Abstract
INTRODUCTION:
Future burden has been modeled from population-based data for several common gastrointestinal diseases. However, as we enter the third decade in the 21st century, there are no such data on diseases of the pancreas holistically. The study aimed to estimate future incidence of pancreatitis, pancreatic cancer, diabetes of the exocrine pancreas (DEP), and exocrine pancreatic dysfunction (EPD) as well as years of life lost (YLL) due to premature death in individuals with those diseases up to 2050.
METHODS:
Historical New Zealand nationwide data on hospital discharge, pharmaceutical dispensing, cancer, and mortality were obtained. Annual incidence of each disease and annual YLLs due to premature death in individuals with each disease were calculated. A time series analysis using the stepwise autoregressive method was conducted.
RESULTS:
Pancreatitis yielded the highest projected incidence (123.7 per 100,000; 95% confidence interval, 116.7–130.7) and YLL (14,709 years; 13,642–15,777) in 2050. The projected incidence and YLL of pancreatic cancer were 18.6 per 100,000 (95% confidence interval, 13.1–24.1) and 14,247 years (11,349–17,144) in 2050, respectively. Compared with pancreatitis and pancreatic cancer, DEP and EPD yielded lower but more steeply increasing projected incidence rates and YLLs.
DISCUSSION:
The findings suggest that the burden of pancreatitis, pancreatic cancer, DEP, and EPD will rise in the next 3 decades unless healthcare systems introduce effective prevention or early treatment strategies for diseases of the pancreas and their sequelae.
INTRODUCTION
Healthcare systems in developed countries face a substantial burden of diseases of the exocrine pancreas (pancreatitis and pancreatic cancer). Globally, the incidence rates of acute pancreatitis (AP), chronic pancreatitis (CP), and pancreatic cancer are 33.7 cases, 9.6 cases, and 8.1 cases per 100,000 person-years, respectively (1). In the United States alone, the total direct cost of AP admissions is at least US$2.2 billion, with the average cost of US$9,870 per admission (2). In the United Kingdom alone, the annual direct and indirect costs of CP are at least equivalent to US$460 million (3). The average direct cost of 1 case with pancreatic cancer is at least US$65,500 in the United States (4). Furthermore, AP, CP, and pancreatic cancer lead to 11%, 23%, and 98% loss of quality of life, respectively (5–7). The average quality-adjusted life year lost to pancreatic cancer is 11.5 years per person in Europe (7). On top of that, individuals with pancreatitis and pancreatic cancer often develop metabolic sequelae (such as diabetes of the exocrine pancreas [DEP] and exocrine pancreatic dysfunction [EPD])—the burden of which is largely unknown.
Estimation of future disease burden has important implications for disease prevention and management. Projection of the total number of patients (i.e., event-based approach) might enable a proactive allocation of healthcare resources to populations in need. It might also facilitate the development of efficient policies and guidelines (8). Mortality is a major contributor to disease burden. The number of years of life lost (YLL) is a measure of premature mortality, considering simultaneously the number of deaths and life expectancy at age of death. Projection of YLL due to premature mortality (i.e., time-based approach) provides a comprehensive outlook of the fatal burden at a population level (9). Future burden of several major diseases in the field of gastroenterology has recently been modeled from population-based data, including inflammatory bowel disease (8), nonalcoholic fatty liver disease (10), and primary liver cancer (11). However, similar data on diseases of the exocrine pancreas are scarce. A projection study on incidence of pancreatic cancer in the United States to 2030 estimated its increase, with the annual average growth of 1.3% in men and 1.4% in women (12). The limitation of that study was that it projected incidence of any pancreatic cancer (i.e., both primary and secondary). It also projected future incidence from past data over 5 years only and assumed the same incidence over the projection period as the historical period. For the most common sequelae of both pancreatitis and pancreatic cancer—DEP and EPD—there is a dearth of data on their future burden. Furthermore, despite the importance of investigating age-specific disease burden with a view to identifying subpopulations with high disease burden, no study has projected burden of diseases of the pancreas and their sequelae in various age groups.
The primary aim of this study was to estimate the burden of pancreatitis, primary pancreatic cancer, DEP, and EPD up to 2050, using both event-based (i.e., incidence) and time-based (i.e., YLL) approaches. The secondary aim was to project age- and sex-specific incidence and YLL.
METHODS
Data sources
Nationwide hospital discharge data of individuals who were admitted for diseases of the exocrine pancreas (pancreatitis and pancreatic cancer) were obtained. This database was linked to pharmaceutical dispensing data, cancer registry data, and mortality data using encrypted identifier, provided by the Ministry of Health Analytical Services (National Health Board, New Zealand). The hospital discharge data contained information on demographics (age and sex), date of admission, and diagnostic codes (Ninth and Tenth revision of the International Classification of Diseases [ICD-9 and ICD-10] codes). The pharmaceutical dispensing data covered primary to tertiary healthcare utilization, with information on chemical name and date of dispensing. The cancer registry data were assembled from a range of data resources (histological data, mortality collection, and hospital discharge data) and included information on date of diagnosis and diagnostic codes. In addition, we used New Zealand age- and sex-specific population data (publicly available from the Infoshare database) (13) to calculate incidence. We also obtained New Zealand period life tables (publicly released by Stats NZ) (14) to calculate YLL due to premature mortality. The period life tables contained expected number of years of life remaining (i.e., life expectancy) at each single year of age, stratified by sex.
Derivation of incidence
This study included individuals aged 20 years or older who had the following diseases between 2006 and 2017: pancreatitis (AP or CP), primary pancreatic cancer, DEP (postpancreatitis diabetes mellitus [PPDM] or pancreatic cancer-related diabetes [PCRD]), and EPD (after pancreatitis or pancreatic cancer). Incidence of AP was defined as first admission with the relevant diagnostic codes (ICD-10, K85) in primary position and that of CP was as first admission with the relevant diagnostic codes (K86.0; K86.1) in any position. Individuals who were diagnosed with both AP and CP during the observation period were regarded as individuals with CP only for the purpose of this study. Incidence of primary pancreatic cancer was defined as having pancreatic cancer (ICD-10, C25) as the first-reported cancer in the cancer registry. The identified cases of AP, CP, and pancreatic cancer were incident as confirmed by tracking data back to 1998.
