Abstract
The cell surface is a mechanobiological unit that encompasses the plasma membrane, its interacting proteins, and the complex underlying cytoskeleton. Recently, attention has been directed to the mechanics of the plasma membrane, and in particular membrane tension, which has been linked to diverse cellular processes such as cell migration and membrane trafficking. However, how tension across the plasma membrane is regulated and propagated is still not completely understood. Here, we review recent efforts to study the interplay between membrane tension and the cytoskeletal machinery and how they control cell form and function. We focus on factors that have been proposed to affect the propagation of membrane tension and as such could determine whether it can act as a global or local regulator of cell behavior. Finally, we discuss the limitations of the available tool kit as new approaches that reveal its dynamics in cells are needed to decipher how membrane tension regulates diverse cellular processes.
Keywords: Membrane tension, Membrane flow and propagation, Membrane-to-cortex attachment, Membrane curvature, Cell migration, Cell surface mechanics
Introduction
The cell surface is a unique and complex physical system. Rapid modulation of mechanical properties and forces at the cell periphery enables cells to change shape and perform specialized functions such as cell motility, where cell surface mechanics control cell polarity [1, 2, 3, 4], cell retraction [5], and cell adhesion to the extracellular environment [6]. Recently, it has also become clear that surface mechanics can affect cell identity [7, 8, 9, 10]. In this review, we focus on membrane tension and discuss whether it can regulate cell behavior locally or globally. Moreover, we highlight the need for cleaner tools to decipher its functions and dynamics.
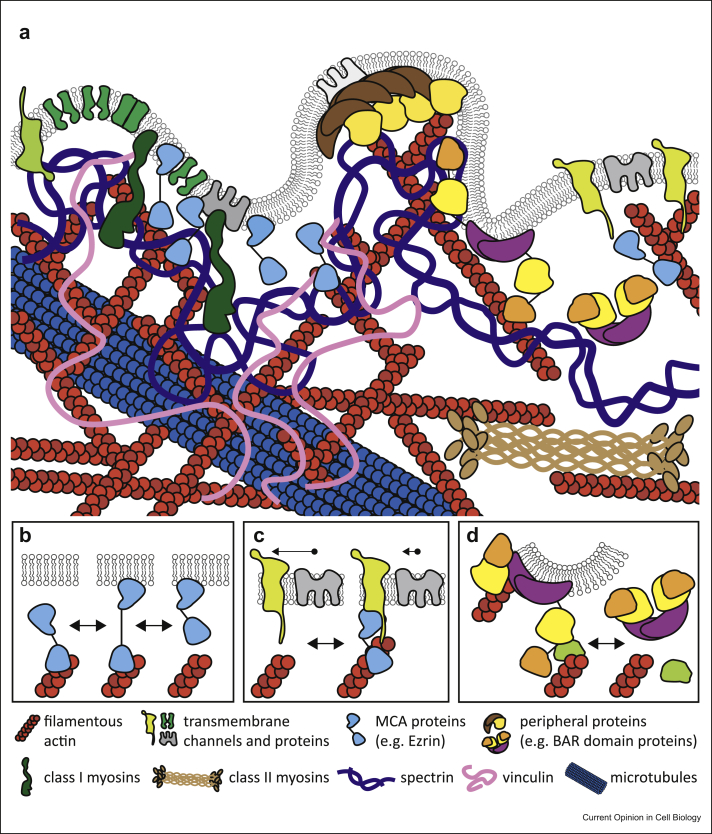
The cell surface comprises the plasma membrane, which is a lipid bilayer with transmembrane and membrane-bound proteins and sugars, as well as an underlying actomyosin cortical cytoskeleton or cell cortex. These components of the cell surface are closely interconnected by nonspecific molecular interactions and a layer of distinct proteins (such as the ERM (Ezrin, Radixin, Moesin), or Myo1 families) whose composition varies in different cell types. Moreover, intermediate filaments and networks of spectrins are also more or less prominent at the cell surface in a cell type–dependent manner (Figure 1a).
Figure 1.
The cell surface interactome. (a) The cell surface encompasses the lipid bilayer (with associated sugars not depicted for simplicity) and a broad variety of proteins (described in the graphical legend at the bottom). Examples of membrane–cytoskeleton interactions governing plasma membrane mechanics: (b) MCA proteins influence cell surface mechanics by dynamically binding to actin and thus affecting the surface viscous drag. (c) Transmembrane proteins can interact directly or indirectly (e.g. via MCA linkers) with the underlying cytoskeleton and affect diffusion of other molecules in the plasma membrane. (d) The modular architecture of BAR (Bin/Amphiphysin/Rvs) domain proteins allows dynamic remodeling of the plasma membrane and actin cytoskeleton by combining a BAR domain that can sense/generate curvature with auxiliary domains (SH3, PX, PH, RhoGEF, RhoGAP domains, reviewed in the study by Carman and Dominguez [11]). MCA, membrane-to-cortex-attachment.
