Abstract
Context
46,XX patients with classic congenital adrenal hyperplasia (CAH) are exposed to elevated androgens in utero causing varying levels of virilization. The majority undergo feminizing genitoplasty early in life, with potential impact on sexual function and health-related quality of life (HRQoL).
Objective
We aimed to determine how sexual and lower urinary tract function, body image, and global HRQoL differs between patients with classic CAH and controls and to characterize how gynecologic anatomy contributes to outcomes.
Methods
36 patients with classic CAH and 27 control women who were matched for age, race, and marital status underwent standardized gynecological examination and validated questionnaires. The responses were analyzed in relation to gynecological measurements, genotype, and disease status.
Results
Compared with controls, patients with CAH were more likely to have sexual dysfunction (P = 0.009), dyspareunia (P = 0.007), and other pelvic pain (P = 0.007); were less likely to be heterosexual (P = 0.013) or ever have been sexually active (P = 0.003); had poorer body image independent of body mass index (P < 0.001); and had worse HRQoL in the areas of general health (P = 0.03) and pain (P = 0.009). The patients with CAH had smaller vaginal calibers and perineal body lengths and larger clitoral indexes when compared with controls (P < 0.001). A larger vaginal caliber in CAH patients was associated with better overall sexual function (P = 0.024), increased sexual satisfaction (P = 0.017), less pain (P < 0.001), and greater number of sexual partners (P = 0.02).
Conclusions
46,XX patients with CAH have increased rates of sexual dysfunction, poor body image, and poor HRQoL, which is mitigated by having a larger vaginal caliber. Management aimed at optimizing vaginal caliber might improve sexual function.
Keywords: sexual function, body image, health-related quality of life, genitourinary surgery, congenital adrenal hyperplasia
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is the most common cause of 46,XX atypical genitalia. Prenatal adrenal androgen excess affects the development of the external genitalia. Findings include a common urogenital sinus in place of separate urethral and vaginal openings, partially fused labia majora, and clitoral enlargement. The degree of virilization is associated with the severity of the disease and degree of in utero androgen exposure [1, 2]. Therefore, the severe salt-wasting form often conveys more virilization than the less severe simple virilizing form, and patients with the mild nonclassic form escape prenatal virilization but may later in life develop clitoromegaly [3].
Traditionally, most 46,XX patients with classic CAH undergo some form of feminizing genitoplasty. This often occurs in infancy, with some degree of clitoral reduction and vaginoplasty, but frequently patients undergo additional reconstruction around the time of puberty, coitarche, or during adult life [4, 5]. The most common surgical sites are the clitoris, labia, vaginal introitus, and lower vagina [6]. Although surgical procedures have been debated regarding timing, indications, and types of procedure, the majority of 46,XX patients with classic CAH do undergo genital surgery. The surgical methods used for separating the urogenital sinus, bringing the vaginal opening to the perineum, introitoplasty, and clitoroplasty have evolved over time.
Previous studies have aimed to determine how disease state and surgery affect sexual function and health-related quality of life (HRQoL) in 46,XX patients with CAH. In a meta-analysis of 29 observational studies, most patients were sexually active, although only 48% reported comfortable intercourse, and overall data on HRQoL were sparse and inconclusive [7]. Of the 29 studies, only 7 were case-control studies. 46,XX patients with classic CAH have been shown to score lower than controls on questionnaires regarding sexual function [5, 8]. Anatomic outcomes have been poorly characterized in these patients; however, clitoral size, level of confluence, and presence of stenosis at the vaginal introitus have been suggested to influence outcomes [5]. Patients with the most severe disease, salt-wasters with a null genotype, are reported to have the lowest sexual function and least sexual satisfaction, possibly due to the extent and difficulty of the surgery performed [1]. It has been suggested that single-stage reconstructive surgery does not have a negative impact on sexual function; however, this was based on 1 study of 12 patients [9].
Sexual function for women is influenced by multiple factors, including gender identity, sexual identity, body image, lower urinary tract function, ability to perform activities of daily life, and general perception of health [1]. 46,XX patients with CAH are more likely to identify as a sexual minority or abstain from intercourse than healthy counterparts, but the degree of this finding has been variable [5, 7, 10]. Studies regarding gender identity have also been incongruent; some found 46,XX patients with CAH more likely to identify as male and other studies reported that 100% of participants identify as female [8, 11, 12]. It has also been suggested that 46,XX patients with CAH are more likely to identify as a sexual minority, with an increased prevalence of same-sex attraction compared with the general population [12]. Body image is a well-known determiner of sexual function and HRQoL, and it is especially important in women with chronic disease requiring surgery that results in visible scars [13, 14]. While studies in the past have focused on few aspects of sexual function, the aim of this study was to examine the multidimensional aspects of sexual function as it relates to overall HRQoL in comparison to age-, race-, and marital status–matched controls, as well as to characterize how postsurgical gynecologic anatomy contributes to outcomes in adult 46,XX patients with classic CAH.