The identification of DEP (PPDM and PCRD, whichever came first) and EPD was based on both hospital discharge and pharmaceutical dispensing records (from all levels, including primary health care). PPDM was identified using the following criteria: diabetes diagnosis in any position (ICD-10, E10; E11; E13) and/or dispensing records of antidiabetic medications 90 days after first admission for pancreatitis (K85; K86.0; K86.1 in any position) (15–17). The purpose of the 90-day rule was, in line with the previous literature (15–17), to minimize the possibility of including stress-induced hyperglycemia during acute illness. Individuals with the diagnostic and/or dispensing records relevant to diabetes before or within 90 days after first admission for pancreatitis were deemed to have preexisting diabetes and, hence, excluded from the PPDM cases. In addition, PPDM was categorized as postacute pancreatitis diabetes mellitus (PPDM-A) and postchronic pancreatitis diabetes mellitus (PPDM-C). The inclusion criteria for PCRD were diabetes diagnosis in any position (ICD-10, E10; E11; E13) and/or dispensing records of antidiabetic medications after diagnosis of pancreatic cancer. EPD was defined as having at least 2 dispensing records of pancreatic enzyme, in which the first record was more than 90 days after first admission for pancreatitis or pancreatic cancer (whichever came first) and the second record was within a 6-month period after the first record. The second dispensing record of pancreatic enzyme was set as the date of incident EPD. To ensure that we included incident cases only, a 1-year washout period was applied; hence, the observation period was set between 2007 and 2017 in the DEP and EPD analyses.
Derivation of YLL
To calculate total YLL due to premature death in individuals with pancreatitis, pancreatic cancer, and their metabolic sequelae, we used the life expectancy table approach (9). Deaths in individuals with each target disease were identified on annual basis. The annual data were aggregated into each single year of age at death, stratified by sex, yielding the number of deaths in each age and sex stratum. The resulting data were matched with period life tables to obtain standard life expectancy at each age of death in years. YLL was calculated by multiplying the number of deaths by the life expectancy at age of death in each age and sex stratum. YLL of each age and sex stratum was summed for each target disease. Because of the availability of mortality data, the observation period was until 2015. The period life tables were available for the following truncated periods: 2005–2007 (used for 2006–2009 YLL calculations), 2010–2012 (for 2010–2012 YLL), and 2012–2014 (for 2013–2015 YLL).
Statistical analysis
A time series approach was used to model future incidence of pancreatitis, pancreatic cancer, and their metabolic sequelae up to 2050. The historical cohort data were aggregated to obtain annual frequency of each target disease. Annual incidence was calculated as annual frequency divided by the number of nationwide populations in the corresponding year. The time series data of the observed annual incidence were used for constructing a forecasting model with the use of stepwise autoregressive method (the “forecast” procedure in SAS version 9.4; SAS Institute, Cary, NC). In this model, autoregressive parameters were automatically selected by a stepwise regression procedure, only including the lags at which the autoregressive parameters were statistically significant (18). This approach was similar to the ones used in other published studies on future disease burden in the field of gastroenterology (8). In the analysis of the main diseases (pancreatitis, pancreatic cancer, DEP, and EPD), the observed and forecasted incidence rates were plotted, with 95% confidence intervals (CIs) for the forecasted values. To provide a summary statistic, we calculated the average annual growth (%) from 2020 to 2050 for the main diseases. To forecast YLL due to premature mortality in individuals with pancreatitis, pancreatic cancer, and their metabolic sequelae up to 2050, the historical YLL data were aggregated to obtain annual YLL of each target disease using the aforementioned modeling method.
Subgroup analyses were conducted to model age- and sex-specific future incidence rates and YLL for the main diseases. We first stratified the annual historical data by age (<40, 40–64, and 65+ years) or sex and then aggregated them into each stratum. The aforementioned modeling method was used.
RESULTS
Projected burden of pancreatitis
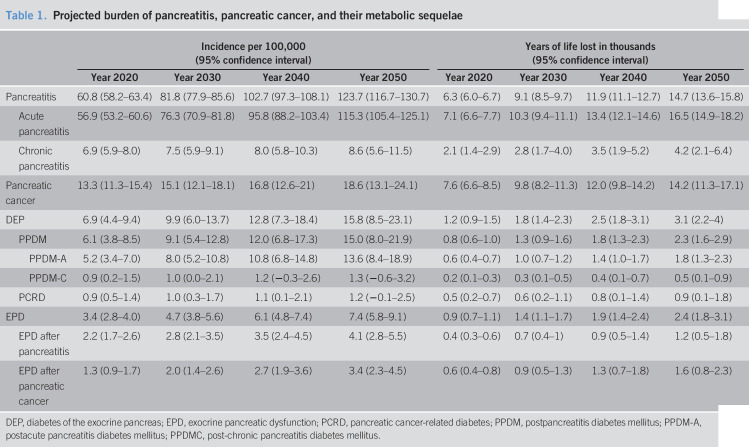
The incidence of pancreatitis was 40.2 per 100,000 (38.7 for AP and 5.6 for CP) in 2010 and was projected to increase to 81.8 (95% CI, 77.9–85.6) in 2030 and 123.7 (95% CI, 116.7–130.7) in 2050, with the average annual growth of 2.4% (Table 1; Figure 1). In the analysis stratified by age, the 65+ years group had the highest projected incidence in 2050 (170.4 per 100,000; 95% CI, 143.2–197.6) (Table 2). The average annual growth was 2.7%, 2.6%, and 1.5% in the <40 years, 40–64 years, and 65+ years groups, respectively. In the analysis stratified by sex, the incidence was projected to increase, with the average annual growth of 2.4% in both sexes (Table 3). The projected incidence rates of AP and CP are presented in Table 1 and Supplementary Figure 1 (see Supplementary Digital Content, http://links.lww.com/CTG/A412).
Table 1.
