnosis of stage I disease. In addition, elevated LDH and Stage M1c disease at the time of diagnosis of metastatic disease continue to negatively impact on metastatic melanoma survival.
There are limited studies investigating the prognostic role of initial tumor stage on post metastatic survival. A study of 58 patients with recurrent Hodgkin’s lymphoma found initial stage of disease to be the most important predictor of survival following relapse.14 Patients who initially presented with stage IA-IIIAS+N- disease had a 4-year post-relapse survival rate of 81% compared with those originally diagnosed with stage IIIAS±N+-IIIB disease who had a survival rate of 42% (p<0.05).14 Similarly, a Danish study of 2427 women with breast cancer demonstrated decreased survival in patients diagnosed with breast cancer at a more advanced disease, indeed, mortality was nearly doubled for patients diagnosed with Stage III breast cancer compared to Stage 1.12 In contrast a study of 598 patients with stage IV colorectal cancer did not find a significant difference in post metastatic OS between patients who initially presented with stage 0-III disease compared with those who presented with distant metastases (HR 0.92, p=0.680).15 The discordance of results is possibly due to the population being studied and study design, retrospective versus prospective. Moreover, it is possible that disease specific factors, such as hormonal factors or other cellular cofactors, can influence tumor biology and disease prognosis.
Thickness is a main determinant of initial stage in primary melanoma. Our data are in concordance with a recent study evaluating the prognostic value of primary tumor characteristics in stage IV melanoma patients. The study included 227 patients diagnosed with stage IV melanoma, though the total melanoma population is not indicated, and demonstrated increasing thickness to be an independent predictor of worse post metastatic survival (HR 1.09, 95% CI 1.02–1.06, p<0.01)13, but the analysis was limited to tumor thickness. Additional characteristics, including ulceration, lymph nodes, and in transit/satellite metastases, also contribute to staging information. The authors hypothesize that thicker tumors have distinct biologic attributes that not only contribute to their aggressive nature but persist throughout disease progression as well.13
In our study, M1c stage, a surrogate for location of metastases, was independently prognostic of worse OS, consistent with previous studies.8–11 Similarly, our study found high LDH levels to be an independent marker of worse OS, which is consistent with previous reports.7,17 Our study also contributes to the ongoing debate of the prognostic significance of a BRAF mutation. 18–20 Our analysis demonstrated that the presence of a BRAF mutation had no effect on the development of metastatic disease and no effect on overall survival once diagnosed with metastatic disease. Our results are consistent with recent findings demonstrating no difference in OS between BRAF-mutant and WT populations from the time of stage IV diagnosis.18 Importantly, BRAF-mutant disease was not predictive of survival on in our study. In our study, we also observed that patients who received adjuvant therapy had improved pmOS. Our patient cohort does represent a smaller sample size than large multicenter studies, and could be reflective of the patient population at our institution, as well as the fact that this is an observational study. Moreover, patients were treated with agents prior to the use of immune checkpoint inhibitors in the adjuvant setting. Adjuvant therapy is used to reduce the risk of recurrence in patients with high-risk local or regional melanoma with a 5-year risk of recurrence greater than 30%.21 Previous clinical trials of adjuvant treatments have shown little benefit with high toxicity22–25, though recent randomized studies demonstrated improvement in disease-free survival when treated with high dose ipilimumab26 and nivolumab.27
Our analysis cannot be appreciated without considering our study’s limitations. This is a single-center study at a large, academic medical center, which may bias the patient population. It is highly likely that patients treated with immunotherapy, targeted therapy, and certain adjuvant treatments received treatment as part of a clinical trial, most of which have strict inclusion criteria related to performance status, metastatic sites, and treatment history, which could influence outcomes. In addition, it is possible that patient-related factors—for example, frequency of routine medical visits—might affect diagnosis of primary and metastatic melanoma. Patients with high risk stage II and III disease are seen more often by oncologists in follow-up and receive more frequent whole body imaging as compared to stage I melanoma patients. Stage I melanoma patients do not require oncology follow up and do not undergo surveillance imaging unless there are symptoms that necessitate scans. This has potential for contributing towards a lead-time bias in the detection of metastatic disease (in the form of asymptomatic metastases) for patients presenting at stage II or III. We do not believe, however, that our results have been affected in this way; our results suggest longer survival for patients diagnosed with stage I disease, where a lead-time bias would presumably influence survival calculations towards longer survival for patients undergoing vigilant disease monitoring.
