Abstract
Chronic low dose arsenic exposure continues to be a worldwide health concern because of its prevalence and link to increased cancer risk, including non-small cell lung cancer (NSCLC). Mortality of NSCLC patients increases with the development of a metastatic lesion compared to when the tumor is localized; however, the exact mechanism for what causes NSCLC cells to metastasize in the context of environmental toxicant exposure has yet to be fully elucidated. One proposed contributor to metastasis in NSCLC is nuclear factor (erythroid-derived 2)-like 2 (NRF2), a transcription factor with known oncogenic properties that has proved to be critical for arsenic carcinogenesis. Here, we demonstrate that chronic arsenic exposure enhances the invasive and migratory capacity of immortalized lung epithelial cells via NRF2-dependent upregulation of SRY-box 9 (SOX9), another transcription factor linked with cell proliferation, epithelial-mesenchymal transition, and metastasis. We identified a functional antioxidant response element (ARE) in the promoter region of SOX9, suggesting that it is an NRF2 target gene, with mutation of the ARE preventing NRF2 binding. Pharmacological induction or inhibition of NRF2 increased or decreased SOX9 expression, respectively. Furthermore, we demonstrate that hyperactivation of NRF2 via knockout of Kelch-like ECH-associated protein 1 (KEAP1), its negative regulator, contributes to proliferation; while, inhibition of NRF2 or direct knockdown of SOX9 slowed the ability of NSCLC cells to proliferate, migrate, and invade. Overall, this study suggests that NRF2-mediated SOX9 upregulation can contribute to the metastatic potential of both environmentally and genetically driven lung tumors.
Keywords: NRF2, SOX9, metastasis, NSCLC, arsenic, cancer
Introduction
Arsenic toxicity remains a global health concern that affects millions of persons each year, however, the disease-specific consequences of chronic low dose arsenic exposure are still emerging. Epidemiological evidence suggests that chronic arsenic exposure via contaminated ground water leads to the onset and progression of various diseases, including cancer (Ghosh, 2013; Shankar et al., 2014; Crinnion, 2017). Specifically, measurement of arsenic concentrations in the toenail clippings of lung cancer patients has shown an association between arsenic exposure and increased risk of lung cancer incidence (Heck et al., 2009). As of 2019, lung cancer remains the leading cause of cancer related mortality in the United States, accounting for nearly 24% of all cancer related deaths (Siegel et al., 2019). Non-small cell lung cancer (NSCLC) constitutes ~85% of all newly diagnosed lung cancer cases and is thus responsible for a significant amount of patient mortality (Zappa and Mousa, 2016). In some cases, increased metastasis of transformed cells from the primary site to other tissues has been linked to increased morbidity in NSCLC patients. As many NSCLC tumors are not diagnosed until Stage III, when metastasis is likely to have already occurred, the 5-year survival rate is only 16%, highlighting the need to understand and prevent metastasis of NSCLC (Urvay et al., 2016). While mounting evidence has begun to elucidate the molecular mechanisms underlying metastasis in NSCLC, much is still unknown in the context of what promotes migration and metastasis during arsenic-induced NSCLC (Perlikos et al., 2013). Therefore, further investigation at both the epidemiological and mechanistic level to identify key drivers of metastasis in NSCLC should prove critical in preventing the progression of somatic mutation- and environmental toxin-induced lung cancers.
One molecular target associated with increased metastasis is SRY-box 9 (SOX9). SOX9 is a transcription factor that is most commonly associated with controlling gene expression responsible for sex determination during embryonic development (Chaboissier et al., 2004). However, evidence has shown that cell proliferation, epithelial-mesenchymal transition (EMT), and cancer cell metastasis are all linked with upregulation and activation of SOX9 (Yang et al., 2019). For example, the involvement of SOX9 in promoting proliferation has been demonstrated, as Sox9-deficient mice lacking Erb-B2 receptor tyrosine kinase 3 (ErbB3) expression exhibit heightened cell differentiation and decreased proliferation (Akiyama et al., 2004). Mechanistically, SOX9 increases metastasis by initiating transcription of Type X collagen (COL10A1), with increased collagen promoting the invasion and migration of gastric cancer cells via enhancement of EMT (Li et al., 2018). Recently, SOX9 was also shown to drive invasion, migration, and metastasis of NSCLC via activation of the Wnt/β-catenin signaling pathway (Huang et al., 2019). However, while evidence suggesting that SOX9 plays a critical role in cancer cell proliferation and metastasis continues to grow, the driving force behind SOX9-driven metastasis, particularly during chronic exposure to environmental toxicants, is still unknown.
One possible regulator of SOX9 is the transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2) (Nabeshima et al., 2020). NRF2 controls the expression of genes containing antioxidant response elements (AREs) (TGANNNNGC) in their promoter regions (Itoh et al., 1997). When activated, NRF2 dimerizes with small musculoaponeurotic fibrosarcoma (sMAF), binds to the ARE, and facilitates the recruitment of transcriptional machinery that activates the synthesis of mRNA encoding various downstream target genes that are involved in DNA repair, redox regulation, phase I, II, and III metabolism, and prevention of apoptosis, all of which help maintain a healthy, proliferating cell (Dodson et al., 2019). Under basal conditions, NRF2 is maintained at low levels by Kelch-like ECH-associated protein 1 (KEAP1): a substrate adaptor protein of a Cullin3-Ring-Box 1 (CUL3-RBX1) E3 ubiquitin ligase complex (Itoh et al., 1999). Key cysteine residues in KEAP1 (i.e. Cys151) are important redox sensors that upon oxidation trigger a conformational change in the complex that prevents the ubiquitination and subsequent degradation of NRF2; this allows the cell to respond to xenobiotic stressors and restore homeostasis (Dinkova-Kostova et al., 2002; Zhang and Hannink, 2003). While the upregulation of these genes helps to maintain/restore homeostasis in normal cells, recent evidence has also outlined the “dark side” of NRF2 in cancer, which refers to mutations in KEAP1, NFE2L2 (NRF2), or CUL3 that lead to constitutive activation of NRF2, with cancer types bearing these mutations being referred to as “NRF2-addicted cancers” (Ooi et al., 2013; Kerins and Ooi, 2018). NRF2 addiction has been observed in ovarian, prostate, head and neck cancers, and NSCLC and is often correlated with a worse prognosis for patients (Singh et al., 2006; Kim et al., 2010; Zhang et al., 2010; Konstantinopoulos et al., 2011; Lawrence et al., 2014). By preventing apoptosis, maintaining reduced conditions, and heightening drug metabolism/efflux, hyperactivation of NRF2 promotes cancer cell survival and leads to resistance to chemo- and radiotherapies (Wang et al., 2008; Frank et al., 2018). Recent evidence has also linked NRF2 to EMT and metastasis, however, the underlying mechanisms remain poorly understood (Wang et al., 2016; Rojo de la Vega et al., 2018; Lignitto et al., 2019).
It has been well-established that arsenic upregulates NRF2, with evidence suggesting that chronic, low dose arsenic exposure causes malignant transformation of healthy lung cells (Stueckle et al., 2012; Lau et al., 2013a). However, very little is known regarding the pathways downstream of NRF2 that mediate arsenic-promoted tumor progression and metastasis. Analysis of the SOX9 gene revealed a putative ARE sequence in its promoter region, indicating that NRF2 could transcriptionally regulate SOX9. Due to its role in metastasis, this suggested that SOX9 may provide a link between arsenic exposure, NRF2, and metastasis. Therefore, the goal of this study was to investigate if increased levels of NRF2 are enhancing the metastatic potential of lung cancer cells (i.e. their ability to migrate/invade) via SOX9. Our results show that chronic arsenic exposure increased the metastatic potential of immortalized lung epithelial cells via an NRF2-SOX9 axis. This NRF2-SOX9-dependent increase in metastatic potential was determined by measuring changes in the ability of arsenic-transformed cells to proliferate, migrate, and invade, and was further validated in an NRF2-addicted KEAP1−/− NSCLC cell line. Specifically, NRF2 and SOX9 are both required for increased NSCLC cell proliferation, as pharmacological inhibition or siRNA-mediated knockdown of these proteins, either individually or jointly, prevents proliferation. Additionally, loss of NRF2 or SOX9 mitigates invasion and migration of NSCLC cells. Taken together, these results demonstrate a novel mechanism by which the NRF2-SOX9 axis drives metastasis of NSCLC in both genetic mutation-induced and chronic arsenic-induced lung cancer models.