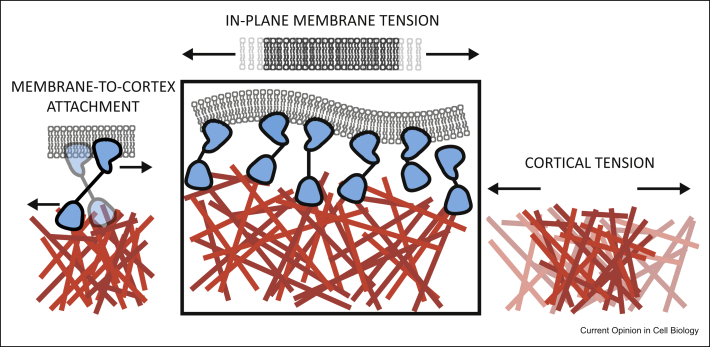
Mechanically, both the membrane and the actomyosin cortex can be characterized by their tension. Cortical tension is reviewed elsewhere in this issue (Charras et al.). The main focus of this review is membrane tension, which is a measure of the energetic cost of increasing the membrane area (measured in J/m2 = N/m). In lipid vesicles, in-plane membrane tension can propagate globally because a lipid bilayer has the properties of a 2D liquid, allowing rapid diffusion of its components. However, in cells, plasma membrane tension can be greater and more local than in pure lipid vesicles because of the following factors: peripheral protein binding, the presence of transmembrane proteins, and interactions with the underlying actomyosin cortex, governed by proteins with ability to interact simultaneously with both the plasma membrane and filamentous actin (membrane-to-cortex adhesion energy or membrane-to-cortex attachment, MCA). These factors provide additional resistance to membrane area changes and can constrain such changes to a localized area of the cell (Figure 2).
Figure 2.
Mechanics of the plasma membrane and the underlying actomyosin cortex. Both the plasma membrane (gray) and the cortex (red) can be described by their tension, a measure of the energetic cost of increasing their area. In cells, membrane tension arises from both in-plane tension (final distance between lipids exaggerated for clarity) and membrane-to-cortex-attachment (MCA, blue).
Current methods used to quantify plasma membrane tension involve using atomic force spectroscopy and optical or magnetic tweezers to measure the force required to pull and hold tubes of the plasma membrane called tethers [12, 13, 14]. During these static tether pulling experiments, the resistive force depends on both the bending rigidity (a measure of the energy associated with membrane bending, measured in kBT or Nm) and the tension of the membrane, with the latter arising from both in-plane tension and MCA in cells. MCA can be characterized by dynamic tether pulling, where force measurements are performed during the tether extraction, before the lipid flow has a chance to equilibrate. MCA proteins then act as moving ‘obstacles’ against the flow of the lipids toward the tether, generating a viscous drag (Figure 1b). By pulling tethers from the same cell at different extraction velocities and fitting purposed continuum models to the force–velocity curve, this viscous drag can be quantified [15,16].
To flow or not to flow?
Plasma membrane tension has been shown to regulate a plethora of cellular processes ranging from membrane trafficking [17,18], cell spreading [19,20], phagocytosis [18], and cell polarity [1,21]. During cell motility, current models suggest that actin polymerization at the leading edge increases membrane tension, which in turn acts as a long-range inhibitor of protrusions elsewhere in the cell. To drive such large-scale cellular organization, membrane tension would need to quickly propagate throughout the surface of a cell.
Whether membrane tension can indeed propagate rapidly in cells and have widespread effects on cell behavior or only results in local perturbations has recently been questioned [22,23]. Several studies suggest that membrane tension could account for long-range communication between distant parts of cells [1,4,∗24, 25, 26]. In neutrophils, increasing surface tension by micropipette aspiration was shown to inhibit leading-edge formation and signaling within seconds, while a decrease resulted in the opposite effect [1]. Along these lines, migrating keratocytes exhibit changes in leading-edge actin dynamics and protrusion velocity upon micropipette aspiration at the rear of a cell [24]. Thus, mechanical communication appears on the timescale of seconds, indicating that membrane tension can propagate globally and rapidly through the plasma membrane of a cell. However, recent data from the Cohen laboratory is challenging this view. Upon measuring mechanical coupling between two membrane tethers being at 5–15 μm apart, they concluded that although changes in membrane tension propagated in blebs (herniations of the plasma membrane), no long-range propagation was observed in cells over a timescale of 10 min [22].
How can these seemingly opposing results be reconciled? Cohen and Shi [23] suggest that the lack of propagation of membrane tension changes can be explained by the impediment of membrane flow caused by transmembrane proteins interacting with the underlying cytoskeleton, which could vary between cell types. Indeed, cortical thickness and architecture vary between cell types and subcellular regions [27,28] and so does the expression of MCA and transmembrane proteins. Moreover, the binding of such proteins to the underlying cytoskeleton can also influence diffusion of other components in the plasma membrane [29] (Figure 1c). Thus, the ability to cluster or immobilize obstacles that could affect the propagation of membrane tension comes in part from biochemical regulation but can also be dynamically influenced by other proteins interacting directly or indirectly with the plasma membrane. Recent evidence highlights how cytoskeletal components such as vinculin, spectrin, microtubules, class I and II myosins, or MCA proteins can regulate membrane tension [6,30, 31, 32, 33, 34, 35]. However, whether and how they contribute to the propagation of membrane tension is still unclear.