In conclusion, we found initial stage, in particular stage I disease at primary diagnosis, to be a positive prognostic factor following diagnosis of metastatic disease, as well as receipt of adjuvant treatment prior to metastatic diagnosis. Our results highlight the need for further investigation of metastatic disease behavior based on initial stage, both clinically and biologically. Vigilant, early detection and adjuvant treatment of disease may be of benefit to patients and should continue to be evaluated.
Supplementary Material
Acknowledgments
Research Support: Funding for the work was provided by the Perlmutter Cancer Center and New York University Langone Medical Center.
The study has been presented in part at the American Society of Clinical Oncology Annual Meeting in Chicago, Illinois on June 3–7, 2016.
Footnotes
All authors have no conflicts of interest to declare.
References
- 1.Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). : SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016, 2016 [Google Scholar]
- 2.Flaherty KT, Infante JR, Daud A, et al. : Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694–703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long GV, Stroyakovskiy D, Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–88, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Chesney J, Pavlick AC, et al. : Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Wolchok JD, et al. : Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–17, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372:2521–32, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Gershenwald JE, Soong SJ, et al. : Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eton O, Legha SS, Moon TE, et al. : Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 16:1103–11, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Manola J, Atkins M, Ibrahim J, et al. : Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18:3782–93, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Bedikian AY, Johnson MM, Warneke CL, et al. : Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest 26:624–33, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Hao M, Zhao G, Du X, et al. : Clinical characteristics and prognostic indicators for metastatic melanoma: data from 446 patients in north China. Tumour Biol, 2016 [DOI] [PubMed]
- 12.Cetin K, Christiansen CF, Svaerke C, et al. : Survival in patients with breast cancer with bone metastasis: a Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open 5:e007702, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luen S, Wong SW, Mar V, et al. : Primary Tumor Thickness is a Prognostic Factor in Stage IV Melanoma: A Retrospective Study of Primary Tumor Characteristics. Am J Clin Oncol, 2015 [DOI] [PubMed]
- 14.Mauch P, Ryback M, Rosenthal D, et al. : The influence of initial pathologic stage on the survival of patients who relapse from Hodgkin’s disease. Blood 56:892–7, 1980 [PubMed] [Google Scholar]
- 15.Seal B, Chastek B, Kulakodlu M, et al. : Differences in survival for patients with metastatic colorectal cancer by lines of treatment received and stage at original diagnosis. Int J Clin Pract 69:251–8, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Garbe C, Leiter U: Melanoma epidemiology and trends. Clin Dermatol 27:3–9, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Erez A, Shental O, Tchebiner JZ, et al. : Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J 16:439–43, 2014 [PubMed] [Google Scholar]
- 18.Carlino MS, Haydu LE, Kakavand H, et al. : Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br J Cancer 111:292–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty KT: Is it good or bad to find a BRAF mutation? J Clin Oncol 29:1229–30, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Long GV, Menzies AM, Nagrial AM, et al. : Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29:1239–46, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Davar D, Kirkwood JM: Adjuvant Therapy of Melanoma. Cancer Treat Res 167:181–208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins MB, Hsu J, Lee S, et al. : Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 26:5748–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty KT, Lee SJ, Zhao F, et al. : Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31:373–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty LE, Othus M, Atkins MB, et al. : Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma--an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol 32:3771–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocellin S, Lens MB, Pasquali S, et al. : Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev 6:CD008955, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 16:522–30, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Weber J, Mandala M, Del Vecchio M, et al. : Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 377:1824–1835, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


