Abstract
The transition from a developmentally arrested mature oocyte to a developing embryo requires a series of highly conserved events, collectively known as egg activation. All of these events are preceded by a ubiquitous rise of intracellular calcium, which results from influx of external calcium and/or calcium release from internal storage. In Drosophila, this calcium rise initiates from the pole(s) of the oocyte by influx of external calcium in response to mechanical triggers. It is thought to trigger calcium responsive kinases and/or phosphatases, which in turn alter the oocyte phospho-proteome to initiate downstream events. Recent studies revealed that external calcium enters the activating Drosophila oocyte through Trpm channels, a feature conserved in mouse. The local entry of calcium raises the question of whether Trpm channels are found locally at the poles of the oocyte or are localized around the oocyte periphery, but activated only at the poles. Here, we show that Trpm is distributed all around the oocyte. This requires that it thus be specially regulated at the poles to allow calcium wave initiation. We show that neither egg shape nor local pressure is sufficient to explain this local activation of Trpm channels.
Keywords: calcium wave, Drosophila development, egg activation, mechanosensitive ion channels, oocyte-to-embryo transition
1 |. INTRODUCTION
Egg activation releases mature oocytes from developmental arrest so that they can proceed into embryogenesis (reviewed in Kashir, Nomikos, Lai, & Swann, 2014; Krauchunas & Wolfner, 2013). Most of this process is conserved in species studied to date. A common feature of egg activation is a rise in levels of intracellular calcium, due to influx of external calcium and/or release of internal calcium from stores (reviewed in Swann & Lai, 2016). The trigger of this calcium rise varies across species, from mechanical stimulation from the reproductive tract in fruit flies and wasps, to the fertilizing sperm in vertebrates, echinoderms, and nematodes (Horner & Wolfner, 2008a; reviewed in Horner & Wolfner, 2008b).
In Drosophila egg activation, environmental calcium enters the oocyte through Transient receptor potential, family M (Trpm) channels in response to mechanical triggers (Hu & Wolfner, 2019), such as pressure from the reproductive tract and/or swelling of the oocyte due to fluid uptake from the oviducts (Heifetz, Yu, & Wolfner, 2001). Drosophila egg activation can be induced in vitro by incubating mature oocytes in hypotonic buffer so that they swell (Horner & Wolfner, 2008a; Page & Orr-Weaver, 1997). The calcium influx initiates a wave of calcium that propagates across the oocyte and can be visualized with the fluorescent calcium sensor GCaMP expressed in the germline (Kaneuchi et al., 2015; York-Andersen et al., 2015). The propagation of this calcium wave requires calcium release from internal stores, through the inositol 1,4,5-trisphosphate receptor (IP3) pathway (Hu,Vélez-Avilés, & Wolfner, 2020; Kaneuchi et al., 2015). Both in vivo and in vitro, calcium waves initiate from one or both poles of the Drosophila oocyte. In vivo, they initiate only from the posterior end 95% of the time (Kaneuchi et al., 2015). The posterior end of the oocyte enters the oviduct first and experiences the pressure from passing through the pedicel and swelling in the oviduct first. In vitro, incubation in a hypotonic buffer results in swelling throughout the oocyte, but calcium waves still initiate from the poles for the majority of the time (Kaneuchi et al., 2015; York-Andersen et al., 2015). These observations raise the question of how the oocyte poles are specially regulated to allow calcium wave initiation. Since the calcium influx occurs through Trpm channels, we were curious to know whether Trpm is found only at the poles of the oocytes, explaining its local activation, or whether it is distributed around the oocyte but only activated at the poles.
Here, we show that Trpm is localized around the mature oocyte. We then test and rule out two models for its activation at the poles: the ellipsoidal shape of the oocyte and differential sensitivity to regional pressure around the oocyte. Our data suggest that there is local special regulation of Trpm at oocyte poles to allow it to open for calcium influx only there.
2 |. RESULTS
2.1 |. Trpm protein is not restricted to the poles of mature oocytes
To examine the cellular distribution of Trpm, we used a validated anti-Trpm antibody (Hofmann et al., 2010) to visualize the distribution of Trpm in mature oocytes, activated but unfertilized eggs, and early embryos from Cas9 control and trpm germline knockout mutants (Hu & Wolfner, 2019). In the Cas9 control, Trpm is not restricted to the poles, but is distributed around the periphery of mature oocytes. (Figure 1a). Trpm channels appeared more diffuse around the plasma membranes after in vivo egg activation and were not seen on the plasma membrane in 1–5 hr early embryos (Figure 1a). Samples from trpm germline knockout females did not show significant staining under the same imaging settings (Figure 1b). To rule out the possible effect of Cas9 on Trpm localization, we repeated the staining on Oregon-R-P2 (ORP2) wildtype mature oocytes. We observed the same pattern (Figure S1). To verify independently the TRPM localization and its distribution change, we used anti-FLAG to probe Trpm in trpm MiMIC insertion lines that carry multi-tag insertion including FLAG in frame with the endogenous trpm gene (Nagarkar-Jaiswal et al., 2015). We observed similar FLAG distributions and pattern changes in mature oocytes and unfertilized eggs (Figure 1c,d).