Another important characteristic of the plasma membrane that could contribute to the differences seen in the propagation of membrane tension by different studies is the fact that it is a 2D structure that occupies a 3D space. Membrane deformations, also referred to as membrane curvature, have been extensively studied in vitro in the context of several membrane binding/remodeling proteins [36], and the relationship between tension and curvature is well understood for these simplified systems [37, 38, 39]. Interestingly, MCA proteins such as Ezrin can change their membrane tethering abilities depending on interactions with actin and curvature-sensing binding partners [40]. Thus, it is plausible that differences in the curvature landscape of a cell could affect the propagation of membrane tension by influencing obstacle distribution and binding (Figure 1d). In cells, the relationship between membrane tension and curvature is more complex than that in vitro. Actin polymerization can deform the plasma membrane and increase its tension. At the same time, curved regions can lead to further actin polymerization by recruiting BAR (Bin/Amphiphysin/Rvs) domain proteins that can bind and activate WASP (Wiskott-Aldrich Syndrome protein) and WAVE (Wiskott-Aldrich Syndrome protein family member), canonical actin polymerization complexes [41, ∗42, ∗43]. Some stereotypic structures, such as endocytic vesicles [43], caveolae [44], and eisosomes [45], act as hubs for mechanoadaptation by translating changes in membrane tension into biochemical signaling. However, the nature and dimensions of plasma membrane deformations exist beyond these well-known structures [46]. The prevalence, shape, size, and dynamics of those noncanonical deformations and how they might affect protein activity that regulates cell surface mechanics remain unexplored because of the limited methods for studying them in living cells.
Furthermore, we should also consider the diversity of cell migration modes and speeds and how that might affect the propagation of membrane tension. Cell types whose behavior is consistent with instantaneous propagation of membrane tension include the fastest migrating cells in the human body (immune cells ∼10 μm/min) and fish keratocytes, which achieve comparable speeds [47,48]. In contrast, other cell types that do not display a long-range propagation of membrane tension changes (HeLa, NIH 3T3, MDCK, hippocampal neurons) migrate at much lower speeds (∼0.1–0.5 μm/min) [22]. These two orders of magnitude difference in migration speed are the consequence of changes in cell surface mechanics and adhesion to the substrate but could also explain the presence or absence of membrane tension propagation. In turn, that propagation could tune these cell surface mechanics that determine cell speed (Table 1).
Table 1.
Summary of tether force values and cell migration speeds for different cell types.
| Cell type | Speed [μm/min] | Tether force [pN] | Methods (tether force) | How fast does membrane tension propagate? |
|---|---|---|---|---|
| Neurons | 0.05–0.7 [50,51] | 7-32 [52, 53, 54] | OT | >10 min [22] |
| HeLa | 0.06 [55] | 13 [56] | OT | >10 min [22] |
| NIH3T3 | 0.08 [57,58] | 7-40 [59, 60, 61, 62] | OT | >10 min [22] |
| MEFs | 0.2 [56] | 10 [63] | OT | |
| COS-1 | 0.28–0.5 [64] | 25 [64,65] | AFS | |
| MDCK | 0.55 [65] | 50 [66] | AFS | >10 min [22] |
| Macrophages/BMDM | 1–2.5 [67,68] | 30-70 [18,53] | OT | |
| Microglia | 1.1 [69] | 60 [53] | OT | |
| A2780 | 1.2 [5] | 50-72 [5] | OT | A front-rear tension gradient in a stiffness gradient suggests a slow (>∼minutes) tension propagationa [5] |
| Zebrafish prechordal plate cells | 5 [70] | 30 [70] | AFS | |
| Neutrophil-like HL60 cells | 7.5 [25] | 37-50 [4,25] | AFS | <∼secondsa [1,4,25,26] |
| Keratocytes | 12 [71] | 55 [47] | OT | <∼secondsa [47,71, 72, 73] |
| T lymphocytes | 15 [74] | 45 [74] | OT |
OT: optical tweezers, AFS: atomic force spectroscopy.
These studies did not directly measure the propagation of membrane tension.
Finally, the mechanics of the environment can also play a role in the propagation of membrane tension. In migrating human ovarian cancer cells, a gradient in membrane tension between the front and rear of the cell can be observed only when cells migrate on a rigidity gradient [5]. However, whether substrate stiffness alone changes membrane tension and/or its propagation is not completely understood [5,49]. Altogether, we need a more detailed and quantitative picture of the curvature landscape and MCA in a broad range of cell types in diverse environments to assess whether and how the composition and cross-linking of the cell surface affects membrane flow, biochemical signaling, and cell function.
