Abstract
Decreases in energy stores requires negative energy balance where caloric expenditure exceeds energy intake, which can induce adaptive thermogenesis—the reduction of energy expenditure (EE) beyond that accounted for by the weight lost. Adaptive thermogenesis varies between individuals. The component of total daily EE responsible for the interindividual variation in adaptive thermogenesis was investigated in this study, using a rat model that differs in obesity propensity and physical activity. Total daily EE and physical activity were examined before and after 21 days of 50% calorie restriction in male and female rats with lean and obesity-prone phenotypes—rats selectively bred for high and low intrinsic aerobic capacity (HCR and LCR, respectively). Calorie restriction significantly decreased EE more than was predicted by loss of weight and lean mass, demonstrating adaptive thermogenesis. Within sex, HCR and LCR did not significantly differ in resting EE. However, the calorie restriction-induced suppression in non-resting EE, which includes activity EE, was significantly greater in HCR than in LCR; this phenotypic difference was significant for both male and female rats. Calorie restriction also significantly suppressed physical activity levels more in HCR than LCR. When VO2max was assessed in male rats, calorie restriction significantly decreased O2 consumption without significantly affecting running performance (running time, distance), indicating increased energy efficiency. Percent weight loss did not significantly differ between groups. Altogether, these results suggest that individual differences in calorie restriction-induced adaptive thermogenesis may be accounted for by variation in aerobic capacity. Moreover, it is likely that activity EE, not resting or basal metabolism, may explain or predict the variation in individuals’ adaptive thermogenesis.
Keywords: Physical activity; energy expenditure; obesity; high- and low-capacity runners (HCR, LCR); Non-exercise activity thermogenesis (NEAT)
INTRODUCTION
Obesity is a serious public health concern with more than one-third of US adults obese and another one-third overweight (1). While weight loss ameliorates the disease susceptibility incurred with obesity (2–5), successful weight loss is difficult to achieve and even more difficult to maintain (6). There are large inter-individual differences in people’s ability to lose weight (7–11). As weight is lost, body mass lowers, resulting in lowered energy expenditure (EE). Moreover, this suppression of EE can exceed what is predicted by the reduced body weight alone, a phenomenon called adaptive thermogenesis which creates resistance to continued weight loss and increases risk of renewed weight and fat accumulation (9–11). As with weight loss, adaptive thermogenesis varies between people (11, 12). One potential mediator or predictor of this varied response to energy deficit is intrinsic aerobic capacity, a heritable trait known to have important consequences for human health (13–16).
Aerobic capacity, defined as the intake and utilization capability of oxygen by the body, is one of the strongest predictors of disease, long-term health, and mortality rate in humans (17–21). This complex trait influences not only physical activity but also the components of EE (22–24). As with human metabolism, EE of laboratory animals can be separated into resting and non-resting EE, with non-resting EE primarily composed of thermic effect of food and activity EE (13). Human activity EE can be separated into the calories burned in voluntary exercise (exercise EE) plus the EE of daily living, called non-exercise activity thermogenesis (NEAT) (13–15, 25–27); in humans, resting EE accounts for about 60%, TEF around 10%, and activity EE accounts for about 30% of TDEE (14, 15, 25, 28). Using the rat model of contrasting high and low intrinsic aerobic capacity (29–31), we have demonstrated differences in EE where HCR show elevated EE, which is attributable primarily to their heightened non-resting EE (22). Some behavioral differences also accompany this divergence in aerobic capacity, including elevated physical activity (23, 32, 33). For both human NEAT and physical activity in laboratory animals, there are biologically determined individual differences in the tendency to move (14, 15) that are consistent over time (34) and not secondary to body size (22, 25, 35). High-NEAT people tend to be lean and resist weight gain (13, 15, 27, 34), and high-activity laboratory animals show this same propensity toward leanness (16). The findings described in this study will be expressed in terms of resting and non-resting EE because we do not measure or factor out thermic effect of food, with the understanding that physical-activity EE is the predominant source of non-resting EE.
The elevated non-resting EE seen with high intrinsic aerobic capacity suggests that components of EE may also be differentially labile as total EE changes with food restriction. Adaptive thermogenesis in total daily EE could stem from suppression of any or all of its components, including activity EE (11, 36). During prolonged calorie restriction, increased muscle work efficiency as well as lower physical activity levels are part of the adaptive thermogenic mechanisms underlying suppressed physical-activity EE and thus overall total EE (8). Here, we sought to identify the component(s) of total daily EE responsible for the differential suppression in EE by adaptive thermogenesis, specifically focusing on the differences associated with intrinsic aerobic capacity. Despite the known predictive power of intrinsic aerobic capacity to human health and longevity (37), surprisingly little consideration is given to this trait when considering human health and response to dietary intervention.
To probe this question, we take advantage of a laboratory rodent model that captures the highest and lowest ends of the distribution of aerobic capacity (38). Using heterogeneous N/NIH stock as founder population developed by Drs. Britton and Koch, (38) two lines of rats were generated through artificial selection based on treadmill running capacity: high-capacity runners (HCR) and low-capacity runners (LCR). To maintain genetic variance, a rotational breeding scheme was used to prevent inbreeding. After generations of artificial selection, HCR and LCR different systematically not only in their intrinsic aerobic capacity but also showed differing body weights, body compositions, and metabolic health profiles (25, 34, 39). HCR were leaner and had significantly higher energy expenditure and physical activity compared to the low-capacity runners (23, 33, 39, 40). We have previously demonstrated that these rats respond to calorie restriction with a short-term rise followed by a prolonged suppression in physical activity levels, similar to what is seen in most human and animal studies (11, 41); moreover, both physical activity and weight loss during calorie restriction vary according to intrinsic aerobic capacity (42). Given the association between metabolic health, aerobic capacity, and physical activity, it would be predicted that differences in physical activity may alter energetic response to energy deficit, yet this hypothesis has not been explicitly tested.
Here, we hypothesize that aerobic capacity influences adaptive thermogenesis by altering the activity EE, the major component of non-resting EE, and that this is partially responsible for individual differences in adaptive thermogenesis. Because a baseline comparison according to aerobic capacity has already been considered (22), here we will focus on the impact of calorie restriction, and assess the contribution of activity EE to adaptive thermogenesis in both male and female rats. Historically, research on male subjects predominated, while females were studied less frequently, leading to a sex bias in health, neuroscience, and behavioral research (43, 44). Investigating female rats presents some advantages; not only can we demonstrate generalizability between sexes, this also has an added advantage in energy-balance research because, unlike males, there is a smaller divergence in body weight and body composition between HCR and LCR in females, facilitating direct comparison of EE between phenotypes (22).