FIGURE 1.
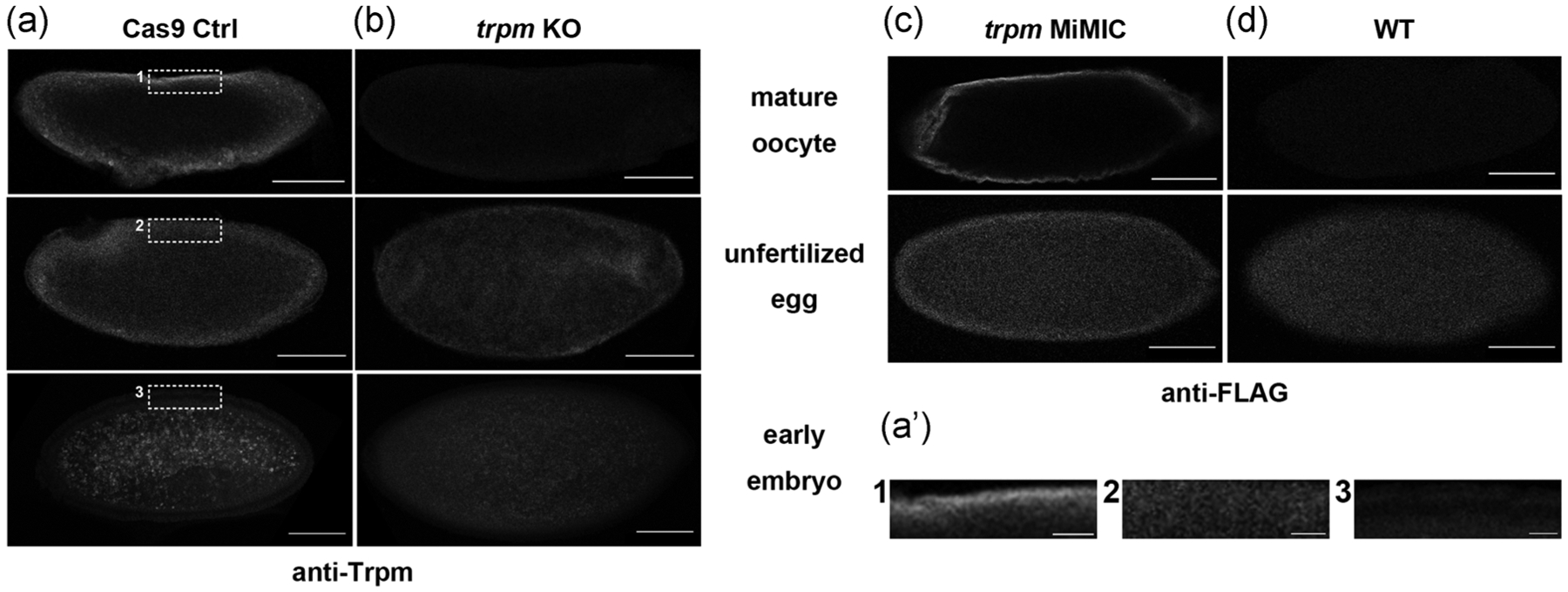
Localization of Trpm before and after egg activation. (a, b) Representative images of anti-Trpm staining of mature oocytes (n = 16 for control [Ctrl], n = 15 for trpm KO), unfertilized eggs (n = 10 for Ctrl, n = 10 for trpm KO) and 1–5 hr early embryos (n = 13 for Ctrl, n = 9 for trpm KO) from (a) matα-GAL4-VP16 > UAS-Cas9 control females and (b) matα-GAL4-VP16 > UAS-Cas9; trpm-gRNA1 trpm germline knockout females (trpm KO); scale bar = 100 μm. (a’) Enlarged view of samples in (a). Dashed lines indicate enlarged region; scale bar = 50 μm. (c,d) Representative images of anti-FLAG staining of mature oocytes (n = 9 for trpm MiMIC, n = 8 for WT) and unfertilized eggs (n = 11 for trpm MiMIC, n = 7 for WT) from trpm MiMIC insertion females and ORP2 wildtype females; scale bar = 100 μm. Images are single optical slices through the center of the oocytes. The oocytes shown were taken from the ovaries of different flies, but results were consistent across all ovaries, in two independent replicates. Similar background signals were seen in unfertilized eggs and early embryos
Taken together, our results suggested that Trpm is not located solely at the poles of mature oocytes and that it gets gradually delocalized from plasma membrane after egg activation, coinciding with the timing of its function in mediating the initial calcium influx at egg activation. The distribution of Trpm channels around mature oocytes rules out the possibility that the calcium wave initiation site is determined by specialized localization of Trpm channels mediating calcium influx. Thus, Trpm channels must be under special regulation or encounter special conditions at the poles to allow them to open only there in response to global mechanical triggers.