A new toolkit to assess and perturb membrane mechanics
We are only at the very beginning of grasping how membrane mechanics regulates cell behavior. This is due in large part because of limited methods to specifically measure and perturb it and because the various mechanical systems at the cell surface are closely interconnected and therefore difficult to measure independently. As discussed in the Introduction, the current gold standard to measure membrane tension and MCA relies on tether pulling. As such, its temporal and spatial resolution might not be sufficient to resolve differences in fast and dynamic processes. Moreover, actin often polymerizes inside tethers within minutes, challenging long-term measurements. In addition, the current models that have been built to interpret the existing data are likely to be incomplete or not sufficiently accurate because they do not consider the mechanics or the dynamics of the underlying cell cortex [16]. To study membrane tension propagation and fluctuations in the context of dynamic processes such as cell migration and cell division, there is a need to develop robust sensors.
In recent work, Colom et al [75] presented a novel fluorescent probe (FliptR, fluorescent lipid tension reporter) that reports changes in lipid packing resulting from a combination of membrane tension and lipid composition changes. FliptR was calibrated to measure membrane tension in cells (MDCK, HeLa) and giant unilamellar vesicles of specific compositions and has already been used in yeast, cancer, and HeLa-derived cells [5,76,77]. As such, fluorescent probes hold a great potential in membrane tension studies by allowing measurements in multicellular environments and with a time resolution previously inaccessible by conventional tether pulling experiments. We envision that the FliptR probe will be used in more systems in the near future; however, it is essential to bear in mind that a rigorous calibration of the probe in every cell type is necessary to obtain accurate membrane tension measurements.
As plasma membrane tension is a collective result of both in-plane lipid bilayer tension and MCA, it would be preferable to control their separate contributions to changes in membrane tension. To that end, our laboratory has engineered a synthetic molecular tool that can directly link the plasma membrane to actin but is inert regarding signaling (inert membrane to cortex linker), and we have recently used it to show that its expression forces mouse embryonic stem cells to retain their naive pluripotent state [10]. Combining similar tools with different diffusion and binding kinetics or specific probes that report changes in lipid composition, with robust and calibrated membrane tension sensors, will further advance our understanding of the dynamics of surface mechanics in cells. Moreover, we will be able to decipher what functions previously associated to some proteins are actually a consequence of their mechanical roles and not a result of their signaling abilities.
Conclusions
Owing to the complex biochemical and biophysical interplay between the plasma membrane and the underlying cytoskeleton, the greatest challenge for cell surface mechanics in the upcoming years lies in understanding how its components, individually and in combination, regulate cell behavior. Specifically, for membrane tension and its propagation, we need to quantify the diffusion and binding kinetics of MCA proteins and grasp how those are affected by membrane topology. To that end, we require new tools with high spatiotemporal control that are verified in a plethora of biological systems, as membrane tension propagation can have multiple roles depending on cell function or state. Moreover, it will be interesting to apply these tools to also study the role of other cytoskeletal components, such as microtubules or intermediate filaments, in membrane mechanics. Together, these will greatly improve our understanding of the role of forces acting at the cell surface.
Credit author statement
Ewa Sitarska: Conceptualization, Writing - original draft, Writing - review & editing, Funding acquisition. Alba Diz-Muñoz: Conceptualization, Writing - original draft, Writing - review & editing, Funding acquisition.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors thank Robert Prevedel, Andela Saric, Marianne Sandvold Beckwith, and Andrea Imle for critical reading of the manuscript. The authors acknowledge the financial support of the European Molecular Biology Laboratory (EMBL), the Human Frontiers Science Program (HFSP) grant number RGY0073/2018, and the Deutsche Forschungsgemeinschaft (DFG) grant numbers DI 2205/2–1 and DI 2205/3–1 to A.D.-M. and the Joachim Herz Stiftung Add-on Fellowship for Interdisciplinary Science to E.S.