Patients and Methods
Patients
46,XX patients CAH who were ≥18 years of age and were enrolled in a Natural History Study (www.clinicaltrials.gov identifier NCT00250159) were invited to participate during routine visits to the National Institutes of Health (NIH) Clinical Center (Bethesda, MD) during 2017 to 2019. Phenotypic classification of either salt-wasting (SW) or simple virilizing (SV) was determined based on clinical and hormonal criteria and review of medical records, as previously described [15], and confirmed by genotyping [16]. Genotype was classified according to expected 21-hydroxylase activity based on published in vitro studies [17]: Group 0 included patients with null mutations, Group A included patients with IVS2-13A/C>G mutation, and Group B included patients with p.I172N mutation. Forty patients with classic CAH were eligible and 36 patients (27 SW, 9 SV) aged between 19 and 67 years chose to participate. Two did not participate due to scheduling conflicts (age 34 SW, age 21 SW), one refused to participate (age 46 SW), and 1 patient was excluded due to a pregnancy (age 27 SV). The majority (94%) had a history of genital surgery. Seven patients had prior pregnancies (3 women with 2 children, 1 woman with 1 child, 1 woman with a miscarriage, and 2 women with elective terminations). All children were full-term and were delivered via cesarean section. Twenty-seven women with no underlying medical conditions matched for age, race, and marital status were recruited from a general gynecology clinic at MedStar Washington Hospital Center (Washington, DC). Three women had prior pregnancies to term, 2 delivered via cesarean section and one vaginal birth. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Development Institutional Review Board and the Medstar Washington Hospital Center Institutional Review Board. All patients provided written informed consent.
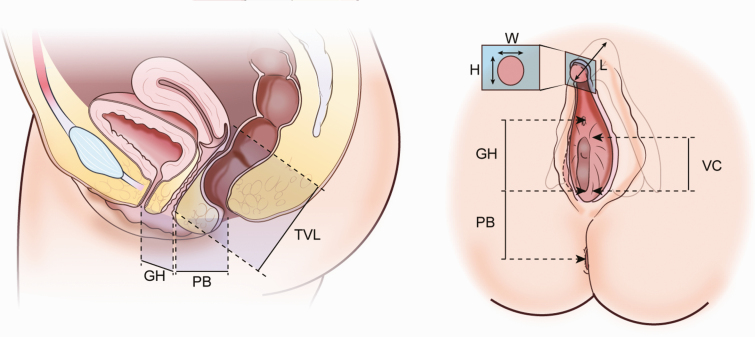
In addition to standard history and physical examination, patients underwent a pelvic examination performed by 1 of 3 gynecologists (K.F., M.D., V.G.L.). Gynecological measurements were performed and included: vaginal length and caliber; clitoral height, width, and length; genital hiatus; and perineal body (Fig. 1). Measurements were obtained using the established Pelvic Organ Prolapse Quantification (POP-Q) system of gynecologic exam [18]. During the exam, initial external measurements were obtained as outlined in Fig. 1. For clitoral measurements, the clitoral hood was retracted to allow for more accurate measurements. Next, a Pedersen speculum was inserted into the vagina and opened as wide as tolerated without patient discomfort. The vaginal caliber was obtained approximately 0.5 cm into the vaginal introitus, or at the expected location of natal hymenal tissue. Vaginal length was then obtained as the distance from this location to the posterior fornix. Comprehensive gynecologic, obstetric, and sexual history was taken. Patients without palpable clitoral tissue on exam were presumed to have undergone clitorectomies.
Figure 1.
Diagram of lower urogenital anatomy in healthy adult women. Abbreviations: GH, genital hiatus; PB, perineal body length; TVL, total vaginal length; VC, vaginal caliber; W, clitoral width; H, clitoral height; L, clitoral length.
Assays
All CAH patients had early morning (at approximately 0800 hours) measurements of 17-hydroxyprogesterone (17-OHP), androstenedione, testosterone, and plasma renin activity prior to medication and within 30 days of gynecological assessment. Androstenedione, 17-OHP, and testosterone were measured by liquid chromatography-tandem mass spectrometry (NIH Clinical Center, Bethesda, MD or Q2 Solutions, Valencia or San Juan Capistrano, CA for n = 4). Plasma renin activity was measured by radioimmunoassay (Mayo Medical Laboratories, Rochester, MN).
Questionnaires
All participants completed 4 validated questionnaires including the Female Sexual Function Index (FSFI), Bristol Female Lower Urinary Tract Symptoms (BFLUTS), 36-Item Short Form Survey (SF-36), and Body Image Scale (BIS). The FSFI assessed 6 domains of sexual function (desire, arousal, orgasm, lubrication, satisfaction, and pain) in regard to penetrative vaginal intercourse. The subscores within each domain range from 0 (no sexual activity in the last month) to 6.0 (high sexual function within the last month), with a total possible score of 36 [19]. This questionnaire was previously cross-validated among women with an array of sexual dysfunction, and a score of ≤26.55 was established as diagnosis of sexual dysfunction [20]. For women who were sexually abstinent, zero values were treated as missing responses to decrease bias towards sexual dysfunction as suggested by Meyer-Bahlburg et al [21]. The BFLUTS questionnaire assessed lower urinary tract symptoms relating to incontinence, other urinary symptoms, sexual function, and HRQoL, with higher values indicating impairment [22]. The SF-36 assessed 8 domains of HRQoL including physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health perception, with scores ranging from 0 to 100 and a higher value corresponding to better self-reported HRQoL [23]. This particular health perception questionnaire has been used in a number of chronic diseases, including in men and women with CAH [24, 25]. The BIS was designed specifically for patients with chronic disease (cancer) and includes 10 items scored from 0 (not at all) to 3 (very much) for a total score of 30, with higher scores indicating greater body image distress [14].
