Abstract
Surgeons perform two primary tasks: operating and engaging patients and caregivers in shared decision-making. Human dexterity and decision-making are biologically limited. Intelligent, autonomous machines have the potential to augment or replace surgeons. Rather than regarding this possibility with denial, ire, or indifference, surgeons should understand and steer these technologies. Closer examination of surgical innovations and lessons learned from the automotive industry can inform this process. Innovations in minimally invasive surgery and surgical decision-making follow classic S-shaped curves with three phases: 1) introduction of a new technology, 2) achievement of a performance advantage relative to existing standards, and 3) arrival at a performance plateau, followed by replacement with an innovation featuring greater machine autonomy and less human influence. There is currently no level I evidence demonstrating improved patient outcomes using intelligent, autonomous machines for performing operations or surgical decision-making tasks. History suggests that if such evidence emerges, and if the machines are cost effective, then they will augment or replace humans, initially for simple, common, rote tasks under close human supervision and later for complex tasks with minimal human supervision. This process poses ethical challenges in assigning liability for errors, matching decisions to patient values, and displacing human workers, but may allow surgeons to spend less time gathering and analyzing data and more time interacting with patients and tending to urgent, critical—and potentially more valuable—aspects of patient care. Surgeons should steer these technologies toward optimal patient care and net social benefit using the uniquely human traits of creativity, altruism, and moral deliberation.
Keywords: Surgery, machine learning, artificial intelligence, innovation, automation
Introduction
Surgeons perform two major, primary tasks: conducting operations and engaging patients and caregivers in shared decision-making. Unfortunately, human dexterity and decision-making are biologically limited. Technical errors are the leading cause of preventable harm in surgical patients; diagnostic and judgement errors follow second.1 Individual surgeon skill is highly variable, fine motor dexterity degrades with age and fatigue, and technical skills affect patient outcomes.2–5 Time constraints and uncertainty impose reliance on cognitive shortcuts that lead to judgement errors, which surgeons themselves identify as the most common cause of major errors.6–8
Innovations in minimally invasive surgery and surgical decision-making have improved surgeons’ abilities to perform operations and exercise sound judgement.9–12 As technologies improve, these innovations rely less on human input and more on intelligent, autonomous machines, i.e. computer systems that learn to perform human tasks and cognitive functions with some degree of independence.13, 14 Currently, intelligent machines can perform manual tasks and make decisions with remarkable efficacy.15–18 History suggests that these abilities will continue to improve.19 If there comes a time when machines perform surgeon’s tasks with greater efficacy and lower cost, then market and patient demand may have machines assume these roles. Rather than regarding this possibility with denial, ire, or indifference, surgeons should seek to understand and steer these technologies toward optimal patient care and net social benefit.
Innovation Curves
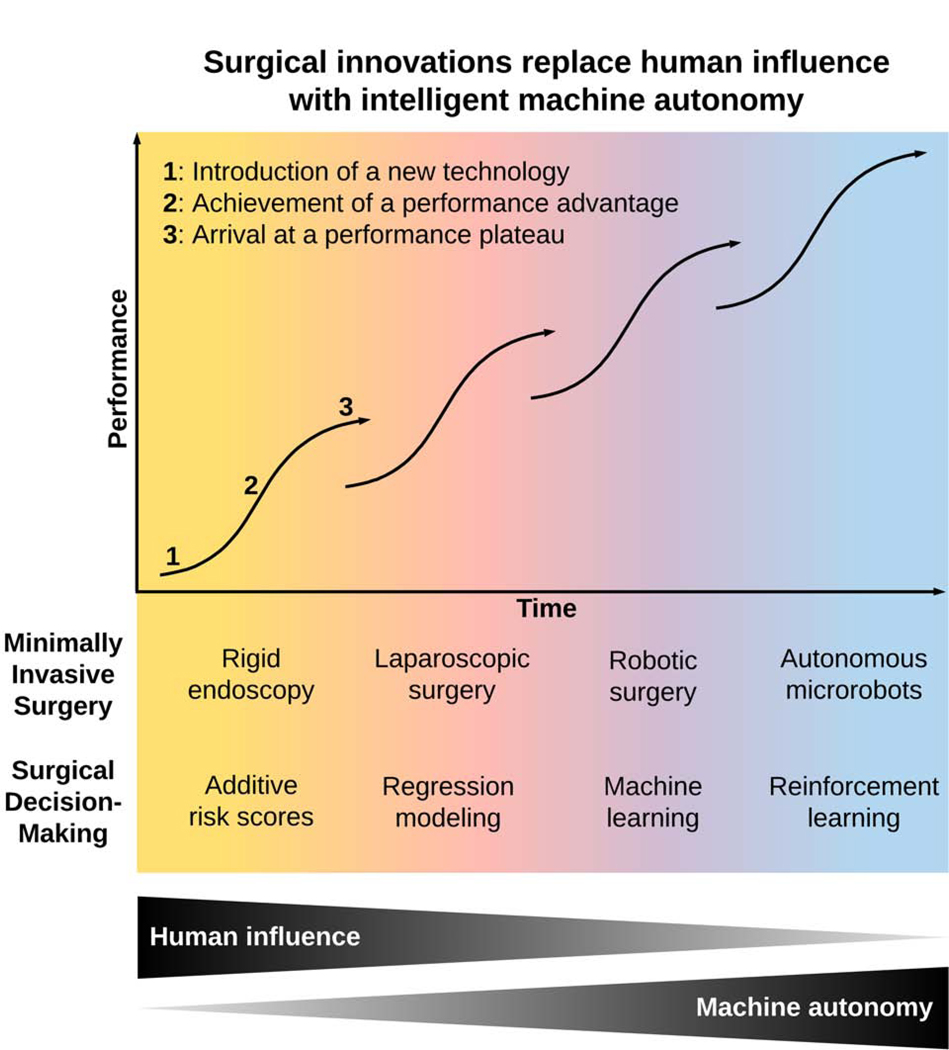
Innovations in minimally invasive surgery and surgical decision-making follow classic S-shaped curves with three phases: 1) introduction of a new technology, 2) achievement of a performance advantage relative to existing standards, and 3) arrival at a performance plateau, followed by augmentation or replacement with an innovation featuring greater machine autonomy and less human influence (Figure 1, Table 1).