This review comes from a themed issue on Cell Dynamics
Edited by Diane Barber and Xavier Trepat
References
- 1.Houk A.R., Jilkine A., Mejean C.O., Boltyanskiy R., Dufresne E.R., Angenent S.B., Altschuler S.J., Wu L.F., Weiner O.D. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabaud M., Heuzé M.L., Bretou M., Vargas P., Maiuri P., Solanes P., Maurin M., Terriac E., Le Berre M., Lankar D. Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells. Nat Commun. 2015;6:7526. doi: 10.1038/ncomms8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruprecht V., Wieser S., Callan-Jones A., Smutny M., Morita H., Sako K., Barone V., Ritsch-Marte M., Sixt M., Voituriez R. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell. 2015;160:673–685. doi: 10.1016/j.cell.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diz-Muñoz A., Thurley K., Chintamen S., Altschuler S.J., Wu L.F., Fletcher D.A., Weiner O.D. Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002474. e1002474–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetmanski J.H.R., de Belly H., Busnelli I., Waring T., Nair R.V., Sokleva V., Dobre O., Cameron A., Gauthier N., Lamaze C. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev Cell. 2019;51:460–475. doi: 10.1016/j.devcel.2019.09.006. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pontes B., Monzo P., Gole L., Le Roux A.-L., Kosmalska A.J., Tam Z.Y., Luo W., Kan S., Viasnoff V., Roca-Cusachs P. Membrane tension controls adhesion positioning at the leading edge of cells. J Cell Biol. 2017;216:2959–2977. doi: 10.1083/jcb.201611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maître J.-L., Turlier H., Illukkumbura R., Eismann B., Niwayama R., Nédélec F., Hiiragi T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan C.J., Costanzo M., Ruiz-Herrero T., Mönke G., Petrie R.J., Bergert M., Diz-Muñoz A., Mahadevan L., Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature. 2019;571:112–116. doi: 10.1038/s41586-019-1309-x. [DOI] [PubMed] [Google Scholar]
- 9.de Belly H., Jones P.H., Paluch E.K., Chalut K.J. Membrane tension mediated mechanotransduction drives fate choice in embryonic stem cells. bioRxiv. 2019 798959. [Google Scholar]
- 10.Bergert M., Lembo S., Milovanović D., Börmel M., Neveu P., Diz-Muñoz A. Cell surface mechanics gate stem cell differentiation. bioRxiv. 2019 doi: 10.1016/j.stem.2020.10.017. 798918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carman P.J., Dominguez R. BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton. Biophys Rev. 2018;10:1587–1604. doi: 10.1007/s12551-018-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sens P., Plastino J. Membrane tension and cytoskeleton organization in cell motility. J Phys Condens Matter. 2015;27:273103. doi: 10.1088/0953-8984/27/27/273103. [DOI] [PubMed] [Google Scholar]
- 13.Pontes B., Monzo P., Gauthier N.C. Membrane tension: a challenging but universal physical parameter in cell biology. Semin Cell Dev Biol. 2017;71:30–41. doi: 10.1016/j.semcdb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Diz-Muñoz A., Weiner O.D., Fletcher D.A. In pursuit of the mechanics that shape cell surfaces. Nat Phys. 2018;14:648–652. doi: 10.1038/s41567-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochmuth F.M., Shao J.Y., Dai J., Sheetz M.P. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys J. 1996;70:358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brochard-Wyart F., Borghi N., Cuvelier D., Nassoy P. Hydrodynamic narrowing of tubes extruded from cells. Proc Natl Acad Sci USA. 2006;103:7660–7663. doi: 10.1073/pnas.0602012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Ren Physiol. 2002;282:F179–F190. doi: 10.1152/ajprenal.2002.282.2.F179. [DOI] [PubMed] [Google Scholar]
- 18.Masters T.A., Pontes B., Viasnoff V., Li Y., Gauthier N.C. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc Natl Acad Sci USA. 2013;110:11875–11880. doi: 10.1073/pnas.1301766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raucher D., Sheetz M.P. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier N.C., Fardin M.A., Roca-Cusachs P., Sheetz M.P. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc Natl Acad Sci USA. 2011;108:14467–14472. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujita K., Takenawa T., Itoh T. Feedback regulation between plasma membrane tension and membrane-bending proteins organizes cell polarity during leading edge formation. Nat Cell Biol. 2015;17:749–758. doi: 10.1038/ncb3162. [DOI] [PubMed] [Google Scholar]
- Shi Z., Graber Z.T., Baumgart T., Stone H.A., Cohen A.E. Cell membranes resist flow. Cell. 2018;175:1769–1779. doi: 10.1016/j.cell.2018.09.054. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]; First paper that challenges membrane tension propagation in cells.
- Cohen A.E., Shi Z. Do cell membranes flow like honey or jiggle like jello? Bioessays. 2019;42 doi: 10.1002/bies.201900142. e1900142. [DOI] [PubMed] [Google Scholar]; "Prospects and overviews" article that suggests possible causes to the lack of membrane tension propagation by the authors of the original paper.
- Mueller J., Szep G., Nemethova M., de Vries I., Lieber A.D., Winkler C., Kruse K., Small J.V., Schmeiser C., Keren K. Load adaptation of lamellipodial actin networks. Cell. 2017;171:188–200. doi: 10.1016/j.cell.2017.07.051. e16. [DOI] [PubMed] [Google Scholar]; Study shows how actin architecture adapts to membrane tension changes.