MATERIALS AND METHODS
Animals
Male rats of generation 31 (12 HCR, 12 LCR) and generation 27 (10 HCR, 6 LCR) and female rats of generation 31 (11 HCR, 13 LCR) were used in these studies. Two separate studies were performed on individually housed rats. Male rats are generally larger than females and show a more marked phenotypic segregation in body weight (22, 30). In a prior study comparing weight-matched HCR and LCR, a weight-matched subset for female HCR and LCR did not significantly differ from the larger population within phenotype, for example in aerobic capacity (22). Thus, for this set of studies we used HCR and LCR females of similar body-weight range. Phenotyping analysis at the University of Michigan confirmed that HCR showed significantly higher treadmill running capacity, with HCR running between about 700% to over 1,000% longer distances than LCR (female generation 31 HCR, 1914 ± 80 m; LCR, 276 ± 32 m; generation 27 male HCR, 1928 ± 133 m; LCR, 198 ± 12 m; generation 31 male HCR, 1806 ± 63 m; LCR, 157 ± 13 m). Generations of artificial selection led to divergence of not only the intrinsic aerobic capacity of the HCR and LCR, but also segregation of body weights, body compositions, and health profiles (25, 34, 39). Similar to people with higher NEAT, our high-NEAT HCR gain less fat on a high-calorie diet (16, 45); like lean people, our lean HCR rats are also more physically active, and both have high intrinsic aerobic capacity (24). Similar to less active people, LCR rats are obesity prone weight loss resistant when on a “diet” (42). Thus, the HCR/LCR rat model system may provide insights into the physioligcal changes in energy balance seen with light and low aerobic capacity.
Males and females were measured separately (i.e., not counterbalanced) and thus a valid direct statistical comparison cannot be made between sexes; male and female rats were used to demonstrate generalizability between sexes (46) rather than to investigate potential sex differences. VO2max was assessed on the generation 27 male rats which were previously calorie restricted in a separate study. A 12:12 hour light: dark cycle was maintained with the light cycle starting at 7AM (EST). Rodent chow (5P00 MRH 3000, T.R. Co. Inc) and water were provided ad libitum to each individually-housed rat before food restriction. Studies were conducted with the approval of the Kent State University Institutional Animal Care and Use Committee (IACUC) and cared for, maintained, and used according to the Guide for Animal Care and Use, 8th edition (47).
Food intake, calorie restriction, and body composition
In HCR and LCR rats, males and females were measured and analyzed separately to examine individual differences in adaptive thermogenesis. Body weight was measured and animals were fed daily at 1200 EST with precision of ±1 hr. In each case, daily food intake of each animal was calculated after measuring their baseline food intake for 8 days, and 50% food consumption of each rat was calculated individually based on the average daily food intake of each rat. 50% calorie restriction was performed on each rat for 21 days. Although 50% may seem severe in comparison to human food-restirction studies, based on experimental food restriction data we found that a milder restriction of 25% resulted in weight maintanance rather than substantial weight loss in Sprague-Dawley rats (48). Body composition was measured before calorie restriction and again on the 21st day of calorie restriction using magnetic resonance spectroscopy with an EchoMRI-700 (EchoMRI LLC, Houston, TX).
Energy expenditure measurement using calorimetry
Rats were acclimated for two days in a dedicated, environmentally controlled area (Environmental Growth Chambers, Chagrin Falls, OH) and calorimetry cage prior to the EE measurement. The calorimetry room was kept at a temperature thermoneutral for rats (25.0–26.1°C) (49). An Oxymax FAST system (Columbus Instruments, Columbus, OH) was used to measure EE and physical activity for each animal with a temporal resolution of 30 seconds, enabling detailed analysis of resting EE and non-resting EE, as described previously (22). Rats were divided into cohorts of 4 to accommodate the 4-chamber calorimetry system, where 2 HCR and 2 LCR rats were measured together and randomly assigned to chamber. Gas exchange and physical activity were measured for ≥25 hours, and data from noon (EST) of day 1 to noon on day 2 were analyzed, with the first 1–2 hours of data eliminated from analyses. Energy expenditure was measured at 30-second intervals for 24 hours except for reference periods of 210 seconds after each 60-sample interval. Physical activity was measured at 10-sec intervals without interruption where the infrared beam breaks were measured in the X, Y, and Z axes, displayed as total horizontal activity counts, ambulatory activity counts (non-repetitive beam breaks), and vertical activity counts as previously described (22). Energy expenditure was separated into resting EE and non-resting EE using CLAX software using a previously validated method (22), with non-resting EE calculated by TDEE = resting EE + non-resting EE (22).
Maximal oxygen consumption (VO2max)
VO2max was measured in male HCR and LCR before and after 21 days of 50% calorie restriction, on the 21st day using a graded treadmill test. Two to three days prior to the experiment, each animal was acclimated to walking on the treadmill for 5 minutes at 10m/min. The treadmills were randomly assigned to HCR and LCR to reduce experimental bias. VO2max was assessed during treadmill running with increasing speed and incline: 2 min at 10 m/min at each 0°, 15°, and 25° incline; then continuing at this slope for 2 min each at 15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35m/min, up to 37m/min. The rats were allowed to run until a respiratory exchange ratio (RER) of 1.0 was reached and/or the animal was unable to continue running. Due to the lack of significant effect of calorie restriction on running performance in males (see Results), this study was not conducted in the females.
Data Analysis
SPSS software was used to analyze data from both male and female rats separately. Repeated-measures ANOVAs were used to compare change in EE and body composition (body weight, lean mass, fat mass) over time between HCR and LCR. For activity and RER, change between baseline and calorie restriction was calculated and compared between HCR and LCR using a 1-tailed t-test as the directionality was demonstrated previously (22), and the effects of calorie restriction on activity and RER are well known (42). Because of the confounding effect of body weight on EE, standard general linear model analysis is not appropriate in this case, so analysis of covariance (ANCOVA) was used to compare groups that differed in body weight and composition, using body weight and lean mass as covariates in separate analyses for males and females. Analysis of residuals, completed to offset potential non-zero intercepts of regression lines for body weight, confirmed ANCOVA results. In general, resting EE varies mostly with lean mass, and activity EE with body weight (50), but here all covariate analyses are reported.
RESULTS
Body composition
In summary as shown in Tables 1–2, rats lost weight with calorie restriction, mobilizing fat while also losing lean mass. There was little phenotypic difference in body composition change, with some differences between HCR and LCR in males secondary to their large phenotypic difference in baseline body weight and composition.
Table 1.
Body weight and composition, physical activity, and energy expenditure in female high- and low-capacity runners (HCR, LCR) subjected to 21 days of 50% calorie restriction (CR), mean±SEM.
| FEMALES | BW (g)† | Fat mass (g)† | Lean mass (g)†‡ | Physical activity (beam breaks/min) | Energy expenditure (kcal/hr) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Horizontal†‡ | Ambulatory†‡ | Vertical†‡ | Total†‡ | Resting†‡ | Non-resting†‡ | |||||
| HCR | Before CR | 245.85 ± 5.63 | 21.45 ± 1.71 | 185.94 ± 4.68 | 5.9 1 ± 0.27 | 2.18 ± 0.13 | 0.90 ± 0.04 | 1.80 ± 0.05 | 1.19 ± 0.02 | 0.61 ± 0.02 |
| After CR | 199.21 ± 4.13 | 9.31 ± 2.07 | 162.06 ± 4.03 | 3.60 ± 0.17 | 1.33 ± 0.08 | 0.39 ± 0.04 | 1.23 ± 0.04 | 0.83 ± 0.01 | 0.40 ± 0.02 | |
| Change | −46.64 ± 3.68** | −12.14 ± 0.72** | −23.88 ± 3.30** | −2.3 ± 0.31** | −0.85 ± 0.14** | −0.51 ± 0.06** | −0.56 ± 0.04** | −0.35 ± 0.02 | −0.21 ± 0.02** | |
| LCR | Before CR | 238.09 ± 3.02* | 26.26 ± 2.56* | 177.18 ± 2.90* | 4.11 ± 0.09* | 1.56 ± 0.04* | 0.60 ± 0.03* | 1.58 ± 0.03* | 1.11 ± 0.02 | 0.46 ± 0.01* |
| After CR | 187.27 ± 3.50* | 6.53 ± 1.19 | 150.67 ± 2.89* | 3.18 ± 0.17* | 1.25 ± 0.08* | 0.40 ± 0.03* | 1.12 ± 0.03* | 0.78 ± 0.03 | 0.35 ± 0.01 (p = 0.068) | |
| Change | −50.82 ± 1.97 | −19.74 ± 1.60 | −26.51 ± 3.06 | −0.92 ± 0.19* | −0.30 ± 0.07* | −0.19 ± 0.05* | −0.45 ± 0.04* | −0.34 ± 0.03 | −0.11 ± 0.02* | |
Main effect of calorie restriction.