Statistics
Data were described by frequency distributions or descriptive statistics and are reported as percentage or as median (interquartile range [IQR]), unless otherwise noted. Depending on the data distribution of the continuous variables, parametric (paired t test) or nonparametric (paired t test with Levene’s test for equal variance correction) tests were used to compare groups. Categorical data were compared by chi-square or Fisher exact tests. Correlation analyses between 2 sets of continuous data used Spearman’s rho. P values ≤ 0.05 were considered statistically significant. Data were analyzed using SPSSS software version 26 (IBM, Armonk, NY).
Results
The CAH cohort consisted of 36 patients with 21-hydroxylase deficiency with genotype classification of 33.3% (n = 12) in Group 0, 41.7% (n = 15) in Group A, and 25% (n = 9) in Group B. The majority (77.8%) of patients were in good disease control, based on androstenedione within the reference range. (Table 1). Compared with controls, patients with CAH had higher body mass index (BMI) (P = 0.013) and more menstrual irregularity (P < 0.001), and they were less likely to be heterosexual (P = 0.013), to have ever been sexually active (P < 0.01) and, if sexually active with men, to use contraception (P = 0.003) (Table 1). All patients with SV CAH identified as having sexual activity with men only, whereas only 48% of patients with SW CAH reported a sexual preference for solely men (P = 0.013). One CAH patient and 1 control self-identified as neither male nor female and 1 patient with CAH identified as male while the remaining CAH patients and controls self-identified as females.
Table 1.
Clinical Characteristics of Patients with Congenital Adrenal Hyperplasia Compared With Controlsa
| CAH Patients (n = 36) | Controls (n = 27) | P-Value | |
|---|---|---|---|
| Age (years) | 28.5 [22.3–35.8] | 28.0 [23.0–31.0] | 0.498 |
| BMI (kg/m2) | 29.4 [22.6–35.5] | 24.5 [21.6–28.4] | 0.013 |
| Marital Status (# married, %) | 9 (25%) | 4 (14.8%) | 0.323 |
| Race (#, %) | |||
| White | 28 (77.8%) | 23 (85.2%) | 0.218 |
| Black or African American | 3 (8.3%) | 4 (14.8%) | |
| Multiple Races | 1 (2.8%) | 0 | |
| Unknown | 4 (11.1%) | 0 | |
| Ethnicity (#, %) | |||
| Not Latino or Hispanic | 29 (80.5%) | 26 (96.3%) | 0.172 |
| Latino or Hispanic | 6 (16.7%) | 1 (3.7%) | |
| Unknown | 1 (2.8%) | 0 | |
| Gender a (#, %) | |||
| Female | 34 (94.4%) | 26 (96.3%) | |
| Male | 1 (2.8%) | 0 | 0.671 |
| Other | 1 (2.8%) | 1 (3.7%) | |
| Disease Control (# in control, %) b | 28 (77.8%) | N/A | |
| Sexual Preference (#, %) | |||
| Men | 22 (61.1%) | 26 (96.3%) | 0.013 |
| Women | 6 (16.7%) | 0 | |
| Both | 6 (16.7%) | 1 (3.7%) | |
| Neither/Asexual | 2 (5.5%) | 0 | |
| Sexual Activity | |||
| Ever Sexually Active (#, %) | 26 (72.2%) | 27 (100%) | 0.003 |
| # of Partners (#) | 1 [0–4] c | 8 [4–12] | 0.013 |
| Age of Menarche (years) | 13 [12–15] | 12 [12–13] | 0.067 |
| Regular Menses (#, %) | 15 (41.7%) | 24 (88.9%) | <0.001 |
| Pregnancy in Sexually Active (#, %) | 6 (23.1%) | 4 (14.8%) | 0.442 |
| Contraception Use in Sexually Active (#, %) | |||
| Past | 22 (84.6%) | 26 (96.3%) | 0.146 |
| Present | 12 (46.2%) | 22 (81.5%) | 0.007 |
| Hormonal | 21 (80.8%) | 25 (92.6%) | 0.204 |
Median [interquartile range] unless otherwise specified.
a Controls were age-, race-, and marital status–matched.
b Good disease control defined as androstenedione within reference range.
c n = 35 patients responded.
Patients with CAH and genital surgery had smaller vaginal calibers and shorter perineal body lengths compared with controls (P < 0.001) (Table 2, Fig. 1). Clitoral length, width, height, and index were all larger in patients with CAH compared with controls (P < 0.001), excluding 4 CAH patients with presumed clitorectomy due to lack of palpable clitoral tissue on exam. The patients with CAH also reported more dyspareunia (P = 0.007), and other pelvic pain compared with controls (P = 0.007).
