Abstract
Purpose
Disruption of proteostasis is a key event in many neurodegenerative diseases. Heat shock proteins (HSPs) participate in multiple functions associated with intracellular transport and proteostasis. We evaluated the effect of augmented HSP70 expression in mutant photoreceptors of mouse retinal degeneration models to test the hypothesis that failure to sustain HSP70 expression contributes to photoreceptor cell death.
Methods
We examined HSP70 expression in retinas of wild-type and mutant mice by RNA and protein analysis. A transgenic mouse line, TgCrx-Hspa1a-Flag, was generated to express FLAG-tagged full-length HSP70 protein under control of a 2.3 kb mouse Crx promoter. This line was crossed to three distinct retinal degeneration mouse models. Retinal structure and function were evaluated by histology, immunohistochemistry, and electroretinography.
Results
In seven different mouse models of retinal degeneration, we detected transient elevation of endogenous HSP70 expression at early stages, followed by a dramatic reduction as cell death ensues, suggesting an initial adaptive response to cellular stress. Augmented expression of HSP70 in RHOT17M mice, in which mutant rhodopsin is misfolded, marginally improved photoreceptor survival, whereas elevated HSP70 led to more severe retinal degeneration in rd10 mutants that produce a partially functional PDE6B. In Rpgrip1−/− mice that display a ciliary defect, higher HSP70 had no impact on photoreceptor survival or function.
Conclusions
HSP70 overexpression has divergent effects in photoreceptors determined, at least in part, by the nature of the mutant protein each model carries. Additional investigations on HSP pathways and associated chaperone networks in photoreceptors are needed before designing therapeutic strategies targeting proteostasis.
Keywords: photoreceptors, retinal degeneration, heat shock protein, proteostasis, chaperone
Inherited retinal degenerative diseases (IRDs), characterized by progressive dysfunction or loss of photoreceptors, constitute a major cause of vision impairment, which severely impacts patients’ quality of life. Although much progress has been achieved with mutations identified in over 200 genes,1 we lack a precise understanding of pathogenic mechanisms and effective treatments for most IRDs. Proteostasis, defined as the appropriate production, folding, trafficking, and degradation of proteins, is essential for cell's health and survival. Given the highly active phototransduction cascade and continuous renewal of outer segments (OS), maintenance of proteostasis is critical for healthy photoreceptors and for the survival of stressed photoreceptors in IRDs. Disrupted cellular proteostasis and resulting apoptosis driven by the unfolded protein response (UPR) are among several hypotheses to explain the mechanism underlying photoreceptor death in degenerating retina.2–5
Molecular chaperones, especially the heat shock protein (HSP) family, act to support cellular proteostasis by restoring protein function under physiological conditions or by triggering programed cell death under stress.6,7 The HSP superfamily is classified by apparent molecular mass into subfamilies. Eight HSP70 proteins identified in humans, belonging to the 70-kDa subfamily, share 52% to 99% amino acid sequence homology and exhibit similar functions yet somewhat distinct characteristics.8 Hereafter we use the term “HSP70” to collectively refer to two proteins, HSP70-1a and HSP70-1b, which only differ from each other by two amino acid residues and are encoded in mice by Hspa1a and Hspa1b genes, respectively.8,9 HSP70 is one of the most studied chaperones, especially in the stressed retina. In addition to the stress-inducible HSP70, a cognate 70-kDa HSP HSC70 (Hspa8)10 is constitutively expressed. Under physiological conditions, HSPs protect and improve folding of newly synthesized proteins, participate in quality control, guide their translocation into distinct organelle destinations, and assist in the assembly/disassembly of multiprotein complexes.11–13 When under stress, transcription regulator heat shock factor 1 (HSF1) activates expression of various HSPs, including HSP70.14 HSP70 binds to misfolded proteins and facilitates their recovery with assistance of other HSPs, such as HSP40 and HSP90, and co-chaperones such as Hip and Hop.15,16 Alternatively, when the damage is not repairable, HSP70 facilitates the degradation of aggregated proteins by delivering them to the ubiquitin proteasome system with assistance of co-chaperone CHIP.17,18
The nexus between disruption in cellular proteostasis and progression of neurodegenerative disease has uncovered an important role for chaperone proteins in regulating the survival or death of stressed neuronal cells.19–21 Disease-causing proteins, such as β-amyloid and tau in Alzheimer disease, huntingtin in Huntington disease, α-synuclein in Parkinson disease, and mutant rhodopsin in retinitis pigmentosa, all interact with the HSP chaperone machinery.20–22 Therefore HSPs are potential therapeutic targets for neurodegenerative diseases.
Although HSP70 deficient mice were generated over a decade ago, the role of HSP70 in retinal development has not been investigated.23 In the adult retina, HSP70 maintains proteostasis and plays an essential role in the maturation of phototransduction proteins.24,25 In cooperation with HSP90 and a photoreceptor specific co-chaperone AIPL1, HSP70 is implicated in facilitating correct folding and assembly of retinal cGMP phosphodiesterase (PDE6) holoenzyme.26–28 Elevated HSP70 expression was observed in photoreceptors following induced retinal detachment and hypothesized to play a protective role.29 HSP70 upregulation was also reported to delay N-methyl-N-nitrosourea-induced photoreceptor death.30 Furthermore, inhibition of HSP90, a negative regulator of HSF1 activation and of HSP70 expression, leads to improved photoreceptor survival and visual function in a rat model with rhodopsin P23H mutation.31 Exogenous expression of HSP70 and HSF1 are also suggested to significantly improve the survival of injured retinal ganglion cells.32,33 Along these lines, HSP70 is suggested to play a protective role in the retina, especially in stressed photoreceptors, in response to either exogenous stress stimuli or inherited mutations.29–32,34
Here we tested the hypotheses that compromised HSP70 expression precedes photoreceptor death in IRDs and that expression of exogenous HSP70 could extend the survival of mutant photoreceptors. We first assessed the expression of HSP70 transcripts in RNA-seq data from rods and S-cone-like photoreceptors35 compared with whole retina36 and then examined HSP70 protein levels in several retinal degeneration mouse models. We augmented HSP70 expression in the photoreceptors of RHOT17M, Rpgrip1−/−, and rd10 mice by crossing them with a transgenic mouse line, TgCrx-Hspa1a-Flag, that overexpresses HSP70. Our studies demonstrate divergent effects of HSP70 expression in degenerating photoreceptors carrying different mutations and highlight the importance of distinct approaches when targeting cellular proteostasis for therapy.