Main effect of line (HCR ≠ LCR).
Significant interaction (different response to CR in HCR vs LCR).
HCR ≠ LCR at the given time point (p<0.05 for all comparisons). HCR, n = 11; LCR, n = 13.
Table 2.
Body weight and composition, physical activity, and energy expenditure in male high- and low-capacity runners (HCR, CR) subjected to 21 days of 50% calorie restriction (CR), mean ± SEM.
| MALES | BW (g)†‡ | Fat mass (g)†‡ | Lean mass (g)†‡ | Physical activity (beam breaks/min) | Energy expenditure (kcal/hr) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Horizontal†‡ | Ambulatory†‡ | Vertical†‡ | Total† | Resting†‡ | Non-resting† | |||||
| HCR | Before CR | 400.04±8.38 | 63.58±3.28 | 280.39±6.54 | 6.84±0.25 | 2.34±0.10 | 0.78±0.05 | 2.32±0.04 | 1.65±0.03 | 0.673±0.02 |
| After CR | 339.05±9.13 | 38.202±3.85 | 252.04±6.43 | 5.70±0.15 | 1.96±0.07 | 0.52±0.04 | 1.63±0.03 | 1.13±0.04 | 0.514±0.02 | |
| Change | −60.98±2.65** | −25.37±1.67** | −28.34±2.16 | −1.13±0.22** | −0.37±0.07** | −0.25±0.04** | −0.69±0.03** | −0.51±0.03 | 0.158±0.01** | |
| LCR | Before CR | 545.81± 19.45* | 139.72± 13.25* | 334.06± 9.83 | 5.23± 0.21* | 1.82± 0.15* | 0.37± 03* | 2.56± 0.07 | 1.883± 0.05 | 0.68± 0.02 |
| After CR | 463.67±19.85* | 98.80±12.58* | 300.40±8.81 | 4.81±0.19* | 1.73± 0.09(p = 0.05065) | 0.38± 0.04* | 1.90± 0.05* | 1.31±0.04 | 0.58±0.02* | |
| Change | −82.14± 2.45* | −40.91± 2.74* | −33.66± 05.52 | −0.42± 0.02* | −0.09± 0.10* | +0.01± 0.02* | −0.65± 0.06* | −0.56± 0.04 | −0.09± 0.02* | |
Main effect of calorie restriction.
Main effect of line (HCR ≠ LCR).
Significant interaction (different response to CR in HCR vs LCR).
HCR ≠ LCR at the given time point (p<0.05 for all comparisons).
HCR, n = 22; LCR, n = 18.
Females.
As shown in Table 1, calorie restriction induced significant weight loss in female rats (in grams; p<0.001) but there was no significant interaction (p=0.307) or difference of body weight between HCR and LCR (p=0.082) observed before or after calorie restriction. No significant differences in percentage of body weight loss (p=0.198) between female HCR and LCR were found. Fat mass loss in grams did not significantly vary between HCR and LCR (p=0.081). There was a significant difference in percentage of fat mass lost (compared to their baseline; p=0.02) where LCR lost a greater percentage of their fat mass compared to HCR. Calorie restriction induced significant lean mass loss (in grams; p<0.001), and lean mass loss in grams was significantly different between HCR and LCR (p<0.038). However, no significant interaction (p=0.566) or differences in percentage of baseline lean mass loss (p=0.396) between HCR and LCR were observed. HCR females lost 14% of their lean mass and 60% of their fat mass, and LCR lost 12% of their lean mass and 76% of their fat mass.
Males.
As shown in Table 2, calorie restriction induced significant weight loss (in grams; p<0.001), with a significant interaction (p<0.001) where body weight loss (in grams) significantly differed between male HCR and LCR due to the LCRs’ larger starting body weight (male LCR > male HCR; p<0.001). However, no significant differences were seen in percentage of body weight loss (p=0.932). Calorie restriction induced significant loss of fat and lean mass (in grams; p<0.001), and there was a significant interaction where LCR lost more fat mass (p<0.001) and lean mass (p<0.023) than HCR. With respect to percent of fat lost, HCR lost 42.3% of their fat mass, which was significantly more than the 32.4% of fat mass lost by LCR (p =0.0276). HCR and LCR did not differ in the percent of their baseline lean mass that they lost (p=0.840); HCR males lost 10.1% of their lean mass while LCR males lost 9.8% of their lean mass.
RER and physical activity
Calorie restriction decreased RER, with minor differences between phenotypes and no interaction between CR and phenotype. Physical activity was consistently higher in HCR compared to LCR, and CR suppressed physical activity more in HCR than in LCR.
Females.
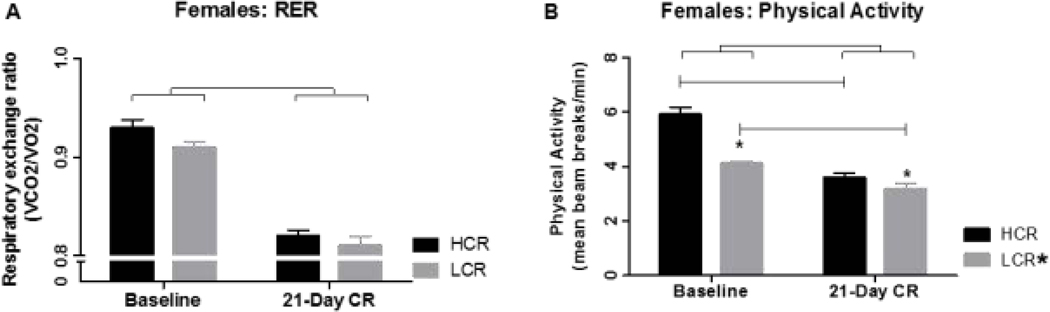
As shown in Figure 1A, respiratory exchange ratio (RER, VCO2/VO2) significantly decreased after calorie restriction compared to baseline in female rats (p <0.001). However, there was no difference in RER between female HCR and LCR or differential effect on these groups due to calorie restriction (Figure 1A). As previously reported (22) baseline physical activity was higher in HCR than LCR females. For physical activity levels in female rats, all components of physical activity (horizontal, ambulatory, and vertical activity) were significantly suppressed by calorie restriction (p<0.001), and there was a significant main effect of line where female HCR were more active than female LCR, regardless of calorie restriction (p<0.001; Figure 1B, Table 1). There was a significant interaction where HCR showed a greater suppression in activity than LCR (p<0.001), though both HCR and LCR were significantly less active after calorie restriction than before. Both before and after calorie restriction, physical activity was greater in HCR than LCR (p<0.000). The change in total, ambulatory, and vertical activity counts between baseline and calorie restriction was significantly greater in HCR compared to LCR (p<0.001).