Table 2.
Gynecological Measurements and Pelvic Pain in Patients with Congenital Adrenal Hyperplasia with Prior Urogenital Surgery Compared With Controlsa
| CAH Patients (n = 34)b | Controls (n = 27) | P-Value | |
|---|---|---|---|
| Vaginal Measurements (cm) | |||
| Vaginal Length | 8.5 [7.0–9.8] | 8.0 [7.5–9.0] | 0.770 |
| Vaginal caliber | 3.0 [1.8–3.6] | 4.0 [4.0–4.5] | <0.001 |
| Perineal body length | 3.4 [2.5–4.1] | 5.0 [4.0–5.5] | <0.001 |
| Genital hiatus | 1.7 [1.0–2.0] c | 2.0 [1.5–2.5] | 0.446 |
| Clitoral Measurements (cm) | |||
| Clitoral length | 1.3 [0.9–2.0] d | 1.0 [0.5–1.0] | <0.001 |
| Clitoral width | 1.1 [0.8–1.5] d | 0.5 [0.2–0.5] | <0.001 |
| Clitoral height | 1.4 [1.0–1.8] d | 0.5 [0.2–1.0] | <0.001 |
| Clitoral Index (cm2) | 1.5 [0.8–3.0] d | 0.25 [0.04–0.5] | <0.001 |
| Self-reported Pelvic Pain (#, %) | |||
| Dysmenorrhea | 9 (26.5%) | 13 (48.1%) | 0.080 |
| Dyspareunia | 12 (50%) e | 4 (14.8%) | 0.007 |
| Other pelvic pain | 8 (23.5%) | 0 | 0.007 |
Median [interquartile range] unless otherwise specified.
Clitoral Index was calculated as clitoral length × width × height.
a Controls were age-, race-, and marital status–matched.
b Excluded 2 patients without genital surgery.
c Unable to measure on 3 patients. n = 31
d Excluded 7 patients (4 no palpable clitoris; 3 buried and unable to measure). n = 27
e Excluded 10 patients who had never been sexually active. n = 24
More severe CAH genotype was associated with shorter perineal body lengths (P < 0.001) with the median lengths for Group 0, A, and B being 2.8 cm (IQR, 2.3-3.3), 3.2 cm (IQR, 2.4-4.0), and 4.5 cm (IQR, 4.0-5.5), respectively. Within the CAH cohort who had undergone urogenital surgery, those with SW CAH had smaller vaginal calibers (P = 0.008) and shorter perineal body lengths (P < 0.001) compared with those with SV CAH. Genotype was not associated with other anatomic measurements, body image, or measures of sexual or lower urinary tract function.
Two patients with SV CAH did not have genital surgery, 14 (57% SW) had 1 surgery, 12 (100% SW) had 2 surgeries, and 8 (87.5% SW) had 3 or more surgeries. In all patients with CAH, the number of genitourinary surgeries was associated with a shorter perineal body (P < 0.001), but not vaginal caliber. Body image, HRQoL, and BFLUTS were not associated with the number of surgeries, but in the 4 patients with clitorectomies, body image was poor in 2 (scores of 28 and 26 out of 30), urinary control was poor in all 4, and sexual function and HRQoL were variable but within range of the other CAH patients. In the 2 patients without urogenital surgery, sexual function was high (total scores of 35 and 35.7 out of 36) and congruent to controls, but body image and HRQoL were comparable to other CAH patients and urinary control varied. Lack of sexual satisfaction was associated with greater number of surgeries (P = 0.046).
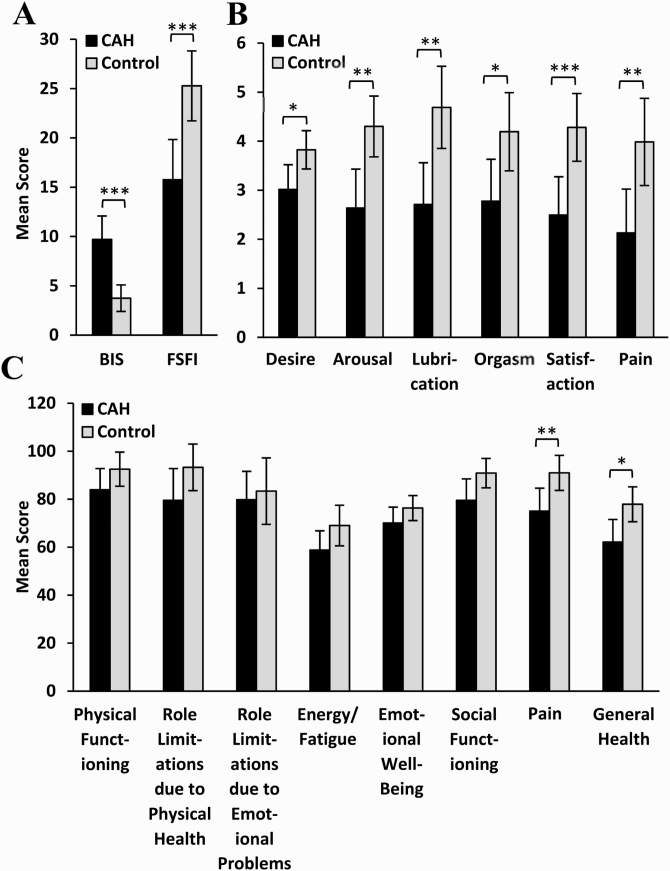
Compared with controls, patients with CAH and prior urogenital surgery had lower total FSFI scores (P < 0.001) signifying poorer overall sexual function (Fig. 2A). Ten controls (37%) were found to have sexual dysfunction (FSFI ≤26.55) and 24 patients (71%) with CAH who had undergone surgery had sexual dysfunction (P = 0.009). When the FSFI subcategories were evaluated, CAH patients had poorer self-reported sexual desire, arousal, lubrication, orgasm, satisfaction, and increased pain compared with controls (Fig. 2B). After excluding 13 patients with CAH and 4 controls due to sexual inactivity, the modified FSFI scores remained lower in patients with CAH compared with controls (P = 0.012), and significant differences remained in the subcategories of arousal (P = 0.043), lubrication (P = 0.003), and satisfaction (P = 0.017).