Methods
Mice
Mice were reared under ∼200 lux white, fluorescent cyclic (12 hr/12 hr light/dark cycle) light conditions following recommendations of the Guide for the Care and Use of Laboratory Animals, the Institute of Laboratory Animal Resources, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. All protocols were approved by the Animal Care and Use Committee of the National Eye Institute (NEI ASP# 650). Animals were fed NIH-31 Open Formula Mouse Diet (7017) (ENVIGO, Frederick, MD, USA). The following retinal degeneration mouse lines were used: TgRHOT17M37 (referred to as RHOT17M), Rpgrip1tm1Tili38 (Rpgrip1−/−), and Pde6brd10/rd10 (rd10) from Jackson Laboratory (Bar Harbor, ME, USA). Other mouse models include: C57BL/6 (wild-type), Pde6brd1/rd1(rd1), Cep290rd16/rd16(rd16),39 Prph2rd2/rd2(rds) (Jackson Laboratory), and Nrl−/−;Nrl-GFP (Nrl−/−).40 Both male and female mice were used in this study.
Generation of TgCrxp-Hspa1a-Flag Mice
A 2.3 kb mouse cone-rod homeobox (Crx) promoter (from −2286 to +72) and the Hspa1a-coding region with an additional Kozak sequence and FLAG-tag were cloned into a modified promoter-less pCl vector (Promega, Madison, WI, USA). The Crx:Hspa1a-Flag fragment was purified and injected into fertilized FVB/N mouse oocytes. Transgenic founder mice and their progeny were identified by PCR. The founders were backcrossed to C57BL/6J mice to establish a stable colony. TgCrxp-Hspa1a-Flag mice are referred to as TgHspa1a+, whereas littermate controls without the transgene are called TgHspa1a-.
Histology of Retinal Cross-Sections
Eyes were enucleated and marked on dorsal side. Eyeballs were fixed in 4% glutaraldehyde followed by 4% paraformaldehyde, embedded in methacrylate and sectioned at 1-µm thickness along the dorsal-ventral axis. Pictures of hematoxylin and eosin (H&E)-stained retinal sections were taken at 20X and 40X magnifications. Outer nuclear layer (ONL) thickness was determined from sections displaying optic nerve head. Only the dorsal half was measured by taking nine measurements at equal spacing from the optic nerve head to the periphery on each retinal section.
Immunoblotting and Immunofluorescence Microscopy
Whole retina was homogenized in radioimmunoprecipitation assay buffer. Retinal proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked with StartingBlock (Thermo Fisher, Rockford, IL, USA) for 1 hour at room temperature and incubated overnight at 4°C with the primary antibody. The following primary antibodies were used: mouse anti-HSP70 (1:1000, Cat. #ADI-SPA-810; Enzo, Farmingdale, NY, USA), rabbit anti-FLAG (1:3000, Cat. #2368; Cell Signaling, Danvers, MA, USA), and mouse anti-β-actin (1:2000, Cat. #MABT825; Millipore Sigma, St. Louis, MO, USA). Blots were washed three times using Tris-buffered saline with Tween-20 (137 mM sodium chloride, 20 mM Tris, 0.1% Tween-20, pH 7.6) and incubated with horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse secondary antibody (Jackson Immunoresearch, West Grove, PA, USA) for 1 hour at room temperature. The membranes were developed using SuperSignal West Pico or Femto Chemiluminescent substrate (Thermo Fisher).
For immunofluorescence, eyes were enucleated and fixed in 4% paraformaldehyde for 30 minutes, cryo-preserved through a series of sucrose gradients, cryo-sectioned at 10-µm thickness, and processed as previously described.41 Primary antibodies were used at the following dilutions: rabbit anti-FLAG (1:600, Cat. #2368; Cell Signaling), mouse anti-HSP70 (1:200, Cat. #ADI-SPA-810; Enzo), mouse anti-rhodopsin (1:600, Cat. #MAB5356; Millipore Sigma), rabbit anti-calreticulin (1:400, Cat. #12238; Cell Signaling), and rabbit anti-PDE6B (1:200; Dr. Tiansen Li). Secondary antibodies conjugated with Alexa Fluor 488 or 594 (Thermo Fisher) were used at a dilution of 1:1000. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Images were acquired using a Zeiss 700 confocal microscope (Carl Zeiss Meditec, Dublin, CA, USA).
Electroretinography (ERG)
ERG recordings were performed with a computer-based system (E2, Diagnosys LLC, Lowell, MA, USA), using flashes produced with LEDs or Xenon bulbs. Mice were dark-adapted overnight and anesthetized with an intraperitoneal injection of ketamine (80 mg/Kg) and xylazine (8 mg/Kg). Pupils were dilated with topical phenylephrine (2.5%) and tropicamide (0.5%). Corneal ERGs were recorded from both eyes using gold wire loop electrodes with a drop of 2.5% hypromellose ophthalmic demulcent solution. Reference was taken with a gold wire loop placed in the mouth. For dark-adapted ERG, mice were stimulated with flashes of increasing light intensity (from 0.0001–1000 cd.s.m−2). Responses were computer-averaged and recorded at 3 to 60 second intervals depending on the stimulus intensity. For light-adapted ERG, mice were light-adapted for 2 minutes and were stimulated with flashes (from 0.3–100 cd.s.m−2) in the presence of a white 32 cd.m−2 rod-suppressing background.