Figure 1. Respiratory exchange ratio (RER) and physical activity in female high- and low-capacity rats (HCR and LCR).
(A) Calorie restriction (CR) significantly reduced RER (VCO2/VO2), with no difference between HCR and LCR. (B) HCR and LCR were less physically active after CR (total activity counts), though HCR showed a significantly greater decrease than LCR; HCR were significantly more active than LCR before and after CR. *different from HCR within condition (above bar), or main effect of selected line (legend); simple brackets signify significant change over CR within line; double brackets signify main effect of CR; p<0.05.
Males.
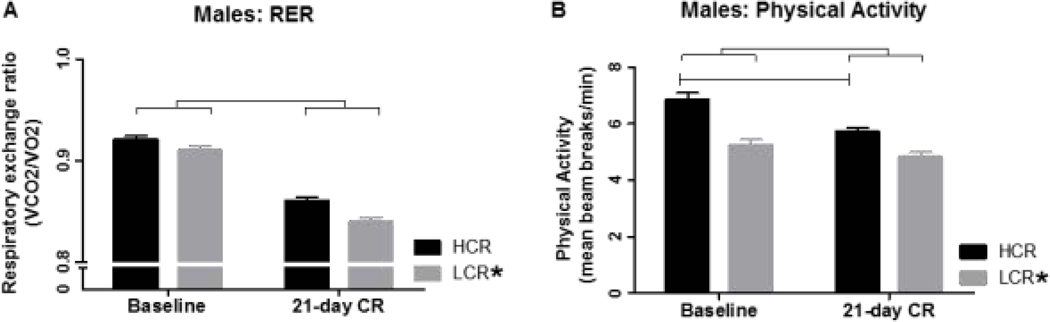
Calorie restriction significantly suppressed RER (p<0.001; Figure 2A), with no significant interaction with phenotype; HCR showed slightly higher RER overall (p=0.032) (Figure 2A). Similar to female rats, calorie restriction significantly suppressed all components of physical activity in male rats (p<0.001; Figure 2B). There was a main effect of line where HCR were more active than LCR overall (p<0.001), and calorie restriction suppressed physical activity more in HCR than in LCR males (Table 2; Figure 2B). When the change in activity from baseline was compared between HCR and LCR, in horizontal (p=0.023), ambulatory p=0.042, and vertical (p<0.001) activity counts were significantly more suppressed in HCR than LCR.
Figure 2: Respiratory exchange ratio (RER) and physical activity in male high- and low-capacity rats (HCR and LCR).
(A) Calorie restriction (CR) significantly reduced RER (VCO2/VO2), with a difference between HCR and LCR but no significant interaction. (B) CR induced a significant decrease in total physical activity counts. HCR were more active than LCR overall. *different from HCR within condition (above bar), or significant main effect of selected line (legend); simple brackets signify significant change over CR within line; double brackets signify main effect of CR; p<0.05.
Energy expenditure
Calorie restriction induced adaptive thermogenesis in all components of EE. The consistent feature distinguishing the HCR from LCR in their response to energy restriction was the change in non-resting EE, where CR induced a greater suppression of non-resting EE in the HCR than in LCR. There was less consistent phenotypic difference in the adaptations in total or resting EE where all showed adaptive thermogenesis, with consistently greater suppression in non-resting EE in HCR, both males and females.
Females.
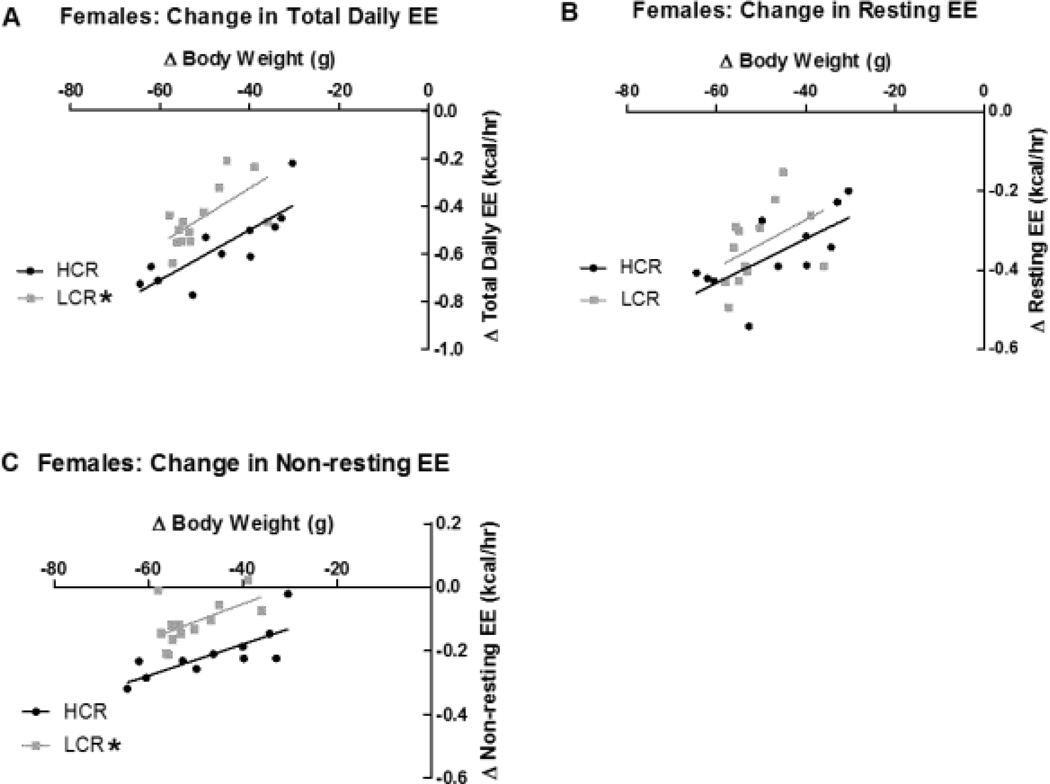
As expected, total daily EE significantly covaried with both lean mass and body weight in HCR and LCR both before (p<0.001 for both lean mass and body weight) and after calorie restriction (p=0.002 for body weight and 0.043 for lean mass). At baseline, when either lean mass or body weight were taken into account in separate analyses, female HCR showed significantly higher total daily EE than female LCR (p<0.001). After a 21-day calorie restriction, on the other hand, no differences were observed between HCR and LCR total daily EE. When analyzing the change in total EE, using change in body weight or change in lean mass as the covariate, HCR and LCR significantly differed, where HCR showed a greater change in total EE for a given change in weight or lean mass (p<0.001; Figure 3A). As a percent of their baseline total daily EE [(baseline total daily EE - post-calorie restriction total daily EE)/baseline total daily EE], HCR suppressed their percent total daily EE by 31.6% whereas LCR showed a 28.6% suppression.