Figure 2.
Validated questionnaire responses from CAH patients with prior urogenital surgery (n = 34) and age-, race-, and marital status–matched controls (n = 27). (A) Total Body Image Scale (BIS) and Female Sexual Function Index (FSFI) scores. Higher BIS value indicates greater body image distress, while higher FSFI value indicates better function. (B) FSFI subcomponent and (C) 36-Item Short Form Survey (SF-36) domain scores. Higher SF-36 value indicates better health-related quality of life. Mean and 95% confidence intervals shown. * P < 0.05, ** P < 0.01, *** P < 0.001.
Compared with controls, patients with CAH and urogenital surgery also had poorer body image (P < 0.001) (Fig. 2A). Five controls (19%) were noted to be overweight (BMI >30), while 15 (44%) of CAH patients who had undergone surgery were overweight (P = 0.034). When accounting for differences in BMI, patients in the CAH group with a BMI >30 did not score differently than those with a BMI ≤30 on BIS, FSFI, BFLUTS, or SF-36.
Based on the SF-36 questionnaires, patients with CAH and genital surgery reported more pain (P = 0.009) and poorer general health (P = 0.03) compared with controls (Fig. 2C). There were no significant differences in lower urinary tract symptomatology based on the BFLUTS questionnaire. In addition, of the 24 patients with CAH who had ever been sexually active, 14 (58%) self-reported having either dyspareunia or another form of pelvic pain.
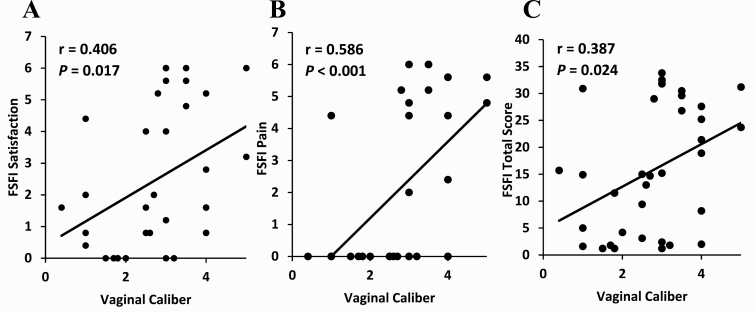
In patients with CAH, a larger vaginal caliber was associated with better sexual function in the categories of satisfaction (P = 0.017), pain (P < 0.001), and overall sexual function (P = 0.024) (Fig. 3A-3C). A larger vaginal caliber was also associated with a greater number of sexual partners (P = 0.02). Having a greater number of sexual partners was associated with better sexual function in all subcategories and in total score (P ≤ 0.01). Vaginal caliber was not associated with number of surgeries. No other components of gynecologic measurements, including clitoral or vaginal measurements, were associated with differences in sexual function. When sexually inactive patients with CAH were excluded, a larger vaginal caliber was associated with less sexual function–related pain (P = 0.002).
Figure 3.
Relationship between parameters of sexual function and vaginal caliber in women with CAH who have undergone urogenital surgery (n = 34). (A) The Female Sexual Function Index (FSFI) subcomponent Satisfaction, (B) FSFI subcomponent Pain, and (C) total FSFI score.
In addition, there was no association between any of the biomarkers of disease control (17-OHP, androstenedione, testosterone, and renin) and body image, sexual function, HRQoL, or any gynecological measurements.
Discussion
In our study of the multidimensional determinants of sexual function in 46,XX patients with classic CAH, we found that our patients with CAH had greater prevalence of dyspareunia and other pelvic pain, poorer self-reported body image, and overall greater impairment of sexual function compared with controls. In patients with CAH, having a larger vaginal caliber was associated with better overall sexual function, especially in the categories of satisfaction and pain. Vaginal caliber was smallest in the most severely affected null group, but it was not associated with the number of urogenital surgeries. Although we were not able to evaluate vaginal caliber in relation to type of urogenital surgery performed, this long-term outcome should be considered in the management of 46,XX patients with CAH and when making surgical decisions.