Results
HSP70 Expression in the Developing and Degenerating Retina
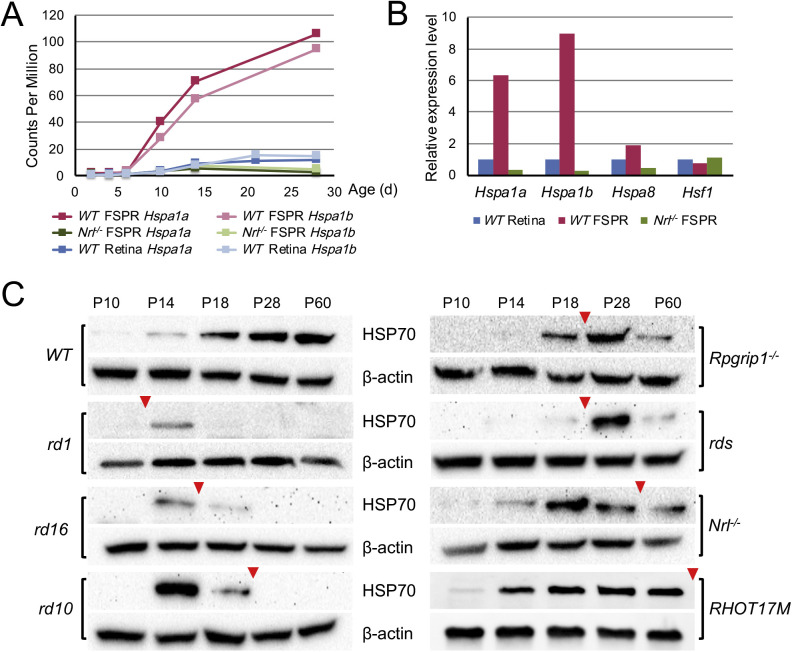
We previously reported developmental transcriptome profiles of rods (flow sorted from Nrl-GFP mice) and S-cone-like photoreceptors (flow sorted from Nrl−/−;Nrl-GFP mice) and of whole retinas.35,36,42 Focusing on chaperone genes, we noticed that expression of the two HSP70 transcripts, Hspa1a and Hspa1b, in rods progressively increased from postnatal day 2 (P2) to P28 (Fig. 1A). Compared with an even distribution of the transcription factor Hsf1, expression of the cognate Hspa8 was somewhat enriched in rods at P28 (Fig. 1B). Notably, Hspa1a and Hspa1b showed dramatically higher expression in rods at P28 compared with S-cone-like photoreceptors and the whole retina (Fig. 1B). Accordingly, HSP70 protein increased in temporal correlation with maturation of the retina (Fig. 1C). Immunoblotting of retinal extracts from seven mouse models (rd1, rd16, rd10, Rpgrip1−/−, rds, Nrl−/−, and RHOT17M) revealed transient expression of HSP70 at early/mid stage of degeneration, except in the RHOT17M retina (Fig. 1C). The timing of HSP70 expression correlated with the severity of disease. In mouse models with a relatively fast degeneration (rd1, rd16, and rd10), HSP70 expression was observed at P14, whereas relatively slow mouse models (Rpgrip1−/−, rds, and Nrl−/−) revealed later expression from P18 to P28. We also noted that HSP70 expression dramatically decreased during the progression of photoreceptor degeneration in the earlier mentioned six models. Distinctively, HSP70 expression was evident at P14 in a slow degeneration model RHOT17M and persisted as the degeneration progressed. In contrast, we observed a stable temporal expression of the constitutively expressed cognate HSC70 and HSP90, a member of the 90-kDa subfamily, in degenerating retinas (data not shown).
Figure 1.
HSP70 expression in developing and degenerating retina. (A) Expression level of the two transcripts of HSP70, Hspa1a and Hspa1b, during retina/photoreceptor maturation from P2 to P28, determined by RNAseq in flow sorted photoreceptors (FSPR) from Nrl-GFP mice (WT FSPR) or Nrl−/− mice (Nrl−/− FSPR) and in whole Nrl-GFP (WT) retinas. Each data point represents the average of two measurements (n = 2). (B) Relative expression levels of Hspa1a and Hspa1b at P28 in FSPR from Nrl-GFP mice or Nrl−/− mice compared with that in whole Nrl-GFP retinas. Also shown are relative expression levels of two other HSP family related genes Hspa8 and Hsf1. (C) Protein level of HSP70 in whole retinas of wild-type (Nrl-GFP) and seven retinal degeneration models, rd1, rd16, rd10, Rpgrip1−/− rds, Nrl−/−, and RHOT17M, at a series of ages from P10 to 2 months. Arrowheads indicate the time point in each model when approximately 50% of photoreceptors are still alive.
Generation and Characterization of TgCrx-Hspa1a-Flag Mice
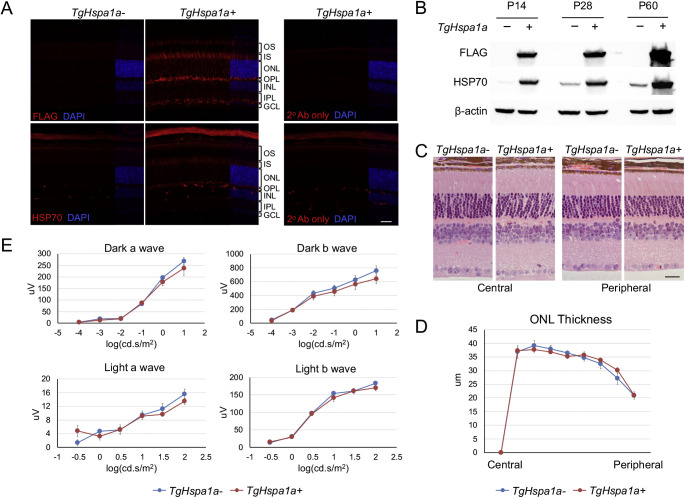
To test the potential neuroprotective effect of HSP70, we generated a transgenic mouse line with augmented HSP70 expression in both rod and cone photoreceptors, controlled by a 2.3 kb mouse Crx promoter. The TgCrx-Hspa1a-Flag (referred to as TgHspa1a+) mouse expresses a FLAG-tagged full-length HSP70 protein. Immunofluorescent labeling of FLAG on TgHspa1a+ retina revealed transgene expression in photoreceptors with strong signals in the inner segment (IS) and synaptic terminals (Fig. 2A). Weaker staining of FLAG was observed in inner plexiform layer, possibly resulting from Crx promoter activity in bipolar cells43 (Fig. 2A). Robust expression of HSP70 protein driven by the transgene was detected by immunoblotting in P14 TgHspa1a+ retinas, and the expression gradually increased at P28 and P60 (Fig. 2B). Eight-month old TgHspa1a+ mice displayed normal retinal morphology (Fig. 2C) with no difference in ONL thickness between TgHspa1a+ (n = 4) and TgHspa1a- (n = 5) littermates (Fig. 2D). ERG response (a- and b-wave amplitude) showed no change between TgHspa1a+ (n = 3) and TgHspa1a- (n = 6) mice in dark- or light-adapted conditions (Fig. 2E). Thus augmented expression of HSP70 does not alter the development, survival, or function of wild-type photoreceptors.
Figure 2.