Figure 1:
Past, present, and projected future innovations in surgery use progressively more computer autonomy and less human influence, augmenting or replacing previous methods once a cost-effective performance advantage is achieved with a new innovation.
Table 1:
Phases of innovation in minimally invasive surgery and surgical decision-making.
| Phases of innovation | ||||
|---|---|---|---|---|
| Surgical innovations | Introduction of new technology | Achievement of a performance advantage | Arrival at a performance plateau | |
| Minimally invasive surgery | Rigid endoscopy | Visualization of internal structures through natural orifices | Light sources, sheaths for instrument insertion | Inability to triangulate, limited working space |
| Laparoscopic Surgery | Trocar used to establish pneumoperitoneum | Improved outcomes for select procedures, higher costs than open surgery | Minimal advantages for natural orifice laparoscopy | |
| Robotic Surgery | Computed tomography-guided brain biopsy | Improved outcomes for select procedures, higher costs than laparoscopic and open surgery | Requires skin and fascial defects to insert instruments | |
| Autonomous microrobots | Ingestible robot repairs a gastric defect in five minutes | Has not yet been demonstrated | Have not yet been observed | |
| Surgical Decision-making | Additive risk scores | Single static variable thresholds can yield high sensitivity | Risk scores using multiple variables can achieve good accuracy | Can underestimate risk for adverse outcomes among high-risk patients |
| Regression modeling | Estimates relationships between inputs and outputs | Patient-specific predictions may affect preoperative risk reduction strategies | Inability to accurately represent complex, non-linear associations | |
| Machine learning | Computer learns from data rather than conforming to rules | Improved predictive accuracy, opportunities for phenotype discovery | Predictions and phenotypes indirectly inform decision-making | |
| Reinforcement learning | Recommends optimal actions for discrete choices and states | Has not yet been demonstrated | Have not yet been observed | |
Minimally Invasive Surgery Innovations
Fatigue, imprecision, and variability in technical skill can adversely affect surgeons and their patients.2–5 Technological advances in minimally invasive surgery improve surgeons’ abilities to perform manual dexterity tasks, and harbor the potential for autonomous robotic surgery.9, 10, 20, 21
Rigid Endoscopy
Endoscopy was first used to inspect the cervix more than one thousand years ago.22 Following a long period of technological stagnation, Phillip Bozzini used a wax candle to illuminate a urologic endoscope, which was branded a “toy” by his contemporaries.23 Problems with thermal injuries from light sources were overcome through use of platinum wires heated with electric currents or light sources encased in metal catheters with ice water cooling. Subsequent development of separate ocular and sheath components allowed of insertion of instruments to perform diagnostic procedures.24, 25 However, interventions were limited by the inability to triangulate instruments and vision, and the intra-abdominal contents could not be inspected. When Hans Christian Jakobaeus disseminated his work regarding the use of a trocar to establish pneumoperitoneum, the transition to laparoscopic surgery began.
Laparoscopic Surgery
Kurt Semm described laparoscopic management of gynecologic disorders in the 1970s.25 These techniques were applied to general surgery when Erich Mühe performed a laparoscopic cholecystectomy in 1985. He obtained pneumoperitoneum with a Veress needle, introduced pistol grip instruments though a large trocar with side-view optics and other small incisions, and removed the gallbladder though the large trocar.26 He was ridiculed for performing “Mickey Mouse surgery” and his technique was summarized as “small brain - small incision.”27 Philippe Mouret, another pioneer of laparoscopic cholecystectomy, remarked that he felt the “weight of medico-legal responsibility for having innovated in a classic operation, which had reached a stage of near perfection.”28 Mouret’s concerns were valid. In an early prospective observational study, the incidence of common bile duct injury was 5.5%, compared with 0–0.25% for open cholecystectomy during the same era.29 The learning curve was short and steep.30 As laparoscopic cholecystectomy gained acceptance and adoption, its safety and efficacy improved, as evidenced by decreased mortality, pneumonia, wound infection, and hospital length of stay.9 However, attempts to make further clinically significant improvements on modern laparoscopic surgery have had limited success.31 As laparoscopic surgery reached a performance plateau, robotic surgery gained acceptance and adoption.