Our control population consisted of women who were matched with our patients with CAH for age, race, and marital status. These important demographics have been previously shown to impact perception of health, sexual function, and body image [26, 27]. The control population only self-identified as female or other, while 1 CAH patient self-identified as male and another self-identified as other. Within the 2 groups, patients with CAH were more likely to have obesity. While obesity is correlated with body dissatisfaction [13] and impaired HRQoL in the general population [28], our study did not find that the obese patients with CAH had poorer scores than nonobese patients with CAH, suggesting that for 46,XX patients with CAH, other factors than weight are more influential. Interestingly, we previously reported that being overweight was the strongest predictor of body image disturbance in adolescent patients with CAH [29]; we did not find similar results in this study of adults.
Similar to other studies [10, 12, 30], our population with CAH was more likely to be sexually attracted to women or both men and women compared with controls. Heterosexually active 46,XX patients with CAH were less likely to use contraception compared with controls. While our study did not address other routine health visits, decreased use of contraceptives might reflect lack of routine reproductive health services sought by patients with CAH or possibly belief by either the patient or physicians that women with CAH are less likely to be fertile. However, normal pregnancy rate has been reported in 46,XX patients with classic CAH who wish to conceive despite overall low fecundity [31]. All 46,XX patients with CAH should receive routine gynecologic standard of care and counseling regarding birth control if sexually active with men.
The FSFI questionnaire was designed to identify all forms of female sexual dysfunction regardless of etiology [19] and has been used to evaluate sexual function in patients with CAH [7, 8]. Although studies using FSFI have included sexually inactive or abstinent patients, the questionnaire has been modified to eliminate responses of no sexual activity to decrease overall bias and ambiguity [21]. This modification was implemented in our patient population where 37.8% of patients had never been sexually active and yielded similar results to the original scoring method.
The majority of studies have found significant sexual dysfunction in 46,XX patients with CAH [7]. Our study was no different, finding that overall, patients with CAH had significantly higher rates of global sexual dysfunction compared with controls. Our findings were also similar to previous studies showing that patients with CAH who had urogenital surgery had more sexual dysfunction than controls based on FSFI [5, 7, 32]. In our cohort, dysfunction was especially significant in arousal and lubrication, but also in global satisfaction. The 2 patients who had not had surgery had similar FSFI scores as controls, but these subjects were only mildly virilized.
Prior studies have aimed to determine the impact of surgical factors on sexual function. One previous study aimed to determine if number of surgeries influenced sexual outcome and found that those who underwent more reoperations were more likely to have sexual dysfunction [5]. Similar to prior studies [1, 5, 6], we found that the severity of disease contributed to the number of surgeries; however, in our study, having multiple surgeries was correlated with poor sexual satisfaction but not global sexual dysfunction.
Clitoral sensitivity has been found to be decreased in 46,XX patients with CAH who had urogenital surgery, and this was correlated with poor sexual function, especially in those who had clitorectomy [1, 6]. In one report, patients who had no surgery had sensitivity and sexual function scores indistinguishable from controls, but numbers were small (n = 6). We did not evaluate sensitivity in the genital region, but the 4 patients in our study with lack of palpable clitoral tissue mostly scored poorly on body image, urinary control was poor in all 4, and sexual function was variable. The treatment of clitoromegaly has evolved over time. Currently, many 46,XX patients with CAH do not undergo clitoral reduction and when clitoroplasty is chosen for severe clitoromegaly, neurovascular-sparing clitoroplasty is recommended [33].
Interestingly, vaginal caliber was the anatomic finding most associated with sexual function, where a narrow diameter correlated with sexual dysfunction. The number of surgeries, however, had no relationship to the vaginal caliber size, suggesting that the smaller vaginal caliber found in many patients with CAH is independent of the number of surgeries. Several studies report a high incidence of vaginal stenosis, a common surgical complication [32, 34, 35]. An unproven surgical advantage of delayed reconstruction is that the risk of vaginal stenosis may be diminished if performed postpubertally in a higher estrogen state [33, 36]. Our study suggests that surgical approaches should be aimed at optimizing vaginal caliber in an attempt to minimize sexual dysfunction.
Urogenital surgery, especially complete mobilization, is a complicated surgery, and risks include urinary dysfunction [37]. Lower urinary tract function is a key element of perception of health and sexual health as well. Previous studies have shown that the overall majority of patients who undergo feminizing genitoplasty for CAH have no residual urinary dysfunction [22, 37]. In fact, most studies show that all patients have urinary continence following surgery [38]. Our study also suggests that urinary dysfunction is not a major contributing factor to the poor sexual function and HRQoL observed in many 46,XX patients with classic CAH. However, the 4 patients with clitorectomies reported worse urinary control compared with the rest of the CAH cohort, and one of the 2 patients with CAH without urogenital surgery had multiple issues with urinary leakage, probably due to vaginal voiding.