Projected burden of pancreatitis, pancreatic cancer, and their metabolic sequelae
| Incidence per 100,000 (95% confidence interval) | Years of life lost in thousands (95% confidence interval) | |||||||
| Year 2020 | Year 2030 | Year 2040 | Year 2050 | Year 2020 | Year 2030 | Year 2040 | Year 2050 | |
| Pancreatitis | 60.8 (58.2–63.4) | 81.8 (77.9–85.6) | 102.7 (97.3–108.1) | 123.7 (116.7–130.7) | 6.3 (6.0–6.7) | 9.1 (8.5–9.7) | 11.9 (11.1–12.7) | 14.7 (13.6–15.8) |
| Acute pancreatitis | 56.9 (53.2–60.6) | 76.3 (70.9–81.8) | 95.8 (88.2–103.4) | 115.3 (105.4–125.1) | 7.1 (6.6–7.7) | 10.3 (9.4–11.1) | 13.4 (12.1–14.6) | 16.5 (14.9–18.2) |
| Chronic pancreatitis | 6.9 (5.9–8.0) | 7.5 (5.9–9.1) | 8.0 (5.8–10.3) | 8.6 (5.6–11.5) | 2.1 (1.4–2.9) | 2.8 (1.7–4.0) | 3.5 (1.9–5.2) | 4.2 (2.1–6.4) |
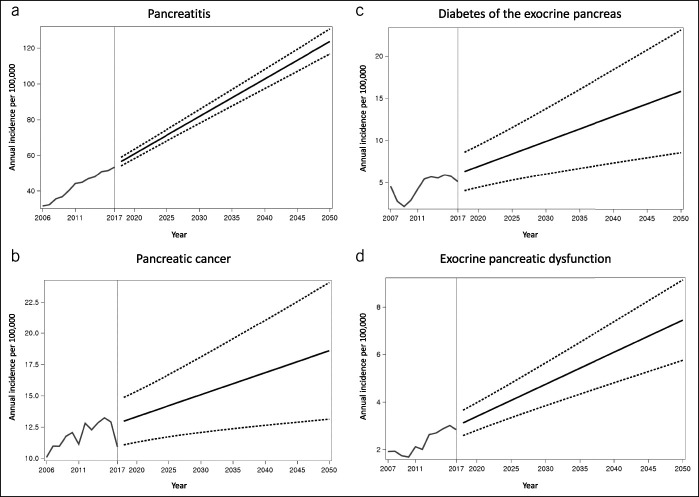
| Pancreatic cancer | 13.3 (11.3–15.4) | 15.1 (12.1–18.1) | 16.8 (12.6–21) | 18.6 (13.1–24.1) | 7.6 (6.6–8.5) | 9.8 (8.2–11.3) | 12.0 (9.8–14.2) | 14.2 (11.3–17.1) |
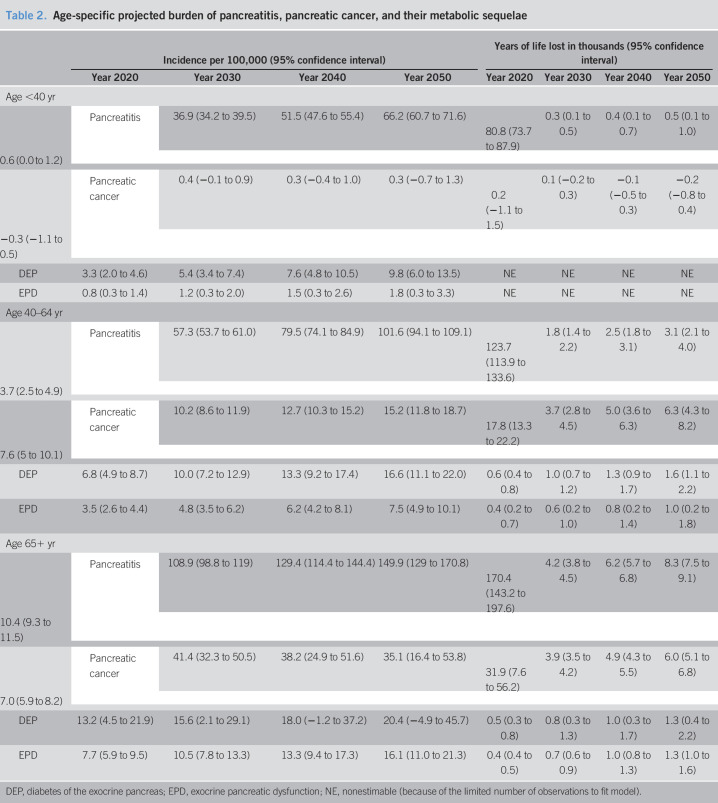
| DEP | 6.9 (4.4–9.4) | 9.9 (6.0–13.7) | 12.8 (7.3–18.4) | 15.8 (8.5–23.1) | 1.2 (0.9–1.5) | 1.8 (1.4–2.3) | 2.5 (1.8–3.1) | 3.1 (2.2–4) |
| PPDM | 6.1 (3.8–8.5) | 9.1 (5.4–12.8) | 12.0 (6.8–17.3) | 15.0 (8.0–21.9) | 0.8 (0.6–1.0) | 1.3 (0.9–1.6) | 1.8 (1.3–2.3) | 2.3 (1.6–2.9) |
| PPDM-A | 5.2 (3.4–7.0) | 8.0 (5.2–10.8) | 10.8 (6.8–14.8) | 13.6 (8.4–18.9) | 0.6 (0.4–0.7) | 1.0 (0.7–1.2) | 1.4 (1.0–1.7) | 1.8 (1.3–2.3) |
| PPDM-C | 0.9 (0.2–1.5) | 1.0 (0.0–2.1) | 1.2 (−0.3–2.6) | 1.3 (−0.6–3.2) | 0.2 (0.1–0.3) | 0.3 (0.1–0.5) | 0.4 (0.1–0.7) | 0.5 (0.1–0.9) |
| PCRD | 0.9 (0.5–1.4) | 1.0 (0.3–1.7) | 1.1 (0.1–2.1) | 1.2 (−0.1–2.5) | 0.5 (0.2–0.7) | 0.6 (0.2–1.1) | 0.8 (0.1–1.4) | 0.9 (0.1–1.8) |
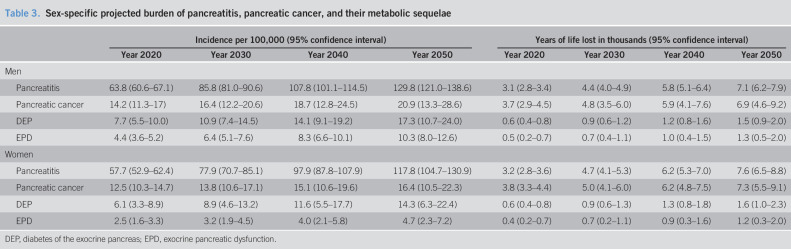
| EPD | 3.4 (2.8–4.0) | 4.7 (3.8–5.6) | 6.1 (4.8–7.4) | 7.4 (5.8–9.1) | 0.9 (0.7–1.1) | 1.4 (1.1–1.7) | 1.9 (1.4–2.4) | 2.4 (1.8–3.1) |
| EPD after pancreatitis | 2.2 (1.7–2.6) | 2.8 (2.1–3.5) | 3.5 (2.4–4.5) | 4.1 (2.8–5.5) | 0.4 (0.3–0.6) | 0.7 (0.4–1) | 0.9 (0.5–1.4) | 1.2 (0.5–1.8) |
| EPD after pancreatic cancer | 1.3 (0.9–1.7) | 2.0 (1.4–2.6) | 2.7 (1.9–3.6) | 3.4 (2.3–4.5) | 0.6 (0.4–0.8) | 0.9 (0.5–1.3) | 1.3 (0.7–1.8) | 1.6 (0.8–2.3) |
DEP, diabetes of the exocrine pancreas; EPD, exocrine pancreatic dysfunction; PCRD, pancreatic cancer-related diabetes; PPDM, postpancreatitis diabetes mellitus; PPDM-A, postacute pancreatitis diabetes mellitus; PPDMC, post-chronic pancreatitis diabetes mellitus.