2.2 |. Oocyte shape does not regulate polar calcium wave initiation
The ellipsoidal shape of Drosophila oocyte could make its poles and waist experience different levels of mechanical pressure when passing through the reproductive tract in vivo or swelling in hypotonic buffer (modified Robb’s buffer [RB]; Hu & Wolfner, 2019) in vitro. To determine if oocyte shape affects the site of calcium wave initiation, we examined the calcium wave phenotype in oocyte from kugelei (kug, Fat2) mutants. kug is required for the follicle cell migration around the oocyte that shapes the oocyte (Horne-Badovinac, Hill, Gerlach, Menegas, & Bilder, 2012; Viktorinová, König, Schlichting, & Dahmann, 2009). Its mutants fail to elongate egg their chambers during oogenesis, resulting in near-spherical oocytes. These oocytes would experience similar pressures at their poles and waistline during swelling in vitro. We crossed into kugN103-2 null mutants the nos-GCaMP6m-attP40 transgene that expresses a fluorescent calcium sensor in the germline and visualized the calcium waves in the spherical mature mutant oocytes in in vitro egg activation. All calcium waves still initiated from the poles in spherical oocytes (Figure 2a and Movie S1), with 80% (12/15) showing calcium waves starting from both ends and 20% (3/15) showing calcium waves starting from the anterior end. Thus, whether calcium waves start from the poles is not determined solely by oocyte shape. The frequency of wave initiation at the anterior end in oocytes from kug mutants in vitro is higher than was reported for the wildtype strains previously studied (Kaneuchi et al., 2015; York-Andersen et al., 2015). Because the kug mutation does not appear to alter A/P patterning in the egg (Viktorinová et al., 2009), we think the difference in preferred pole of calcium wave initiation reflects differences in strain background, but we cannot rule out an effect of the near-spherical shape of mature oocytes from kug mutants on which pole is preferred in vitro.
FIGURE 2.
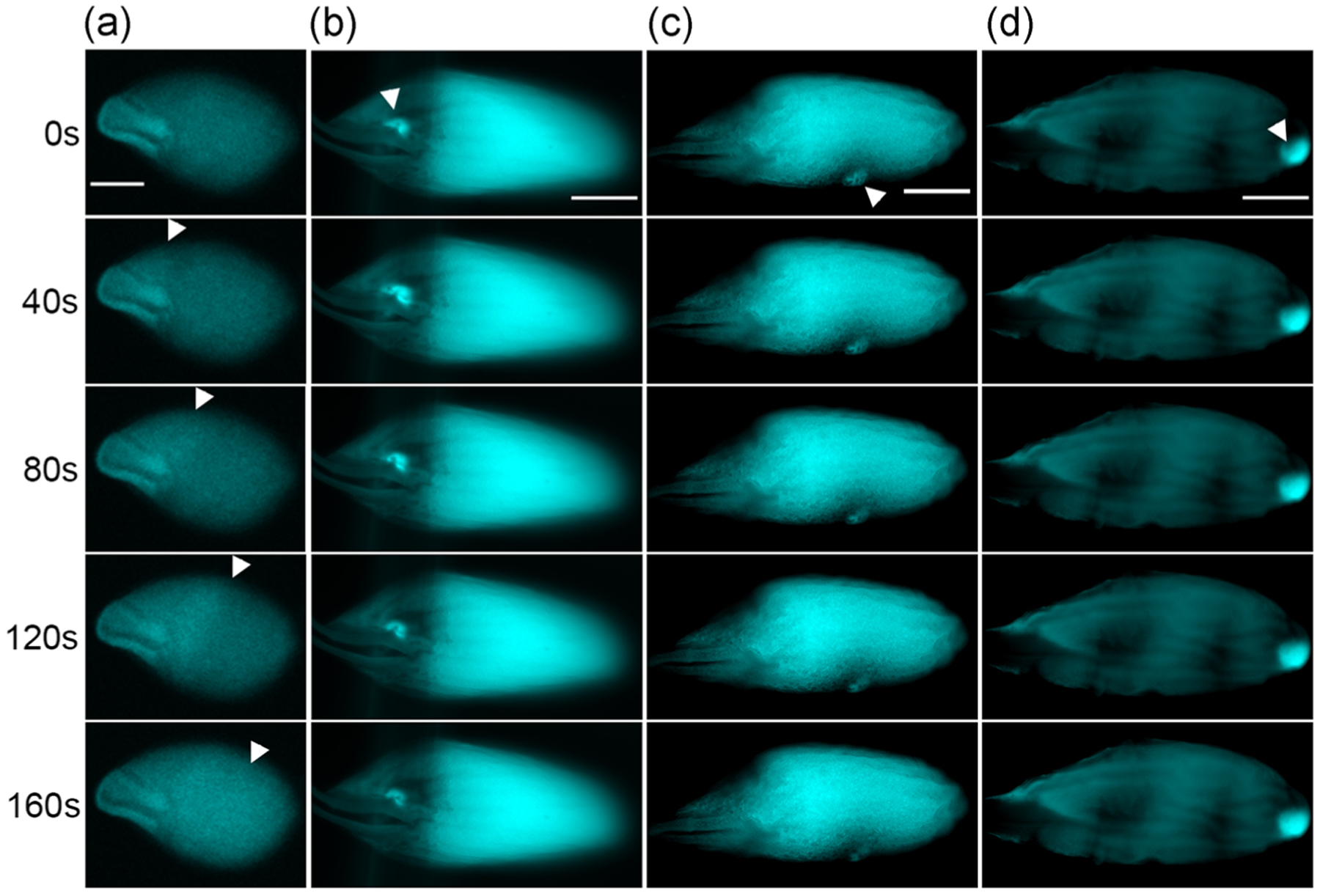
Regional calcium rise induced by microneedles in wildtype oocytes and calcium waves in kug mutant oocytes. (a) Representative images showing calcium waves propagating from the ends of kug mutant near-spherical shaped mature oocytes (n = 15). Arrowheads: wavefront of the calcium wave. (b–d) Representative images showing regional calcium rises that do not propagate into waves induced by microneedle pressing at (b) anterior (n = 7), (c) waist (n = 4), and (d) posterior (n = 4) regions of wildtype mature oocytes. Arrowheads: pressing site of microneedles. The background level of calcium varies among mature oocytes (Hu & Wolfner, 2019); scale bar = 100 μm
2.3 |. Oocyte poles and waist display similar sensitivity to regional mechanical pressure
We asked if oocyte poles and waist display similar sensitivity to mechanical pressure. In our previous studies, we showed that a regional calcium rise can be induced with a microneedle in mature oocytes incubated in hypertonic isolation buffer (IB), which prevents oocytes from swelling and activation (York-Andersen, Hu, Wood, Wolfner, & Weil, 2019). This calcium rise stays regional without propagation. Here, we extended this experiment by applying microneedle pressure at the anterior end, waist and posterior end of mature oocytes, respectively. We observed that regardless of the pressing site, a regional calcium rise can always be induced (Figure 2b–d and Movies S2–4), but it did not spread into a wave. This suggested that the oocyte poles and waist display similar responses to regional pressure. When exposed to global pressure such as that from swelling in RB, oocytes are likely to use other mechanisms to regulate calcium rises to cause calcium waves to initiate only from the poles.