Materials and Methods
Cell culture, cell lines, antibodies, and materials
Sulforaphane (Santa Cruz Biotechnology), brusatol, and sodium arsenite (Sigma Aldrich) were all dissolved in autoclaved water or DMSO prior to treatment. H1299 (NSCLC cell line), A549 (NSCLC cell line), H838 (NSCLC cell line), and BEAS-2B (immortalized lung epithelial cell line) cells were all purchased from the American Type Culture Collection (ATCC). H1299 cells were selected to demonstrate proliferation, invasion, and migration as this cell line is derived from a metastatic NSCLC. A549 and H838 cell lines were selected for biotin pulldown as both contain a KEAP1 mutation that allows for constitutively active/upregulated NRF2. BEAS-2B cells were selected for transformation experiments as previous work has indicated that these cells display carcinogenic behavior when exposed to toxicants. CRISPR-Cas9 technology was used to generate NRF2 knockout A549, H838, and BEAS-2B cell lines, as well as KEAP1 knockout H1299 cells. All cells were cultured in DMEM containing L-Glutamine, 4.5 g/L glucose and sodium pyruvate (Corning Cellgro) with 10% FBS (Genesee Scientific) and 100 unit/mL Pen Strep (Gibco). All antibodies were purchased from Santa Cruz Biotechnology (GAPDH, KEAP1, MAF, NRF2, SOX9, NQO1). For the chronic arsenic (As(iii)) treatment experiments, BEAS-2B cells were cultured for 3 months with either no treatment or 0.5 μM As(iii); colony formation was measured via a soft agar assay as described previously (Borowicz et al., 2014)
Transfection
Transfection of DNA plasmids was carried out using Lipofectamine 3000 (Invitrogen) as per the manufacturer’s protocol. Briefly, H1299 cells were cotransfected with 500 ng of plasmid encoding a SOX9-ARE-driven firefly luciferase and 500 ng of a plasmid encoding TK-driven-Renilla-luciferase for 24 hours prior to treatment with 10 μM sulforaphane or 40 nM brusatol for an additional 16 hours. Luciferase activity was then measured via a luciferase reporter assay (Promega). Transfection of small interfering RNA (siRNA) was done using HiPerfect (Qiagen) and 40 nM of either a non-targeted (Qiagen) or SOX9-targeted (Ambion) siRNA construct for 48–72 hours.
Immunoblot and Pulldown
All immunoblot samples were collected in 1X sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM dithiothreitol [DTT], 0.1% bromophenol blue) and boiled for 10 minutes. Cells were sonicated for 20 minutes using the Biorupter system (Diagenode). Lysates were run on a 7.5% SDS-PAGE gel, then transferred to a nitrocellulose membrane (Prometheus). Membranes were blocked in 5% milk for 1 hour prior to overnight incubation with primary antibody at 4 degrees Celsius. The following day, membranes were washed 4 times for 15 minutes in 1X phosphate-buffered saline (PBS) plus 0.1% Tween-20 (Sigma Aldrich), then incubated with secondary antibody in 5% milk for 1 hour. Next, membranes were washed in PBS with Tween-20 again 6 times for 10 minutes each. For development, membranes were coated with enhanced chemiluminescent (ECL) solution and imaged using the Azure c600 (Azure Biosystems).
For the biotin pulldown experiments, cells were lysed in RIPA buffer (10 mM sodium phosphate [pH 7.2], 150 mM NaCl, 1% sodium deoxycholate, 2 mM EDTA, 0.1% SDS, 1% NP-40), and then precleared with streptavidin beads (Invitrogen) and incubated with 2 μg biotinylated DNA probes. Pull down of the biotinylated SOX9-ARE or SOX9-mARE (mutated) was done by incubating with streptavidin beads and then either A549, A549 NRF2−/−, H838, or H838 NRF2−/− cell lysate overnight. The DNA-protein complexes were washed three times and eluted from beads by boiling in 1X sample buffer. Proteins were resolved on a 7.5% SDS-PAGE gel and subjected to immunoblot analysis.
qRT-PCR
BEAS-2B NRF2+/+ cells were treated for 3 months with 0.5 μM As(iii) prior to collection (Fig. 1) and H1299 cells were treated with 10 μM sulforaphane for 10 hours prior to collection (Fig. 2). Quantitative real time-polymerase chain reaction (qRT-PCR) was performed using the LightCycler 480 system (Roche) to determine SOX9, NQO1, and GAPDH mRNA transcript levels. Primers for qRT-PCR are outlined in Table 1.
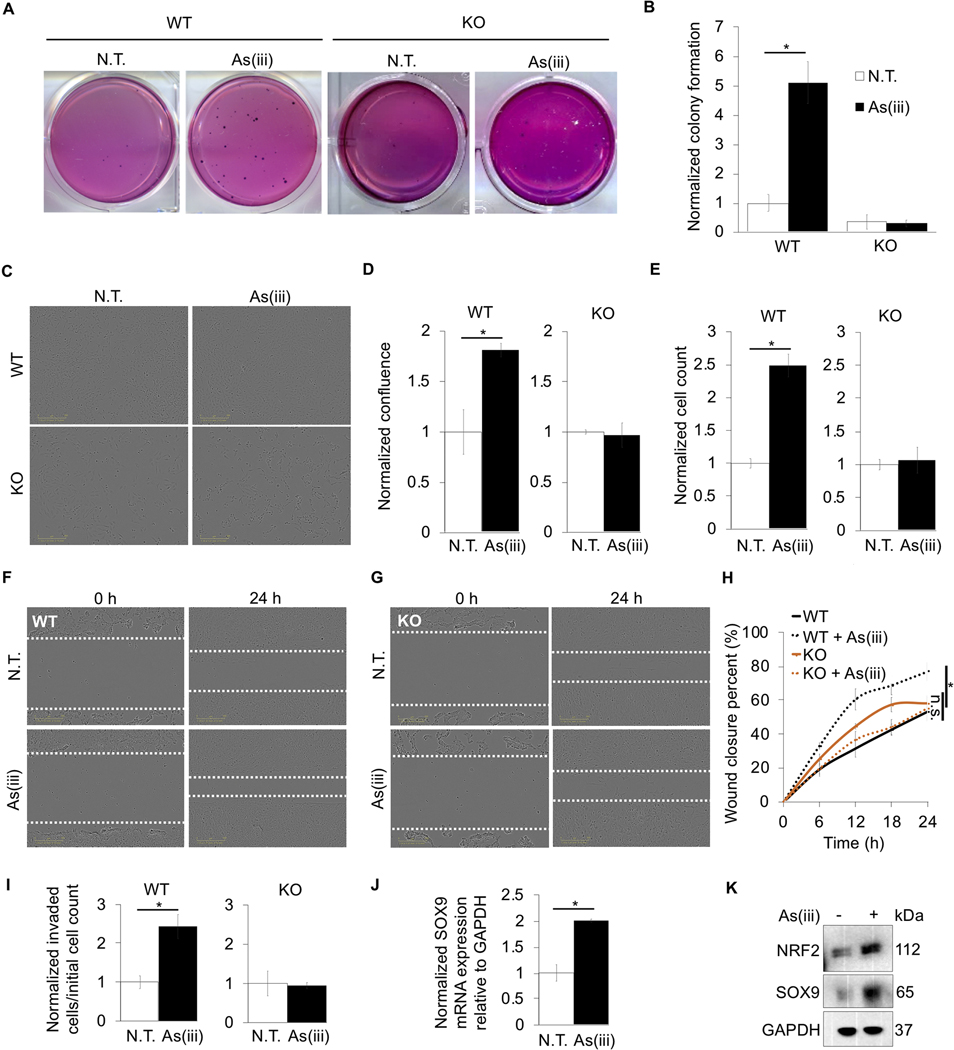
Figure 1: Chronic arsenic treatment causes malignant transformation of immortalized lung epithelial cells and increases their metastatic potential via NRF2.
