Abstract
Context
Gait biomechanics are linked to biochemical changes that contribute to the development of posttraumatic knee osteoarthritis in individuals with anterior cruciate ligament reconstruction (ACLR). It remains unknown if modifying peak loading during gait using real-time biofeedback will result in acute biochemical changes related to cartilage metabolism.
Objective
To determine if acutely manipulating peak vertical ground reaction force (vGRF) during gait influences acute changes in serum cartilage oligomeric matrix protein concentration (sCOMP) among individuals with ACLR.
Design
Crossover study.
Patients or Other Participants
Thirty individuals with unilateral ACLR participated (70% female, age = 20.43 ± 2.91 years old, body mass index = 24.42 ± 4.25, months post-ACLR = 47.83 ± 26.97). Additionally, we identified a subgroup of participants who demonstrated an increase in sCOMP after the control or natural loading condition (sCOMPCHANGE > 0 ng/mL, n = 22, 70% female, age = 20.32 ± 3.00 years old, body mass index = 24.73 ± 4.33, months post-ACLR = 47.27 ± 29.32).
Main Outcome Measure(s)
Serum was collected both prior to and immediately after each condition to determine sCOMPchange.
Intervention(s)
All participants attended 4 sessions that involved 20 minutes of walking on a force-measuring treadmill consisting of a control condition (natural loading) followed by random ordering of 3 loading conditions with real-time biofeedback: (1) symmetric vGRF between limbs, (2) a 5% increase in vGRF (high loading) and (3) a 5% decrease in vGRF (low loading). A general linear mixed model was used to determine differences in sCOMPCHANGE between altered loading conditions and the control group in the entire cohort and the subgroup.
Results
The sCOMPCHANGE was not different across loading conditions for the entire cohort (F3,29 = 1.34, P = .282). Within the subgroup, sCOMPCHANGE was less during high loading (1.95 ± 24.22 ng/mL, t21 = −3.53, P = .005) and symmetric loading (9.93 ± 21.45 ng/mL, t21 = −2.86, P = .025) compared with the control condition (25.79 ± 21.40 ng/mL).
Conclusions
Increasing peak vGRF during gait decreased sCOMP in individuals with ACLR who naturally demonstrated an increase in sCOMP after 20 minutes of walking.
Trial Registry
ClinicalTrials.gov (NCT03035994)
Keywords: cartilage oligomeric matrix protein, vertical ground reaction force, posttraumatic osteoarthritis, biomarkers
Key Points
Among all participants with anterior cruciate ligament reconstruction, serum cartilage oligomeric matrix protein (sCOMP) did not differ between the control and the 3 experimental (symmetric, high, low) loading conditions.
In a subgroup of participants whose sCOMP increased during the control condition, the high-loading condition resulted in decreased sCOMP. Thus, greater mechanical loading during walking may decrease the acute sCOMP response in individuals who normally demonstrate increased sCOMP after walking.
Real-time biofeedback was useful in acutely altering joint tissue biochemistry in those with an increased acute sCOMP response to walking, which may indicate that modifying gait biomechanics is beneficial in preventing posttraumatic osteoarthritis after anterior cruciate ligament reconstruction.
Approximately one-third of individuals who sustained an anterior cruciate ligament (ACL) injury and underwent reconstruction (ACLR) developed radiographic posttraumatic osteoarthritis (PTOA) of the knee within the first decade after injury.1 The development of PTOA is multifaceted, and previous work2 supported the hypothesis that both biomechanical and biochemical changes occur after ACL injury and ACLR, and the interplay between these factors likely contributes to the early development of PTOA. Animal models demonstrated that alterations in mechanical loading, both excessive3 and insufficient,4 led to articular cartilage degradation consistent with the development of PTOA. In individuals with idiopathic knee osteoarthritis, excessive joint loading during gait was associated with increased serum concentrations of biochemical markers linked to cartilage breakdown5 and increased tibiofemoral osteoarthritis severity.6 Conversely, in individuals with ACLR, lesser peak vertical ground reaction force (vGRF) produced by the ACLR limb was associated with deleterious changes in cartilage metabolism that were hypothesized to contribute to PTOA development.7,8 Therefore, increasing peak vGRF in the ACLR limb may result in more beneficial changes in joint tissue biochemistry among individuals with ACLR who are at greater risk of PTOA. Although persistent alterations in mechanical loading may influence tissue metabolism after ACLR,7,8 whether acutely increasing or decreasing mechanical loading can influence joint tissue biochemistry in those with ACLR is unknown.
Serum cartilage oligomeric matrix protein (COMP) is a biomarker of cartilage breakdown,9 and resting levels of COMP are elevated in individuals with ACLR compared with healthy controls.10 The serum COMP concentration (sCOMP) is mechanosensitive and increases in a dose-dependent manner after acute bouts of loading.11,12 A variety of factors influence the acute COMP response, including the duration of loading,13 intensity of loading,14 and increases in body weight.15 Additionally, greater increases in sCOMP after acute bouts of loading have been associated with greater cartilage thinning over 5 years.5 Assessing sCOMP after manipulation of peak vGRF may provide insight into the acute biochemical response to modifying mechanical loading in individuals with ACLR.
Our primary purpose was to determine differences in the change in sCOMP of individuals with ACLR after 20 minutes of walking while using real-time biofeedback (RTBF) to elicit bouts of high loading (increased vGRF), low loading (decreased vGRF), and symmetric loading when compared with the control condition (participant's natural loading). Evidence suggests individuals with a history of a knee injury16 or knee osteoarthritis5,12 and uninjured populations14,17 demonstrated a heterogeneous change in sCOMP after an acute bout of running. Therefore, we secondarily conducted a post hoc analysis to determine the difference in the change in sCOMP between loading conditions in a subgroup of participants who displayed a homogeneous increase in sCOMP after the control condition. Based on the results of previous cross-sectional studies7,8 that showed lesser mechanical loading was associated with more deleterious cartilage metabolism after ACLR, we proposed that low loading would be associated with a greater increase in sCOMP (greater biochemical response), whereas high loading would be associated with a lesser increase in sCOMP (lesser biochemical response) compared with the control condition.