2.4 |. Loss of Kramer does not affect Trpm localization or calcium wave initiation
As our data, above, suggest that the polar initiation of calcium waves is due neither to pole-localized TRPM, nor to greater curvature of the oocyte at the poles, we believe that the ubiquitously distributed Trpm channels may be under special regulation at the poles. Among several potential candidates for potential regulators of Trpm, we tested Kramer (kmr). According to the BioGRID protein interaction database (Stark et al., 2006), Kmr, a regulator of Wnt signaling pathway (Shami Shah et al., 2019) potentially interacts with Trpm. We visualized Trpm localization in oocytes of kmr null females; Trpm was still distributed around oocytes’ periphery, as in wildtype (Figure S2). We also visualized calcium waves in oocytes from kmr null females. Calcium waves still initiates primarily from the poles of these oocytes (Movies S5 and S6). Thus, Kmr is unlikely to regulate Trpm localization or its activity for calcium wave initiation.
3 |. DISCUSSION
Egg activation is a conserved process that is needed to initiate embryogenesis. It is accompanied by a rise in the egg’s level of free intracellular calcium, in most species (reviewed in Horner & Wolfner, 2008b; Krauchunas & Wolfner, 2013; Swann & Lai, 2016). Trpm channels mediate the influx of environmental calcium to initiate a calcium rise that nucleates into a wave as the Drosophila oocyte activates (Hu & Wolfner, 2019); disruption of the other two Trp channels expressed in the ovary (Pain and Trpml) does not affect the calcium wave. Homologues of Trpm also induce calcium rises in activating mouse eggs (Bernhardt et al., 2018). The Drosophila calcium wave initiates from the oocyte poles, primarily the posterior pole (Kaneuchi et al., 2015; York-Andersen et al., 2015). It was unknown whether this local calcium wave initiation reflected the localization of Trpm channels at the poles, or local activation of otherwise uniformly distributed Trpm channels. Here, we showed that Trpm channels are distributed around the periphery of the mature oocyte, indicating that the second model is correct (Figure 3). The localization of Trpm channels around the Drosophila oocyte periphery raises the question of how these channels are only activated at the poles for the initial influx of calcium. Previous data showed that mechanical force external to, or by swelling of, the oocyte caused the Trpm-mediated calcium influx (Hu & Wolfner, 2019). Yet, the fluid nature of cytoplasm determines that different regions of the plasma membrane should experience equivalent pressure around the oocyte when it swells in response to the activation trigger, regardless of its shape (Fitzpatrick, 2017a). Consistent with this model, our observation that the wave initiates at the poles of kug mutant oocytes, whose curvature is significantly different from wildtype (Horne-Badovinac et al., 2012; Viktorinová et al., 2009), argues against a model that the curvature of the oocyte regulates the likelihood of Trpm channel activation, at least in a form that can initiate a calcium wave. Our data also argue against a model that localized pressure alone (such as that exerted by a microneedle) is sufficient to activate mechanosensitive channels in a way that can nucleate a calcium wave. In an ideal situation, a cell containing fluid cytoplasm would adopt a spherical shape to minimize its surface tension (Fitzpatrick, 2017b). The ellipsoid shape of mature oocytes indicates that there must be structural differences between the poles and the waist (e.g., cytoskeletal network, membrane proteins, or lipid composition) that hold the otherwise spherical cell in an elongated shape. Therefore, it is possible that structural molecules or molecular differences at the oocyte poles and/or their plasma membrane cause local activation of Trpm channels.