BEAS-2B NRF2+/+ (WT) and BEAS-2B NRF2−/− (KO) cells were treated with 0.5 μM arsenic (As(iii)) for 30 passages (~3 months); (*p<0.05). (A) Representative images of colony formation in soft agar that was quantified in (B) (n=3) (N.T. = no treatment). (C) Representative images of cell confluence at 24 hours post seeding. (D) Quantification of (C) (n=3). (E) Cell number was quantified 24 hours post seeding (n=3). (F) Images of WT cells with As(iii) chronic exposure at time 0 and 24 hours post scratch (white dashed lines indicate scratch edges). (G) Same conditions as (F) with KO cells. (H) Wound closure percent was quantified every 6 hours up to 24 hours in both untreated and As(iii) treated cells from images in (F) and (G) (n=3) (n.s. = no significance). (I) Invasion was measured using an invasion assay, and the ratio of cells in the bottom chamber vs. the initial number in the top chamber was determined (n=3). (J) Relative mRNA expression of SOX9 in WT cells chronically treated with As(iii) (n=3). (K) Immunoblot analysis of NRF2 and SOX9 in untreated and chronic As(iii) treated WT cells.
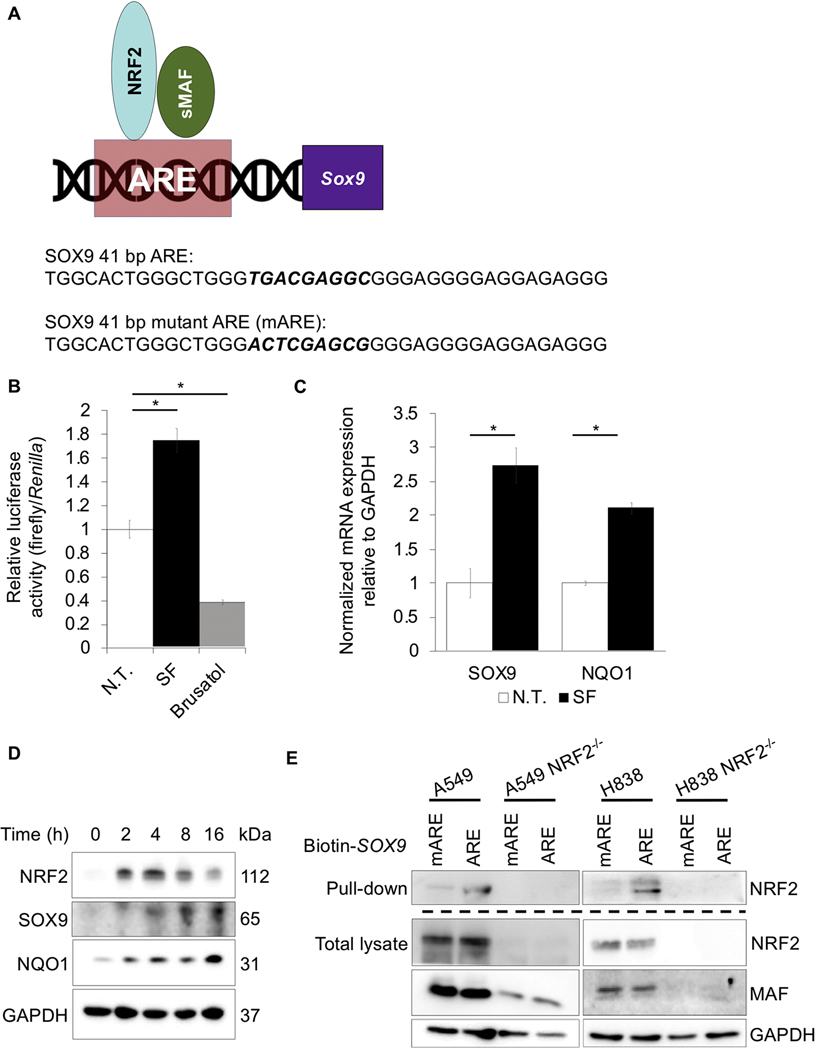
Figure 2: SOX9 is a novel NRF2 target gene.
(A) Model of NRF2 regulation of the ARE upstream of SOX9 gene (top); wildtype and mutant ARE sequences of SOX9 are indicated (bottom). (B) H1299 cells were cotransfected with 500 ng of a plasmid encoding a SOX9-ARE-driven firefly luciferase and 500 ng of a TK-driven-Renilla-luciferase plasmid for 24 hours prior to treatment with 10 μM sulforaphane or 40 nM brusatol for 16 hours, then relative luciferase/Renilla activity was measured (n=3) (N.T. = no treatment). (C) Relative SOX9 and NQO1 mRNA levels in H1299 cells treated with 10 μM sulforaphane for 10 hours (n=6). (D) Immunoblot analysis of NRF2, SOX9, and NQO1 protein levels at 0, 2, 4, 8, and 16 hours levels post 10 μM sulforaphane treatment. (E) Biotin labeled SOX9-ARE and SOX9-mARE were incubated with streptavidin beads and then incubated with A549, A549 NRF2−/−, H838, or H838 NRF2−/− cell lysates; immunoblot analysis of NRF2 (top) and NRF2 and MAF protein levels in total lysates (bottom); (*p<0.05).
Table 1:
qRT-PCR Primers
| Name | Sequences (5’−3’) |
|---|---|
| SOX9-F | GCGGTACCTCCTAGTCTAGACACACACAC |
| SOX9-R | GCAAGCTTGCCACTGAAGTTTCCAGTCAG |
| NQO1-F | ATGTATGACAAAGGACCCTTCC |
| NQO1-R | TCCCTTGCAGAGAGTACATGG |
| GAPDH-F | CTGACTTCAACAGCGACACC |
| GAPDH-R | TGCTGTAGCCAAATTCGTTGT |
Proliferation, Invasion, and Migration assays
All images used for proliferation and scratch assays were taken using the IncuCyte system (Essen Biosciences). Analysis of images for measurement of percent confluence and the invasion (FBS-driven) assay was conducted using the IncuCyte ZOOM software. For BEAS-2B experiments, cells were counted then seeded at 15,000 cells per well in a 96 well plate and monitored for confluence and cell number.
For brusatol experiments, H1299 wildtype and H1299 KEAP1−/− cells were treated with 40 nM brusatol overnight prior to assessing confluence, cell number, invasiveness, and migration for 24 hours. For siRNA experiments, cells were transfected with 40 nM control or SOX9 siRNA for 48–72 hours prior to beginning experiments for confluence, count, invasion, and migration for 24 hours. For determination of cell number via cell count and confluence, H1299 wild type and H1299 KEAP1−/− cells were seeded in a 96 well plate at 10,000 cells per well. Following treatment, cells were trypsinized and counted via a hemocytometer using the trypan blue exclusion method.