Robotic Surgery
In 1985, a robotic brain biopsy platform, using stereotactic coordinates derived from computed tomography brain scans, successfully navigated a robotic arm to its target.32, 33 Ten years passed before results from human studies were reported.34 Subsequent technological improvements offered high magnification three-dimensional views, minimization or elimination of hand tremors, instruments that articulate to extreme angles, comfortable ergonomics, and platforms allowing surgeons to operate more than two robotic arms thus obviating the need for skilled assistants. Robotic surgery has a short, steep learning curve—similar to laparoscopy—and surgeons have reported lower blood loss, shorter hospital length of stay, fewer complications, and earlier return to work relative to laparoscopic and open approaches across several surgical specialties, but with higher operative costs, and limited high-level evidence demonstrating significant performance advantages.10, 20, 21, 35, 36 In a large randomized trial, robotic-assisted rectal cancer resection yielded no significant advantages over laparoscopic resection.37 Machine learning models can assess robotic operative performance and predict patient outcomes.38, 39 Further technological advances could offer haptic feedback, eye-tracking cameras, visualization of sub-surface anatomy, predictive navigation, and virtual constraints that protect anatomic structures such as vessels and nerves, offering potential advantages for the safe, effective performance of technically demanding tasks.40 A recent pilot randomized trial demonstrated the feasibility of robot-assisted lymphovenous microanastomosis (8 mm diameter or less) for women with breast cancer-related lymphedema.41 Compared with manual techniques, there were no significant differences in lymphedema-related outcomes at one- and three-month follow-up. Autonomous robots can perform end-to-end sutured bowel anastomoses with significantly higher leak pressures than laparoscopic and open anastomoses sewn by surgeons.18 However, in addition to cost constraints, many of the factors that hinder laparoscopic surgery also hinder robotic surgery, such as the need to create skin and fascial defects to insert instruments, incurring risk for injury during trocar and instrument insertion, wound infection, and hernia. Autonomous microrobots could mitigate these risks.
Autonomous Microrobots
In the 1966 film Fantastic Voyage, scientists shrink a submarine and drive it through blood vessels to remove clot from an injured colleague’s brain, popularizing a notion credited to Albert Hibbs: “it would be interesting in surgery if you could swallow the surgeon.” Emerging technologies suggest that autonomous surgical microrobots are feasible. In 2016, an ETH Zurich team described a hydrogel microrobot that propels itself through viscous solutions with corkscrew motions by whipping a flagellum-like tail.42 The same year, a MIT team described a biodegradable origami-like robot that folds into an ingestible pill, unfolds in the body, sticks to tissues by friction, and moves in response to external magnetic fields by redistributing its weight.15 In a 3D printed silicone representation of a human esophagus and stomach, the microrobot dislodged a battery embedded in the stomach wall and patched the defect in approximately five minutes. Other groups have used bull sperm and cardiac myocytes for propulsion, magnetic field-guided steering, DNA-protein orientations that allow robots to maneuver autonomously in response to their environment, and magnetotactic bacteria loaded with nanoliposomes that hone to hypoxic signals.43–46 The authors are unaware of any studies reporting the use of autonomous microrobots for surgery on humans, much less a performance advantage over current technologies. However, history and emerging evidence suggest that as technologies improve, autonomous microrobots have the potential to transform surgery.47
Surgical Decision-Making Innovations
Surgeons frequently engage patients in high-stakes shared decision-making under both time constraints and uncertainty imposed by acute surgical disease and busy clinic schedules. These circumstances promote reliance on dogma and heuristics, which can lead to bias, cognitive errors, and preventable harm.8, 48 Innovations in surgical decision-making can mitigate these challenges.