- 25.Graziano B.R., Town J.P., Sitarska E., Nagy T.L., Fošnarič M., Penič S., Iglič A., Kralj-Iglič V., Gov N.S., Diz-Muñoz A. Cell confinement reveals a branched-actin independent circuit for neutrophil polarity. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000457. e3000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai T.Y.C., Collins S.R., Chan C.K., Hadjitheodorou A., Lam P.-Y., Lou S.S., Yang H.W., Jorgensen J., Ellett F., Irimia D. Efficient front-rear coupling in neutrophil chemotaxis by dynamic myosin II localization. Dev Cell. 2019;49:189–205.e6. doi: 10.1016/j.devcel.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chugh P., Clark A.G., Smith M.B., Cassani D.A.D., Dierkes K., Ragab A., Roux P.P., Charras G., Salbreux G., Paluch E.K. Actin cortex architecture regulates cell surface tension. Nat Cell Biol. 2017;19:689–697. doi: 10.1038/ncb3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia S., Lim Y.B., Zhang Z., Wang Y., Zhang S., Lim C.T., Yim E.K.F., Kanchanawong P. Nanoscale Architecture of the cortical actin cytoskeleton in embryonic stem cells. Cell Rep. 2019;28:1251–1267.e7. doi: 10.1016/j.celrep.2019.06.089. [DOI] [PubMed] [Google Scholar]
- Freeman S.A., Vega A., Riedl M., Collins R.F., Ostrowski P.P., Woods E.C., Bertozzi C.R., Tammi M.I., Lidke D.S., Johnson P. Transmembrane pickets connect cyto- and pericellular skeletons forming barriers to receptor engagement. Cell. 2018;172:305–312. doi: 10.1016/j.cell.2017.12.023. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests how MCA could affect diffusion of proteins at the plasma membrane.
- 30.Liu Y., Belkina N.V., Park C., Nambiar R., Loughhead S.M., Patino-Lopez G., Ben-Aissa K., Hao J.-J., Kruhlak M.J., Qi H. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 2012;119:445–453. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brückner B.R., Janshoff A. Elastic properties of epithelial cells probed by atomic force microscopy. BBA - Molecular Cell Research. 2015;1853:3075–3082. doi: 10.1016/j.bbamcr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Smith A.S., Nowak R.B., Zhou S., Giannetto M., Gokhin D.S., Papoin J., Ghiran I.C., Blanc L., Wan J., Fowler V.M. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc Natl Acad Sci USA. 2018;115:E4377–E4385. doi: 10.1073/pnas.1718285115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barger S.R., Reilly N.S., Shutova M.S., Li Q., Maiuri P., Heddleston J.M., Mooseker M.S., Flavell R.A., Svitkina T., Oakes P.W. Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat Commun. 2019;10:1249. doi: 10.1038/s41467-019-09104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datar A., Ameeramja J., Bhat A., Srivastava R., Mishra A., Bernal R., Prost J., Callan-Jones A., Pullarkat P.A. The roles of microtubules and membrane tension in axonal beading, retraction, and atrophy. Biophys J. 2019;117:880–891. doi: 10.1016/j.bpj.2019.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal K., Pogoda K., Nandi S., Mathieu S., Kasri A., Klein E., Radvanyi F., Goud B., Janmey P.A., Manneville J.-B. Role of a kinesin motor in cancer cell mechanics. Nano Lett. 2019;19:7691–7702. doi: 10.1021/acs.nanolett.9b02592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simunovic M., Evergren E., Callan-Jones A., Bassereau P. Curving cells inside and out: roles of BAR domain proteins in membrane shaping and its cellular implications. Annu Rev Cell Dev Biol. 2019;35:111–129. doi: 10.1146/annurev-cellbio-100617-060558. [DOI] [PubMed] [Google Scholar]
- 37.Sorre B., Callan-Jones A., Manzi J., Goud B., Prost J., Bassereau P., Roux A. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc Natl Acad Sci USA. 2012;109:173–178. doi: 10.1073/pnas.1103594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prévost C., Zhao H., Manzi J., Lemichez E., Lappalainen P., Callan-Jones A., Bassereau P. IRSp53 senses negative membrane curvature and phase separates along membrane tubules. Nat Commun. 2015;6:8529. doi: 10.1038/ncomms9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simunovic M., Evergren E., Golushko I., Prévost C., Renard H.-F., Johannes L., McMahon H.T., Lorman V., Voth G.A., Bassereau P. How curvature-generating proteins build scaffolds on membrane nanotubes. Proc Natl Acad Sci USA. 2016;113:11226–11231. doi: 10.1073/pnas.1606943113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F.-C., Bertin A., Bousquet H., Manzi J., Senju Y., Tsai M.-C., Picas L., Miserey-Lenkei S., Lappalainen P., Lemichez E. Ezrin enrichment on curved membranes requires a specific conformation or interaction with a curvature-sensitive partner. Elife. 2018;7:129. doi: 10.7554/eLife.37262. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests how membrane curvature could affect MCA proteins.