For the scratch migration assay of both BEAS-2B cells and H1299 cells, cells were seeded in a 96 well plate, serum starved, then scratched when cells were at >90% confluence using the WoundMaker (Essen Biosciences). Images were taken every 6 hours up to 24 hours, and then wound closure percent was calculated by quantifying the change in wound area at each timepoint using ImageJ (NIH). For the invasion assay of both BEAS-2B cells and H1299 cells, cells were serum starved overnight prior to trypsinization and seeding in a 2.13 mg/mL Matrigel solution (in serum free DMEM) in the IncuCyte Clearview insert in a 96 well plate. DMEM containing 10% FBS was added to the bottom chamber, and cells were seeded into the top chamber and allowed to invade through the membrane. Images were quantified as the ratio of cells that migrate to the bottom chamber compared to the starting number of cells in the top chamber.
Statistics
All statistics were done using an unpaired Student’s t-test. Significance is indicated by * and signifies p < 0.05. Mean values were calculated, and error was reported as the Standard Error of the Mean (SEM). Outliers were removed using the interquartile rule for outliers. All values were normalized to control as indicated on the y-axis.
Results
1. Chronic arsenic exposure causes malignant transformation of immortalized lung epithelial cells and increases their metastatic potential via NRF2
To understand the role of NRF2 in arsenic-mediated cell transformation, BEAS-2B NRF2−/− cells were established using CRISPR-Cas9 technology. BEAS-2B NRF2+/+ (WT) and NRF2−/− (KO) cells were treated with As(iii) for 30 passages (~3 months) at environmentally relevant doses (0.5 μM), and the anchorage-independent growth ability of cells was measured using the soft agar colony formation assay. Arsenic exposure significantly enhanced colony formation (~5-fold) in WT cells, while KO cells exposed to As(iii) exhibited almost no colony formation (Fig. 1A-B). To further assess the role of NRF2 in promoting chronic As(iii)-induced tumorigenesis, the proliferation, migration, and invasion of WT and KO cells was analyzed following chronic As(iii) exposure. As expected, WT cells chronically treated with As(iii) exhibited an ~1.75-fold increase in confluence over a 24 hour period as compared to the untreated control cells. Conversely, KO cells chronically exposed to As(iii) exhibited a similar change in confluence as their non-As(iii) exposed counterparts (Fig. 1C-D). Additionally, chronic As(iii) treatment increased WT cell proliferation over 24 hours ~2.5-fold, whereas KO cells divided similarly regardless of As(iii) exposure (Fig. 1E). Next, cell migration was assessed using a scratch migration assay. WT cells that were chronically treated with As(iii) migrated into the scratch ~2X faster than untreated cells; however, As(iii) treated KO cells showed no difference in their ability to migrate as compared to the untreated control (Fig. 1F-H). Finally, the role of NRF2 in mediating arsenic-promoted metastasis was tested via an invasion assay. Similar to the proliferation and migration experiments, WT cells chronically exposed to As(iii) displayed increased metastatic potential, invading ~2.5-fold more than untreated cells, while KO cells invaded to a similar extent regardless of As(iii) exposure (Fig. 1I). To identify putative NRF2 target effectors that might be responsible for the increased proliferation and migration observed in WT cells chronically exposed to As(iii), a DNA microarray analysis of gene differences between WT and KO cells was performed (data not shown). Of the potential candidates identified, SOX9 was selected for further study due to its previously established role in lung cancer metastasis. NRF2-dependent induction of SOX9 by chronic As(iii) exposure was verified in As(iii) treated WT cells (Fig. 1J-K). Together, this data indicates that chronic As(iii) exposure could not only cause malignant transformation of lung epithelial cells, but also increases the metastatic potential of the transformed tumor cells in an NRF2-dependent manner, and that NRF2-mediated downstream activation of SOX9 could be a critical effector of this response.
2. SOX9 is a novel NRF2 target gene
As increased NRF2 expression correlated with increased SOX9 mRNA transcript and protein levels, whether or not NRF2 is transcriptionally regulating SOX9 was tested. Analysis of the SOX9 promoter indicated the presence of a putative ARE sequence 153 bp upstream of the transcriptional start site (Fig. 2A). To verify that the NRF2-SOX9 axis was important in environmental- and genetic- driven lung cancer models, we utilized a variety of NSCLC cell lines (H1299, A549, and H838). To confirm whether or not this ARE is functional, a dual luciferase reporter assay was performed in H1299 cells co-expressing a firefly luciferase under the control of the putative ARE and a Renilla-luciferase controlled by the thymidine kinase (TK) promoter as an internal control. Luciferase activity increased ~1.7-fold following treatment with the NRF2-inducer sulforaphane (SF), as compared to an ~0.4-fold decrease after brusatol treatment, an inhibitor of the NRF2 pathway (Fig. 2B). Consistent with the luciferase results, SOX9 mRNA transcript levels increased ~2.5-fold, while well-established NRF2 target gene NQO1 also increased ~2-fold following SF treatment (Fig. 2C). These results were confirmed at the protein level, as SF induced NRF2, SOX9, and NQO1 proteins levels in a time-dependent manner (Fig. 2D). Finally, to confirm that NRF2 binds to the putative ARE in the SOX9 promoter, a biotin labeled wildtype (SOX9-ARE) or mutated (SOX9-mARE) ARE-containing oligonucleotide (41 bp) (sequences in Fig. 2A) was incubated with cell lysates from A549, A549 NRF2−/−, H838, and H838 NRF2−/− cells. Wildtype SOX9-ARE was able to pulldown NRF2, whereas SOX9-mARE could not; additionally, in CRISPR generated NRF2−/− cells, neither the SOX9-ARE nor the SOX9-mARE could pull down NRF2 (Fig. 2E). Collectively, these results demonstrate that the ARE sequence in the promoter region of SOX9 is functional, and since SOX9 is well characterized as being pro-metastatic, this suggests that increased NRF2 levels could drive metastasis via SOX9 upregulation.
3. Inhibition of NRF2 decreases NSCLC cell proliferation
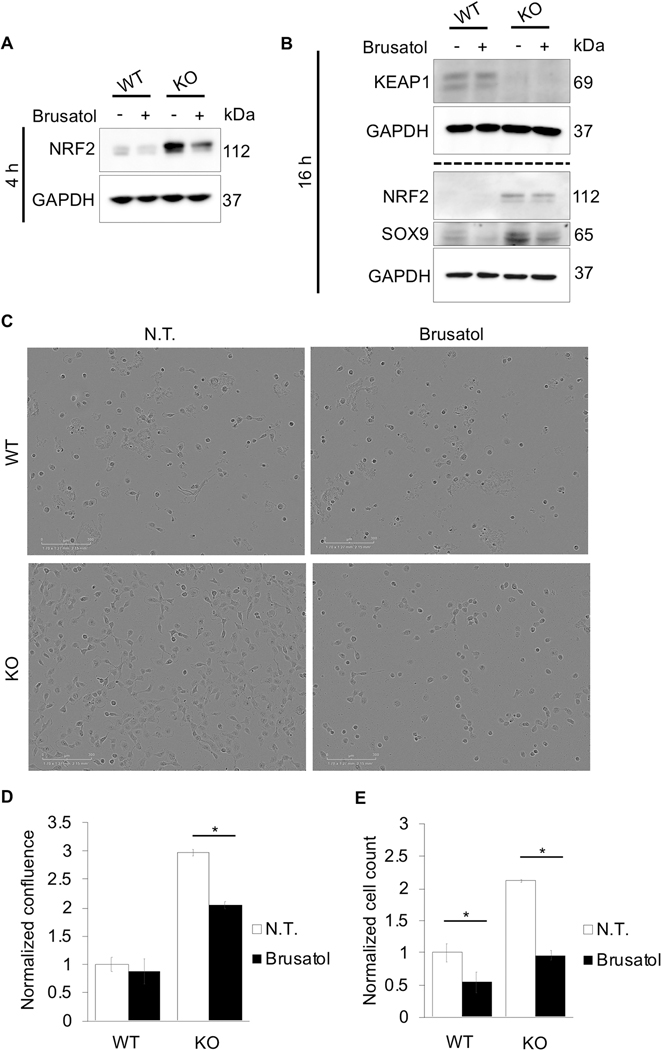
Next, the effect of NRF2 on NSCLC cell proliferation was investigated. Using CRISPR-Cas9 technology, an H1299 KEAP1−/− (KO) cell line with constitutively active NRF2 was generated. Using this newly generated cell line, the effect of NRF2 inhibition on NRF2 and SOX9 protein levels, as well as cell proliferation, was tested relative to H1299 cells (WT). Brusatol decreased NRF2 protein levels in both WT and KO cells (Fig. 3A). In addition, basal protein levels of SOX9 were significantly higher in KO cells, with brusatol still significantly reducing the protein levels of SOX9 (Fig. 3B). WT and KO cells were also tested for cell proliferation. KO cells treated with brusatol exhibited a ~33% decrease in cell confluence compared to their untreated controls (Fig. 3C-D). As seen in panel (E), KO cell proliferation was ~2X that of WT cells over a 24 hour period; however, when treated with brusatol, both WT and KO cell number decreased ~0.5-fold compared to their respective controls (Fig 3E). Thus, in both WT and KO cells, inhibition of NRF2 decreases total cell number, indicating that NRF2 is critical for NSCLC cell proliferation.