Additive Risk Scores
One of the simplest ways to support decisions is risk stratification by additive scores using static variable thresholds. High blood levels of C-reactive protein (CRP) are associated with anastomotic leak following colorectal surgery. Postoperative day three CRP levels less than 172 mg/L has 97% negative predictive value for anastomotic leak, ruling out leak in nearly all cases, but a positive predictive value of only 21%, such that high levels lack clinical utility.49 Incorporating multiple variables can improve predictive performance. Strate et al.50, 51 used seven risk factors to predict severe acute lower intestinal bleeding (0 risk factors = low risk [9%], 1–3 factors = moderate risk [43%], ≥4 factors = high risk [84%]). External validation demonstrated good discrimination with area under receiver operating characteristic curve (AUC) of 0.75.51 Clinicians can use these predictions to guide decisions regarding the urgency of diagnostic testing and the utility of close patient monitoring. Low-risk patients may be appropriate candidates for outpatient management, avoiding unnecessary use of inpatient resources. However, additive risk scores can underestimate risk for adverse outcomes among high-risk patients. Regression modeling techniques were used to identify static variable thresholds and generate scoring systems for many additive risk scores; direct application of regression modeling may be less prone to prediction errors among high-risk patients.52
Regression modeling
Regression modeling estimates relationships between predictor and outcome variables to predict outcomes or explain associations. The National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator uses data from over four million surgeries—including procedure type, demographics, and comorbidities—to predict outcomes such as morbidity, mortality, hospital length of stay, and discharge disposition within 30 days of surgery.11 The calculator makes accurate, patient specific predictions, and may increase the likelihood that patients will participate in risk reduction strategies, e.g. prehabilitation.12 Among 150 preoperative patients who reviewed their Surgical Risk Calculator results, 70% stated that they would participate in prehabilitation and 40% stated that they would delay surgery to participate. Patients often want to be knowledgeable, engaged members of the healthcare team; without the use of decision-support tools, such as the NSQIP calculator, this desire is often unfulfilled, and an opportunity to augment shared decision-making is lost.53–55 Despite these advantages, data from 4 million surgeries may be insufficient to represent rare but important pathophysiology in a cohort of more than 60 million patients undergoing surgery in the US each year, and regression model accuracy may suffer from an inability to accurately represent the complex, non-linear associations among predictor variables.56 Machine learning techniques are adept at this task.
Machine Learning
In 1970, Dr. William Schwartz wrote in the New England Journal of Medicine, “Computing science will probably exert its major effects by augmenting and, in some cases, largely replacing the intellectual functions of the physician.”57 Schwartz held that human disease is too broad and complex to be explained and interpreted by rules; machine learning algorithms learn from data rather than conforming to rules.58 Fifty years later, computers have not replaced physicians’ intellectual functions, but have demonstrated potential to augment decisions with varying levels of autonomy. Machine learning models can predict risk for several postoperative complications with accuracy greater than that of physicians, but often lack electronic and clinical workflow integration, limiting their use in routine clinical practice.59, 60
Supervised algorithms learn from data labeled by humans, then classify or make predictions on new unseen data; unsupervised algorithms create their own output categories—often agnostic of any human-attributed labels—allowing discovery of patterns and associations. Supervised algorithms can predict sepsis more than 24 hours prior to onset with AUC 0.83.61 However, predictions are only as useful as the outcomes they predict. Seymour et al.62 suggest that the overly broad definition of sepsis impairs the development of targeted interventions. They used unsupervised learning to phenotype sepsis patients, assigning points on a scatterplot as cluster centroids, assigning all other points to the nearest centroid, then iteratively recalculating centroids and cluster assignments to form the tightest clusters possible. This method identified four unique sepsis phenotypes, potentially representing subgroups with different responses to targeted therapies. These techniques require time-intensive hand-crafted feature engineering using human domain knowledge whereas deep models autonomously learn feature representations from raw data. Deep models can use electronic health record data to predict mortality among ICU patients with greater accuracy than the sequential organ failure assessment (SOFA) score, even when limited to the same input data used to calculate SOFA.63 Deep learning and statistical modeling can also use characters, words, and other expressions of natural language as model inputs. This technique, termed natural language processing, can generate oncologic decision-support tools predicting germline mutations.64, 65 This approach can leverage the availability of large volumes of genetic data and medical literature to produce personalized cancer prevention management strategies.66 Deep model interpretation mechanisms elucidate the relative importance of individual input features in determining model outputs, providing opportunities to assess whether associations between inputs and outputs are biologically plausible.63, 67 Despite these advantages, predictions and classifications can only indirectly inform discrete choices facing clinicians, limiting their clinical utility. Reinforcement learning directly informs discrete choices.
Reinforcement Learning
In reinforcement learning, an agent learns that specific actions under certain conditions lead to rewards and penalties, using this knowledge to identify actions that achieve an ultimate goal. Two characteristics distinguish reinforcement learning from machine learning: 1) trial-and-error search to identify the best action, and 2) delayed reward, i.e. choosing actions that achieve the ultimate goal rather than short-term rewards.68 For example, a model developed by Komorowski et al.16 recommends vasopressor doses and intravenous fluid volumes for septic patients, assigning rewards and penalties relative to 90-day survival. The model favored higher vasopressor doses and lower intravenous fluid volumes, consistent with evidence that volume overload harms sepsis patients, and that a one-size-fits-all approach to resuscitation is suboptimal.69, 70 On retrospective analysis, when actions taken by clinicians were concordant with model recommendations, mortality was slightly less than 20%. As clinician actions deviated from model recommendations, mortality significantly increased, up to 60%. Notably, clinicians may have deviated from model recommendations based on data not available to the model (e.g. physical exam findings, symptoms), and the same findings contributed to a worse prognosis, making clinician decision-making seem less effective. Therefore, available evidence does not support causal inference between model recommendations and decreased mortality.