Figure 3: Energy expenditure (EE) in female high- and low-capacity rats (HCR and LCR) before and after calorie restriction (CR).
Change in body weight was taken into account using analysis of covariance. When analyzed against change in body weight, change in total daily EE (A) significantly differed between female HCR and LCR. Change in resting EE (B) did not differ between HCR and LCR. When analyzed using change in body weight as the covariate, change in non-resting EE (C) significantly differed between HCR and LCR. *(in the legend) LCR significantly different from HCR within condition; p<0.05.
For resting EE in females, covariance analyses showed no significant difference in lean mass- or body weight-corrected resting EE between female HCR and LCR before or after calorie restriction. When examining the decrease in resting EE using change in weight (Figure 3B) or change in lean mass as the covariate, however, resting EE was significantly lowered after CR (p=0.004 and 0.002, respectively), with no significant difference between HCR and LCR There was no significant difference between HCR and LCR female rats in the percent suppression of resting EE compared to baseline [(baseline resting EE - post-calorie restriction resting EE)/baseline resting EE] between HCR and LCR; HCR suppressed their resting EE by 29.7% and LCR suppressed it by 30.2%.
In female rats, when either lean mass or body weight was taken into account, HCR had significantly higher non-resting EE before calorie restriction (p=0.011 for BW and p=0.004 for lean mass), but these differences were not observed after 21 days of calorie restriction). Similarly, when the decrease in non-resting EE was compared between HCR and LCR using change in weight or lean mass as a covariate, there was a significant group difference where HCR showed a greater suppression in non-resting EE for a given change in weight or lean mass (Figure 3C; p<0.001). There was a significant difference between the percent suppression of non-resting EE compared to baseline non-resting EE [(baseline non-resting EE - post-calorie restriction non-resting EE)/baseline non-resting EE] between HCR and LCR; HCR suppressed their non-resting EE by 34.6%, whereas LCR showed a 23.5% suppression (p=0.040). Analyses of residuals reinforced this conclusion that female HCR had higher total and non-resting, but not resting, EE before 50% CR, but not after CR, resulting in a greater decrease in total and non-resting EE in HCR.
Males.
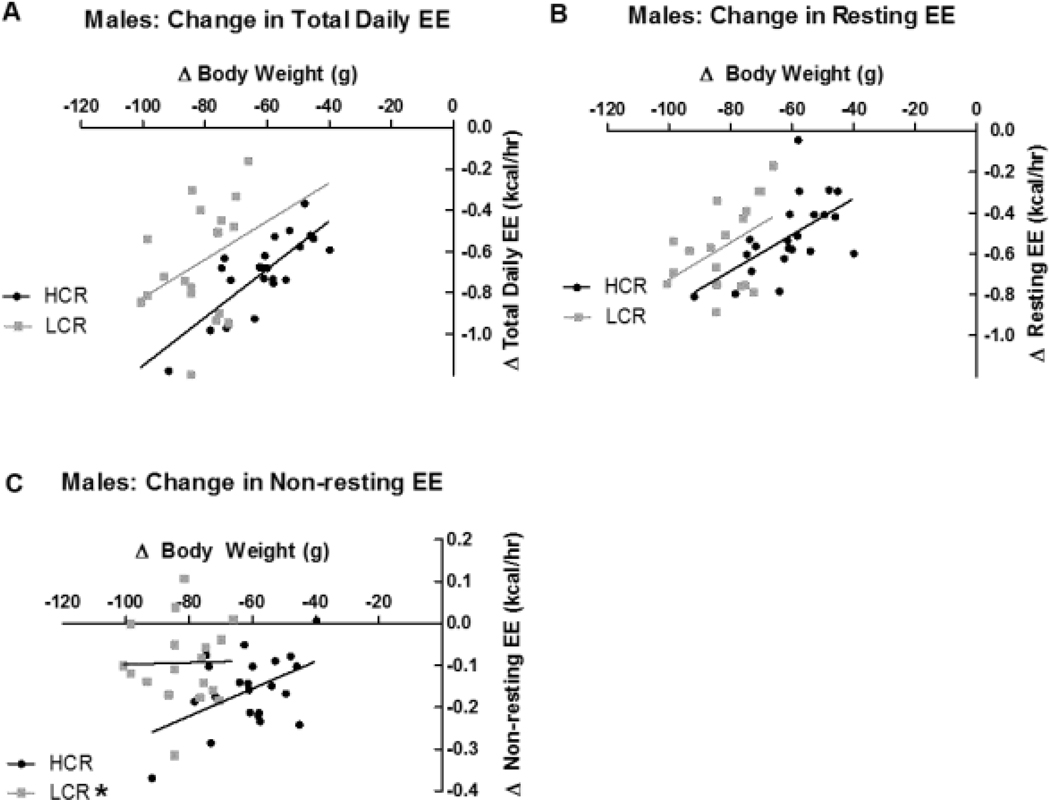
Total daily EE was higher in LCR due to their much greater mass; once body weight or lean mass were taken into account as covariates, there were no differences in total EE between HCR and LCR either before or after restriction. In both HCR and LCR, calorie restriction significantly suppressed total daily EE ( HCR, p<0.001; LCR, p<0.001) when either body weight or lean mass was taken into account. In males, once individual changes in body weight were taken into account, HCR and LCR showed similar suppression of total EE for a given weight loss (i.e., no significant difference between phenotypes); with change in lean mass as the covariate, however, HCR showed a significantly greater suppression in total EE than LCR (p=0.029). HCR suppressed their total daily EE by 30.1 % and LCR suppressed it by 25.7%.
As with total EE, resting EE was higher in LCR due to their larger size, but no phenotypic differences were seen once covariates (weight or lean mass) were considered, either before or after food restriction. In both HCR and LCR males, calorie restriction significantly suppressed resting EE compared to their respective baselines in both covariate analyses (p<0.001 for both HCR and LCR), with similar resting EE suppression (compared to baseline) of 31.2% in HCR and 30.% in LCR, with no significant difference between HCR and LCR at either time point. When the change in resting EE was calculated and compared using change in weight or lean mass as a covariate, no significant phenotypic differences were found.
There were no phenotypic differences in non-resting EE, despite the larger body size of LCR. When the suppression of non-resting EE was compared using change in weight or lean mass as a covariate, HCR showed a significantly greater change in non-resting EE than LCR for a given decrease in weight (Figure 4C; p=0.014) or lean mass (p=0.002). In univariate analysis without covariance, calorie restriction significantly suppressed non-resting EE (p <0.001), with a significant interaction (p=0.030) where HCR showed a greater suppression of non-resting EE, even carrying less load (i.e., weight) and with less overall change in weight and lean mass. HCR suppressed their non-resting EE by 23.6% whereas LCR showed a 13.8% suppression. Analyses of residuals reinforced the conclusion that male HCR showed a greater change in total and non-resting EE.
Figure 4: Energy expenditure (EE) in male high- and low-capacity rats (HCR and LCR) before and after calorie restriction (CR).