FIGURE 3.
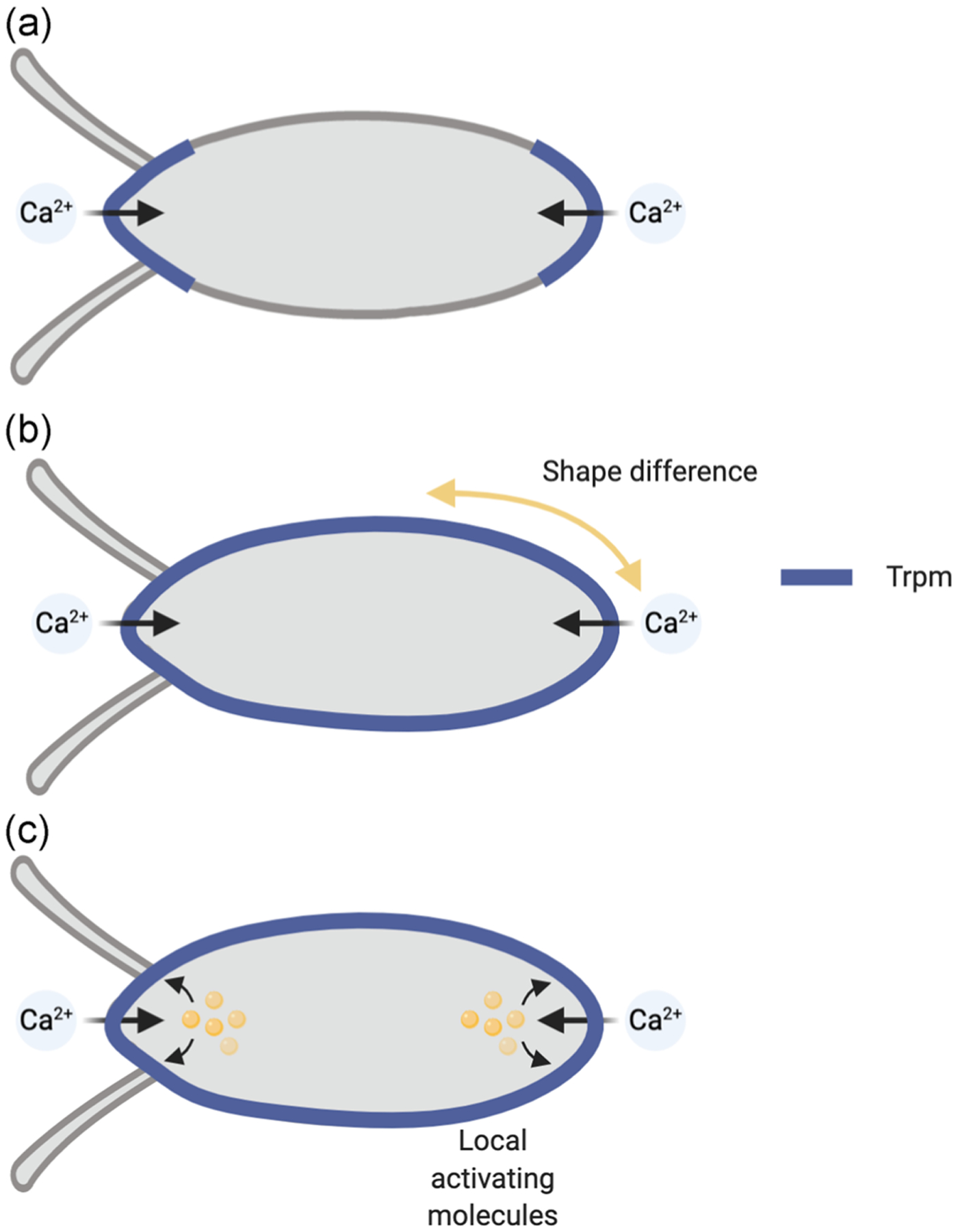
Models of Trpm activation and calcium wave initiation. (a) Trpm distribution is restricted at oocyte poles to allow calcium wave initiation only from there. (b) Trpm distribution is not restricted to the oocyte poles, but the shape and structure of the oocyte make the poles experience pressure in a way that allows Trpm activation and calcium wave initiation there. (c) Trpm distribution is not restricted to the oocyte poles, but special regulatory mechanisms or molecules exist at the poles to allow localized Trpm activation and calcium wave initiation. Our data support the model in (c)
The location of TRPM channels around the entire periphery of the oocyte leads to two, not mutually exclusive, models for how the wave could propagate, after initial calcium entry at the pole(s). In one model, Trpm channels are only activated at the poles. Following calcium influx at this site, propagation of the wave relies on the release of calcium from internal stores, through the IP3R/PLC pathway (Hu et al., 2020; Kaneuchi et al., 2015). In the other model, Trpm channels initially open only at the pole(s) due to the mechanical trigger, but then also open sequentially along with the oocyte through a spreading signal, such as re-organization of the actin cytoskeleton (York-Andersen et al., 2019) and/or propagating calcium signaling via IP3R/PLC-mediated release from stores (Hu et al., 2020; Kaneuchi et al., 2015). In this second model, propagation of the calcium wave would be due to the combined effects of calcium release from internal stores and further influx of external calcium.