For more complex decision-making scenarios in high-volume, high-dimension datasets, exhaustive searches for optimal actions can be prohibitive or impossible, but deep representation of the agent’s environment can mitigate these challenges. The Go board game has 32,490 possible first moves.71 A deep reinforcement model first learned from a human Go expert, then defeated the European Go champion five games to zero. Subsequently, a completely autonomous model trained on self-play only defeated the human input model 100 games to zero.17 Similar approaches have the potential to transform surgical decision-making, but in the absence of high-level evidence for medical applications, this potential remains theoretical.72 In addition, reinforcement learning models require large training datasets to maintain effective sample sizes in sequential decision-making tasks, and such data are not available for many surgical diseases, especially rare ones.73
Lessons Learned from Automotive Innovations
The automotive industry adopts intelligent, autonomous machine innovations that achieve performance advantages according to consumer demands and business advantages. Similar market forces will likely drive surgery toward machine autonomy. Currently, there is no level I evidence demonstrating that intelligent, autonomous machines improve patient outcomes compared with existing standards for performing operations or surgical decision-making tasks, specifically (see the Supplement describing an Embase, MEDLINE, and PubMed search performed by the authors 10/30/2019,). These technologies remain on the initial, flat portion of the innovation S-curve (Figure 1). However, if future hospitals can purchase robotic surgical platforms that autonomously perform operations with lower costs and higher quality than human surgeons, or deep reinforcement learning models that consistently make better decisions than clinicians, then it seems likely that these technologies will gain adoption. History suggests that intelligent, autonomous machines will initially be used for simple, common, rote tasks under close human supervision, and then for complex tasks with minimal human supervision. Automation of programmable tasks may allow surgeons to spend less time gathering and analyzing data and more time interacting with patients and tending to urgent, critical—and potentially more valuable—aspects of patient care.
Lessons learned from automotive innovations reveal opportunities to capitalize on the performance advantages of new technologies without disenfranchising the people that use and benefit from them. When robotic arms largely replaced human assembly line workers in performing rote mechanical tasks, automobile prices fell within reach of the middle class, but many assembly line workers lost their jobs. As in the industrial revolution, there was a lag time between incorporation of autonomous machines and redistribution of the human workforce. Perhaps if this transition were anticipated, a smoother transition could be achieved. Anticipating a similar transition in surgery seems prudent. Eventually, the automotive industry evolved to use human effort and expertise in designing and overseeing robotic arm assembly lines. Automotive workforces also pivoted toward tasks requiring creativity, long-term planning, and moral deliberation, which are especially relevant in designing self-driving cars that sense the environment and respond accordingly. Responses are programmable and have important moral implications. Awad et al.74 created online simulations in which participants identify preferences for how self-driving cars should behave when distributing harm in unavoidable collisions, e.g. the car can maintain its course and hit a jaywalking teenager, or swerve and crash, harming its elderly passenger. The authors collected data on nearly 40 million decisions by participants in 233 countries and found significant cross-cultural variation in preferences for moral dilemmas facing self-driving cars, precluding a one-size-fits-all approach to morally sound programming.
Moral and ethical dilemmas also challenge the adoption of intelligent, autonomous machines in surgery. Management of a patient with both pulmonary edema and pre-renal azotemia could proceed with either diuresis or volume resuscitation. The tradeoff between respiratory failure requiring mechanical ventilator support versus renal failure requiring renal replacement therapy depends in part on the desires and values of the patient and their caregivers. An autonomous reinforcement learning platform trained to optimize an arbitrary end-point such as 90-day mortality could make a recommendation or decision that is medically sound, but contrary to patient values. Also, algorithms trained on biased datasets are likely to produce biased outputs, as demonstrated for crime recidivism predictions.75 Similar problems could occur in machine learning healthcare applications. For example, associations between cardiovascular risk factors and adverse cardiovascular events differ by race and ethnicity; a model trained on data from the Framingham Heart Study, which primarily included white subjects, could produce racially and ethnically biased outputs.76 Algorithms used for allocating liver transplants may disenfranchise female organ recipient candidates by prioritizing serum creatinine, which is lower among women.77 Therefore, investigators must align training dataset and target population demographics and other characteristics that have potential to introduce bias. In addition, judicial systems have limited experience assigning liability for errors made by intelligent machines and differentiating between human and machine error. In making a critical management decision for a life-threatening postoperative complication, a surgeon could be privy to history and physical exam information that is unavailable to an autonomous decision-support platform, take a different course of action than recommended by a model with proven efficacy, and be subject to unwarranted scrutiny when the patient suffers a poor outcome. Similarly, robotic surgical platforms with virtual constraints intended to protect anatomic structures could delay or prevent a surgeon from gaining control of an injured blood vessel, harming a patient and pitting human versus machine in assigning liability. Surgeons must meet these challenges with creativity, altruism, moral deliberation, and emotional intelligence, i.e., the ability to recognize emotional states and act accordingly. These traits remain inaccessible to machines. The surgeon’s role may evolve to interpreting decision-support tools and offering wisdom for patients and caregivers facing complex, high-stakes surgical decisions, using and overseeing semi- and fully-autonomous surgical instruments and robotic platforms in the operating room, and ensuring the safe and effective integration of intelligent, autonomous machines with surgical care.