We also evaluated global HRQoL by administering the SF-36. This questionnaire has been validated to pertain to all degrees of health in the general population, and focuses on patient-perceived aspects of health in areas important to conduct activities of daily living such as functional status, well-being, and overall evaluation of health [23]. In a population-based study in Norway, men and women with CAH were found to have lower SF-36 scores when compared with the general population [24]. Impairment was most prominent in the vitality, social functioning, role limitations due to emotional problems, and general health components. Similarly, a study in the UK reported diminished HRQoL in patients with CAH using SF-36, with poor HRQoL related to long-acting glucocorticoids, adiposity, and insulin resistance [25]. Improvement in vitality, as measured by SF-36, was observed in adults with CAH in poor disease control following 6 to 18 months of treatment with circadian glucocorticoid replacement via a pump [39]. In our case-controlled study, patients with CAH were mostly in good hormonal control and scored lower than controls in the areas of general health and pain. Interestingly, many participants answered affirmatively to a history of pelvic pain, but we did not find this to be associated with sexual function. However, perception of poor general health is likely a contributing factor in the determination of sexual health.
Our study’s strengths lie in the multidimensional aspects of our evaluations encompassing body image, sexual function, and gynecological anatomy providing a comprehensive view of the CAH patient. In addition, the inclusion of age- and race- and marital status–matched controls increases the robustness of our findings. Limitations include our inability to obtain surgical reports, our small sample size for patients with CAH who had not undergone urogenital surgery, and lack of preoperative description of the degree of virilization.
Sexual function and HRQoL in 46,XX patients with CAH are impaired, and the cause is likely multifactorial. When determining overall sexual health and HRQoL, consideration should include a broad range of factors including sexual function, body image, gender identity, sexual orientation, lower urinary tract function, social functions and limitations, and general perception of health, as well as presence or absence of surgery and anatomic outcomes. Our study shows that 46,XX patients with CAH have increased rates of sexual dysfunction when compared with age-, race-, and marital status–matched controls, and factors influencing this finding include body dissatisfaction, poor perception of health, and surgical outcomes in regards to vaginal caliber. While we were unable to conclude to what extent surgery or the type of procedure affects outcome, shared decision making and appropriate counseling on the possibility of sexual dysfunction and decreased HRQoL is vital to the management of every patient with CAH. Our findings support that surgical and medical management of 46,XX patients with classic CAH aimed at optimizing vaginal caliber might improve sexual function.
Acknowledgments
We thank the patients for their participation in this study. We acknowledge Lauren Damle, Tamika Auguste, Felicia Hammilton, and Renuka Darolia at MedStar at Washington Hospital Center for opening their clinic patients for study controls, and Kathleen Bren for her assistance in data input during the study.
Financial Support: This work was supported, in part, by MedStar Graduate Medical Education Grant (grant Z1A HD008985) and, in part, by the Intramural Research Program of the National Institutes of Health.
Glossary
Abbreviations
- 17-OHP
17-hydroxyprogesterone
- BFLUTS
Bristol Female Lower Urinary Tract Symptoms
- BIS
Body Image Scale
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- FSFI
Female Sexual Function Index
- HRQoL
health-related quality of life
- IQR
interquartile range
- SF-36
36-Item Short Form Survey
- SV
simple virilizing
- SW
salt-wasting
Additional Information
Disclosure Summary: D.P.M. received unrelated research funds from Diurnal Limited. All other authors report no potential conflicts of interest in this work.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Nordenström A, Frisén L, Falhammar H, et al. Sexual function and surgical outcome in women with congenital adrenal hyperplasia due to CYP21A2 deficiency: clinical perspective and the patients’ perception. J Clin Endocrinol Metab. 2010;95(8):3633-3640. [DOI] [PubMed] [Google Scholar]
- 2. Lee P, Schober J, Nordenström A, et al. Review of recent outcome data of disorders of sex development (DSD): emphasis on surgical and sexual outcomes. J Pediatr Urol. 2012;8(6):611-615. [DOI] [PubMed] [Google Scholar]
- 3. Nordenström A, Falhammar HMANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180(3):R127-R145. [DOI] [PubMed]
- 4. Eckoldt-Wolke F. Timing of surgery for feminizing genitoplasty in patients suffering from congenital adrenal hyperplasia. Endocr Dev. 2014;27:203-209. [DOI] [PubMed] [Google Scholar]
- 5. van der Zwan YG, Janssen EH, Callens N, et al. ; Dutch Study Group on DSD . Severity of virilization is associated with cosmetic appearance and sexual function in women with congenital adrenal hyperplasia: a cross-sectional study. J Sex Med. 2013;10(3):866-875. [DOI] [PubMed] [Google Scholar]
- 6. Crouch NS, Liao LM, Woodhouse CR, Conway GS, Creighton SM. Sexual function and genital sensitivity following feminizing genitoplasty for congenital adrenal hyperplasia. J Urol. 2008;179(2):634-638. [DOI] [PubMed] [Google Scholar]
- 7. Almasri J, Zaiem F, Rodriguez-Gutierrez R, et al. Genital reconstructive surgery in females with congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4089-4096. [DOI] [PubMed] [Google Scholar]