Change in body weight was taken into account using analysis of covariance. When analyzed against change in body weight, change in total daily EE (A) did not significantly differ between male HCR and LCR. Change in resting EE (B) did not differ between HCR and LCR. When analyzed using change in body weight as the covariate, change in non-resting EE (C) significantly differed between HCR and LCR. *different from HCR within condition; p<0.05.
Maximal oxygen consumption (VO2max)
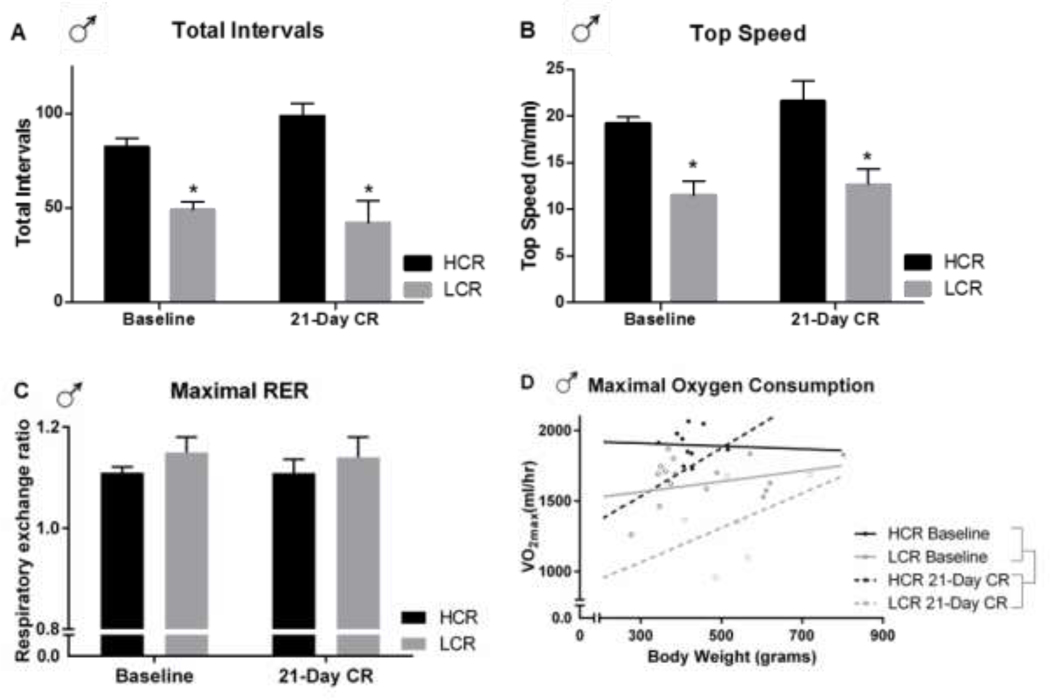
Male HCR and LCR were assessed for VO2max before and after their second exposure to calorie restriction. With respect to running performance, calorie restriction did not significantly impair either the distance run (the number of 10-second running intervals completed, p=0.376) or the maximal running speed achieved (p=0.068; Figure 5A, B), with no main effects of calorie restriction. There was no significant interactions in either the number of running intervals completed (p=0.051 with a trend towards an interaction for an increase in HCR and a decrease in LCR) or the top speed attained (p=0.500) between the HCR/LCR and calorie restriction. Moreover, the trend observed in the top speed attained (p=0.068) suggested an increase, not a decrease, in running endurance after CR matching the trend towards increase in running interval in HCR. As expected, there was a significant main effect of line (HCR>LCR, p<0.001) where HCR ran more intervals and achieved higher speeds both before and after calorie restriction (p<0.001 for both), consistent with their artificially selected phenotypes (38) and consistent with their running performance during phenotyping. RER during the VO2max treadmill test was not significantly affected by calorie restriction in either HCR or LCR (p=0.259; Figure 5C). There was no significant interaction (p=0.864) or main effect of line (p=0.975) observed for RER.
Figure 5: Running performance and VO2max in male high- and low-capacity rats (HCR, LCR) before and after calorie restriction (CR).
(A) The total number of 10-sec running intervals completed and (B) the top speed attained in a VO2max treadmill test were both significantly higher in HCR than LCR, both before and after CR. CR did not significantly suppress running performance, either top speed or total intervals completed. (C) At both baseline and after 21-day CR in male HCR and LCR, there was no significant suppression of, or group difference in, the maximal respiratory exchange ratio (RER; VCO2/VO2) reached during the VO2max treadmill test. (D) At both baseline and after 21-day CR, maximal oxygen consumption (VO2max in ml/hr) was significantly higher in male HCR compared to LCR, and VO2max was suppressed in both HCR and LCR after CR compared to their respective baselines. *difference between HCR and LCR at the given time point; double brackets signify main effect of CR; p<0.05
The maximal oxygen consumption reached (VO2max, ml/hr) significantly decreased after calorie restriction compared to baseline when either body weight or lean mass were taken into account as covariates (Figure 5D; p=0.001); The significant reduction of VO2max suggests an increase in energy efficiency of running with food restriction. Compared to LCR, HCR had higher VO2max (ml/hr) both before (p=0.032) and after (p=0.010) calorie restriction (Figure 5D), again, there was a main effect of line (p=0.001) where HCR had higher O2 consumption.
DISCUSSION
Maintenance of weight loss is a common struggle (10, 51), made even more difficult because of adaptive thermogenesis (12), yet the factors or traits that influence or predict the magnitude of adaptive thermogenesis have yet to be identified. For example, physical activity and the associated EE would be predicted to lessen adaptive thermogenesis during negative energy balance (10, 42), but this contribution has not been quantified. We investigated the impact of an important health-related trait—intrinsic aerobic capacity (10, 14, 37)—on adaptive thermogenesis using the only known laboratory model of this trait, HCR and LCR rats artificially selected for high and low intrinsic aerobic capacity (38). Suppression of non-resting EE is the key factor differentiating the adaptive thermogenic response of HCR and LCR to food restriction. The high baseline non-resting EE typically associated with high intrinsic aerobic capacity, stemming from high physical activity levels and low locomotor efficiency (22, 42), held a greater capacity for suppression during energy restriction. The greater suppression in non-resting EE during energy restriction was identified in both males and females, despite the considerable sex difference in body size. Thus, focusing on non-resting EE of rats with high and low aerobic capacity as the potential differentiating factor in response to energy restriction could help identify potential mechanisms underlying differential metabolic adaptation to food restriction.
Adaptive thermogenic responses to energy restriction were seen in both male and female HCR/LCR rats. Consistent with our previous findings (22), at baseline, female HCR and LCR showed no difference in resting EE, with HCR having higher non-resting EE. However, after calorie restriction, covariance analysis identified no significant difference between female HCR and LCR in either resting or non-resting EE. The HCR suppressed the non-resting EE thereby lowering their non-resting EE to levels seen in LCR (Figure 3C). For resting EE in males, HCR and LCR responded to calorie restriction similarly, with no phenotypic difference either before or after calorie restriction. As seen in females, in male rats, calorie restriction was more effective in suppressing non-resting energy expenditure of male HCR compared to male LCR. Thus, in both males and females, calorie restriction differentially affected resting and non-resting EE between phenotypes, and the factor differentiating HCR from LCR was the extent of adaptation in non-resting EE. Although food restriction impacted resting EE in both male and female rats, the extent of this suppression was consistently similar between phenotypes. Adaptive thermogenesis in non-resting EE, on the other hand, occurred proportional to rats’ baseline levels, suggesting that LCR already have a relatively blunted non-resting EE even without food restriction or reduced weight. Thus, calorie restriction suppressed non-resting EE to reach similar levels in HCR and LCR (Figure 3C and 4C). This was also reflected in rats’ physical activity levels. HCR were more active than LCR before calorie restriction, consistent with established findings (22–24, 42). Similarly, in both males and females, calorie restriction significantly suppressed physical activity levels, with greater suppression seen in the HCR than LCR (Figures 1B and 2B). Thus, the relatively greater suppression of the non-resting EE seen in HCR is at least partially attributable to suppressed physical activity. Increased locomotor efficiency (23) may also factor into the suppressed non-resting EE; this was assessed here only in the context of a VO2max treadmill endurance test.