Conclusions
As technologies improve, intelligent, autonomous machines may gain the capacity to augment or outperform humans in operative and decision-making tasks. History suggests that intelligent, autonomous machines will be used in surgery initially for simple, common, rote tasks under close human supervision, and then for complex tasks with minimal human supervision. Automation of programmable tasks may allow surgeons to spend less time gathering and analyzing data and more time interacting with patients and tending to urgent, critical—and potentially more valuable—aspects of patient care. This process poses ethical challenges in assigning liability for errors, distributing harm, and displacing human workers. Surgeons should assume active roles in guiding these technologies toward optimal patient care and net social benefit, channeling human creativity, moral deliberation, and altruism.
Supplementary Material
Acknowledgments
Disclosure
AB and PR were supported by R01 GM110240 from the National Institute of General Medical Sciences (NIGMS) and by Sepsis and Critical Illness Research Center Award P50 GM-111152 from the NIGMS. PR was supported by CAREER award, NSF-IIS 1750192, from the National Science Foundation (NSF), Division of Information and Intelligent Systems (IIS) and by NIH NIBIB R21EB027344-01. PTJ was supported by R01GM114290 from the NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healey MA, Shackford SR, Osler TM, Rogers FB, Burns E. Complications in surgical patients. Arch Surg 2002; 137: 611–617; discussion 617–618. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013; 369: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 3.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med 2002; 346: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 4.Furuya S, Tominaga K, Miyazaki F, Altenmuller E. Losing dexterity: patterns of impaired coordination of finger movements in musician’s dystonia. Sci Rep 2015; 5: 13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci 2003; 58: 146–152. [DOI] [PubMed] [Google Scholar]
- 6.Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010; 251: 995–1000. [DOI] [PubMed] [Google Scholar]
- 7.Groopman JE How doctors think. Boston: Houghton Mifflin, 2007. [Google Scholar]
- 8.Wolf FM, Gruppen LD, Billi JE. Differential diagnosis and the competing-hypotheses heuristic. A practical approach to judgment under uncertainty and Bayesian probability. JAMA 1985; 253: 2858–2862. [PubMed] [Google Scholar]
- 9.Coccolini F, Catena F, Pisano M, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg 2015; 18: 196–204. [DOI] [PubMed] [Google Scholar]
- 10.Hohwu L, Akre O, Pedersen KV, et al. Open retropubic prostatectomy versus robot-assisted laparoscopic prostatectomy: a comparison of length of sick leave. Scand J Urol Nephrol 2009; 43: 259–264. [DOI] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217: 833–842 e831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond BL, Wanderer JP, Hawkins AT, et al. Use of the American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator During Preoperative Risk Discussion: The Patient Perspective. Anesth Analg 2018. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial Intelligence in Surgery: Promises and Perils. Ann Surg 2018; 268: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haidegger T Autonomy for Surgical Robots: Concepts and Paradigms. IEEE Transactions on Medical Robotics and Bionics 2019; 1: 65–76. [Google Scholar]
- 15.Miyashita S, Guitron S, Yoshida K, et al. Ingestible, controllable, and degradable origami robot for patching stomach wounds. 2016 IEEE International Conference on Robotics and Automation (ICRA)2016; 909–916. [Google Scholar]
- 16.Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med 2018; 24: 1716–1720. [DOI] [PubMed] [Google Scholar]
- 17.Silver D, Schrittwieser J, Simonyan K, et al. Mastering the game of Go without human knowledge. Nature 2017; 550: 354–359. [DOI] [PubMed] [Google Scholar]
- 18.Shademan A, Decker RS, Opfermann JD, et al. Supervised autonomous robotic soft tissue surgery. Sci Transl Med 2016; 8: 337ra364. [DOI] [PubMed] [Google Scholar]
- 19.Kurzweil R The singularity is near : when humans transcend biology. New York: Viking, 2005. [Google Scholar]
- 20.Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 2008; 111: 407–411. [DOI] [PubMed] [Google Scholar]
- 21.Altieri MS, Yang J, Telem DA, et al. Robotic approaches may offer benefit in colorectal procedures, more controversial in other areas: a review of 168,248 cases. Surg Endosc 2016; 30: 925–933. [DOI] [PubMed] [Google Scholar]
- 22.Spaner SJ, Warnock GL. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J Laparoendosc Adv Surg Tech A 1997; 7: 369–373. [DOI] [PubMed] [Google Scholar]