In Drosophila early embryos, developmental studies provide precedent for molecular differences between the oocyte poles and the rest of the oocyte. For example, some molecules that specify the terminal region of embryos are pole-localized, such as Fork head (fkh) (Weigel, Jürgens, Küttner, Seifert, & Jäckle, 1989). Some other molecules are non-localized but are locally activated at the poles, such as Torso (tor; Brönner & Jäckle, 1991), after egg activation and fertilization. Our data indicate that the latter is the case for Trpm, but at an earlier time: during egg activation. To begin testing this hypothesis, we tested Kmr, a predicted physical interactor of Trpm. However, we found that Trpm localization and calcium wave incidence were unaffected by lack of Kmr in the oocyte; thus Kmr is not essential for the activation of TRPM. The BioGRID database (Stark et al., 2006) suggests several other candidates for TRPM-interacting proteins that can be tested in the future; these include the target of rapamycin (TOR) pathway component S6K and phosphatidylinositol 3-kinase Pi3K92E. Both the TOR pathway and PI3K pathway are capable of responding to mechanical cues (Danciu, Adam, Naruse, Freeman, & Hauschka, 2003; You et al., 2014). In addition, The MAPK pathway can also activate in response to mechanical cues (Komuro et al., 1996) and its activity changes during Drosophila egg activation (Sackton, Buehner, & Wolfner, 2014). Whether the MAPK pathway is involved in local activation of Trpm remains to be elucidated.
The mammalian ortholog of Drosophila Trpm, TRPM7, is also involved in calcium influx during egg activation (Bernhardt et al., 2018). TRPM7 displays mechanosensitivity (Liu et al., 2015; Xiao et al., 2014) and can interact with cytoskeleton with non-muscle myosin II (Clark et al., 2006). Actin may be involved in the structure of the ellipsoid oocyte. We recently reported that the cortical actin is less concentrated at the posterior end of Drosophila mature oocytes. It is possible that lack of actin rigidity at the pole allows or facilitates the opening of calcium channels in response to the mechanical trigger (York-Andersen et al., 2019). Given these observations, we believe the actin cytoskeleton may also be a candidate for local activation of Trpm at the oocyte poles. Future studies are needed to determine how ubiquitously localized mechanically gated channels can be activated only in certain parts of a large cell-like Drosophila oocyte.
4 |. MATERIALS AND METHODS
4.1 |. Fly strains and maintenance
All Drosophila strains and crosses were maintained or performed on standard yeast-glucose-agar media at 25°C and a 12/12 light/dark cycle. matα-GAL4-VP16>UAS-GCaMP3, nos-GCaMP6m, matα-GAL4-VP16>UAS-Cas9, and matα-GAL4-VP16>UAS-Cas9; trpm-gRNA1 transgenic line were as previously described (Hu & Wolfner, 2019;Kaneuchi et al., 2015). The kugeleiN103-2 strain (Horne-Badovinac et al., 2012) was a gift from Dr. Sally Horne-Badovinac at the University of Chicago. The kramer2 strain (Shami Shah et al., 2019) was a gift from Dr. Jeremy Baskin at Cornell University. The trpm MiMIC insertion strain (Nagarkar-Jaiswal et al., 2015) (64467) was obtained from Bloomington Drosophila Stock Center.
4.2 |. Microneedle manipulation
Microneedles were fabricated and manipulated as previously described (York-Andersen et al., 2019). Indicated regions of wildtype mature oocytes carrying matα-GAL4-VP16 > UAS-GCaMP3 transgene were pressed with the microneedle until a calcium rise occurred as visible with GCaMP3 signal increase. Propidium iodide was used as an indicator of plasma membrane integrity to prevent false calcium signals due to plasma membrane damage (York-Andersen et al., 2019). Oocyte calcium dynamics were observed for 20 min.