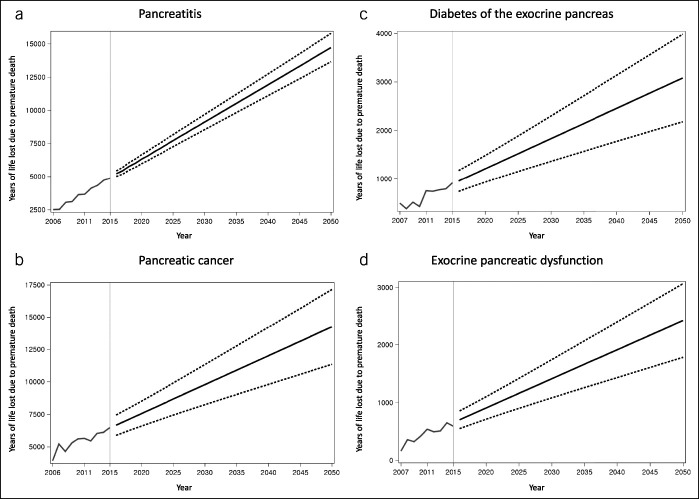
Figure 1.
Observed and projected incidence of (a) pancreatitis, (b) pancreatic cancer, (c) diabetes of the exocrine pancreas, and (d) exocrine pancreatic dysfunction. Grey and black lines indicate observed and projected values, respectively. Dashed lines indicate upper and lower 95% confidence intervals of projected values.
Table 2.
Age-specific projected burden of pancreatitis, pancreatic cancer, and their metabolic sequelae
| Incidence per 100,000 (95% confidence interval) | Years of life lost in thousands (95% confidence interval) | |||||||
| Year 2020 | Year 2030 | Year 2040 | Year 2050 | Year 2020 | Year 2030 | Year 2040 | Year 2050 | |
| Age <40 yr | ||||||||
| Pancreatitis | 36.9 (34.2 to 39.5) | 51.5 (47.6 to 55.4) | 66.2 (60.7 to 71.6) | 80.8 (73.7 to 87.9) | 0.3 (0.1 to 0.5) | 0.4 (0.1 to 0.7) | 0.5 (0.1 to 1.0) | 0.6 (0.0 to 1.2) |
| Pancreatic cancer | 0.4 (−0.1 to 0.9) | 0.3 (−0.4 to 1.0) | 0.3 (−0.7 to 1.3) | 0.2 (−1.1 to 1.5) | 0.1 (−0.2 to 0.3) | −0.1 (−0.5 to 0.3) | −0.2 (−0.8 to 0.4) | −0.3 (−1.1 to 0.5) |
| DEP | 3.3 (2.0 to 4.6) | 5.4 (3.4 to 7.4) | 7.6 (4.8 to 10.5) | 9.8 (6.0 to 13.5) | NE | NE | NE | NE |
| EPD | 0.8 (0.3 to 1.4) | 1.2 (0.3 to 2.0) | 1.5 (0.3 to 2.6) | 1.8 (0.3 to 3.3) | NE | NE | NE | NE |
| Age 40–64 yr | ||||||||
| Pancreatitis | 57.3 (53.7 to 61.0) | 79.5 (74.1 to 84.9) | 101.6 (94.1 to 109.1) | 123.7 (113.9 to 133.6) | 1.8 (1.4 to 2.2) | 2.5 (1.8 to 3.1) | 3.1 (2.1 to 4.0) | 3.7 (2.5 to 4.9) |
| Pancreatic cancer | 10.2 (8.6 to 11.9) | 12.7 (10.3 to 15.2) | 15.2 (11.8 to 18.7) | 17.8 (13.3 to 22.2) | 3.7 (2.8 to 4.5) | 5.0 (3.6 to 6.3) | 6.3 (4.3 to 8.2) | 7.6 (5 to 10.1) |
| DEP | 6.8 (4.9 to 8.7) | 10.0 (7.2 to 12.9) | 13.3 (9.2 to 17.4) | 16.6 (11.1 to 22.0) | 0.6 (0.4 to 0.8) | 1.0 (0.7 to 1.2) | 1.3 (0.9 to 1.7) | 1.6 (1.1 to 2.2) |
| EPD | 3.5 (2.6 to 4.4) | 4.8 (3.5 to 6.2) | 6.2 (4.2 to 8.1) | 7.5 (4.9 to 10.1) | 0.4 (0.2 to 0.7) | 0.6 (0.2 to 1.0) | 0.8 (0.2 to 1.4) | 1.0 (0.2 to 1.8) |
| Age 65+ yr | ||||||||
| Pancreatitis | 108.9 (98.8 to 119) | 129.4 (114.4 to 144.4) | 149.9 (129 to 170.8) | 170.4 (143.2 to 197.6) | 4.2 (3.8 to 4.5) | 6.2 (5.7 to 6.8) | 8.3 (7.5 to 9.1) | 10.4 (9.3 to 11.5) |
| Pancreatic cancer | 41.4 (32.3 to 50.5) | 38.2 (24.9 to 51.6) | 35.1 (16.4 to 53.8) | 31.9 (7.6 to 56.2) | 3.9 (3.5 to 4.2) | 4.9 (4.3 to 5.5) | 6.0 (5.1 to 6.8) | 7.0 (5.9 to 8.2) |
| DEP | 13.2 (4.5 to 21.9) | 15.6 (2.1 to 29.1) | 18.0 (−1.2 to 37.2) | 20.4 (−4.9 to 45.7) | 0.5 (0.3 to 0.8) | 0.8 (0.3 to 1.3) | 1.0 (0.3 to 1.7) | 1.3 (0.4 to 2.2) |
| EPD | 7.7 (5.9 to 9.5) | 10.5 (7.8 to 13.3) | 13.3 (9.4 to 17.3) | 16.1 (11.0 to 21.3) | 0.4 (0.4 to 0.5) | 0.7 (0.6 to 0.9) | 1.0 (0.8 to 1.3) | 1.3 (1.0 to 1.6) |
DEP, diabetes of the exocrine pancreas; EPD, exocrine pancreatic dysfunction; NE, nonestimable (because of the limited number of observations to fit model).