- 23.Gomella LG, Albala DM. Laparoscopic urological surgery: 1994. Br J Urol 1994; 74: 267–273. [DOI] [PubMed] [Google Scholar]
- 24.Gaskin TA, Isobe JH, Mathews JL, Winchester SB, Smith RJ. Laparoscopy and the general surgeon. Surg Clin North Am 1991; 71: 1085–1097. [DOI] [PubMed] [Google Scholar]
- 25.Blum CA, Adams DB. Who did the first laparoscopic cholecystectomy? J Minim Access Surg 2011; 7: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhe E [Laparoscopic cholecystectomy]. Z Gastroenterol Verh 1991; 26: 204–206. [PubMed] [Google Scholar]
- 27.Litynski GS. Erich Muhe and the rejection of laparoscopic cholecystectomy (1985): a surgeon ahead of his time. JSLS 1998; 2: 341–346. [PMC free article] [PubMed] [Google Scholar]
- 28.Mouret P How I developed laparoscopic cholecystectomy. Ann Acad Med Singapore 1996; 25: 744–747. [PubMed] [Google Scholar]
- 29.Kum CK, Eypasch E, Lefering R, et al. Laparoscopic cholecystectomy for acute cholecystitis: is it really safe? World J Surg 1996; 20: 43–48; discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 30.Peters JH, Ellison EC, Innes JT, et al. Safety and efficacy of laparoscopic cholecystectomy. A prospective analysis of 100 initial patients. Ann Surg 1991; 213: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaitzberg SD, Roberts K, Romanelli JR, et al. The NOVEL trial: natural orifice versus laparoscopic cholecystectomy-a prospective, randomized evaluation. Surg Endosc 2018; 32: 2505–2516. [DOI] [PubMed] [Google Scholar]
- 32.Koyama H, Uchida T, Funakubo H, Takakura K, Fankhauser H. Development of a new microsurgical robot for stereotactic neurosurgery. Stereotact Funct Neurosurg 1990; 54–55: 462–467. [DOI] [PubMed] [Google Scholar]
- 33.Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988; 35: 153–160. [DOI] [PubMed] [Google Scholar]
- 34.Glauser D, Fankhauser H, Epitaux M, Hefti JL, Jaccottet A. Neurosurgical robot Minerva: first results and current developments. J Image Guid Surg 1995; 1: 266–272. [DOI] [PubMed] [Google Scholar]
- 35.Kane WJ, Charles EJ, Mehaffey JH, et al. Robotic compared with laparoscopic cholecystectomy: A propensity matched analysis. Surgery 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janjua H, Cousin-Peterson E, Barry TM, et al. The paradox of the robotic approach to inguinal hernia repair in the inpatient setting. Am J Surg 2019. [DOI] [PubMed] [Google Scholar]
- 37.Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017; 318: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung AJ, Chen J, Gill IS. Automated Performance Metrics and Machine Learning Algorithms to Measure Surgeon Performance and Anticipate Clinical Outcomes in Robotic Surgery. JAMA Surg 2018; 153: 770–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung AJ, Chen J, Jarc A, et al. Development and Validation of Objective Performance Metrics for Robot-Assisted Radical Prostatectomy: A Pilot Study. J Urol 2018; 199: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cha J, Broch A, Mudge S, et al. Real-time, label-free, intraoperative visualization of peripheral nerves and micro-vasculatures using multimodal optical imaging techniques. Biomed Opt Express 2018; 9: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Mulken TJM, Schols RM, Scharmga AMJ, et al. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun 2020; 11: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang HW, Sakar MS, Petruska AJ, Pane S, Nelson BJ. Soft micromachines with programmable motility and morphology. Nat Commun 2016; 7: 12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magdanz V, Sanchez S, Schmidt OG. Development of a sperm-flagella driven micro-bio-robot. Adv Mater 2013; 25: 6581–6588. [DOI] [PubMed] [Google Scholar]
- 44.Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012; 335: 831–834. [DOI] [PubMed] [Google Scholar]
- 45.Williams BJ, Anand SV, Rajagopalan J, Saif MT. A self-propelled biohybrid swimmer at low Reynolds number. Nat Commun 2014; 5: 3081. [DOI] [PubMed] [Google Scholar]
- 46.Felfoul O, Mohammadi M, Taherkhani S, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol 2016; 11: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier-Hein L, Vedula SS, Speidel S, et al. Surgical data science for next-generation interventions. Nat Biomed Eng 2017; 1: 691–696. [DOI] [PubMed] [Google Scholar]
- 48.Bekker HL. Making choices without deliberating. Science 2006; 312: 1472; author reply 1472. [DOI] [PubMed] [Google Scholar]
- 49.Singh PP, Zeng IS, Srinivasa S, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014; 101: 339–346. [DOI] [PubMed] [Google Scholar]
- 50.Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol 2008; 6: 1004–1010; quiz 1955-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strate LL, Saltzman JR, Ookubo R, Mutinga ML, Syngal S. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol 2005; 100: 1821–1827. [DOI] [PubMed] [Google Scholar]