Augmented expression of HSP70 from TgCrx-Hspa1a-Flag transgene in wild-type retina. (A) Immunofluorescent labeling of FLAG and HSP70 in retinal cross-sections from TgHspa1a+ mice and TgHspa1a- littermates at P28. (B) Immunoblots showing elevated expression of HSP70 protein driven by the TgCrx-Hspa1a-Flag transgene at P14, P28, and P60. (C) Representative picture of H&E stained retinal cross-sections, and (D) plot of ONL thickness measurements from TgHspa1a+ (n = 4) and TgHspa1a- littermates (n = 5) at 8 months. (E) ERG response in TgHspa1a+ (n = 3) and TgHspa1a- littermates (n = 6) at 8 months. Scale bar for all panels: 25 μm. Error bar: SEM. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Improved Photoreceptor Survival but not ERG Response by Augmented HSP70 Expression in RHOT17M Retina
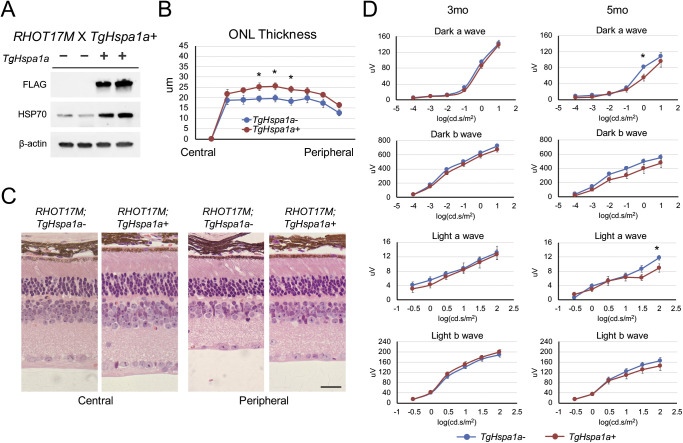
TgHspa1a+ mice were crossed to RHOT17M mice, which express a class II mutant rhodopsin displaying defects in stability and subcellular localization.37,44 RHOT17M mice exhibit slow degeneration with complete photoreceptor loss by 8 months of age.37 Immunoblotting confirmed robust expression of HSP70 from the transgene in 3-month-old RHOT17M;TgHspa1a+ retinas (Fig. 3A). A marginal but significant increase in ONL thickness was observed from center to periphery in cross-sections of 5-month-old RHOT17M;TgHspa1a+ (n = 8) retina compared with littermate controls (n = 6) (Figs. 3B, 3C). An increase in inner nuclear layer and inner plexiform layer thickness was also evident. However, transgene expression in mutant retina did not translate into a significant improvement of scotopic or photopic ERG amplitudes (Fig. 3D).
Figure 3.
Augmented expression of HSP70 in RHOT17M mice. (A) Immunoblots confirm the expression of TgCrx-Hspa1a-Flag transgene and elevated total HSP70 protein level in RHOT17M mice at 3 months of age. (B) Marginally increased ONL thickness was observed in RHOT17M; TgHspa1a+ (n = 8) mice at 5 months of age when compared with RHOT17M; TgHspa1a- (n = 6) littermates. (C) Representative pictures of central and peripheral region of H&E stained retinal cross-sections from RHOT17M;TgHspa1a- and RHOT17M;TgHspa1a+ mice at 5 months. (D) Plots of scotopic and photopic ERG amplitudes of RHOT17M; TgHspa1a- (n = 16 and 11, respectively) and RHOT17M; TgHspa1a+ (n = 16 and 9, respectively) mice at 3 and 5 months. Scale bar: 25 μm for all panels. Error bar: SEM. Student's t-test, *P < 0.05.
No Effect on Photoreceptor Survival but Earlier Impairment of ERG Response by Augmented HSP70 Expression in Rpgrip1−/− Retina
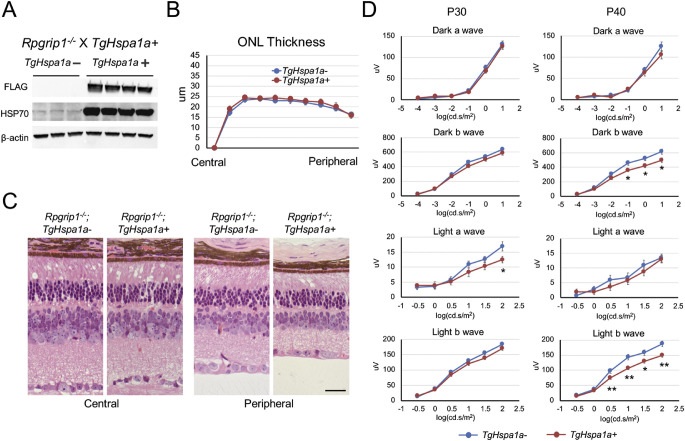
TgHspa1a+ mice were crossed to Rpgrip1−/− mice, which exhibit a ciliary defect with a moderate degeneration rate, showing near complete photoreceptor death at 3 months of age.38 Immunoblotting confirmed robust expression of HSP70 from the transgene in P40 Rpgrip1−/−;TgHspa1a+ retina (Fig. 4A). No difference in ONL thickness was detected between Rpgrip1−/−;TgHspa1a- (n = 7) and Rpgrip1−/−;TgHspa1a+ (n = 8) retina (Figs. 4B, 4C). Scotopic and photopic ERG amplitudes were not significantly different at P30 (Fig. 4D, left panels) but showed reduction at P40 in mice expressing the transgene (Fig. 4D, right panels).
Figure 4.
Augmented expression of HSP70 in Rpgrip1−/− mice. (A) Immunoblots confirm the expression of TgCrx-Hspa1a-Flag transgene and elevated total HSP70 protein level in Rpgrip1−/− mice at P40. (B) ONL thickness is not different between Rpgrip1−/−; TgHspa1a- (n = 7) and Rpgrip1−/−; TgHspa1a+ (n = 8) mice at P40. (C) Representative picture of central and peripheral region of H&E stained retinal cross-sections from Rpgrip1−/−; TgHspa1a- and Rpgrip1−/−; TgHspa1a+ mice at P40. (D) Plots of scotopic and photopic ERG amplitudes of Rpgrip1−/−; TgHspa1a- (n = 14), and Rpgrip1−/−; TgHspa1a+ (n = 18) mice at P30 and P40. Scale bar: 25 μm for all panels. Error bar: SEM. Student's t-test, *P < 0.05, **P < 0.01.