Table 3.
Sex-specific projected burden of pancreatitis, pancreatic cancer, and their metabolic sequelae
| Incidence per 100,000 (95% confidence interval) | Years of life lost in thousands (95% confidence interval) | |||||||
| Year 2020 | Year 2030 | Year 2040 | Year 2050 | Year 2020 | Year 2030 | Year 2040 | Year 2050 | |
| Men | ||||||||
| Pancreatitis | 63.8 (60.6–67.1) | 85.8 (81.0–90.6) | 107.8 (101.1–114.5) | 129.8 (121.0–138.6) | 3.1 (2.8–3.4) | 4.4 (4.0–4.9) | 5.8 (5.1–6.4) | 7.1 (6.2–7.9) |
| Pancreatic cancer | 14.2 (11.3–17) | 16.4 (12.2–20.6) | 18.7 (12.8–24.5) | 20.9 (13.3–28.6) | 3.7 (2.9–4.5) | 4.8 (3.5–6.0) | 5.9 (4.1–7.6) | 6.9 (4.6–9.2) |
| DEP | 7.7 (5.5–10.0) | 10.9 (7.4–14.5) | 14.1 (9.1–19.2) | 17.3 (10.7–24.0) | 0.6 (0.4–0.8) | 0.9 (0.6–1.2) | 1.2 (0.8–1.6) | 1.5 (0.9–2.0) |
| EPD | 4.4 (3.6–5.2) | 6.4 (5.1–7.6) | 8.3 (6.6–10.1) | 10.3 (8.0–12.6) | 0.5 (0.2–0.7) | 0.7 (0.4–1.1) | 1.0 (0.4–1.5) | 1.3 (0.5–2.0) |
| Women | ||||||||
| Pancreatitis | 57.7 (52.9–62.4) | 77.9 (70.7–85.1) | 97.9 (87.8–107.9) | 117.8 (104.7–130.9) | 3.2 (2.8–3.6) | 4.7 (4.1–5.3) | 6.2 (5.3–7.0) | 7.6 (6.5–8.8) |
| Pancreatic cancer | 12.5 (10.3–14.7) | 13.8 (10.6–17.1) | 15.1 (10.6–19.6) | 16.4 (10.5–22.3) | 3.8 (3.3–4.4) | 5.0 (4.1–6.0) | 6.2 (4.8–7.5) | 7.3 (5.5–9.1) |
| DEP | 6.1 (3.3–8.9) | 8.9 (4.6–13.2) | 11.6 (5.5–17.7) | 14.3 (6.3–22.4) | 0.6 (0.4–0.8) | 0.9 (0.6–1.3) | 1.3 (0.8–1.8) | 1.6 (1.0–2.3) |
| EPD | 2.5 (1.6–3.3) | 3.2 (1.9–4.5) | 4.0 (2.1–5.8) | 4.7 (2.3–7.2) | 0.4 (0.2–0.7) | 0.7 (0.2–1.1) | 0.9 (0.3–1.6) | 1.2 (0.3–2.0) |
DEP, diabetes of the exocrine pancreas; EPD, exocrine pancreatic dysfunction.
The total YLL due to premature death in individuals with pancreatitis was 3,636 years (4,019 for AP and 1,561 for CP) in 2010 and was projected to increase to 9,117 (95% CI, 8,547–9,686) in 2030 and 14,709 (95% CI, 13,642–15,777) in 2050, with the average annual growth of 2.9% (Table 1; Figure 2). In the analysis stratified by age, the 65+ years group had the highest projected YLL in 2050 (10,385 years; 95% CI, 9,304–11,465) (Table 2). The average annual growth was 2.1%, 2.4%, and 3.1% in the <40 years, 40–64 years, and 65+ years groups, respectively. In the analysis stratified by sex, the YLL was projected to increase, with the average annual growth of 2.8% in men and 2.9% in women (Table 3). The projected YLLs of AP and CP are presented in Table 1 and Supplementary Figure 1 (see Supplementary Digital Content, http://links.lww.com/CTG/A412).
Figure 2.
Observed and projected years of life lost due to premature mortality in individuals with (a) pancreatitis, (b) pancreatic cancer, (c) diabetes of the exocrine pancreas, and (d) exocrine pancreatic dysfunction. Grey and black lines indicate observed and projected values, respectively. Dashed lines indicate upper and lower 95% confidence intervals of projected values.
Projected burden of pancreatic cancer
The incidence of pancreatic cancer was 12.1 per 100,000 in 2010 and was projected to increase to 15.1 (95% CI, 12.1–18.1) in 2030 and 18.6 (95% CI, 13.1–24.1) in 2050, with the average annual growth of 1.1% (Table 1; Figure 1). In the analysis stratified by age, the 65+ years group had the highest projected incidence in 2050 (31.9 per 100,000; 95% CI, 7.6–56.2) (Table 2). The average annual growth was −1.8%, 1.9%, and −0.9% in the <40 years, 40–64 years, and 65+ years groups, respectively. In the analysis stratified by sex, the incidence was projected to increase, with the average annual growth of 1.3% in men and 0.9% in women (Table 3).
The total YLL due to premature death in individuals with pancreatic cancer was 5,604 years in 2010 and was projected to increase to 9,784 (95% CI, 8,236–11,332) in 2030 and 14,247 (95% CI, 11,349–17,144) in 2050, with the average annual growth of 2.1% (Table 1; Figure 2). In the analysis stratified by age, the 40–64 years group had the highest projected YLL in 2050 (7,588 years; 95% CI, 5,042–10,133) (Table 2). The average annual growth was 2.4% and 2.0% in the 40–64 years and 65+ years groups, respectively. In the analysis stratified by sex, the YLL was projected to increase, with the average annual growth of 2.1% in men and 2.2% in women (Table 3).