METHODS
Design
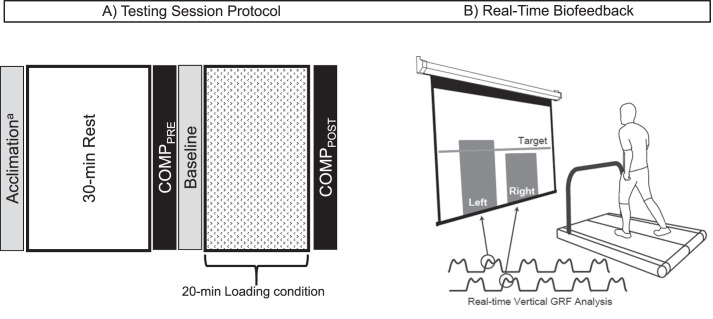
For this randomized crossover study, each participant completed 4 testing sessions (control, symmetric loading, high loading, low loading). The sCOMP remains stable during normal daytime hours18; thus, we did not standardize the time of testing among participants. We did standardize the time of day each session was collected between conditions for each person (0.29 ± 0.48 [mean ± SD] hours, difference between sessions) with at least a 7-day interval (9 ± 2 days) between sessions. The control condition was always conducted first, which allowed us to determine the target values for the remaining RTBF sessions. All testing procedures remained consistent across sessions (Figure 1). Participants first rested for 30 minutes before collection of the baseline blood sample. Next, they walked on the treadmill for 20 minutes during the loading condition. As this study was part of a larger trial that also determined the acute effect of altering peak vGRF on lower extremity biomechanics (clinical trial NCT03035994), we chose to limit the duration of the loading condition to 20 minutes to limit fatigue based on our pilot testing. A 20-minute loading condition was similar to that of an earlier investigation19 that used RTBF to acutely alter various loading characteristics during treadmill gait. The postloading blood sample was collected immediately upon completion of the 20-minute loading condition. At the beginning of the control-condition session only, self-selected over-ground walking speed was identified and used to set the treadmill speed for all subsequent testing sessions.8 Additionally, during the control condition, participants walked for 5 minutes to acclimate to the treadmill before beginning the 30-minute resting period. Before enrollment, the order of the altered loading conditions elicited via RTBF was block randomized using a Latin square. The university's Biomedical Institutional Review Board approved all methods, and all participants provided written informed consent before data collection.
Figure 1.
Testing session protocol and real-time biofeedback (RTBF). A, All 4 testing sessions followed an identical protocol, with 1 of 4 loading conditions prescribed during the 20-minute loading condition. On arrival to the laboratory, participants rested for 30 minutes. A baseline blood sample was collected after the 30-minute rest to assess baseline concentration of cartilage oligomeric matrix protein (sCOMPPRE). Participants were then instructed to walk in a normal gait pattern for a baseline walking trial. After the baseline trial data were collected, RTBF was displayed to the participant during the 20-minute loading condition. No biofeedback was provided during the control session. Immediately after the loading condition, a second blood sample was collected (sCOMPPOST). aAt the beginning of the control session only, self-selected over-ground walking speed was collected and used to set the treadmill speed for all testing sessions. Additionally, during the control session, participants walked for 5 minutes before beginning the 30-minute resting period to allow for acclimation to the treadmill. B, The RTBF displayed a vertical bar graph for each limb, which represented the magnitude of the first peak of the vGRF. A target line was placed in the center of the screen, and participants were instructed to alter their movement to match each vertical bar (ie, peak vGRF) with the target line during each step. Abbreviations: GRF, ground reaction force; vGRF, vertical ground reaction force.
Participants
We enrolled a convenience sample of 30 individuals between 18 and 35 years of age from the university community who underwent primary, unilateral ACLR using either a patellar tendon or hamstrings autograft (Table 1). All participants were engaging in unrestricted physical activity, which included at least 30 minutes of physical activity 3 times per week. We excluded individuals with a history of (1) a musculoskeletal injury to either leg within 6 months of the study, (2) a lower extremity surgery other than ACLR, (3) a previous diagnosis of or current self-reported symptoms related to knee osteoarthritis (pain, swelling, stiffness), or (4) cardiovascular restrictions limiting pursuit of any physical activity. We also excluded pregnant females. Each person self-reported the ACLR graft type used and date of surgery. All participants completed the subjective portion of the International Knee Documentation Committee index to measure self-reported disability and the Tegner Activity Scale to measure physical activity level. Previous authors14 reported a moderate difference in sCOMP between an acute bout of running and a loading protocol consisting of deep knee bends in healthy individuals (Cohen d effect size = 0.5). Therefore, we estimated 26 participants would be needed to detect a moderate effect between conditions (Cohen d = 0.5) with 80% power and an α level of .05 (version 3.1, G*Power Statistical Power Analysis Software; Heinrich-Heine-University Düsseldorf, Germany). We enrolled an additional 4 participants in the event of implausible statistical outliers or missing values due to an inability to obtain all blood samples.
Table 1.