- 52.Jin R, Grunkemeier GL, Providence Health System Cardiovascular Study G. Additive vs. logistic risk models for cardiac surgery mortality. Eur J Cardiothorac Surg 2005; 28: 240–243. [DOI] [PubMed] [Google Scholar]
- 53.Uldry E, Schafer M, Saadi A, Rousson V, Demartines N. Patients’ preferences on information and involvement in decision making for gastrointestinal surgery. World J Surg 2013; 37: 2162–2171. [DOI] [PubMed] [Google Scholar]
- 54.de Mik SML, Stubenrouch FE, Balm R, Ubbink DT. Systematic review of shared decision-making in surgery. Br J Surg 2018; 105: 1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson A, Ronnekleiv-Kelly SM, Pawlik TM. Regret in Surgical Decision Making: A Systematic Review of Patient and Physician Perspectives. World J Surg 2017; 41: 1454–1465. [DOI] [PubMed] [Google Scholar]
- 56.Bagnall NM, Pring ET, Malietzis G, et al. Perioperative risk prediction in the era of enhanced recovery: a comparison of POSSUM, ACPGBI, and E-PASS scoring systems in major surgical procedures of the colorectal surgeon. Int J Colorectal Dis 2018; 33: 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz WB. Medicine and the computer. The promise and problems of change. N Engl J Med 1970; 283: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz WB, Patil RS, Szolovits P. Artificial intelligence in medicine. Where do we stand? N Engl J Med 1987; 316: 685–688. [DOI] [PubMed] [Google Scholar]
- 59.Brennan M, Puri S, Ozrazgat-Baslanti T, et al. Comparing clinical judgment with the MySurgeryRisk algorithm for preoperative risk assessment: A pilot usability study. Surgery 2019; 165: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leeds IL, Rosenblum AJ, Wise PE, et al. Eye of the beholder: Risk calculators and barriers to adoption in surgical trainees. Surgery 2018; 164: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henry KE, Hager DN, Pronovost PJ, Saria S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med 2015; 7: 299ra122. [DOI] [PubMed] [Google Scholar]
- 62.Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019; 321: 2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shickel B, Loftus TJ, Adhikari L, et al. DeepSOFA: A Continuous Acuity Score for Critically Ill Patients using Clinically Interpretable Deep Learning. Sci Rep 2019; 9: 1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes KS, Zhou J, Bao Y, et al. Natural language processing to facilitate breast cancer research and management. Breast J 2020; 26: 92–99. [DOI] [PubMed] [Google Scholar]
- 65.Braun D, Yang J, Griffin M, Parmigiani G, Hughes KS. A Clinical Decision Support Tool to Predict Cancer Risk for Commonly Tested Cancer-Related Germline Mutations. J Genet Couns 2018; 27: 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng Z, Yin K, Bao Y, et al. Validation of a Semiautomated Natural Language Processing-Based Procedure for Meta-Analysis of Cancer Susceptibility Gene Penetrance. JCO Clin Cancer Inform 2019; 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shamout FE, Zhu T, Sharma P, Watkinson PJ, Clifton DA. Deep Interpretable Early Warning System for the Detection of Clinical Deterioration. IEEE J Biomed Health Inform 2019. [DOI] [PubMed] [Google Scholar]
- 68.Sutton RS, Barto AG Reinforcement learning : an introduction. Cambridge, Massachusetts: The MIT Press, 2018. [Google Scholar]
- 69.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259–265. [DOI] [PubMed] [Google Scholar]
- 70.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364: 2483–2495. [DOI] [PubMed] [Google Scholar]
- 71.Silver D, Huang A, Maddison CJ, et al. Mastering the game of Go with deep neural networks and tree search. Nature 2016; 529: 484–489. [DOI] [PubMed] [Google Scholar]
- 72.Topol EJ. A decade of digital medicine innovation. Sci Transl Med 2019; 11. [DOI] [PubMed] [Google Scholar]
- 73.Gottesman O, Johansson F, Komorowski M, et al. Guidelines for reinforcement learning in healthcare. Nat Med 2019; 25: 16–18. [DOI] [PubMed] [Google Scholar]
- 74.Awad E, Dsouza S, Kim R, et al. The Moral Machine experiment. Nature 2018; 563: 59–64. [DOI] [PubMed] [Google Scholar]
- 75.Angwin J, Larson J, Mattu S, Kirchner L. Machine Bias. ProPublica 2016 May 23. Accessed 24 January 2019 Available at: https://www.propublica.org/article/machinebias-risk-assessments-in-criminal-sentencing.
- 76.Gijsberts CM, Groenewegen KA, Hoefer IE, et al. Race/Ethnic Differences in the Associations of the Framingham Risk Factors with Carotid IMT and Cardiovascular Events. PLoS One 2015; 10: e0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol 2011; 54: 462–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.