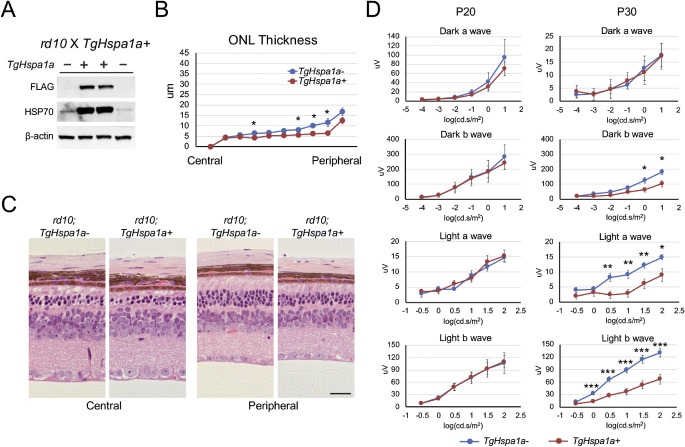
Accelerated Photoreceptor Degeneration by Augmented HSP70 Expression in rd10 Retina
TgHspa1a+ mice were crossed to rd10 mice, which carry a missense mutation in the Pde6b gene and display relatively fast degeneration with complete photoreceptor loss at 2 months.45 Robust expression of HSP70 from the transgene was detected by immunoblotting in P20 rd10;TgHspa1a+ retinas (Fig. 5A). More severe degeneration was observed in the peripheral retina of rd10;TgHspa1a+ (n = 5) mice at P30 compared with rd10;TgHspa1a- (n = 5) littermates (Figs. 5B, 5C). At P30, and not earlier, we observed significantly reduced scotopic b-wave amplitude and dramatically reduced photopic a- and b-wave amplitudes in mice expressing the transgene (Fig. 5D).
Figure 5.
Augmented expression of HSP70 in rd10 mice. (A) Immunoblots confirm the expression of TgCrx-Hspa1a-Flag transgene and elevated total HSP70 protein level in rd10 mice at P20. (B) Decreased ONL thickness, more pronounced in the retinal periphery, was observed in rd10; TgHspa1a+ (n = 5) mice at P30 when compared with rd10; TgHspa1a- (n = 5) littermates. (C) Representative picture of central and peripheral region of H&E stained retinal cross-sections from rd10;TgHspa1a- and rd10;TgHspa1a+ mice at P30. (D) Plots of scotopic and photopic ERG amplitudes of rd10; TgHspa1a- (n = 5 and 10) and rd10; TgHspa1a+ (n = 10 for both ages) mice at P20 and P30, respectively. Scale bar: 25 μm for all panels. Error bar: SEM. Student's t-test, *P < 0.05, **P < 0.01, ***P < 0.001.
Altered Rhodopsin and PDE6B Expression with Augmented HSP70 Expression in RHOT17M and rd10 Retina
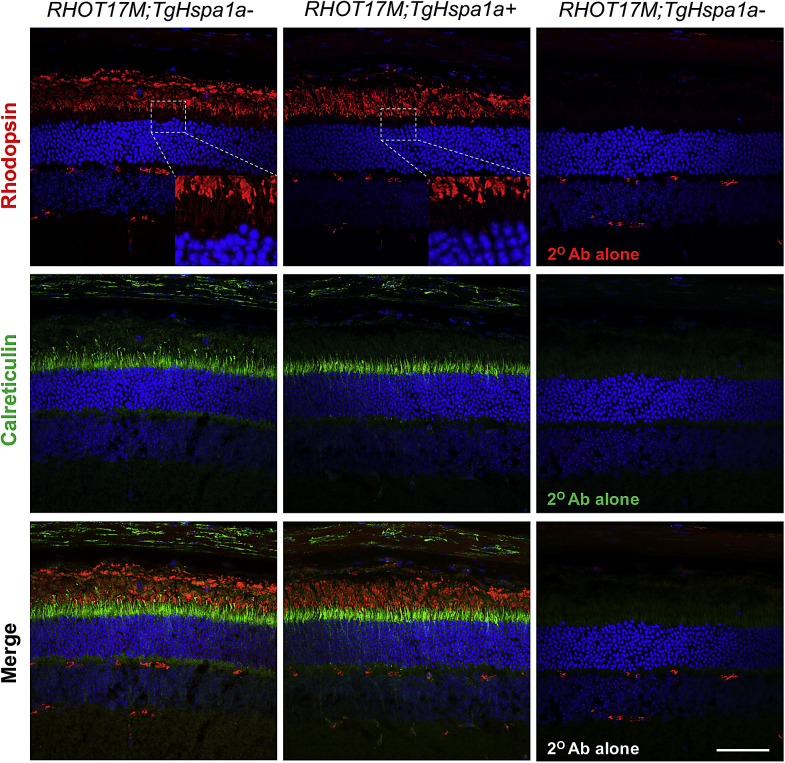
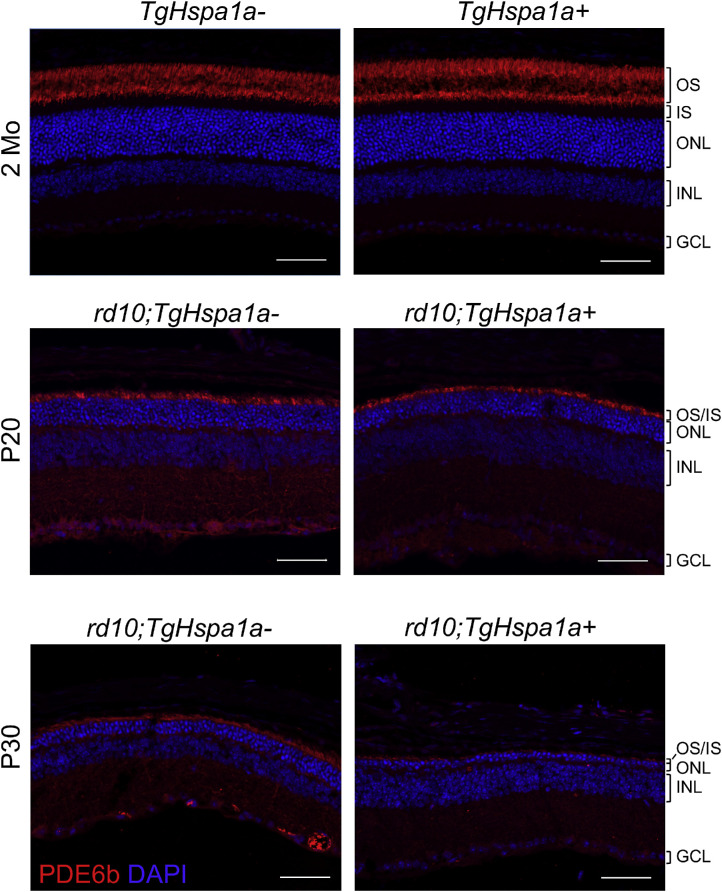
Immunolabeling revealed robust staining of rhodopsin in the OS of 3-month-old RHOT17M;TgHspa1a- and RHOT17M;TgHspa1a+ retinas (Fig. 6). We observed weaker staining of rhodopsin in the IS area (marked by calreticulin) in RHOT17M;TgHspa1a+ retina (Fig. 6 insert box), suggesting reduced retention of mutant rhodopsin. High expression of HSP70 in 2-month-old retina under a nondegeneration background (C57BL/6) does not affect expression or localization of PDE6B protein (Fig. 7). In the rd10 retinas, PDE6B expression level at P20 is comparable with or without augmented HSP70 expression, whereas reduced staining of PDE6B was detected in P30 rd10;TgHspa1a+ mice compared with controls (Fig. 7).