Participant Demographics and the Percentage Change in Peak Vertical Ground Reaction Force (vGRF) in Each Conditiona
| No. (%) |
||
| Characteristic |
Entire Cohort (n = 30) |
Subgroup (n = 22) |
| Sex, No. (%) females | 21 (70) | 16 (73) |
| Graft type, No. (%) patellar tendon autograft | 14 (47) | 11 (50) |
| Concomitant meniscal surgery | 17 (56.7) | 12 (55) |
| Mean ± SD |
||
| Age, y | 20.43 ± 2.91 | 20.32 ± 3.0 |
| Height, cm | 172.70 ± 10.81 | 171.77 ± 10.27 |
| Mass, kg | 73.16 ± 16.10 | 73.24 ± 15.27 |
| Body mass index | 24.42 ± 4.25 | 24.73 ± 4.33 |
| Months since ACLR (range) | 47.83 ± 26.97 (6–118) | 47.27 ± 29.32 (6–118) |
| International Knee Documentation Committee score | 86.49 ± 9.51 | 87.53 ± 7.75 |
| Tegner Scale score | 7.47 ± 1.33 | 7.64 ± 1.40 |
| Baseline vGRF outcomes | ||
| ACLR-limb vGRF, % BW | 1.11 ± 0.06 | 1.09 ± 0.04 |
| Contralateral-limb vGRF, % BW | 1.12 ± 0.06 | 1.10 ± 0.04 |
| vGRF Limb symmetry index, % | 98.72 ± 2.62 | 98.66 ± 2.86 |
| Loading-condition change in ACLR-limb peak vGRF, % | ||
| Control | 1.35 ± 2.37 | 1.41 ± 1.99 |
| Symmetric | 1.31 ± 3.77 | 0.55 ± 2.57 |
| High | 5.04 ± 2.33 | 4.56 ± 2.31 |
| Low | −2.31 ± 2.28 | −2.33 ± 2.43 |
| Loading-condition change in contralateral-limb peak vGRF, % | ||
| Control | 1.56 ± 2.49 | 1.79 ± 2.60 |
| Symmetric | 1.23 ± 4.42 | 0.19 ± 2.44 |
| High | 5.20 ± 3.18 | 5.06 ± 3.50 |
| Low | −1.81 ± 3.43 | −1.79 ± 3.95 |
| Loading-condition average strides/min | ||
| Control | 54.93 ± 3.29 | 54.86 ± 3.28 |
| Symmetric | 55.93 ± 4.18 | 55.95 ± 3.99 |
| High | 55.67 ± 4.20 | 55.59 ± 3.58 |
| Low | 55.67 ± 5.08 | 54.54 ± 5.23 |
Abbreviations: % BW, percentage of body weight; ACLR, anterior cruciate ligament reconstruction.
The percentage change in peak vGRF was calculated as the percentage change score from baseline (peak vGRF during the final minute of the loading condition/baseline peak vGRF] * 100).
Real-Time Biofeedback Conditions
Participants walked on a dual-belt, force-measuring treadmill with two 178- × 102-cm force plates (model S020008; Bertec Corp, Columbus, OH) to allow for collection of peak vGRF during each loading condition. During the baseline trial of the control condition, a custom MATLAB program (The MathWorks, Inc, Natick, MA) processed and extracted bilateral peak vGRF from the first 50% of the stance phase, which was used to determine the RTBF targets for the 3 experimental loading conditions (symmetric loading, high loading, and low loading) conducted in the subsequent sessions. A 72-in (183-cm) projection screen directly in front of the treadmill displayed the RTBF during the experimental loading conditions. A second custom MATLAB script continuously computed the average of the previous 4 peak vGRFs for each limb, which was visually displayed as right and left bar graphs on the projection screen with a horizontal target line across the center (Figure 1 and Supplementary Video File). The target line for the symmetric loading condition corresponded to the mean peak vGRF between the ACLR and contralateral limbs. The target line for the high-loading and low-loading conditions corresponded with a 5% increase or decrease, respectively, in the baseline peak vGRF for each limb. Hence, the high-loading and low-loading conditions cued a relative change in the vGRF magnitude of each limb individually. Target values for the high-loading and low-loading conditions were determined individually for the left and right leg according to the baseline value of each limb.
Our pilot work demonstrated that a 5% change in vGRF was feasible for participants to achieve during a 20-minute session, which resulted in changes in peak vGRF, instantaneous vGRF loading rate, peak internal knee-extension moment, and knee-flexion excursion during the high-loading and low-loading conditions.20 Additionally, earlier researchers21 suggested an approximate 5% difference in peak vGRF between symptomatic and asymptomatic individuals with ACLR.
Before the RTBF intervention, we provided each participant with a brief presentation explaining the peak vGRF and how the RTBF continuously displayed peak vGRF. Participants were instructed to match the height of each vertical bar graph, which represented the peak vGRF for each limb, to the target line. They could self-select the manner in which they altered their gait pattern to effectively manipulate peak vGRF and match each bar graph to the target line. To maximize the likelihood that participants would consistently reach the target, all were provided with 1 strategy that focused on manipulating the vertical displacement of their center of mass (CoM). Specifically, the examiner told them that increasing or decreasing the vertical displacement of their CoM might result in a subsequent increase or decrease in peak vGRF. However, participants were instructed that any strategy could be used to successfully increase or decrease peak vGRF to match the target line and that manipulating the CoM vertical displacement was not the only strategy that could be used.
Collection and Analysis of sCOMP
We used a standard vacutainer serum collection tube with a 21-gauge needle to collect 5 mL of antecubital venous blood before (sCOMPPRE) and immediately after (sCOMPPOST) each loading condition. The serum collection tubes were placed on ice until they were centrifuged at 4°C for 10 minutes at 3000g.8 Serum was pipetted equally into two 1.5-mL cryovials and stored in a −80°C freezer for batch analysis after the study. A commercially available specific enzyme-linked immunosorbent assay (ELISA; BosterBio, Pleasanton, CA) with an assay detection sensitivity of <10 ng/mL was used to determine sCOMP. For all assays, unknown samples were diluted 33 times, and all standards and unknown samples were evaluated in triplicate determinations. We ensured that all samples from a single participant were analyzed on a single ELISA plate. The overall average intra-assay variability was 4.71%, and the individual tests demonstrated intra-assay variabilities <10%.