Projected burden of DEP
The incidence of DEP was 2.8 per 100,000 (1.8 for PPDM-A, 0.5 for PPDM-C, and 0.7 for PCRD) in 2010 and was projected to increase to 9.9 (95% CI, 6.0–13.7) in 2030 and 15.8 (95% CI, 8.5–23.1) in 2050, with the average annual growth of 2.8% (Table 1; Figure 1). In the analysis stratified by age, the 65+ years group had the highest projected incidence in 2050 (20.4 per 100,000; 95% CI, −4.9 to 45.7) (Table 2). The average annual growth was 3.7%, 3.0%, and 1.5% in the <40 years, 40–64 years, and 65+ years groups, respectively. In the analysis stratified by sex, the incidence was projected to increase, with the average annual growth of 2.7% in men and 2.9% in women (Table 3). The projected incidence rates of PPDM-A and PPDM-C are presented in Table 1 and Supplementary Figure 2 (see Supplementary Digital Content, http://links.lww.com/CTG/A412).
The total YLL due to premature death in individuals with DEP was 427 years (75 for PPDM, 72 for PPDM-C, and 280 for PCRD) in 2010 and was projected to increase to 1,832 (95% CI, 1,364–2,300) in 2030 and 3,081 (95% CI, 2,179–3,983) in 2050, with the average annual growth of 3.2% (Table 1; Figure 2). In the analysis stratified by age, the 40–64 years group had the highest projected YLL in 2050 (1,632 years; 95% CI, 1,068–2,196) (Table 2). The average annual growth was 3.3% and 2.9% in the 40–64 years and 65+ years groups, respectively. In the analysis stratified by sex, the YLL was projected to increase, with the average annual growth of 3.0% in men and 3.4% in women (Table 3). The projected YLLs of PPDM-A and PPDM-C are presented in Table 1 and Supplementary Figure 2 (see Supplementary Digital Content, http://links.lww.com/CTG/A412).
Projected burden of EPD
The incidence of EPD was 1.7 per 100,000 (1.3 after pancreatitis and 0.5 after pancreatic cancer) in 2010 and was projected to increase to 4.7 (95% CI, 3.8–5.6) in 2030 and 7.4 (95% CI, 5.8–9.1) in 2050, with the average annual growth of 2.6% (Table 1; Figure 1). In the analysis stratified by age, the 65+ years group had the highest projected incidence in 2050 (16.1 per 100,000; 95% CI, 11.0–21.3) (Table 2). The average annual growth was 2.6%, 2.6%, and 2.5% in the <40 years, 40–64 years, and 65+ years groups, respectively. In the analysis stratified by sex, the incidence was projected to increase, with the average annual growth of 2.9% in men and 2.2% in women (Table 3).
The total YLL due to premature death in individuals with EPD was 420 years (130 years after pancreatitis and 337 years after pancreatic cancer) in 2010 and was projected to increase to 1,413 (95% CI, 1,082–1,744) in 2030 and 2,428 (95% CI, 1,790–3,065) in 2050, with the average annual growth of 3.3% (Table 1; Figure 2). In the analysis stratified by age, the 65+ years group had the highest projected YLL in 2050 (1,311 years; 95% CI, 1,007–1,616) (Table 2). The average annual growth was 3.0% and 3.7% in the 40–64 years and 65+ years groups, respectively. In the analysis stratified by sex, the YLL was projected to increase, with the average annual growth of 3.4% in men and 3.2% in women (Table 3).
DISCUSSION
This study used several New Zealand nationwide databases from the sole publicly funded health system covering the entire country. It was the first study to estimate the future incidence (an event-based measure of burden due to morbidity) and YLL (a time-based measure of burden due to premature mortality) of not only diseases of the exocrine pancreas but also their metabolic sequelae. The modeling method used in this study was a time series analysis approach, which had been widely used in both nonmedical fields (such as economic forecasting) (19) and medical fields (including gastroenterology) (8,20). We reported that the incidence rates and YLLs of pancreatitis, pancreatic cancer, DEP, and EPD were projected to increase in the upcoming decades. Among the 4 diseases, pancreatitis yielded the highest projected incidence (123.7 per 100,000) and YLL (14,709 years) in 2050. The projected incidence of pancreatic cancer (18.6 per 100,000) was much lower than that of pancreatitis, whereas the projected YLL of pancreatic cancer (14,247 years) was similar to that of pancreatitis in 2050. Compared with pancreatitis and pancreatic cancer, DEP and EPD yielded lower projected incidence rates and YLLs but higher average annual growth percentages.
The increasing incidence of AP in this study is consistent with earlier temporal trends studies from the United States and Europe (21–23), and this trend might have been driven by multiple factors. One possible factor is an increase in testing of pancreatic enzymes levels, which uncovers milder cases of AP. A previous study from 2 hospitals in the United States demonstrated that serum pancreatic enzyme testing increased from 4.6% in 1996 to 9.5% in 2005, and this increase was correlated with a rising fraction of AP out of emergency department visits (24). However, there are no published data on temporal trends in pancreatic enzyme testing after that—which would correspond to the observation period in this study (i.e., 2006 and beyond). Because it is unknown whether the testing of pancreatic enzymes continued to increase after 2005, it is impossible to determine whether this factor explains the rising incidence of AP observed in this study. Another possible contributor is an increase in the prevalence of obesity, which is a strong risk factor for AP in the Western world (25). Although the prevalence of other risk factors for AP (such as alcohol consumption or tobacco smoking) generally did not increase over the past 2 decades, that of obesity increased from 26.5% in 2006 to 30.9% in 2018 (according to New Zealand data) (26). Moreover, a previous study using the United States nationwide inpatient sample reported that the prevalence of obesity (as an AP-associated diagnosis) was 3.1-times higher during the period of 2009–2012 compared with that of 2002–2005 (21). It is noteworthy that the studied metabolic sequelae (DEP and EPD) also displayed an increasing trend in incidence in the present study. One might argue that the increasing incidence rates of DEP and EPD were driven by the rising incidence of AP. This might have been the case in this study as most cases of DEP were PPDM-A. However, the relationship between pancreatitis, PPDM, and EPD is complex (27,28); hence, an incidence trend of pancreatitis and that of its metabolic sequelae might not necessarily run in parallel or show a linear relationship. In this study, the increasing trend was prominent in projected incidence rates of CP and pancreatic cancer but not in those of PPDM-C and PCRD. Furthermore, the development of DEP is known to be influenced by a range of factors. In addition to the conventional risk factors for diabetes (e.g., central obesity) (29,30), recurrent episodes of AP—a pancreas-specific risk factor for DEP—(31) might have differentially contributed to the incidence of DEP during the observation period.