- 41.Stanishneva-Konovalova T.B., Kelley C.F., Eskin T.L., Messelaar E.M., Wasserman S.A., Sokolova O.S., Rodal A.A. Coordinated autoinhibition of F-BAR domain membrane binding and WASp activation by Nervous Wreck. Proc Natl Acad Sci USA. 2016;113:E5552–E5561. doi: 10.1073/pnas.1524412113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P., Hansen S.D., Akin O., Li T.-D., Hayden C.C., Fletcher D.A., Mullins R.D. WH2 and proline-rich domains of WASP-family proteins collaborate to accelerate actin filament elongation. EMBO J. 2018;37:102–121. doi: 10.15252/embj.201797039. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests how the interplay between membrane curvature ad WASP could affect actin polymerization.
- Almeida-Souza L., Frank R.A.W., García-Nafría J., Colussi A., Gunawardana N., Johnson C.M., Yu M., Howard G., Andrews B., Vallis Y. A flat BAR protein promotes actin polymerization at the base of clathrin-coated pits. Cell. 2018;174:325–337. doi: 10.1016/j.cell.2018.05.020. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests how membrane curvature and BAR domain proteins could affect actin polymerization.
- 44.Echarri A., Pavón D.M., Sánchez S., García-García M., Calvo E., Huerta-López C., Velázquez-Carreras D., Viaris de Lesegno C., Ariotti N., Lázaro-Carrillo A. An Abl-FBP17 mechanosensing system couples local plasma membrane curvature and stress fiber remodeling during mechanoadaptation. Nat Commun. 2019;10:5828. doi: 10.1038/s41467-019-13782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appadurai D., Gay L., Moharir A., Lang M.J., Duncan M.C., Schmidt O., Teis D., Vu T.N., Silva M., Jorgensen E.M. Plasma membrane tension regulates eisosome structure and function. Mol Biol Cell. 2020;31:287–303. doi: 10.1091/mbc.E19-04-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren C., Yuan Q., Braun M., Zhang X., Petri B., Zhang J., Kim D., Guez-Haddad J., Xue W., Pan W. Leukocyte cytoskeleton polarization is initiated by plasma membrane curvature from cell attachment. Dev Cell. 2019;49:206–219.e7. doi: 10.1016/j.devcel.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieber A.D., Yehudai-Resheff S., Barnhart E.L., Theriot J.A., Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr Biol. 2013;23:1409–1417. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 48.Mogilner A., Barnhart E.L., Keren K. Experiment, theory, and the keratocyte: an ode to a simple model for cell motility. Semin Cell Dev Biol. 2019:143–151. doi: 10.1016/j.semcdb.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Mandal K., Raz-Ben Aroush D., Graber Z.T., Wu B., Park C.Y., Fredberg J.J., Guo W., Baumgart T., Janmey P.A. Soft hyaluronic gels promote cell spreading, stress fibers, focal adhesion, and membrane tension by phosphoinositide signaling, not traction force. ACS Nano. 2019;13:203–214. doi: 10.1021/acsnano.8b05286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okujeni S., Egert U. Self-organization of modular network architecture by activity-dependent neuronal migration and outgrowth. Elife. 2019;8:13. doi: 10.7554/eLife.47996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dos-Santos Carvalho S., Moreau M.M., Hien Y.E., Garcia M., Aubailly N., Henderson D.J., Studer V., Sans N., Thoumine O., Montcouquiol M. Vangl2 acts at the interface between actin and N-cadherin to modulate mammalian neuronal outgrowth. Elife. 2020;9 doi: 10.7554/eLife.51822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai J., Sheetz M.P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys J. 1995;68:988–996. doi: 10.1016/S0006-3495(95)80274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pontes B., Ayala Y., Fonseca A.C.C., Romão L.F., Amaral R.F., Salgado L.T., Lima F.R., Farina M., Viana N.B., Moura-Neto V. Membrane elastic properties and cell function. PloS One. 2013;8 doi: 10.1371/journal.pone.0067708. e67708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanda H., Gu J.G. Membrane mechanics of primary afferent neurons in the dorsal root ganglia of rats. Biophys J. 2017;112:1654–1662. doi: 10.1016/j.bpj.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratcliffe C.D.H., Siddiqui N., Coelho P.P., Laterreur N., Cookey T.N., Sonenberg N., Park M. HGF-induced migration depends on the PI(3,4,5)P3-binding microexon-spliced variant of the Arf6 exchange factor cytohesin-1. J Cell Biol. 2018;218:285–298. doi: 10.1083/jcb.201804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thottacherry J.J., Kosmalska A.J., Kumar A., Vishen A.S., Elosegui-Artola A., Pradhan S., Sharma S., Singh P.P., Guadamillas M.C., Chaudhary N. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat Commun. 2018;9:4217. doi: 10.1038/s41467-018-06738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangukiya H.B., Negi H., Merugu S.B., Sehar Q., Mashausi D.S., Yunus F.-U.-N., Wu Z., Li D. Paracrine signalling of AGR2 stimulates RhoA function in fibroblasts and modulates cell elongation and migration. Cell Adhes Migrat. 