The sCOMPPRE and sCOMPPOST concentrations were used to calculate the absolute change in sCOMP (sCOMPCHANGE = sCOMPPOST – sCOMPPRE) during each testing session. We also identified the subgroup of participants to be included in our secondary analysis, which comprised only those participants who demonstrated increased sCOMP (sCOMPCHANGE > 0 ng/mL) during the control condition.
Statistical Analysis
All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). We obtained blood samples at the preloading and postloading timepoints for all 4 loading conditions in 26 of the 30 participants; a sufficient volume of blood was not obtained at all timepoints from 4 participants. Before analyses, we assessed changes in sCOMP for outliers in each condition, which were characterized as any value greater than 3 SDs from the mean. No statistical outliers were present for sCOMPCHANGE in any condition. Two of the participants with missing sCOMP values were missing both premeasures and postmeasures for 2 conditions (symmetric and low loading for one and high loading and low loading for the other); these values were not imputed for analysis but were included in the mixed-model analysis under a missing-at-random paradigm. The remaining 2 participants were only missing sCOMP values for the postmeasure of the control condition; multiple imputation procedures (via SAS PROC MI and PROC MIANALYZE) were used to estimate these 2 COMP concentrations through a monotone regression specification of explanatory variables being baseline sCOMP concentrations within the same testing session, as well as sex, age, body mass index, and time since ACLR.22 A general linear mixed model (via SAS PROC MIXED) was calculated with the difference in sCOMPCHANGE across the 4 loading conditions (control, symmetric, high loading, low loading) specified as the response vector, an unstructured correlation structure to account for the within-person correlation across the 4 conditions, and the 4-level condition effect as the primary explanatory variable of interest. As sCOMPPRE varied across the 4 testing sessions (Table 2), this variable was included as a covariate in the general linear mixed model; the general linear mixed model further assessed the period and carryover effects, with nonsignificant carryover effects and then nonsignificant period effects being removed from the final model. Post hoc comparisons from this final model were performed with the Dunnett-Hsu adjustment for multiple comparisons to determine the adjusted (for sCOMPPRE) difference in sCOMPCHANGE between altered loading conditions (symmetric, high loading, and low loading) and the control condition. We then calculated 6 Cohen d effect sizes23 with corresponding 95% confidence intervals (CIs) to determine the magnitude of difference in sCOMPCHANGE between loading conditions and the control condition. Cohen d effect sizes were classified as strong (≤0.80), moderate (0.79 to 0.50), or small (≥0.49).23 Statistical analyses were first conducted in the entire cohort and were then repeated in the subgroup of participants demonstrating an increased sCOMP that was >0 ng/mL during the control condition. Statistical significance was set a priori at .05.
Table 2.
Serum Cartilage Oligomeric Matrix Protein Concentration (sCOMP), ng/mL (Mean ± SD)
| sCOMP |
|||
| Group and Loading Condition |
Pretest |
Posttest |
Raw Change |
| All participants (n = 30) | |||
| Control | 149.50 ± 53.17 | 167.15 ± 53.49 | 17.14 ± 26.41 |
| Symmetric | 158.52 ± 63.98 | 169.13 ± 66.37 | 10.61 ± 19.59 |
| High | 162.72 ± 64.58 | 168.71 ± 62.89 | 6.23 ± 23.24 |
| Low | 154.84 ± 60.08 | 170.04 ± 58.87 | 14.95 ± 22.75 |
| Participants with increased sCOMP after normal walking (n = 22) | |||
| Control | 144.08 ± 46.99 | 169.86 ± 49.71 | 25.79 ± 21.40 |
| Symmetric | 147.36 ± 52.69 | 157.29 ± 50.77 | 9.93 ± 21.45 |
| High | 158.61 ± 58.44 | 160.56 ± 55.62 | 1.95 ± 24.22 |
| Low | 150.59 ± 51.63 | 163.98 ± 52.19 | 13.39 ± 22.01 |
RESULTS
We screened 34 individuals with ACLR for the study; 31 were included in the initial cohort. One individual dropped out before finishing all conditions. Therefore, the entire cohort consisted of 30 individuals, and 22 individuals constituted a subgroup of participants who demonstrated increased sCOMP during the control condition (Figure 2).
Figure 2.
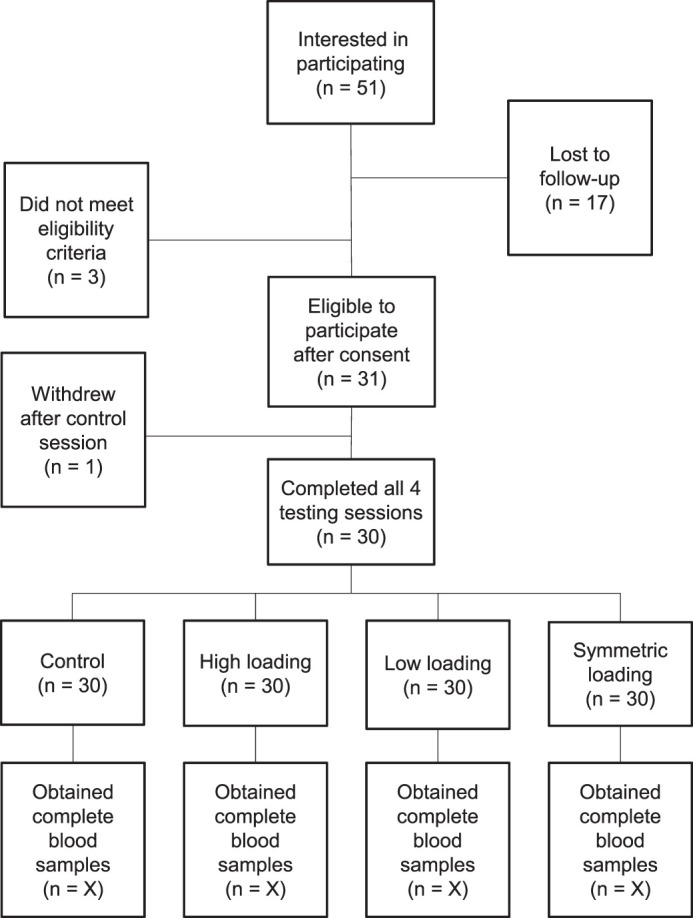
Study flowchart.