Figure 3: Inhibition of NRF2 decreases NSCLC cell proliferation.
Immunoblot analysis of NRF2, KEAP1, and SOX9 in H1299 (WT) and H1299 KEAP1−/− (KO) at (A) 4 or (B) 16 hours post 40 nM brusatol treatment. (C) Representative images of cell confluence of WT and KO cells 24 hours post 40 nM brusatol treatment. (D) Quantification of images in (C) (n=3). (E) Cell number was quantified 24 hours post seeding (n=3); (*p<0.05).
4. Loss of SOX9 decreases NSCLC cell proliferation
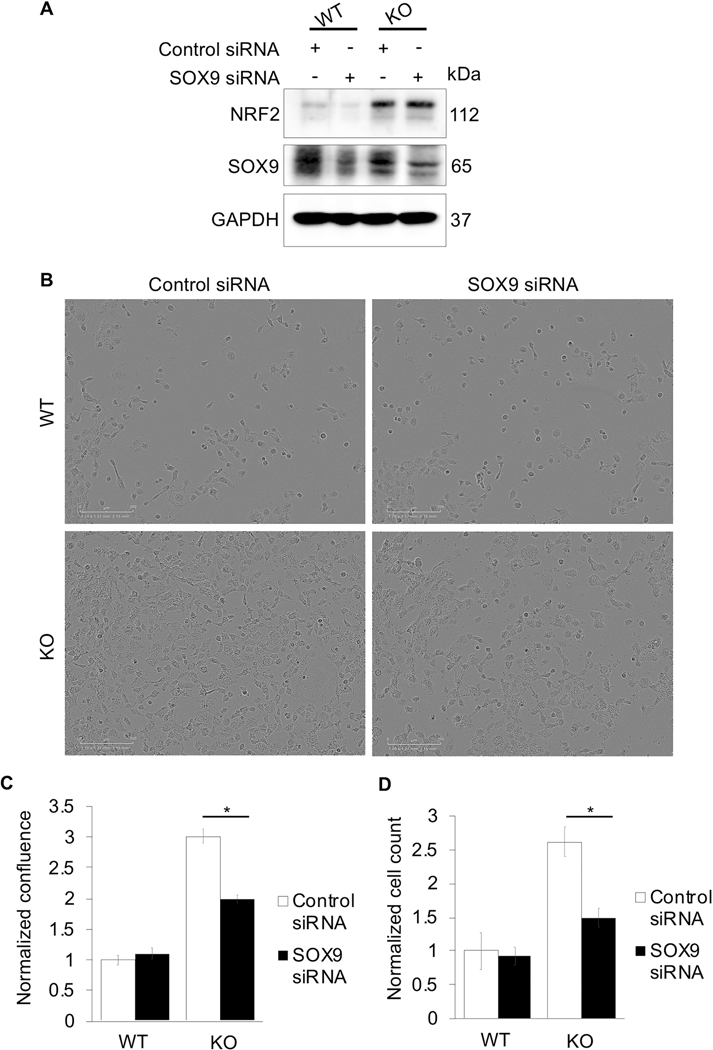
To establish an axis between NRF2, SOX9, and cell proliferation, the effect of loss of SOX9 on cell growth in a high NRF2 setting was tested. H1299 (WT) and H1299 KEAP1−/− (KO) cells transiently transfected with SOX9 siRNA showed a decrease in the protein levels of SOX9 without affecting NRF2 protein levels. (Fig. 4A). SOX9 knockdown decreased cell proliferation ~33% in KO cells, but not in WT cells (Fig. 4B-C). Similarly, cell counts revealed no significant difference in total cell number following SOX9 knockdown in WT cells; yet, in KO cells, SOX9 knockdown resulted in a ~0.6-fold decrease in total cell number (Fig. 4D). This supports the notion that basal levels of SOX9 in WT cells are very low; however, in KO cells, where NRF2 is hyperactivated, there is a higher basal level of SOX9, explaining why knockdown of SOX9 significantly decreased cell proliferation in this cell line. Overall, this indicates that cancers with constitutively active NRF2 could have increased metastatic potential via increased SOX9 expression, and that inhibition of SOX9 may be a viable therapeutic approach to treat these cancers.
Figure 4: Loss of SOX9 decreases NSCLC cell proliferation.
H1299 (WT) and H1299 KEAP1−/− (KO) cells were transiently transfected with 40 nM control or SOX9 siRNA for 72 hours. (A) Immunoblot analysis of NRF2 and SOX9 protein levels. (B) Representative images of cell confluence. (C) Quantification of images in (B) (n=3). (D) Cells were counted 24 hours post seeding (n=3); (*p<0.05).
5. Pharmacological inhibition of NRF2 slows NSCLC cell migration and invasion
The role of NRF2 on NSCLC cell migration was determined next. H1299 (WT) and H1299 KEAP1−/− (KO) cells were treated with brusatol overnight, then scratched, and migration was monitored over a 24 hour period (Fig. 5A-B). The percent change in wound area following brusatol treatment was then calculated every 6 hours for 24 hours. Brusatol treatment decreased the rate and overall migration of both WT and KO cells into the scratch. As expected, KO cells were more sensitive to brusatol treatment (~65% decrease in wound closure, compared to a ~25% decrease in wildtype cells at 24 hours) (Fig. 5C-D). Invasiveness of WT and KO cells treated with brusatol was also assessed (Fig. 5E). In both cell lines, brusatol decreased the total number of cells able to invade into the bottom chamber ~0.5-fold, indicating that NRF2 is crucial for NSCLC cell invasion.
Figure 5: Pharmacological inhibition of NRF2 slows NSCLC cell migration and invasion.
Representative images at 24 hours post scratch of (A) H1299 (WT) and (B) H1299 KEAP1−/− (KO) cells that were treated with 40 nM brusatol for 16 hours prior to scratch (white dashed lines indicate scratch edges). Wound closure percent of (C) WT and (D) KO cells at 0, 6, 12, 18, and 24 hours post scratch (n=3). (E) WT and KO cells were treated with 40 nM brusatol and serum starved overnight prior to measuring invasive behavior via number of cells that migrated to bottom chamber 24 hours post seeding (n=5–6); (*p<0.05).