4.3 |. Immunostaining
Mature oocytes from the indicated genotype were fixed and stained following established protocols (Radford & McKim, 2016). Embryosor eggs from Oregon-R-P2 (ORP2) wildtype females mated with either ORP2 males (for early embryos) or with the spermless male offspring of bw sp tud1 females (Mahowald & Boswell, 1983; for activated but unfertilized eggs) were collected from grape juice/agar plates. 1–5hr old embryos and eggs were dechorionated in 50% sodium hypochlorite (commercial Clorox bleach), fixed in methanol/heptane, and stored at 4°C until use. Fixed embryos and eggs were washed with phosphate-buffered saline, 0.1% Tween (PBST) three times for 5 min each and blocked with PBST containing 5% vol of normal goat serum (PBST-NGS). Embryos were then incubated overnight at 4°C in PBST-NGS containing primary antibody (rabbit anti-Trpm [Hoffman et al., 2010], a kind gift from Dr. Craig Montell at University of California, Santa Barbara; 1:500; or mouse anti-FLAG, Millipore Sigma; 1:500). Embryos and eggs were then washed with PBST three times for 5 min each and incubated at room temperature in PBST-NGS containing the corresponding secondary antibody (AlexaFluor 594 anti-rabbit, AlexaFluor 594 anti-mouse or AlexaFluor 488 anti-rabbit, Thermo Fisher Scientific) for 4 hr. Samples were then washed with PBST three times and mounted on glass microscope slides in anti-fade mounting buffer.
4.4 |. Imaging
Fixed and stained oocytes, embryos, and eggs were imaged using a Zeiss LSM880 Confocal Multiphoton Microscope with a ×10 lens and Zen software. The detection wavelength was set to 585–733 nm for AlexaFluor 594 signals and 493–56 nm for AlexaFluor 488 signals. Mature oocytes were dissected from females of indicated genotypes. They were activated in vitro as previously described (Hu & Wolfner, 2019; Kaneuchi et al., 2015; Page &Orr-Weaver, 1997). During in vitro activation, oocytes were imaged using a Zeiss Elyra Super Resolution Microscope with a ×5 or ×10 lens and Zen software. The detection wavelength was set to 493–556 nm for GCaMP6m signal. Images were processed with the ImageJ software when needed.
Supplementary Material
ACKNOWLEDGMENTS
Imaging data were acquired on microscopes in the Cornell University Biotechnology Resource Center, with NYSTEM (CO29155) and NIH (S10OD018516) funding for the shared Zeiss LSM880 Confocal Multiphoton Microscope. We thank Drs. Sally Horne-Badovinac and Jeremy Baskin for fly strains and Dr. Craig Montell for anti-Trpm. We thank Drs. Jan Lammerding and Gregory Fedorchak for equipment and guidance in the microneedle experiments. We thank Dr. Itai Cohen for discussion about physical forces and oocyte shape, and Drs. C. Han, J. Liu, S. Horne-Badovinac, and two anonymous reviewers for helpful comments and suggestions. We thank NIH grants R21-HD088744 and R03-HD101732 for supporting this study.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Bernhardt ML, Stein P, Carvacho I, Krapp C, Ardestani G,Mehregan A, … Williams CJ (2018). TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proceedings of the National Academy of Sciences of the United States of America, 115(44), E10370–E10378. http://www.pnas.org/lookup/doi/10.1073/pnas.1810422115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brönner G, & Jäckle H (1991). Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mechanisms of Development, 35(3), 205–211. 10.1016/0925-4773(91)90019-3 [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, vanLeeuwen B, Ran L, Ryazanov AG,Figdor CG, … van Leeuwen FN (2006). TRPM7, a novel regulator of actomyosin contractility and cell adhesion. The EMBO Journal, 25(2), 290–301. 10.1038/sj.emboj.7600931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danciu TE, Adam RM, Naruse K, Freeman MR, & Hauschka PV(2003). Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Letters, 536(1–3), 193–197. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R (2017a). Hydrostatics. Theoretical Fluid Mechanics, 2–39. 10.1088/978-0-7503-1554-8ch2 [DOI] [Google Scholar]
- Fitzpatrick R (2017b). Surface tension. Theoretical Fluid Mechanics, 3–21. 10.1088/978-0-7503-1554-8ch3 [DOI] [Google Scholar]
- Heifetz Y, Yu J, & Wolfner MF (2001). Ovulation triggers activation of Drosophila oocytes. Developmental Biology, 234, 416–424. 10.1006/dbio.2001.0246 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Chen X, Dietz AS, Gudermann T, & Montell C (2010). Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLOS One, 5(5), 10519 10.1371/journal.pone.0010519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Hill J, Gerlach G, Menegas W, & Bilder D (2012). A screen for round egg mutants in Drosophila identifies tricornered, furry, and misshapen as regulators of egg chamber elongation. G3 (Bethesda, MD), 2(3), 371.–. 10.1534/g3.111.001677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner VL, & Wolfner MF (2008a). Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Developmental Biology, 316(1), 100–109. 10.1016/j.ydbio.2008.01.014.Mechanical [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner VL, & Wolfner MF (2008b). Transitioning from egg to embryo:Triggers and mechanisms of egg activation. Developmental Dynamics, 237(3), 527–544. 10.1002/dvdy.21454 [DOI] [PubMed] [Google Scholar]