Figure 6.
Reduced rhodopsin retention in IS of RHOT17M; TgHSP70+ retinas. Immunofluorescent labeling of rhodopsin and calreticulin in retinal cross-sections from 3-month-old RHOT17M; TgHspa1a- versus RHOT17M; TgHspa1a+ mice. Scale bar: 50 μm for all panels.
Figure 7.
Expression of PDE6B is reduced in rd10; TgHSP70+ retinas. Immunofluorescent labeling of PDE6b in retinal cross-sections from 2-month-old TgHspa1a- versus TgHspa1a+ mice and rd10;TgHspa1a- vs rd10; TgHspa1a+ mice at P20 and P30. Scale bar: 50 μm for all panels. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Discussion
High turnover of proteins makes the photoreceptors especially vulnerable to disruptions of proteostasis, a feature commonly observed in neurodegenerative diseases. Several retinal disease-causing mutations produce misfolded or mistargeted proteins, accumulation of which can trigger cellular responses that eventually lead to demise of the photoreceptors.46,47 Thus manipulation of chaperone system and UPR pathways offers an attractive, potential therapeutic strategy to treat retinal degeneration irrespective of the original mutation. The HSP family of proteins control many aspects of cellular proteostasis including nascent protein folding, trafficking to organelles, repair of damaged proteins, and assembly of multiprotein complexes. In doing so, they promote cellular survival and longevity against proteotoxic stress implying a likely role in neuroprotection; yet there is still no detailed investigation of their therapeutic effects in retinal degenerative conditions.
In this study, we observed transient high expression of HSP70 protein as a common feature in early stages of degenerating retinas. Unexpectedly, augmented HSP70 expression through a transgenic approach manifested divergent effects in the three retinal degeneration mouse models we tested. A protective effect on augmentation of HSP70 expression was only attained in the RHOT17M mice. Rhodopsin mutations can be classified into several subgroups based on the mutant protein's structure, stability, function, and intracellular localization.48,49 T17M rhodopsin at low concentration displays altered post-translational modification and reduced stability; but the majority can fold well enough to exit endoplasmic reticulum (ER) and translocate to the OS.49 In the RHOT17M mice and in transiently transfected cell cultures, T17M rhodopsin manifests reduced thermal stability, resulting in partially misfolded proteins and moderate ER retention, lacking full ability of regeneration with the chromophore 11-cis-retinal, thereby failing to integrate in the plasma membrane.37,49,50 Induction of ER stress was reported in RHOT17M retinas,51 and manipulating ER stress-related UPR pathway could improve photoreceptor survival in RHOT17M mice.52,53 Previous studies examined different methods to improve chaperone activity in treating retinal degeneration related to rhodopsin aggregation.49,54 In this study, improvement in ONL thickness indicated protective effects in the RHOT17M retina with augmented HSP70 expression. Reduced retention of mutant rhodopsin in the IS suggests that HSP70 facilitates folding and translocation of mutant rhodopsin to the OS. Further studies are however needed to elucidate the precise mechanism of HSP70's protective effect, with quantitative analysis of the changes in mutant opsin localization to the OS versus retention at ER or degradation at proteosome.
In Rpgrip1−/− and rd10 mice, augmented HSP70 expression did not demonstrate a protective effect; instead, we observed an accelerated degeneration in the rd10 retina and no apparent impact in the Rpgrip1−/− retina. RPGRIP1 functions as a docking site for ciliary proteins at the base of the connecting cilium and likely facilitates trafficking of nascent proteins from the IS into the OS.38,55–57 With loss of RPGRIP1, RPGR fails to anchor at the base of the connecting cilium and the proper transport and localization of rod and cone opsin are affected.38 Enhancing the chaperone system does not rescue the phenotype, and no protective effect was observed. The rd10 mice carry a missense mutation in the gene encoding β-subunit of cGMP phosphodiesterase 6 (Pde6b).45 The rd10 allele produces a hypomorphic PDE6B that is partially defective in assembling into the PDE6αβγ2 holoenzyme with decreased transport to the OS, leading to higher free cGMP, increased channel opening and Ca2+ influx, and rapid photoreceptor death.58–60 We were intrigued by the accelerated photoreceptor degeneration in rd10 retina by HSP70 overexpression. The mutant PDE6B is expressed at low levels in rd10 retina, exhibits residual catalytic activity, and is partially mislocalized, consistent with a misfolding defect.58 Increased expression of HSP70 would enhance the quality control mechanisms, which target misfolded proteins,61 as evident by decreased staining of PDE6B in rd10;TgHspa1a+ mice (Fig. 7). Photoreceptor specific chaperone AIPL127,28 directly interacts with HSP70 and HSP90,25,62 forming a chaperone heterocomplex that regulates biosynthesis, stability, and translocation of PDE6 holoenzyme.26 HSP70 and HSP90 may compete for binding to the client protein PDE6B. We suggest that overexpression of one arm of the chaperone heterocomplex may lead to imbalance of the system, enhancing PDE6B degradation and accelerating photoreceptor degeneration. Thus augmenting chaperone activity might have limited or no effect when dealing with protein-null defects such as Rpgrip1−/−, and overstimulating the chaperone machinery may accelerate degeneration in specific cases such as rd10 mice.
It is unclear why improved rod survival in RHOT17M mice was not accompanied by a corresponding improvement in dark-adapted ERG amplitudes. HSP70 may assist mutant rhodopsin folding and/or trafficking to the correct subcellular compartment (i.e., the OS). Although likely relieving ER stress and thereby promoting cell survival, increased mutant rhodopsin in the OS would interfere with phototransduction. Misfolded mutant rhodopsin that fails to fully regenerate with the chromophore can stimulate phototransduction via transducin activation in the absence of light and reduce rods sensitivity because full dark adaptation is not achievable, as if they are constantly exposed to light.49 We hypothesize that increased trafficking of mutant rhodopsin to the OS may at least in part account for the lack of ERG improvement in this model. Surprisingly, we observed drastically reduced photopic a- and b-wave amplitude in rd10 mice at P30 and significantly reduced photopic b-wave amplitude in Rpgrip1−/− mice at P40 with augmented HSP70 expression, suggesting compromised cone function or survival. As the TgHspa1a transgene has no deleterious effect on cone function and survival in wild-type background, a likely scenario in rd10 retina might be that accelerated rod decline secondarily impacted cone viability.