6. Knockdown of SOX9 decreases metastatic potential of NSCLC
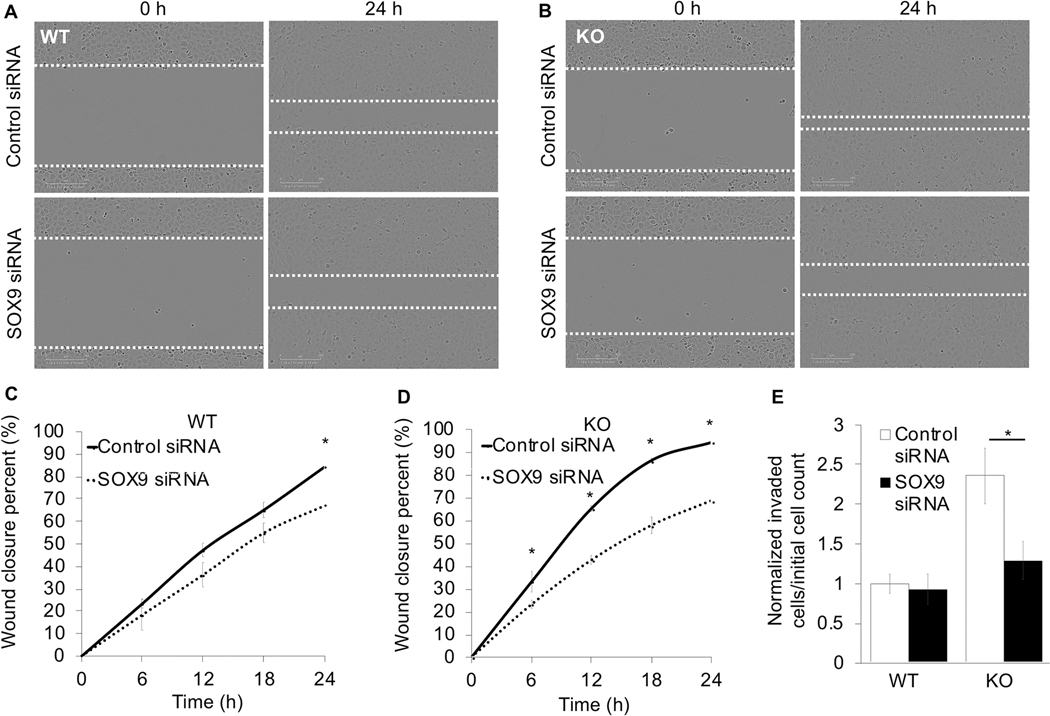
To test whether or not increased SOX9 expression underlies the enhanced NRF2-driven migration and invasion capacity of NSCLC cells, SOX9 siRNA knockdown experiments in H1299 (WT) and H1299 KEAP1−/− (KO) cells were conducted. Following SOX9 knockdown, the ability of both cell lines to migrate into the scratch was hindered, as wound closure decreased ~17% in WT cells and ~26% in KO cells at 24 hours (Fig. 6A-D); although, the effects of SOX9 knockdown were significantly more prevalent in KO cells from 6 hours post scratch onward. Additionally, KO cells were able to invade significantly more (~2.5-fold greater) than WT cells; however, loss of SOX9 completely negated the ability of these cells to invade (Fig. 6E). Once again, this indicates that in NRF2 overexpressing cancer cells, increased SOX9 expression drives migration; thus, cancers with high levels of NRF2 may have increased risk of metastasis due to increased SOX9 levels. Therefore, SOX9 is a potential therapeutic target to immobilize NRF2 hyperactive cancers.
Figure 6: Knockdown of SOX9 decreases metastatic potential of NSCLC.
Representative images of (A) H1299 (WT) or (B) H1299 KEAP1−/− (KO) cells at 0 and 24 hours post scratch that were transfected with either 40 nM control or SOX9 siRNA for 72 hours prior to scratching (white dashed lines indicate scratch edges). Using the conditions from (A) and (B), wound closure percent was measured at 0, 6, 12, 18, and 24 hours post scratch in (C) WT and (D) KO cells (n=3). (E) At 72 hours post siRNA transfection with 40 nM control or SOX9 siRNA, invasive behavior of serum starved WT and KO cells was measured via cells that migrated into bottom chamber at 24 hours per initial number of cells in top chamber (n=4–6); (*p<0.05).
7. Loss of SOX9 negates the metastatic potential of arsenic-transformed lung epithelial cells
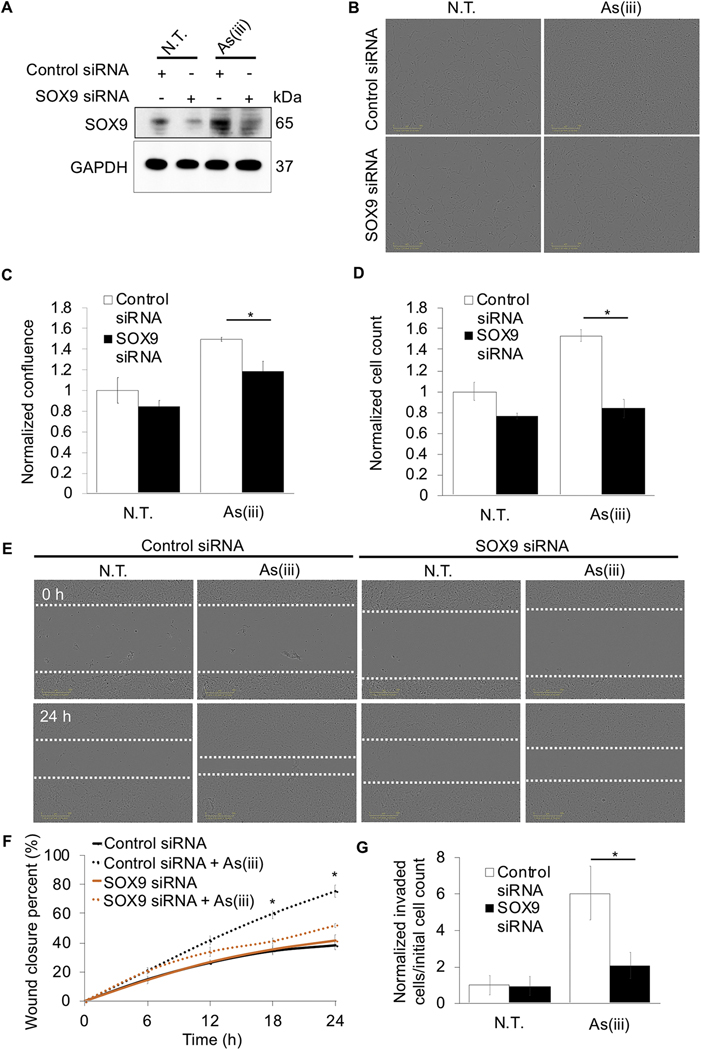
Finally, to demonstrate that SOX9 inhibition can be used as a strategy to slow down metastasis of lung cancer cells in an environmental-toxin carcinogenesis model, SOX9 was knocked down in As(iii)-transformed BEAS-2B NRF2+/+ cells, and proliferation, migration, and invasion were measured. Consistent with Fig. 1K, chronic As(iii) treatment increased SOX9 protein expression in BEAS-2B cells; however, following siRNA transfection, SOX9 levels were reduced to nearly non-As(iii) treated levels (Fig. 7A). Similar to Figure 1, As(iii) treatment increased confluence of cells ~1.5-fold, however this increase was significantly less in As(iii) treated cells where SOX9 was knocked down (~1.1-fold increase compared to non-As(iii) treated control cells) (Fig.7B-C). Moreover, while chronic As(iii) treatment increased cell number ~1.5-fold in control siRNA cells, knockdown of SOX9 decreased this effect ~0.5-fold (Fig. 7D). In addition, control siRNA transfected BEAS-2B cells chronically treated with As(iii) exhibited an ~80% wound closure 24 hours post-scratch, while untreated BEAS-2B with control or SOX9 siRNA, or SOX9 siRNA transfected BEAS-2B cells chronically treated with As(iii), exhibited an ~30–40% wound closure (Fig. 7E-F). BEAS-2B cells that received chronic As(iii) treatment showed ~6-fold more cells able to migrate into the bottom chamber compared to untreated control; meanwhile knockdown of SOX9 in chronic As(iii) treated BEAS-2B cells reduced the amount of cells able to invade to the bottom chamber by ~66% (Fig. 7G). Together with Figure 1, this data supports the hypothesis that SOX9 is a critical factor in promoting the increased proliferative, migratory, and invasive properties of lung cancer cells transformed by chronic As(iii) exposure.