- Hu Q, Vélez-Avilés AN, & Wolfner MF (2020). Drosophila Plc21C is involved in calcium wave propagation during egg activation. MicroPublication Biology, 2020 10.17912/micropub.biology.000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, & Wolfner MF (2019). The Drosophila Trpm channel mediates calcium influx during egg activation. Proceedings of the National Academy of Sciences, 116(38), 18994–19000. 10.1073/pnas.1906967116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, & Wolfner MF (2015). Calcium waves occur as Drosophila oocytes activate. Proceedings of the National Academy of Sciences of the United States of America, 112(3), 791–796. 10.1073/pnas.1420589112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Nomikos M, Lai FA, & Swann K (2014). Sperm-induced Ca2+ release during egg activation in mammals. Biochemical and Biophysical Research Communications, 450(3), 1204–1211. 10.1016/j.bbrc.2014.04.078 [DOI] [PubMed] [Google Scholar]
- Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, & Yazaki Y (1996). Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. The FASEB Journal, 10(5), 631–636. 10.1096/fasebj.10.5.8621062 [DOI] [PubMed] [Google Scholar]
- Krauchunas AR, & Wolfner MF (2013). Molecular changes during egg activation. Current Topics in Developmental Biology, 102, 267–292. 10.1016/B978-0-12-416024-8.00010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-S, Liu Y-A, Huang C-J, Yen M-H, Tseng C-T, Chien S, & Lee OK (2015). Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Scientific Reports, 5(October), 16522 10.1038/srep16522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP, & Boswell RE (1983). Germ plasma and germ cell development in invertebrates In McLaren A & Wylie CC (Eds.), Current problems in germ cell differentiation/the seventh symposium of the British society for developmental biology. Cambridge: Cambridge University Press. [Google Scholar]
- Nagarkar-Jaiswal S, Lee PT, Campbell ME, Chen K, Anguiano-Zarate S, Gutierrez MC, … Bellen HJ (2015). A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife, 2015(4), 1–28. 10.7554/eLife.05338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AW, & Orr-Weaver TL (1997). Activation of the meiotic divisions in Drosophila oocytes. Developmental Biology, 183, 195–207. 10.1006/dbio.1997.8506 [DOI] [PubMed] [Google Scholar]
- Radford SJ, & McKim KS (2016). Techniques for imaging prometaphase and metaphase of meiosis I in fixed Drosophila oocytes. Journal of Visualized Experiments, 116, 54666 10.3791/54666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackton KL, Buehner NA, & Wolfner MF (2014). Modulation of MAPK activities during egg activation in Drosophila. Fly, 1(4), 222–227. Retrieved from: http://www.tandfonline.com/doi/abs/10.4161/fly.5200 [DOI] [PubMed] [Google Scholar]
- Shami Shah A, Batrouni AG, Kim D, Punyala A, Cao W, Han C, …Baskin JM (2019). PLEKHA4/kramer attenuates dishevelled ubiquitination to modulate Wnt and planar cell polarity signaling. Cell Reports, 27(7), 2157–2170. 10.1016/j.celrep.2019.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C, Breitkreutz B-J, Reguly T, Boucher L, Breitkreutz A, & Tyers M (2006). BioGRID: A general repository for interaction datasets. Nucleic Acids Research, 34(Database issue), D535–D539. 10.1093/nar/gkj109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, & Lai FA (2016). Egg activation at fertilization by a soluble sperm protein. Physiological Reviews, 96(1), 127–149. 10.1152/physrev.00012.2015 [DOI] [PubMed] [Google Scholar]
- Viktorinová I, König T, Schlichting K, & Dahmann C (2009). The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development, 136(24), 4123–4132. 10.1242/dev.039099 [DOI] [PubMed] [Google Scholar]
- Weigel D, Jürgens G, Küttner F, Seifert E, & Jäckle H (1989). The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell, 57(4), 645–658. 10.1016/0092-8674(89)90133-5 [DOI] [PubMed] [Google Scholar]
- Xiao E, Yang H, Gan Y-H, Duan D-H, He L-H, Guo Y, … Zhang Y(2014). TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells, 33(2), 615–621. 10.1002/stem.1858 [DOI] [PubMed] [Google Scholar]
- York-Andersen AH, Hu Q, Wood BW, Wolfner MF, & Weil TT(2019). A calcium-mediated actin redistribution at egg activation in Drosophila. Molecular Reproduction and Development, 87, 293–304. 10.1002/mrd.23311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Andersen AH, Parton RM, Bi CJ, Bromley CL, Davis I, & Weil TT (2015). A single and rapid calcium wave at egg activation in Drosophila. Biology Open, 4(4), 553–560. 10.1242/bio.201411296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J-S, Lincoln HC, Kim C-R, Frey JW, Goodman CA, Zhong X-P, & Hornberger TA (2014). The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. Journal of Biological Chemistry, 289(3), 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