Entire Cohort
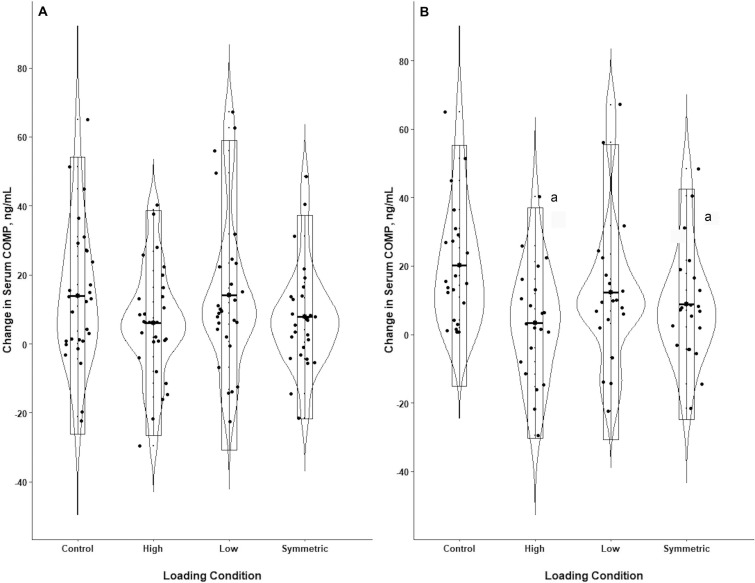
On average, participants could achieve a 5% increase in peak vGRF during the high-loading condition (mean ACLR limb change = 5.04% ± 2.33%, range = −0.49% to 9.94%; mean contralateral limb change = 5.20% ± 3.18%, range = 0.73% to 14.17%; Table 1). During the low-loading condition, participants could decrease their peak vGRF, although the decrease was less than the 5% cued via RTBF (mean ACLR limb change = −2.31% ± 2.28%, range = −7.77% to 3.12%; mean contralateral limb change = −1.81% ± 3.45%, range = −9.22% to 10.57%; Table 1). For our entire cohort (N = 30), sCOMPCHANGE was not different across the 4 loading conditions (F3,29 = 1.34, P = .282; Figure 3). Effect sizes for differences between conditions for sCOMPCHANGE were small and demonstrated inconclusive CIs (Table 3).
Figure 3.
Changes in serum cartilage oligomeric matrix protein concentration (sCOMP). A, displays the results from the entire cohort of participants (n = 30), whereas B, displays the results from the subgroup of participants who demonstrated increased sCOMP during the control condition (n = 22); data are presented as the mean changes in sCOMP with corresponding SDs. a Indicates smaller increase in sCOMP than in the control condition.
Table 3.
Cohen d Effect Sizes Between Loading Conditions
| Loading Conditions |
Effect Size, d |
95% Confidence Interval |
| All participants included (n = 30) | ||
| Control versus symmetric | 0.33 | −0.19, 0.83 |
| Control versus high | 0.48 | −0.04, 0.99 |
| Control versus low | 0.06 | −0.44, 0.57 |
| Participants with increased sCOMP after the control (n = 22) | ||
| Control versus symmetric | 0.78 | 0.16, 1.38 |
| Control versus high | 0.99 | 0.35, 1.60 |
| Control versus low | 0.61 | 0.00, 1.20 |
Post Hoc Test on Subgroup
Participants in the subgroup also demonstrated an increase (mean ACLR limb change = 5.04% ± 2.33%, range = −0.49% to 8.69%; mean contralateral limb change = 5.06% ± 3.36%, range = 0.73% to 14.17%) and decrease (mean ACLR limb change = −2.33% ± 2.43%, range = −7.77% to 3.12%; contralateral limb = −1.80% ± 3.95%, range = −9.22% to 10.57%) in peak vGRF during the high-loading and low-loading conditions, respectively. In the subgroup of participants who exhibited an increase in sCOMP during the control condition (n = 22), sCOMPCHANGE was different among conditions (F3,21 = 4.63, P = .012; Figure 3). Versus the control condition, sCOMPCHANGE was less during the symmetric (t21 = −2.86, adjusted P = .025) and high-loading (t21 = −3.53, adjusted P = .006); Table 3) conditions. The difference in sCOMPCHANGE between the low-loading condition and the control condition was not significant (t21 = −2.22, adjusted P = .094). A strong effect was present for a smaller increase in sCOMP during the high-loading condition and a moderate effect was present for the symmetric loading condition compared with the control condition; the conclusive CIs did not include zero (Table 3). Neither carryover nor period effects were significant for the entire cohort or the subgroup.