Figure 7: Loss of SOX9 negates the metastatic potential of arsenic-transformed lung epithelial cells.
BEAS-2B NRF2+/+ cells that were either untreated or received 0.5 μM arsenic (As(iii)) treatment for 30 passages (~3 months) were transiently transfected with either 40 nM control or 40 nM SOX9 siRNA for 72 hours. (A) Immunoblot analysis of SOX9 protein levels. (B) Representative images of cell confluence. (C) Quantification of images in (B) (n=3). (D) Cells were counted 24 hours post seeding (n=3). (E) Representative images of cells at 0 and 24 hours post scratch; cells were scratched at 72 hours post siRNA transfection (white dashed lines indicate scratch edges). (F) Wound closure percent was calculated at 0, 6, 12, 18, and 24 hours post scratch using same conditions at (E) (n=3). (G) Cells were serum starved overnight and at 72 hours post transfection were measured for invasion via number of cells that migrated into bottom chamber 24 hours later compared to initial starting number of cells (n=4–6); (*p<0.05).
Discussion
The role of chronic arsenic exposure in the onset and progression of lung cancer has been well studied; however, the mechanisms underlying arsenic-induced metastasis are still relatively unknown (Chiu et al., 2004). This study not only validated that arsenic exposure causes malignant transformation of immortalized lung epithelial cells, but also further demonstrated that chronic exposure increases metastatic potential via the NRF2 signaling pathway. Furthermore, we demonstrated that the NRF2-SOX9 axis was important in increasing metastatic potential in a KEAP1−/− NSCLC cell line. While NRF2 activation by chemopreventive compounds is traditionally thought to be protective against acute toxicant exposure, chronic activation of NRF2 by arsenic in a non-canonical manner has been characterized as the “dark-side” of NRF2, which could promote malignant transformation; once cells are transformed, the protective downstream effects of NRF2 would help the now cancerous cells to survive and proliferate (Lau et al., 2013b). Whereas in an NRF2−/− setting, cells that acquired enough damage and/or mutations to malignantly transform would be unable to survive as the accumulation of damage without NRF2-mediated protection would ultimately trigger cell death. Our results showcase how chronic As(iii) exposure increases the proliferation, migration, and invasion of these malignantly transformed cells via the transcriptional upregulation of SOX9 by NRF2 (Fig. 1). Furthermore, knockdown of SOX9 in these chronic As(iii) treated cells negates their ability to proliferate, migrate, or invade, thus supporting the claim that chronic arsenic exposure increases metastatic potential via the NRF2-SOX9 axis (Fig. 7). SOX9 was chosen as a candidate target gene from a DNA microarray that compared fold change of several genes across BEAS-2B NRF2+/+ and NRF2−/− cells (data not shown). Of the candidate genes identified in this array, genes with a positive fold change similar to previously identified NRF2 target genes were chosen; from these, SOX9 was selected due to its involvement in metastasis. By better understanding how key pro-metastatic cascades, including the NRF2-SOX9 axis, increase the metastatic potential of cells transformed by chronic arsenic exposure, we can begin to generate better intervention strategies to target arsenic-driven lung cancers.
As previously outlined, NRF2 levels are kept basally low in cells via a KEAP1-CUL3-RBX1 complex that continuously facilitates the ubiquitination and subsequent proteasomal degradation of NRF2 (Villeneuve et al., 2010). ROS and electrophilic pharmacological compounds can induce NRF2 by oxidizing key cysteine residues in KEAP1, causing a conformational change in the KEAP1-NRF2 complex that ultimately prevents ubiquitination and degradation of NRF2 (Hu et al., 2011; Uruno and Motohashi, 2011; Kensler et al., 2013; Dinkova-Kostova et al., 2017). Interestingly, arsenic also induces NRF2, but via autophagic dysfunction-dependent sequestration of KEAP1 by p62/SQSTM1 into autophagosomes/aggresomes, thus allowing newly synthesized NRF2 to accumulate and activate transcription in a prolonged manner (Lau et al., 2013b). Herein, we demonstrate that chronic arsenic exposure causes upregulation of the NRF2-SOX9 axis in lung epithelial cells, indicating a potential relationship between arsenic-induced tumorigenesis and the metastatic potential of tumors. Experimentally, we chose to utilize BEAS-2B cells as these are an immortalized bronchial epithelial cell line that upon exposure to arsenic can undergo malignant transformation, thus making these cells an ideal model to understand the role of arsenic on tumorigenesis and metastasis. However, BEAS-2B cells as a model of environmentally-induced lung cancer are limited as spontaneous transformation can occur, therefore an NSCLC genetic-based model (H1299 cells) was used to show the mechanistic relationship of NRF2-SOX9 and metastasis in an already transformed metastatic cell line. H1299 cells as a NSCLC model were advantageous to use as these are not NRF2-addicted, but by generating a KEAP1−/− model, the relationship between NRF2-addiction, SOX9, and metastasis could be assessed. Still, the role of the NRF2-SOX9 axis in various cancers and its contribution to increased metastasis warrants further investigation.
While recent evidence has shown a link between NRF2 and metastasis (Long et al., 2016; Kitamura and Motohashi, 2018; Lignitto et al., 2019), our data specifically demonstrate that NRF2 directly controls gene expression of a pro-metastatic protein (SOX9) via an ARE sequence in its promoter region (Fig. 2). Previously, it was reported that NRF2 could suppress E-cadherin, thus contributing to EMT (Arfmann-Knubel et al., 2015). Previous work from our lab has shown that NRF2 activation facilitates cell migration during diabetic wound healing (Long et al., 2016), with the new data presented here indicating that NRF2 also plays a critical role in migration and invasion of cancer cells. While it is well known that NRF2-addicted cancers are resistant to therapies, thus worsening patient prognosis, our data indicates that these tumors could be even more aggressive due to increased metastatic potential. This also suggests that induction of NRF2 in patients with non-NRF2-addicted cancers could be detrimental as this would increase SOX9 expression and the risk of metastasis of the existing tumor. Future investigations should consider the interplay between NRF2-SOX9 and other metastasis-related pathways to fully elucidate the drivers of increased metastatic potential in not only NSCLC, but other NRF2-addicted cancers as well.
Overall, our study demonstrates that both chronic arsenic exposure and loss of KEAP1 function can increase metastatic potential of NSCLC due to increased NRF2/SOX9 levels. Mutations in KEAP1 are frequently observed in NSCLC, and thus these tumors have constitutively active NRF2 and become NRF2-addicted; these patients may then be at an increased risk for metastasis due to transcriptional upregulation of SOX9. Our data indicates that loss of KEAP1 exacerbates the aggressiveness of the tumor, as when KEAP1 is functional, NSCLC cells proliferated, migrated, and invaded less (Fig. 3, 5). This demonstrates that regardless of the upstream cause (i.e. chronic arsenic exposure or genetic mutations), induction of the NRF2 signaling pathway has negative effects on cancer patient outcome via increasing metastatic potential of malignantly transformed cells. Additionally, knockdown of SOX9 slowed the ability of NSCLC cells to proliferate, migrate, and invade, thus suggesting the critical role of SOX9 in enhancing NSCLC metastatic potential (Fig. 4, 6). In both cases, increased NRF2 activity drives upregulation of SOX9 and its pro-metastatic behavior, thus indicating the significance of this mechanism and our need to understand how to manipulate this pathway in cancer therapy.