Non-resting EE depends on a combination of activity level, load, and locomotor efficiency. Weight loss results in a decrease in the total energy or kilocalories (Kcal) required to perform the daily activities of living due to increased skeletal muscle work efficiency (8), or increased fuel economy of activity (23). Locomotor efficiency is adaptable, for example activation of hypothalamic melanocortin receptors increases the number of kcal needed for the same amount of work (52), and caloric expenditure during moderate-level physical activity is higher in the lean phonotype (22, 23). HCR and LCR were selected based on their aerobic capacity, and the lower locomotor efficiency in HCR contributes to their higher non-resting EE (22, 23), but how locomotor efficiency was impacted by calorie restriction was unknown. Here, we used the VO2max graded treadmill exercise test, running the rats to exhaustion while measuring O2 consumption and EE. Consistent with their phenotype, the HCR had higher VO2max and ran significantly longer compared to LCR in terms of both time and distance, both before and after calorie restriction. Interestingly, calorie restriction did not significantly compromise the running performance—the maximal speed reached or intervals run—in either phenotype, and there was even a trend toward an increase in top speed after calorie restriction (p=0.068). The maximal respiratory exchange ratio (RER) during the VO2max test also remained unaltered by food restriction. On the other hand, calorie restriction significantly impacted running efficiency by suppressing O2 consumption after calorie restriction, even after the lower body weight was taken into consideration (Figure 5D). Thus, calorie restriction made running more efficient without compromising endurance, conserving energy and making rats less susceptible to weight loss while protecting running ability. Most phenotypic difference and plasticity in locomotor efficiency is found at lower speeds or work intensities in both rats (22, 52) and also in humans (10, 53). It would be interesting to further investigate potential differences in the locomotor-efficiency response to CR at lower workloads, and determine how this might vary with intrinsic aerobic capacity.
The findings presented here imply that resting and non-resting EE, being differentially modulated by calorie restriction, are likely to be physiologically distinct processes with at least somewhat separable underlying mechanisms. This has implications with respect to promoting and maintaining a decreased body weight. Both high- and low-capacity rats showed flexibility in their resting EE, however, marked flexibility in non-resting EE was observed only in the HCR, similar to what is seen with physical activity (Figures 1 & 2). Thus, the adaptable nature of non-resting EE appears to be diminished in the LCR. In both male and female rats, HCR showed a significantly greater absolute suppression of NREE by calorie restriction. The higher baseline non-resting EE seen in HCR, at least in females, could allow for greater adaptation during food restriction. Thus, unlike HCR, LCR have optimized their physical activity and activity-related EE to survive conditions of restricted food availability even under free-fed conditions, making LCR a thrifty phenotype. This is consistent with the idea that LCR are better suited to an environment with unreliable access to food where energy restriction is an ever-present risk.
Contrasting the variability observed in adaptive thermogenesis in humans to that seen here in rats, people who were resistant to weight loss showed more suppression of their non-resting EE with weight loss (11, 12, 54). In the context of the HCR/LCR rat model system, the suppression of non-resting EE in the more physically active HCR makes sense; because HCR had a higher activity EE at baseline compared to LCR, they were able to conserve energy through lowering their activity-related EE to adapt to limited food availability. With respect to energy balance, HCR and LCR may employ different strageies to optimize survival depending on the energy availability in their environment, with LCR maintaining relatively low non-resting EE under normal conditions and HCR suppressing their non-resting EE in the face of energy restriction. Differences in fuel utilization during exercise, where HCR are more efficient in fatty acid oxidation than LCR (55), could also have adaptive significance. Although the obesity-prone human and HCR/LCR strategies or approaches to thriftiness differ, they are similar in that they both rely on flexibility in physical activity and non-resting EE. Given that calorie restriction increases skeletal muscle efficiency in humans (8), changes in muscle energy use could impact non-resting EE, and likely resting EE as well. Hence, targeting appropriate mechanisms— behavioral and skeletal muscle—to offset the suppression of activity EE could be an effective strategy to counter the tendency to re-gain weight, especially in more energetically “thrifty” individuals. Hypothetically, HCR and LCR may show different short-term adaptive thermogenic responses during positive energy balance as well, with increases in EE. LCR gain more weight and display hyperphagia when on a high-fat diet (45, 56). The LCR also gain more weight over time compared to HCR at normal ad libitium food intake conditions (23). With just 3 days of access to a high-fat diet, LCR show more weight gain and energy intake, higher RER with lower indices of lipid utilization, and lower weight-adjusted resting EE (57). With respect to human health, those who show less adaptability during negative energy balance also show higher adaptability during positive energy balance (12). Flexibility in NEAT (part of non-resting EE) also predicts fat gain during overfeeding in people (16), supporting the role of non-resting or activity-related EE in adaptive thermogenesis even during positive energy balance. Given the evident importance of flexibility in non-resting EE to human obesity (10, 54), the underlying mechanisms linking this to aerobic capacity may give insights relevant to human health. Moreover, aerobic capacity could potentially account for unexplained variance in human studies.
The phenotypic difference in body weights between HCR and LCR is robust and consistent between studies (22). Moreover, the differences in energy expenditure components at baseline in HCR and LCR are similar to what has been seen in our previously published studies using rats of a different generation of selection (22). Previous studies have shown that calorie restriction has resulted in a greater proportional weight loss in HCR compared to LCR (42). Here, however, we observed a significant overall weight loss in HCR and LCR without this group differences. This is unlikely to be due to genetic drift in the selected lines because the generations of selection from which rats from the different studies were drawn overlapped between these studies (42). The disparity between studies with respect to food restriction-induced weight loss could stem from differences in animal handling (58), temperature variations, cage size, or subtle differences in the severity of the calorie restriction inflicted.
In summary, when high- and low-capacity rats are subjected to calorie restriction, inherently lean HCR adapted by suppressing their normally elevated levels of spontaneous physical activity and non-resting EE, making their metabolism equally energy efficient to the LCR. These results are consistent across male and female rats. These results suggest that individual differences in calorie restriction-induced adaptive thermogenesis may be accounted for by variations in NEAT that are mechanistically linked to aerobic capacity. Specifically, it is likely activity EE, not resting or basal metabolism, accounts for individual variation in adaptive thermogenesis.