DISCUSSION
Overall, sCOMPCHANGE was not different between the control condition (participant's natural loading) and the altered loading conditions (symmetric, high loading, and low loading) in the entire cohort. When evaluating a subgroup of 22 participants who demonstrated a homogeneous sCOMP response to the control condition (ie, increase >0 ng/mL), we found a difference in sCOMPCHANGE for some of the loading conditions versus the control condition. Here, our hypothesis was supported as the increase in sCOMP was less during the high-loading condition than during the control condition. Although relatively modest, the increase in sCOMP was also less during symmetric loading compared with the control condition. These results are important as they suggest that manipulation of peak vGRF during walking may acutely influence the biochemical response in individuals with ACLR. Therefore, manipulating lower extremity loading during gait using RTBF may become a beneficial intervention for optimizing joint tissue biochemistry after ACLR and mitigating the future development of PTOA.
Overall, the greatest increase in sCOMP was in the control condition, followed by the low-loading, symmetric loading, and high-loading conditions (Table 2). Our results suggest that typical gait patterns used by individuals with ACLR in the control condition might be associated with the greatest cartilage breakdown and that manipulation of joint loading in any direction (high, low, or symmetric) may be helpful in attenuating the acute sCOMP response to walking. It is also possible that individuals will demonstrate varying magnitudes of an sCOMP response to walking by manipulating vGRF in different directions. Our cohort demonstrated a heterogeneous COMP response after 20 minutes of walking at a self-selected speed during the control condition; 22 of 30 participants displayed an increase in sCOMP (COMPCHANGE > 0 ng/mL). The change in serum COMP in our study is consistent with previous research17 that showed an increase in sCOMP after an acute walking bout in healthy participants who were of similar age as our participants. Greater increases in sCOMP immediately after loading likely reflect an efflux of COMP from lower extremity joints into the bloodstream,14,24 whereas assessment of the delayed sCOMP response may be more indicative of biochemical processes associated with cartilage health.5 It remains unknown if an acute increase or decrease in sCOMP after loading is beneficial or detrimental to long-term joint health. Although our small sample size precluded us from identifying differences between the 2 subgroups, the subgroup of 22 participants demonstrated lower baseline vGRF bilaterally (peak vGRF on the ACLR limb = 1.09 ± 0.04 % BW, contralateral limb = 1.10 ± 0.04 % BW) than the remaining 8 participants who demonstrated decreased sCOMP after the control condition (peak vGRF on the ACLR limb = 1.15 ± 0.07 % BW, contralateral limb = 1.17 ± 0.08 % BW). Future studies with larger sample sizes are needed to determine differences between individuals who exhibit an increase or decrease in COMP after loading and how the acute COMP response is associated with long-term changes in joint health.
In the subset of individuals who demonstrated increased sCOMP after the control condition (n = 22), the increase in serum COMP was less during high loading, which we interpreted as lesser efflux of sCOMP from the lower extremity joints into the blood stream. The raw change in sCOMP during the high-loading condition was small (1.95 ± 24.22 ng/mL) versus the change during the control condition (25.79 ± 21.40 ng/mL). We considered the difference in sCOMPCHANGE between the high-loading and control conditions to be meaningful because of the moderate between-conditions effect size with conclusive CIs that did not cross zero (Cohen d = 0.99, 95% CI = 0.35, 1.60; Table 3). Our results build on those of earlier cross-sectional studies that showed greater mechanical loading during walking was associated with lesser resting levels of collagen turnover8 and deleterious metabolic changes7 in individuals with ACLR. Evidence from animal models suggested large magnitude, high-rate impact loading may result in cartilage degradation,3 yet our RTBF cued only a small (ie, 5%) increase in peak vGRF during 20 minutes of walking. Differences between our results and animal experiments may be due to possibly substantial differences in the magnitude or rate of loading.
The difference in sCOMPCHANGE between the low-loading condition and the control condition was not significant (t21 = −2.22, adjusted P = .094, Cohen d effect size = −0.56). Although 63% and 73% of patients achieved the 5% increase in peak vGRF within 1 SD on the ACLR and contralateral limbs, respectively, during the high-loading condition, only 40% and 37% achieved the 5% decrease in peak vGRF on the ACLR limb and contralateral limb, respectively. The inability of participants to achieve the 5% decrease in peak vGRF during the low-loading condition may have influenced the change in sCOMP after this loading condition and produced nonsignificant differences in sCOMPCHANGE between the low-loading and control conditions. As we maintained a constant treadmill speed across all loading conditions (ie, each person's self-selected walking speed was determined during the control condition testing session), it was likely more difficult for participants to decrease peak vGRF during the low-loading condition without being able to decrease their walking speed. Most individuals also demonstrated lower peak vGRF on the ACLR limb compared with the contralateral limb (67% demonstrated a limb symmetry index <100). As these participants may have been chronically offloading the ACLR limb during their natural gait pattern, it might have been more difficult to achieve a further decrease in peak vGRF during the low-loading condition. In contrast to our hypothesis that low loading would result in a greater increase in sCOMP, we found that the change in sCOMP was less than in the control condition, although sCOMPCHANGE was not different between the conditions. The low-loading condition may have cued a decrease in the overall load applied to the entire limb, but individuals may have used different joint kinematics that caused increased loading locally or knee-specific joint loading. Biomechanical factors other than peak vGRF likely influenced the change in sCOMP after an acute bout of loading. Future research is needed to identify additional biomechanical outcomes that are associated with sCOMPCHANGE and how altering various lower extremity biomechanical factors is associated with changes in sCOMP.