In addition to emphasizing the need to limit exposure to arsenic, this evidence further affirms the need for NRF2 targeted therapies in the treatment of cancer. Currently, there are no NRF2-specific inhibitors in clinical trials, yet patients with NRF2-addicted cancer have a significantly worse prognosis. We explored the mechanism by which chronic arsenic toxicity increases metastatic potential to elucidate a potential therapeutic target in highly aggressive cancers. As we have demonstrated NRF2 transcriptional regulation of SOX9 in both a genetic and arsenic-transformation-based model of lung cancer, this suggests that that SOX9 is a key factor in promoting metastatic potential of both genetically and environmentally-induced NRF2-addicted cancers. This study thus introduces the possible value in developing therapies to target SOX9, along with other downstream effectors of NRF2, to mitigate NSCLC progression and metastasis. Overall, our findings verify that chronic arsenic exposure has the potential to not only induce malignant transformation, but also exacerbate the aggressiveness of tumor by inducing the NRF2 signaling pathway, and subsequently its pro-metastatic downstream target SOX9; therefore, by understanding this mechanism, a better therapeutic regimen can be designed to target these oncoproteins in the treatment of aggressive lung cancers.
Highlights 1.
Chronic arsenic exposure increases metastatic potential of immortalized lung epithelial cells 2
SOX9 is a transcriptional target gene of NRF2 3
NRF2 increases metastatic potential of NSCLC via SOX9 4
Loss of SOX9 negates metastatic potential caused by chronic arsenic exposure and genetic upregulation 5 of NRF2
Funding and Acknowledgments
The authors are funded by the following National Institutes of Health grants: T32ES007091 [C.J.S.], R35ES031575 [D.D.Z.], and P42ES004940 [D.D.Z.].
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B, 2004. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A 101, 6502–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfmann-Knubel S, Struck B, Genrich G, Helm O, Sipos B, Sebens S, Schafer H, 2015. The Crosstalk between Nrf2 and TGF-beta1 in the Epithelial-Mesenchymal Transition of Pancreatic Duct Epithelial Cells. PLoS One 10, e0132978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, Winn RA, 2014. The soft agar colony formation assay. J Vis Exp, e51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A, 2004. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131, 1891–1901. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Ho SC, Yang CY, 2004. Lung cancer mortality reduction after installation of tap-water supply system in an arseniasis-endemic area in Southwestern Taiwan. Lung Cancer 46, 265–270. [DOI] [PubMed] [Google Scholar]
- Crinnion W, 2017. Arsenic: The Underrecognized Common Disease-inducing Toxin. Integr Med (Encinitas) 16, 8–13. [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW, 2017. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol 69, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P, 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99, 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD, 2019. Modulating NRF2 in Disease: Timing Is Everything. Annu Rev Pharmacol Toxicol 59, 555–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R, Scheffler M, Merkelbach-Bruse S, Ihle MA, Kron A, Rauer M, Ueckeroth F, Konig K, Michels S, Fischer R, Eisert A, Fassunke J, Heydt C, Serke M, Ko YD, Gerigk U, Geist T, Kaminsky B, Heukamp LC, Clement-Ziza M, Buttner R, Wolf J, 2018. Clinical and Pathological Characteristics of KEAP1- and NFE2L2-Mutated Non-Small Cell Lung Carcinoma (NSCLC). Clin Cancer Res 24, 3087–3096. [DOI] [PubMed] [Google Scholar]
- Ghosh A, 2013. Evaluation of chronic arsenic poisoning due to consumption of contaminated ground water in West Bengal, India. Int J Prev Med 4, 976–979. [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ, 2009. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect 117, 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Eggler AL, Mesecar AD, van Breemen RB, 2011. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol 24, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, Wei FK, Xu XL, Ye SX, Song JW, Ding PK, Zhu J, Li HF, Luo XP, Gong H, Su L, Yang L, Gong LY, 2019. SOX9 drives the epithelial-mesenchymal transition in non-small-cell lung cancer through the Wnt/beta-catenin pathway. J Transl Med 17, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y, 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M, 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, Talalay P, 2013. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 329, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins MJ, Ooi A, 2018. A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci Rep 8, 12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH, 2010. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol 220, 446–451. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Motohashi H, 2018. NRF2 addiction in cancer cells. Cancer Sci 109, 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL, Cannistra SA, 2011. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res 71, 5081–5089. [DOI] [PubMed] [Google Scholar]
- Lau A, Whitman SA, Jaramillo MC, Zhang DD, 2013a. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol 27, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD, 2013b. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol 33, 2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G, 2014. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Huang H, Shi G, Zhao L, Li T, Zhang Z, Liu R, Hu Y, Liu H, Yu J, Li G, 2018. TGF-beta1-SOX9 axis-inducible COL10A1 promotes invasion and metastasis in gastric cancer via epithelial-to-mesenchymal transition. Cell Death Dis 9, 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B, Sayin VI, Papagiannakopoulos T, Pagano M, 2019. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 178, 316–329 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, Zheng H, Zhang DD, 2016. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 65, 780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Hamada S, Taguchi K, Tanaka Y, Matsumoto R, Yamamoto M, Masamune A, 2020. Keap1 deletion accelerates mutant K-ras/p53-driven cholangiocarcinoma. Am J Physiol Gastrointest Liver Physiol 318, G419–G427. [DOI] [PubMed] [Google Scholar]
- Ooi A, Dykema K, Ansari A, Petillo D, Snider J, Kahnoski R, Anema J, Craig D, Carpten J, Teh BT, Furge KA, 2013. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res 73, 2044–2051. [DOI] [PubMed] [Google Scholar]
- Perlikos F, Harrington KJ, Syrigos KN, 2013. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol 87, 1–11. [DOI] [PubMed] [Google Scholar]
- Rojo de la Vega M, Chapman E, Zhang DD, 2018. NRF2 and the Hallmarks of Cancer. Cancer Cell 34, 21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Shanker U, Shikha, 2014. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. ScientificWorldJournal 2014, 304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2019. Cancer statistics, 2019. CA Cancer J Clin 69, 7–34. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S, 2006. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3, e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueckle TA, Lu Y, Davis ME, Wang L, Jiang BH, Holaskova I, Schafer R, Barnett JB, Rojanasakul Y, 2012. Chronic occupational exposure to arsenic induces carcinogenic gene signaling networks and neoplastic transformation in human lung epithelial cells. Toxicol Appl Pharmacol 261, 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno A, Motohashi H, 2011. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25, 153–160. [DOI] [PubMed] [Google Scholar]
- Urvay SE, Yucel B, Erdis E, Turan N, 2016. Prognostic Factors in Stage III Non-Small-Cell Lung Cancer Patients. Asian Pac J Cancer Prev 17, 4693–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve NF, Lau A, Zhang DD, 2010. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal 13, 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu X, Long M, Huang Y, Zhang L, Zhang R, Zheng Y, Liao X, Wang Y, Liao Q, Li W, Tang Z, Tong Q, Wang X, Fang F, Rojo de la Vega M, Ouyang Q, Zhang DD, Yu S, Zheng H, 2016. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci Transl Med 8, 334ra351. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD, 2008. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liang R, Liu C, Liu JA, Cheung MPL, Liu X, Man OY, Guan XY, Lung HL, Cheung M, 2019. SOX9 is a dose-dependent metastatic fate determinant in melanoma. J Exp Clin Cancer Res 38, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa C, Mousa SA, 2016. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5, 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Hannink M, 2003. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23, 8137–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S, 2010. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther 9, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]