Published data on projected burden of diseases of the exocrine pancreas are scarce and limited to pancreatic cancer only. A study from the United States projected the incidence of pancreatic cancer up to 2030 and found the average annual growth of 1.3% in men and 1.4% in women (12), which was similar to our estimate of 1.1% (notwithstanding the differences in methodology with our study). That study assumed that the incidence was constant over the historical period and projection period and the authors based extrapolation on population projections (12). The present study also used extrapolation but it was based on time series data. This enabled us to account for changes in incidence over time. This is important because the incidence of pancreatic cancer tended to increase in more than 10 developed countries between 1998 and 2007, as a global report demonstrated (32). This study provides a grim outlook that incidence of pancreatic cancer is likely to continue to increase in the upcoming decades in (unless effective prevention strategies or early treatment options are introduced). Furthermore, the global report mentioned above also showed that most developed countries had the increasing trend in mortality from pancreatic cancer (32), which is consistent with our estimate of the increasing YLL due to premature mortality in individuals with pancreatic cancer.
As far as age-specific projected burden is concerned, the 65+ years group had the highest projected incidence rates of pancreatitis, pancreatic cancer, DEP, and EPD among the studied age groups. This is consistent with earlier studies, suggesting that older age is related to higher incidence rates of pancreatitis, pancreatic cancer, DEP, and EPD (1,16,33,34). However, the average annual growth percentages were higher in the <40 years and 40–64 years groups than that in the 65+ years group in the analyses of pancreatitis, pancreatic cancer, and DEP. In particular, only the 40–64 years group had the increasing trend in the projected incidence of pancreatic cancer. Furthermore, the 40–64 years group had the highest projected YLL of pancreatic cancer (and its average annual growth) among the studied age groups. The above-mentioned findings, coupled with the fact that pancreatic cancer is the most lethal disease of the exocrine pancreas (the 5-year survival rate <10%) (35), suggest that healthcare policies might need to prioritize the middle-aged population (40–64 years) as a target group with a view to tackling the expected increase in burden of pancreatic cancer and DEP.
Several limitations of this study are to be acknowledged. First, there is an intrinsic issue of uncertainty because we estimated future disease burden based on past data. To address this issue, to the extent used in modern literature, we provided 95% CIs of the projected burden. Second, in the time series analysis, we fit a linear trend model while assuming that the incidence and YLL were stable over the study period, though our historical data displayed fluctuations (which might have been larger in pancreatic cancer, DEP, and EPD than that in pancreatitis). We also assumed that demographic distribution of the study population did not change over time. Although population aging over time might affect future burden of diseases of the exocrine pancreas, the assumption is unlikely to undermine our projection because we provided age-specific estimates of the projected burden. Third, the model for the data was derived wholly from New Zealand (which has a relatively small population). However, countries with large populations could not be used as a general model because integrated population-based data (pharmaceutical dispensing, cancer, mortality, and hospital discharge) are typically not available at present to project the burden of sequelae of pancreatic diseases—EPD and DEP. Hence, our research sets a benchmark for projecting the burden of EPD and DEP before larger populations are analyzed in the future. Fourth, the use of ICD codes for pancreatitis might have overestimated its actual incidence in this study. A meta-analysis of 24 cohorts suggested that the overall diagnostic accuracy of ICD codes for pancreatitis is suboptimal, with the pooled estimate of positive predictive value of ICD codes for AP being 71% (36). However, the estimate improves to 78% when the study population is constrained to incident AP in adults, which was the case in this study. Last, one could argue that the results of this study from New Zealand might not be generalizable to other geographical regions. In addition, the ethnic/racial composition of New Zealand is somewhat different from the United States with 17% indigenous (Māori) population and less than 1% Black people (both are high-risk ethnic/racial groups for AP incidence) (37). However, the incidence rates of pancreatitis and pancreatic cancer from our historical data in New Zealand were similarly high to the pooled estimates from a meta-analysis covering 21 countries (including the American, European, and Asian regions) (1). Specifically, the pooled incidence of AP was 33.7 (95% CI, 23.3–48.8) per 100,000 in the meta-analysis (1), and it was 38.7 in our historical data of the year 2010. In addition, the 2018 GLOBOCAN data showed a negligible difference in the age-standardized incidence of pancreatic cancer between New Zealand (11.3) and other developed countries (e.g., 12.9 in the United States, Sweden, and Denmark; 11.7 in the United Kingdom and South Korea) (38).
In conclusion, at the start of the 2020s decade, this study quantified the future burden of pancreatitis, pancreatic cancer, DEP, and EPD for the next 3 decades. This study adds the projected burden of pancreatitis and its metabolic sequelae to the existing literature, which to date has been limited to pancreatic cancer only. Given that the elderly population had the highest projected incidence of pancreatitis and its metabolic sequelae, healthcare systems need to be sufficiently prepared to accommodate for the forthcoming increase in the burden, specifically in aging societies. In addition, taking into account that the highest projected YLLs due to premature mortality was observed in middle-aged adults with pancreatic cancer and/or DEP, effective prevention of premature mortality due to pancreatic cancer, PCRD, and PPDM might need to primarily target middle-aged people.
CONFLICTS OF INTEREST
Guarantor of the article: Maxim S. Petrov, MD, MPH, PhD.
Specific author contributions: M.S.P.: supervised the study. J.C. and M.S.P.: designed the study. J.C.: analyzed data and drafted the manuscript. M.S.P.: provided significant intellectual input and critically reviewed the manuscript. All authors approved the final version of this manuscript.
Financial support: This study was part of the Clinical and epidemiOlogical inveStigations in Metabolism, nutritiOn, and pancreatic diseaseS (COSMOS) program. COSMOS is supported, in part, by the Royal Society of New Zealand (Rutherford Discovery Fellowship to M.S.P).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ The burden of pancreatic cancer and pancreatitis has been poorly studied.
✓ The burden of their sequelae (diabetes of the exocrine pancreas and exocrine pancreatic dysfunction) has never been studied.
WHAT IS NEW HERE
✓ A time series analysis was applied, for the first time, to project the burden of diseases of the pancreas.
✓ Both event-based and time-based approaches were used.