On average, our participants walked with less peak vGRF on the ACLR limb compared with the contralateral limb, resulting in a limb symmetry index of less than 100% (Table 1; approximately 73% of participants in the subgroup of 22 demonstrated a limb symmetry index <100 [data not shown]). Albeit small, the difference in peak vGRF between the ACLR and contralateral limbs was significant (t29 = 2.780, P = .009; Cohen d effect size = −0.25), and most individuals in the subgroup were required to increase peak vGRF on the ACLR limb during the symmetric condition. The small increase in peak vGRF on the ACLR limb induced by symmetric loading may have had the same overall effect, ie, less of an increase in sCOMP, as the high-loading condition. Previous investigators5 have suggested that acute increases in sCOMP after exercise reflect cartilage breakdown; however, whether 20 minutes of loading would have been enough time to measure a change in metabolism is unclear. Future authors should determine if acutely altering peak vGRF during walking results in changes in inflammatory mediators and biochemical markers of cartilage turnover over time. Although the mean change in peak vGRF during the symmetric loading condition was similar to the mean change in peak vGRF during the control condition (Table 1), we observed a difference in sCOMPCHANGE between these conditions. During the symmetric loading condition, the target lines for each participant depended on the magnitude of asymmetry present at baseline: therefore, some participants had to increase peak vGRF on their ACLR limb, whereas others had to decrease it. The vGRF interlimb asymmetry in our cohort ranged from 95% to 105%, indicating that some participants were required to increase and others were required to decrease peak vGRF on the ACLR limb to reach the target line. Variations in the magnitude and direction of change in peak vGRF across our cohort may have contributed to an overall average change in peak vGRF that was small and similar to the control condition (Table 1), despite the potential for larger individual differences in the change in peak vGRF between the control condition and the symmetric loading condition. Future authors may seek to enroll larger cohorts of participants with ACLR in order to identify individualized biomechanical and biochemical responses to alterations in peak vGRF.
LIMITATIONS
Our findings provide evidence that acutely altering peak vGRF during walking influenced sCOMP in individuals with ACLR, yet certain limitations to this novel study can inform the design of future research. We chose to assess sCOMP because this marker has been reported to increase immediately after acute loading11,12,14; evaluating the delayed sCOMP response to loading may be useful, as a delayed increase in sCOMP (ie, 3.5–5.5 hours after loading) may be more indicative of a change in cartilage metabolism due to its association with cartilage thinning.5 Although a greater increase in sCOMP after an acute bout of loading may be related to greater cartilage breakdown,11,13 we were unable to specifically determine if acutely altering peak vGRF would result in an acute change in the concentrations of other biomarkers that may adversely affect cartilage health. Previous investigators24 have suggested that increased sCOMP after an acute loading protocol indicates an efflux of sCOMP from the synovial fluid of lower extremity joints into the blood, but sCOMP may also reflect a systemic response to loading by other joints or the involvement of tissues other than cartilage (eg, subcondral bone25). Even though our altered loading conditions (symmetric, high loading, and low loading) were block randomized before enrollment, the control condition was always completed first. We also assessed self-selected walking speed and allowed 5 minutes of treadmill acclimation before the 30-minute rest period during the control condition, which may have influenced sCOMPPRE during the control condition. Age,12 physical activity level,26 and sex27 have been linked with resting sCOMP in different patient populations. Despite our use of a within-subject crossover design to account for baseline differences between individual participants, our initial study was not powered to determine if age, physical activity level, and sex affected the sCOMP response to each loading condition. Future researchers should evaluate the effects of age, physical activity level, and sex on the sCOMP response to manipulating loads. Furthermore, our relatively small cohort of individuals with ACLR was young (mean age = 20.43 ± 2.91 years) and physically active at least 3 times per week. Whether our results are generalizable to the larger population of individuals with ACLR remains unknown. Other participant characteristics, such as the type and location of concomitant meniscal procedures, may have also influenced our results. Future studies are needed to determine how surgical characteristics influence the sCOMPCHANGE after alterations in loading.
Our cohort comprised individuals with a relatively wide range of time post-ACLR (6 to 118 months post-ACLR). Previous authors21 indicated that time post-ACLR may influence the relationship between vGRF and clinically relevant knee symptoms, suggesting that low loading was associated with worse symptoms in those <12 months post-ACLR, and high loading was associated with worse symptoms in those >24 months post-ACLR. It is important to learn if the sCOMP responses to high-loading and low-loading conditions differ based on time post-ACLR. Additionally, we did not restrict participants' physical activity before each testing session; thus, we cannot determine how physical activity may have influenced our results. To account for variations in resting levels of sCOMP across the 4 testing sessions, we collected a new baseline sample at each testing session and included sCOMPPRE as a covariate in the general linear mixed model. Alterations in lower extremity joint kinetics and kinematics also occur after ACLR28 and may lead to PTOA. Other loading factors, including step frequency and total number of loading cycles, may affect the sCOMP response in addition to peak vGRF. Further research is needed to determine if biomechanical outcomes other than peak vGRF influence sCOMP during walking. Although individuals with ACLR can acutely change peak vGRF after a 20-minute RTBF intervention,20 we did not evaluate the number of steps needed to consistently achieve the desired change in vGRF. We need to understand if individuals who can achieve the desired change in vGRF with a smaller number of steps demonstrate a different acute sCOMP response than those who take more steps. We did not assess the difference in sCOMP responses to symmetric, high, and low loading between our ACLR cohort and uninjured control participants; future work should address whether uninjured individuals respond differently to loading conditions than those with ACLR.
CONCLUSIONS
For the entire cohort, no differences occurred in the change in sCOMP between the control condition and each of 3 experimental loading conditions among individuals with ACLR. After evaluating a subgroup (n = 22) of individuals who demonstrated increased sCOMP during the control condition, we determined that manipulating the magnitude of peak vGRF during 20 minutes of walking acutely influenced the sCOMP response. Individuals who increased sCOMP during the control condition demonstrated less of an increase in sCOMP during the high-loading condition that cued a 5% increase in peak vGRF and the symmetric loading condition versus the control condition. Therefore, greater mechanical loading during walking may decrease the acute sCOMP response in individuals with ACLR who demonstrate an increase in sCOMP during 20 minutes of walking using their natural loading strategy in the control condition. These data suggest that RTBF can be used to acutely alter joint tissue biochemistry and that modifying gait biomechanics may be an important intervention for mitigating the development of PTOA in individuals after ACLR.