Conclusions
Members of HSP70 family usually work together with HSP40 (DNAJ), HSP90, and HSP110 family co-chaperones, forming a large and complex chaperone network in maintaining cellular proteostasis. DNAJs, which regulate HSP70's function as co-chaperones, play an important role in suppressing disease-causing protein aggregations.63 DNAJB6b and DNAJB8 are shown to be superior suppressors than HSP70 in treating aggregates associated with polyglutamine expansion.64 Thus HSP70 may not be the only or best candidate for therapies targeting the chaperone system. We had previously demonstrated retinal expression of the small HSP α-crystallin, traditionally believed to localize in the ocular lens.65 Expression of α-crystallin is elevated in diseased retina where it may play a protective role.66,67 In some cases, augmentation of HSP function can be a double-edged sword, and augmentation of different HSPs might result in unintended consequences. A member of the 90 KDa family, HSP90α, predominantly expressed in the retina and brain,68 has also been targeted for treating retinal degeneration.69 Although inhibition of HSP90 protected dying photoreceptors in vivo,31,70 prolonged HSP90 inhibition led to photoreceptor death in beagle dogs,71 and HSP90α-deficient mice displayed retinal degeneration.68 Given that HSP70 augmentation in our studies showed distinct impact on degenerating photoreceptors from different mutants, we suggest that a better understanding of the chaperone machinery and especially HSPs in degenerating retinas is required and that various chaperone candidates should be considered when developing treatment paradigms focused on photoreceptor protein homeostasis.
Acknowledgments
The authors thank the University of Michigan transgenic core facility for generating TgCrx-Hspa1a-Flag mice; Megan Kopera and Yide Mi for maintaining the animal colony; Jessica Gumerson and Robert Fariss for advice in immunostaining and microscopy; Haohua Qian and NEI Visual Function Core for assistance in ERG; and NEI histology core for histology of retina samples.
Supported by the Intramural Research Program of the National Eye Institute (EY00450, EY000546). The authors alone are responsible for the content and writing of the article.
Disclosure: K. Jiang, None; E. Fairless, None; A. Kanda, None; N. Gotoh, None; T. Cogliati, None; T. Li, None; A. Swaroop, None
References
- 1. Retina Information Network (RetNet) Houston, TX: University of Texas-Houston Health Science Center. Available at: https://sph.uth.edu/retnet. Accessed June, 2020. [Google Scholar]
- 2. Chapple JP, Grayson C, Hardcastle AJ, Saliba RS, van der Spuy J, Cheetham ME. Unfolding retinal dystrophies: a role for molecular chaperones? Trends Mol Med. 2001; 7: 414–421. [DOI] [PubMed] [Google Scholar]
- 3. Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010; 33: 441–472. [DOI] [PubMed] [Google Scholar]
- 4. Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010; 11: 273–284. [DOI] [PubMed] [Google Scholar]
- 5. Gorbatyuk M, Gorbatyuk O. Review: retinal degeneration: focus on the unfolded protein response. Mol Vis. 2013; 19: 1985–1998. [PMC free article] [PubMed] [Google Scholar]
- 6. Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006; 125: 443–451. [DOI] [PubMed] [Google Scholar]
- 7. Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016; 353: aac4354. [DOI] [PubMed] [Google Scholar]
- 8. Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007; 581: 3702–3710. [DOI] [PubMed] [Google Scholar]
- 9. Kampinga HH, Hageman J, Vos MJ, et al.. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009; 14: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012; 136: 354–374. [DOI] [PubMed] [Google Scholar]
- 11. Doyle SM, Genest O, Wickner S. Protein rescue from aggregates by powerful molecular chaperone machines. Nat Rev Mol Cell Biol. 2013; 14: 617–629. [DOI] [PubMed] [Google Scholar]
- 12. Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016; 21: 379–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019; 20: 665–680. [DOI] [PubMed] [Google Scholar]
- 14. Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010; 11: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J. The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem. 2015; 78: 243–273. [DOI] [PubMed] [Google Scholar]
- 16. Genest O, Wickner S, Doyle SM. Hsp90 and Hsp70 chaperones: collaborators in protein remodeling. J Biol Chem. 2019; 294: 2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edkins AL. CHIP: a co-chaperone for degradation by the proteasome. Subcell Biochem. 2015; 78: 219–242. [DOI] [PubMed] [Google Scholar]
- 18. Quintana-Gallardo L, Martin-Benito J, Marcilla M, Espadas G, Sabido E, Valpuesta JM. The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Sci Rep. 2019; 9: 5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014; 15: 233–249. [DOI] [PubMed] [Google Scholar]
- 20. Smith HL, Li W, Cheetham ME. Molecular chaperones and neuronal proteostasis. Semin Cell Dev Biol. 2015; 40: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindberg I, Shorter J, Wiseman RL, Chiti F, Dickey CA, McLean PJ. Chaperones in neurodegeneration. J Neurosci. 2015; 35: 13853–13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015; 55: 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt CR, Dix DJ, Sharma GG, et al.. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004; 24: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosmaoglou M, Schwarz N, Bett JS, Cheetham ME. Molecular chaperones and photoreceptor function. Prog Retin Eye Res. 2008; 27: 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sacristan-Reviriego A, van der Spuy J. The Leber congenital amaurosis-linked protein AIPL1 and its critical role in photoreceptors. Adv Exp Med Biol. 2018; 1074: 381–386. [DOI] [PubMed] [Google Scholar]
- 26. Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, van der Spuy J. The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Invest Ophthalmol Vis Sci. 2008; 49: 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramamurthy V, Niemi GA, Reh TA, Hurley JB. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci USA 2004; 101: 13897–13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Bulgakov OV, Wen XH, et al.. AIPL1, the protein that is defective in Leber congenital amaurosis, is essential for the biosynthesis of retinal rod cGMP phosphodiesterase. Proc Natl Acad Sci USA. 2004; 101: 13903–13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kayama M, Nakazawa T, Thanos A, et al.. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am J Pathol. 2011; 178: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koriyama Y, Sugitani K, Ogai K, Kato S. Heat shock protein 70 induction by valproic acid delays photoreceptor cell death by N-methyl-N-nitrosourea in mice. J Neurochem. 2014; 130: 707–719. [DOI] [PubMed] [Google Scholar]