Highlights:
Food restriction suppresses energy expenditure, inducing adaptive thermogenesis
We compared rats selectively bred for low & high aerobic exercise capacity—LCR/HCR
Calorie restriction suppressed both resting and non-resting energy expenditure
Food restriction induced a greater suppression of non-resting EE in HCR than LCR
Aerobic capacity may determine metabolic response to energy restriction
ACKNOWLEDGEMENTS
This work was funded by National Institutes of Health (NIH) grants NIH R01NS055859 and NIH R15DK097644. We would also like to acknowledge the care taken by the animal facility and Kent State University and all the current and previous graduate (Dr. Charu Shukla, Dr. Chaitanya Kumar Gavini, Lydia Heemstra, Dr. Mark E Smyers) and undergraduate (Addison Spriggs, Emily Hitchens, Derek Lapp, Emily Cosentino, Eric Dyne) students in the laboratory for providing us with their help. We would like to thank David Costello for his help with statistical analysis.The LCR-HCR rat model system was funded by the Office of Research Infrastructure Programs grant P40OD021331 (to LGK and SLB) from the National Institutes of Health. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. The LCR-HCR rat models were funded in part by the NIH P40OD021331 (LGK and SLB) and are maintained as an animal model resource for researchers at the University of Toledo, Toledo, OH. Contact LGK Lauren.Koch2@UToledo.Edu or SLB brittons@umich.edu for information.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–8. [DOI] [PubMed] [Google Scholar]
- 3.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108(13):1541–5. [DOI] [PubMed] [Google Scholar]
- 4.Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR. Obesity and quality of life: mediating effects of pain and comorbidities. Obes Res. 2003;11(2):209–16. [DOI] [PubMed] [Google Scholar]
- 5.Riboli E. The cancer-obesity connection: what do we know and what can we do? BMC biology. 2014;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24(8):1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond). 2010;34 Suppl 1:S47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucet E, McInis K, Mahmoodianfard S. Compensation in response to energy deficits induced by exercise or diet. Obes Rev. 2018;19 Suppl 1:36–46. [DOI] [PubMed] [Google Scholar]
- 12.Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, et al. A Human Thrifty Phenotype Associated With Less Weight Loss During Caloric Restriction. Diabetes. 2015;64(8):2859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotz CM, Levine JA. Role of nonexercise activity thermogenesis (NEAT) in obesity. Minn Med. 2005;88(9):54–7. [PubMed] [Google Scholar]
- 14.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab. 2004;286(5):E675–85. [DOI] [PubMed] [Google Scholar]
- 15.Levine JA. Nonexercise activity thermogenesis--liberating the life-force. J Intern Med. 2007;262(3):273–87. [DOI] [PubMed] [Google Scholar]
- 16.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. [DOI] [PubMed] [Google Scholar]
- 17.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. The American journal of clinical nutrition. 1999;69(3):373–80. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr., et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA : the journal of the American Medical Association. 1996;276(3):205–10. [PubMed] [Google Scholar]
- 19.Fredriksen PM, Veldtman G, Hechter S, Therrien J, Chen A, Warsi MA, et al. Aerobic capacity in adults with various congenital heart diseases. The American journal of cardiology. 2001;87(3):310–4. [DOI] [PubMed] [Google Scholar]
- 20.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. Jama-J Am Med Assoc. 1999;282(16):1547–53. [DOI] [PubMed] [Google Scholar]
- 21.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Archives of internal medicine. 2005;165(18):2114–20. [DOI] [PubMed] [Google Scholar]
- 22.Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58(3):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, et al. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One. 2009;4(6):e5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine JA, Kotz CM. NEAT--non-exercise activity thermogenesis--egocentric & geocentric environmental factors vs. biological regulation. Acta physiologica Scandinavica. 2005;184(4):309–18. [DOI] [PubMed] [Google Scholar]
- 26.Levine JA. Non-exercise activity thermogenesis (NEAT). Nutrition reviews. 2004;62(7 Pt 2):S82–97. [DOI] [PubMed] [Google Scholar]
- 27.Levine JA. Non-exercise activity thermogenesis (NEAT). Best Pract Res Clin Endocrinol Metab. 2002;16(4):679–702. [DOI] [PubMed] [Google Scholar]
- 28.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Current opinion in clinical nutrition and metabolic care. 2004;7(6):599–605. [DOI] [PubMed] [Google Scholar]
- 29.Britton SL, Koch LG. Animal models of complex diseases: an initial strategy. IUBMB life. 2005;57(9):631–8. [DOI] [PubMed] [Google Scholar]
- 30.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52. [DOI] [PubMed] [Google Scholar]
- 31.Koch LG, Meredith TA, Fraker TD, Metting PJ, Britton SL. Heritability of treadmill running endurance in rats. Am J Physiol. 1998;275(5):R1455–60. [DOI] [PubMed] [Google Scholar]
- 32.Burghardt PR, Flagel SB, Burghardt KJ, Britton SL, Gerard-Koch L, Watson SJ, et al. Risk-assessment and coping strategies segregate with divergent intrinsic aerobic capacity in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(2):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109(10):1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–6. [DOI] [PubMed] [Google Scholar]
- 35.Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol. 2007;19(12):923–40. [DOI] [PubMed] [Google Scholar]
- 36.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest. 1995;95(3):980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladenvall P, Persson CU, Mandalenakis Z, Wilhelmsen L, Grimby G, Svardsudd K, et al. Low aerobic capacity in middle-aged men associated with increased mortality rates during 45 years of follow-up. Eur J Prev Cardiol. 2016;23(14):1557–64. [DOI] [PubMed] [Google Scholar]
- 38.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends in cardiovascular medicine. 2012;22(2):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch LG, Britton SL. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity (Silver Spring). 2008;16 Suppl 3:S28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290(2):E396–403. [DOI] [PubMed] [Google Scholar]
- 42.Smyers ME, Bachir KZ, Britton SL, Koch LG, Novak CM. Physically active rats lose more weight during calorie restriction. Physiol Behav. 2015;139:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465(7299):690. [DOI] [PubMed] [Google Scholar]
- 45.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293(1):E31–41. [DOI] [PubMed] [Google Scholar]
- 46.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academies Press; 2011. xxv, 220 p. p. [Google Scholar]
- 48.Gorrell E, Shemery A, Kowalski J, Bodziony M, Mavundza N, Titus AR, et al. Skeletal muscle thermogenesis induction by exposure to predator odor. J Exp Biol. 2020;223(Pt 8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda). 2014;29(6):413–20. [DOI] [PubMed] [Google Scholar]
- 50.Schoeller DA, Jefford G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes Relat Metab Disord. 2002;26(1):97–101. [DOI] [PubMed] [Google Scholar]
- 51.Hall KD. Diet versus exercise in “the biggest loser” weight loss competition. Obesity (Silver Spring). 2013;21(5):957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavini CK, Jones WC 2nd, Novak CM. Ventromedial hypothalamic melanocortin receptor activation: Regulation of activity energy expenditure and skeletal muscle thermogenesis. The Journal of physiology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–92. [DOI] [PubMed] [Google Scholar]
- 54.Most J, Redman LM. Impact of calorie restriction on energy metabolism in humans. Exp Gerontol. 2020;133:110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015;21(3):468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35(2):151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2014;307(4):E355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature methods. 2014;11(6):629–32. [DOI] [PubMed] [Google Scholar]