REFERENCES
- 1.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: a systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33(7):939–947. doi: 10.1002/jor.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ewers BJ, Weaver BT, Sevensma ET, Haut RC. Chronic changes in rabbit retro-patellar cartilage and subchondral bone after blunt impact loading of the patellofemoral joint. J Orthop Res. 2002;20(3):545–550. doi: 10.1016/S0736-0266(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 4.Leong DJ, Li YH, Gu XI, et al. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25(1):182–191. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erhart-Hledik JC, Favre J, Asay JL, et al. A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis Cartilage. 2012;20(11):1309–1315. doi: 10.1016/j.joca.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Clark AG, Jordan JM, Vilim V, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–2297. doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrosimone B, Blackburn JT, Harkey MS, et al. Greater mechanical loading during walking is associated with less collagen turnover in individuals with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(2):425–432. doi: 10.1177/0363546515618380. [DOI] [PubMed] [Google Scholar]
- 9.Garvican ER, Vaughan-Thomas A, Innes JF, Clegg PD. Biomarkers of cartilage turnover. Part 1: Markers of collagen degradation and synthesis. Vet J. 2010;185(1):36–42. doi: 10.1016/j.tvjl.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri-Smith RM, Wojtys EM, Potter HG. Early cartilage changes after anterior cruciate ligament injury: evaluation with imaging and serum biomarkers-a pilot study. Arthroscopy. 2016;32(7):1309–1318. doi: 10.1016/j.arthro.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Andersson ML, Thorstensson CA, Roos EM, Petersson IF, Heinegård D, Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2006;7:98. doi: 10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mündermann A, King KB, Smith RL, Andriacchi TP. Change in serum COMP concentration due to ambulatory load is not related to knee OA status. J Orthop Res. 2009;27(11):1408–1413. doi: 10.1002/jor.20908. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Lee YH, Kim CK. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol. 2009;105(5):765–770. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 14.Niehoff A, Kersting UG, Helling S, et al. Different mechanical loading protocols influence serum cartilage oligomeric matrix protein levels in young healthy humans. Eur J Appl Physiol. 2010;110(3):651–657. doi: 10.1007/s00421-010-1529-0. [DOI] [PubMed] [Google Scholar]
- 15.Denning WM, Winward JG, Pardo MB, Hopkins JT, Seeley MK. Body weight independently affects articular cartilage catabolism. J Sports Sci Med. 2015;14(2):290–296. [PMC free article] [PubMed] [Google Scholar]
- 16.Cattano NM, Driban JB, Barbe MF, Tierney RT, Amin M, Sitler MR. Biochemical response to a moderate running bout in participants with or without a history of acute knee injury. J Athl Train. 2017;52(6):567–574. doi: 10.4085/1062-6050-51.5.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harkey MS, Blackburn JT, Hackney AC, Lewek MD, Schmitz RJ, Pietrosimone B. Acute serum cartilage biomarker response following walking and drop landing. Med Sci Sports Exerc. 2018;50(7):1465–1471. doi: 10.1249/MSS.0000000000001585. [DOI] [PubMed] [Google Scholar]
- 18.Andersson ML, Petersson IF, Karlsson KE, et al. Diurnal variation in serum levels of cartilage oligomeric matrix protein in patients with knee osteoarthritis or rheumatoid arthritis. Ann Rheum Dis. 2006;65(11):1490–1494. doi: 10.1136/ard.2005.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowell HP, Milner CE, Hamill J, Davis IS. Reducing impact loading during running with the use of real-time visual feedback. J Orthop Sports Phys Ther. 2010;40(4):206–213. doi: 10.2519/jospt.2010.3166. [DOI] [PubMed] [Google Scholar]
- 20.Luc-Harkey BA, Franz JR, Blackburn JT, Padua DA, Hackney AC, Pietrosimone B. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. J Biomech. 2018;76:94–102. doi: 10.1016/j.jbiomech.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Blackburn JT. Walking ground reaction force post-ACL reconstruction: analysis of time and symptoms. Med Sci Sports Exerc. 2019;51(2):246–254. doi: 10.1249/MSS.0000000000001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y. Missing data analysis using multiple imputation: getting to the heart of the matter. Circ Cardiovasc Qual Outcomes. 2010;3(1):98–105. doi: 10.1161/CIRCOUTCOMES.109.875658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for Behavioral Sciences 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 24.Hyldahl RD, Evans A, Kwon S, et al. Running decreases knee intra-articular cytokine and cartilage oligomeric matrix concentrations: a pilot study. Eur J Appl Physiol. 2016;116(11–12):2305–2314. doi: 10.1007/s00421-016-3474-z. [DOI] [PubMed] [Google Scholar]
- 25.Kong L, Tian Q, Guo F, et al. Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol. 2010;29(4):276–286. doi: 10.1016/j.matbio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattano NM, Driban JB, Cameron KL, Sitler MR. Impact of physical activity and mechanical loading on biomarkers typically used in osteoarthritis assessment: current concepts and knowledge gaps. Ther Adv Musculoskelet Dis. 2017;9(1):11–21. doi: 10.1177/1759720X16670613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan J, Luta G, Stabler T, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48(3):675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 28.Davis-Wilson HC, Pfeiffer SJ, Johnston CD, et al. Bilateral gait 6 and 12 months post-anterior cruciate ligament reconstruction compared with controls. Med Sci Sports Exerc. 2020;52(4):785–794. doi: 10.1249/MSS.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]