- 31. Aguila M, Bevilacqua D, McCulley C, et al.. Hsp90 inhibition protects against inherited retinal degeneration. Hum Mol Genet. 2014; 23: 2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwong JM, Gu L, Nassiri N, et al.. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene Ther. 2015; 22: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu W, Xia F, Ha Y, et al.. Neuroprotective effects of HSF1 in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2019; 60: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Athanasiou D, Aguila M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. The cell stress machinery and retinal degeneration. FEBS Lett. 2013; 587: 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JW, Yang HJ, Brooks MJ, et al.. NRL-regulated transcriptome dynamics of developing rod photoreceptors. Cell Rep. 2016; 17: 2460–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brooks MJ, Chen HY, Kelley RA, et al.. Improved retinal organoid differentiation by modulating signaling pathways revealed by comparative transcriptome analyses with development in vivo. Stem Cell Reports. 2019; 13: 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li T, Sandberg MA, Pawlyk BS, et al.. Effect of vitamin A supplementation on rhodopsin mutants threonine-17 –>methionine and proline-347 –>serine in transgenic mice and in cell cultures. Proc Natl Acad Sci USA. 1998; 95: 11933–11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y, Hong DH, Pawlyk B, et al.. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci USA. 2003; 100: 3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang B, Khanna H, Hawes N, et al.. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006; 15: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mears AJ, Kondo M, Swain PK, et al.. Nrl is required for rod photoreceptor development. Nat Genet. 2001; 29: 447–452. [DOI] [PubMed] [Google Scholar]
- 41. Assawachananont J, Kim SY, Kaya KD, Fariss R, Roger JE, Swaroop A. Cone-rod homeobox CRX controls presynaptic active zone formation in photoreceptors of mammalian retina. Hum Mol Genet. 2018; 27: 3555–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida S, Mears AJ, Friedman JS, et al.. Expression profiling of the developing and mature Nrl-/- mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum Mol Genet. 2004; 13: 1487–1503. [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Xu S, Rivolta C, et al.. Barrier to autointegration factor interacts with the cone-rod homeobox and represses its transactivation function. J Biol Chem. 2002; 277: 43288–43300. [DOI] [PubMed] [Google Scholar]
- 44. Sung CH, Schneider BG, Agarwal N, Papermaster DS, Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991; 88: 8840–8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang B, Hawes NL, Pardue MT, et al.. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007; 47: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin JH, Li H, Yasumura D, et al.. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007; 318: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lobanova ES, Finkelstein S, Skiba NP, Arshavsky VY. Proteasome overload is a common stress factor in multiple forms of inherited retinal degeneration. Proc Natl Acad Sci USA. 2013; 110: 9986–9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sung CH, Davenport CM, Nathans J. Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa. Clustering of functional classes along the polypeptide chain. J Biol Chem. 1993; 268: 26645–26649. [PubMed] [Google Scholar]
- 49. Athanasiou D, Aguila M, Bellingham J, et al.. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog Retin Eye Res. 2018; 62: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang H, Xiong S, Xia X. Retinitis pigmentosa-associated rhodopsin mutant T17M induces endoplasmic reticulum (ER) stress and sensitizes cells to ER stress-induced cell death. Mol Med Rep. 2014; 9: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 51. Kunte MM, Choudhury S, Manheim JF, et al.. ER stress is involved in T17M rhodopsin-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 3792–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choudhury S, Nashine S, Bhootada Y, et al.. Modulation of the rate of retinal degeneration in T17M RHO mice by reprogramming the unfolded protein response. Adv Exp Med Biol. 2014; 801: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhootada Y, Kotla P, Zolotukhin S, et al.. Limited ATF4 expression in degenerating retinas with ongoing ER stress promotes photoreceptor survival in a mouse model of autosomal dominant retinitis pigmentosa. PLoS One. 2016; 11: e0154779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Griciuc A, Aron L, Ueffing M. ER stress in retinal degeneration: a target for rational therapy? Trends Mol Med. 2011; 17: 442–451. [DOI] [PubMed] [Google Scholar]
- 55. Hong DH, Yue G, Adamian M, Li T. Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem. 2001; 276: 12091–12099. [DOI] [PubMed] [Google Scholar]
- 56. Won J, Gifford E, Smith RS, et al.. RPGRIP1 is essential for normal rod photoreceptor outer segment elaboration and morphogenesis. Hum Mol Genet. 2009; 18: 4329–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012; 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang T, Reingruber J, Woodruff ML, et al.. The PDE6 mutation in the rd10 retinal degeneration mouse model causes protein mislocalization and instability and promotes cell death through increased ion influx. J Biol Chem. 2018; 293: 15332–15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barhoum R, Martinez-Navarrete G, Corrochano S, et al.. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008; 155: 698–713. [DOI] [PubMed] [Google Scholar]
- 60. Sundar JC, Munezero D, Bryan-Haring C, Saravanan T, Jacques A, Ramamurthy V. Rhodopsin signaling mediates light-induced photoreceptor cell death in rd10 mice through a transducin-independent mechanism. Hum Mol Genet. 2020; 29: 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fernandez-Fernandez MR, Gragera M, Ochoa-Ibarrola L, Quintana-Gallardo L, Valpuesta JM. Hsp70–a master regulator in protein degradation. FEBS Lett. 2017; 591: 2648–2660. [DOI] [PubMed] [Google Scholar]
- 62. Yadav RP, Majumder A, Gakhar L, Artemyev NO. Extended conformation of the proline-rich domain of human aryl hydrocarbon receptor-interacting protein-like 1: implications for retina disease. J Neurochem. 2015; 135: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kakkar V, Prins LC, Kampinga HH. DNAJ proteins and protein aggregation diseases. Curr Top Med Chem. 2012; 12: 2479–2490. [DOI] [PubMed] [Google Scholar]
- 64. Hageman J, Rujano MA, van Waarde MA, et al.. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell. 2010; 37: 355–369. [DOI] [PubMed] [Google Scholar]
- 65. Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis. 2003; 9: 410–419. [PubMed] [Google Scholar]
- 66. Fort PE, Lampi KJ. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res. 2011; 92: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dou G, Sreekumar PG, Spee C, et al.. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012; 53: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Y, Zheng X, Ding Y, et al.. The molecular chaperone Hsp90alpha deficiency causes retinal degeneration by disrupting Golgi organization and vesicle transportation in photoreceptors. J Mol Cell Biol. 2020; 12: 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aguila M, Cheetham ME. Hsp90 as a potential therapeutic target in retinal disease. Adv Exp Med Biol. 2016; 854: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tam LC, Kiang AS, Campbell M, et al.. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90). Hum Mol Genet. 2010; 19: 4421–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kanamaru C, Yamada Y, Hayashi S, et al.. Retinal toxicity induced by small-molecule Hsp90 inhibitors in beagle dogs. J Toxicol Sci. 2014; 39: 59–69. [DOI] [PubMed] [Google Scholar]