2019;13:332–344. doi: 10.1080/19336918.2019.1685928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W.Y., Davidson C.D., Lin D., Baker B.M. Actomyosin contractility-dependent matrix stretch and recoil induces rapid cell migration. Nat Commun. 2019;10:1186. doi: 10.1038/s41467-019-09121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niebuhr K., Giuriato S., Pedron T., Philpott D.J., Gaits F., Sable J., Sheetz M.P., Parsot C., Sansonetti P.J., Payrastre B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002;21:5069–5078. doi: 10.1093/emboj/cdf522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gauthier N.C., Rossier O.M., Mathur A., Hone J.C., Sheetz M.P. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell. 2009;20:3261–3272. doi: 10.1091/mbc.E09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pontes B., Viana N.B., Salgado L.T., Farina M., Neto V.M., Nussenzveig H.M. Cell cytoskeleton and tether extraction. Biophys J. 2011;101:43–52. doi: 10.1016/j.bpj.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayala Y.A., Pontes B., Hissa B., Monteiro A.C.M., Farina M., Moura-Neto V., Viana N.B., Nussenzveig H.M. Effects of cytoskeletal drugs on actin cortex elasticity. Exp Cell Res. 2017;351:173–181. doi: 10.1016/j.yexcr.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Schneider L., Cammer M., Lehman J., Nielsen S.K., Guerra C.F., Veland I.R., Stock C., Hoffmann E.K., Yoder B.K., Schwab A. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masuda T., Baba K., Nomura T., Tsujita K., Murayama T., Itoh T., Takatani-Nakase T., Sokabe M., Inagaki N., Futaki S. An influenza-derived membrane tension-modulating peptide regulates cell movement and morphology via actin remodeling. Communications Biology. 2019;2:243. doi: 10.1038/s42003-019-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samson S.C., Elliott A., Mueller B.D., Kim Y., Carney K.R., Bergman J.P., Blenis J., Mendoza M.C. p90 ribosomal S6 kinase (RSK) phosphorylates myosin phosphatase and thereby controls edge dynamics during cell migration. J Biol Chem. 2019;294:10846–10862. doi: 10.1074/jbc.RA119.007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietuch A., Janshoff A. Mechanics of spreading cells probed by atomic force microscopy. Open Biol. 2013;3:130084. doi: 10.1098/rsob.130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Neill P.R., Castillo-Badillo J.A., Meshik X., Kalyanaraman V., Melgarejo K., Gautam N. Membrane flow drives an adhesion-independent amoeboid cell migration mode. Dev Cell. 2018;46:9–22.e4. doi: 10.1016/j.devcel.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roddie H.G., Armitage E.L., Coates J.A., Johnston S.A., Evans I.R. Simu-dependent clearance of dying cells regulates macrophage function and inflammation resolution. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.2006741. e2006741–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herzog C., Pons Garcia L., Keatinge M., Greenald D., Moritz C., Peri F., Herrgen L. Rapid clearance of cellular debris by microglia limits secondary neuronal cell death after brain injury in vivo. Development. 2019;146 doi: 10.1242/dev.174698. dev174698–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diz-Muñoz A., Krieg M., Bergert M., Ibarlucea-Benitez I., Muller D.J., Paluch E., Heisenberg C.-P. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000544. e1000544–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keren K., Pincus Z., Allen G.M., Barnhart E.L., Marriott G., Mogilner A., Theriot J.A. Mechanism of shape determination in motile cells. Nature. 2008;453:475–480. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozlov M.M., Mogilner A. Model of polarization and bistability of cell fragments. Biophys J. 2007;93:3811–3819. doi: 10.1529/biophysj.107.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieber A.D., Schweitzer Y., Kozlov M.M., Keren K. Front-to-Rear membrane tension gradient in rapidly moving cells. Biophys J. 2015;108:1599–1603. doi: 10.1016/j.bpj.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gérard A., Patino-Lopez G., Beemiller P., Nambiar R., Ben-Aissa K., Liu Y., Totah F.J., Tyska M.J., Shaw S., Krummel M.F. Detection of rare antigen-presenting cells through T cell-intrinsic meandering motility, mediated by Myo1g. Cell. 2014;158:492–505. doi: 10.1016/j.cell.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom A., Derivery E., Soleimanpour S., Tomba C., Molin M.D., Sakai N., Gonzalez-Gaitan M., Matile S., Roux A. A fluorescent membrane tension probe. Nat Chem. 2018;10:1118–1125. doi: 10.1038/s41557-018-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; First probe to image membrane tension and lipid composition changes in cells.
- 76.Riggi M., Bourgoint C., Macchione M., Matile S., Loewith R., Roux A. TORC2 controls endocytosis through plasma membrane tension. J Cell Biol. 2019;218:2265–2276. doi: 10.1083/jcb.201901096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coomer C.A., Carlon-Andres I., Iliopoulou M., Dustin M.L., Compeer E.B., Compton A.A., Padilla-Parra S. Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008359. e1008359. [DOI] [PMC free article] [PubMed] [Google Scholar]