✓ The burden of pancreatic cancer, pancreatitis, diabetes of the exocrine pancreas, and exocrine pancreatic dysfunction is projected to rise differentially.
TRANSLATIONAL IMPACT
✓ Gastroenterologists (and other health professionals) need to be prepared for the expected increase in the burden of diseases of the exocrine pancreas and their metabolic sequelae.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A412
REFERENCES
- 1.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1(1):45–55. [DOI] [PubMed] [Google Scholar]
- 2.Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007;35(4):302–7. [DOI] [PubMed] [Google Scholar]
- 3.Hall TC, Garcea G, Webb MA, et al. The socio-economic impact of chronic pancreatitis: A systematic review. J Eval Clin Pract 2014;20(3):203–7. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill CB, Atoria CL, O'Reilly EM, et al. Costs and trends in pancreatic cancer treatment. Cancer 2012;118(20):5132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pendharkar SA, Salt K, Plank LD, et al. Quality of life after acute pancreatitis: A systematic review and meta-analysis. Pancreas 2014;43(8):1194–200. [DOI] [PubMed] [Google Scholar]
- 6.Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality of life in chronic pancreatitis: A case-control study from the North American pancreatitis study 2 cohort. Pancreas 2013;42(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrato A, Falcone A, Ducreux M, et al. A systematic review of the burden of pancreatic cancer in Europe: Real-world impact on survival, quality of life and costs. J Gastrointest Cancer 2015;46(3):201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156(5):1345–53.e4. [DOI] [PubMed] [Google Scholar]
- 9.Martinez R, Soliz P, Caixeta R, et al. Reflection on modern methods: Years of life lost due to premature mortality—A versatile and comprehensive measure for monitoring non-communicable disease mortality. Int J Epidemiol 2019;48(4):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valery PC, Laversanne M, Clark PJ, et al. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018;67(2):600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 13.Stats NZ. Infoshare, estimated resident population by age and sex, New Zealand. (http://archive.stats.govt.nz/infoshare). Accessed July 22, 2020.
- 14.Stats NZ. New Zealand period life tables. (http://archive.stats.govt.nz/browse_for_stats/health/life_expectancy/nz-period-life-tables-info-releases.aspx). Accessed July 22, 2020.
- 15.Petrov MS. Diabetes of the exocrine pancreas: American Diabetes Association-compliant lexicon. Pancreatology 2017;17(4):523–6. [DOI] [PubMed] [Google Scholar]
- 16.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019;16(3):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen HN, Yang CC, Chang YH, et al. Risk of diabetes mellitus after first-attack acute pancreatitis: A national population-based study. Am J Gastroenterol 2015;110(12):1698–706. [DOI] [PubMed] [Google Scholar]
- 18.Brocklebank JC, Dickey DA, Choi B. SAS for Forecasting Time Series. SAS Institute: Cary, NC, 2018. [Google Scholar]
- 19.Granger CWJ, Newbold P. Forecasting Economic Time Series. Academic Press: Cambridge, MA, 2014. [Google Scholar]
- 20.Xue JL, Ma JZ, Louis TA, et al. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 2001;12(12):2753–8. [DOI] [PubMed] [Google Scholar]
- 21.Krishna SG, Kamboj AK, Hart PA, et al. The changing epidemiology of acute pancreatitis hospitalizations: A decade of trends and the impact of chronic pancreatitis. Pancreas 2017;46(4):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gapp J, Hall AG, Walters RW, et al. Trends and outcomes of hospitalizations related to acute pancreatitis: Epidemiology from 2001 to 2014 in the United States. Pancreas 2019;48(4):548–54. [DOI] [PubMed] [Google Scholar]
- 23.Roberts SE, Morrison-Rees S, John A, et al. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017;17(2):155–65. [DOI] [PubMed] [Google Scholar]
- 24.Yadav D, Ng B, Saul M, et al. Relationship of serum pancreatic enzyme testing trends with the diagnosis of acute pancreatitis. Pancreas 2011;40(3):383–9. [DOI] [PubMed] [Google Scholar]
- 25.Petrov MS. Abdominal fat: A key player in metabolic acute pancreatitis. Am J Gastroenterol 2013;108(1):140–2. [DOI] [PubMed] [Google Scholar]
- 26.New Zealand Health Survey Annual Data Explorer. Ministry of Health: New Zealand, 2019. (https://minhealthnz.shinyapps.Io/nz-health-survey-2018-19-annual-data-explorer/_w_47e63e4c/#!/home). Accessed July 22, 2020. [Google Scholar]
- 27.Petrov MS. Metabolic trifecta after pancreatitis: Exocrine pancreatic dysfunction, altered gut microbiota, and new-onset diabetes. Clin Transl Gastroenterol 2019;10(10):e00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho J, Scragg R, Pandol SJ, et al. Exocrine pancreatic dysfunction increases the risk of new-onset diabetes mellitus: Results of a nationwide cohort study. Clin Transl Sci 2020. [Epub ahead of print July 21, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrov MS. Panorama of mediators in postpancreatitis diabetes mellitus. Curr Opin Gastroenterol 2020;36(5):443–51. [DOI] [PubMed] [Google Scholar]
- 30.Bharmal SH, Cho J, Alarcon Ramos GC, et al. Trajectories of glycaemia following acute pancreatitis: A prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol 2020;55(8):775–88. [DOI] [PubMed] [Google Scholar]
- 31.Ho TW, Wu JM, Kuo TC, et al. Change of both endocrine and exocrine insufficiencies after acute pancreatitis in non-diabetic patients: A nationwide population-based study. Medicine (Baltimore) 2015;94(27):e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong MCS, Jiang JY, Liang M, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 2017;7(1):3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohr JM, Panic N, Vujasinovic M, et al. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J Intern Med 2018;283(5):446–60. [DOI] [PubMed] [Google Scholar]
- 34.Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig Liver Dis 2017;49(5):540–4. [DOI] [PubMed] [Google Scholar]
- 35.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J Oncol 2019;10(1):10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao AY, Tan ML, Plana MN, et al. The use of international classification of diseases codes to identify patients with pancreatitis: A systematic review and meta-analysis of diagnostic accuracy studies. Clin Transl Gastroenterol 2018;9(10):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cervantes A, Waymouth EK, Petrov MS. African-Americans and indigenous peoples have increased burden of diseases of the exocrine pancreas: A systematic review and meta-analysis. Dig Dis Sci 2019;64(1):249–61. [DOI] [PubMed] [Google Scholar]
- 38.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.