- 8. Fagerholm R, Santtila P, Miettinen PJ, Mattila A, Rintala R, Taskinen S. Sexual function and attitudes toward surgery after feminizing genitoplasty. J Urol. 2011;185(5):1900-1904. [DOI] [PubMed] [Google Scholar]
- 9. Lesma A, Bocciardi A, Corti S, Chiumello G, Rigatti P, Montorsi F. Sexual function in adult life following Passerini-Glazel feminizing genitoplasty in patients with congenital adrenal hyperplasia. J Urol. 2014;191(1):206-211. [DOI] [PubMed] [Google Scholar]
- 10. Gondim R, Teles F, Barroso U Jr. Sexual orientation of 46, XX patients with congenital adrenal hyperplasia: a descriptive review. J Pediatr Urol. 2018;14(6):486-493. [DOI] [PubMed] [Google Scholar]
- 11. de Jesus LE, Costa EC, Dekermacher S. Gender dysphoria and XX congenital adrenal hyperplasia: how frequent is it? Is male-sex rearing a good idea? J Pediatr Surg. 2019;54(11):2421-2427. [DOI] [PubMed] [Google Scholar]
- 12. Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, Okopien B. Sexual function and depressive symptoms in young women with nonclassic congenital adrenal hyperplasia. J Sex Med. 2016;13(1):34-39. [DOI] [PubMed] [Google Scholar]
- 13. Weinberger NA, Kersting A, Riedel-Heller SG, Luck-Sikorski C. Body dissatisfaction in individuals with obesity compared to normal-weight individuals: a systematic review and meta-analysis. Obes Facts. 2016;9(6):424-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189-197. [DOI] [PubMed] [Google Scholar]
- 15. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finkielstain GP, Chen W, Mehta SP, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96(1):E161-E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Speiser PW, Dupont J, Zhu D, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90(2):584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q)–a new era in pelvic prolapse staging. J Med Life. 2011;4(1):75-81. [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. [DOI] [PubMed] [Google Scholar]
- 20. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1-20. [DOI] [PubMed] [Google Scholar]
- 21. Meyer-Bahlburg HF, Dolezal C. The female sexual function index: a methodological critique and suggestions for improvement. J Sex Marital Ther. 2007;33(3):217-224. [DOI] [PubMed] [Google Scholar]
- 22. Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol Female Lower Urinary Tract Symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol. 2004;191(1):73-82. [DOI] [PubMed] [Google Scholar]
- 23. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nermoen I, Husebye ES, Svartberg J, Løvås K. Subjective health status in men and women with congenital adrenal hyperplasia: a population-based survey in Norway. Eur J Endocrinol. 2010;163(3):453-459. [DOI] [PubMed] [Google Scholar]
- 25. Han TS, Krone N, Willis DS, et al. ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE) . Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur J Endocrinol. 2013;168(6):887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chithambo TP, Huey SJ. Black/white differences in perceived weight and attractiveness among overweight women. J Obes. 2013;2013:320326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang AJ, Subak LL, Thom DH, et al. Sexual function and aging in racially and ethnically diverse women. J Am Geriatr Soc. 2009;57(8):1362-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han TS, Tijhuis MA, Lean ME, Seidell JC. Quality of life in relation to overweight and body fat distribution. Am J Public Health. 1998;88(12):1814-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ning C, Green-Golan L, Stratakis CA, et al. Body image in adolescents with disorders of steroidogenesis. J Pediatr Endocrinol Metab. 2008;21(8):771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer-Bahlburg HF, Dolezal C, Baker SW, New MI. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch Sex Behav. 2008;37(1):85-99. [DOI] [PubMed] [Google Scholar]
- 31. Casteràs A, De Silva P, Rumsby G, Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf). 2009;70(6):833-837. [DOI] [PubMed] [Google Scholar]
- 32. Gastaud F, Bouvattier C, Duranteau L, et al. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007;92(4):1391-1396. [DOI] [PubMed] [Google Scholar]
- 33. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merke DP, Poppas DP. Management of adolescents with congenital adrenal hyperplasia. Lancet Diabetes Endocrinol. 2013;1(4):341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krege S, Walz KH, Hauffa BP, Körner I, Rübben H. Long-term follow-up of female patients with congenital adrenal hyperplasia from 21-hydroxylase deficiency, with special emphasis on the results of vaginoplasty. BJU Int. 2000;86(3):253-8; discussion 258. [DOI] [PubMed] [Google Scholar]
- 36. Ludwikowski BM, González R. The surgical correction of urogenital sinus in patients with DSD: 15 years after description of total urogenital mobilization in children. Front Pediatr. 2013;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nidal S, Kocherov S, Jaber J, Levi-Khademi F, Farkas A, Chertin B. Sexual function and voiding status following one stage feminizing genitoplasty. J Pediatr Urol. 2020;16(1):97.e1-97.e6. [DOI] [PubMed] [Google Scholar]
- 38. Dangle PP, Lee A, Chaudhry R, Schneck FX. Surgical complications following early genitourinary reconstructive surgery for congenital adrenal hyperplasia-interim analysis at 6 years. Urology. 2017;101:111-115. [DOI] [PubMed] [Google Scholar]
- 39. Mallappa A, Nella AA, Sinaii N, et al. Long-term use of continuous subcutaneous hydrocortisone infusion therapy in patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2018;89(4):399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.