Abstract
Background
Functional reconstruction after resection of pelvic malignancies involving the acetabulum remains challenging. Numerous reconstruction methods have been proposed, but they are generally associated with mechanical and nonmechanical complications. To improve the function of patients with primary malignancies of the acetabulum after internal hemipelvectomy and reduce the complication rate after this procedure, we designed a series of three-dimensionally (3D) printed, custom-made, integrative hemipelvic endoprostheses with a porous structure and wanted to present the early results of using this construct to determine whether it could be considered a reasonable reconstruction option.
Questions/purposes
We performed this study to (1) evaluate, in a small group of patients, whether the new endoprosthesis restores short-term lower-limb function; (2) identify short-term complications associated with the use of this endoprosthesis; and (3) assess osseointegration between the host bone and the 3D-printed integrative hemipelvic endoprosthesis with a porous structure.
Methods
Between October 2016 and May 2017, our center treated 26 patients with malignancies involving the acetabulum. Thirteen of these patients received hemipelvic replacement with a 3D-printed, custom-made, integrative endoprosthesis, six received hemipelvic replacement with a modular endoprosthesis, four received radiotherapy, and three received external hemipelvectomy. Resection and reconstruction with a 3D-printed, custom-made, integrative endoprosthesis were indicated if the resection margin was the same as that achieved in hemipelvectomy, if reconstruction would preserve reasonable function after resection, if the patient had a good physical status and life expectancy longer than 6 months, and if the patient was willing to accept the potential risk of a 3D-printed, custom-made, endoprosthesis. The exclusion criteria were an inability to achieve a satisfactory surgical margin with limb salvage, inability to preserve the function of the limb because of tumor involvement of the sacral nerve or sciatic nerve, and unresectable and/or widely metastatic disease on presentation. Pain and function were evaluated with the 10-cm VAS score (range 0 to 10; a lower score is desirable), the 1993 version of the Musculoskeletal Tumor Society (MSTS-93) score (range 0 to 30; a higher score is desirable), and the Harris hip score ([HHS]; range 0 to 100; a higher score is desirable) were evaluated preoperatively and at a median of 27 months after reconstruction (range 24 to 31 months). The functional scores and complications were recorded after reviewing the patients’ records. Osseointegration was assessed with digital tomosynthesis by two senior surgeons. We observed the trabecular structures connected to the implant surface to assess whether there was good osseointegration.
Results
The median preoperative VAS score, MSTS-93 score, and HHS were 5 (range 2 to 8), 14 (range 3 to 18), and 64 (range 20 to 76) points, respectively. At the latest follow-up interval, the median VAS score, MSTS-93 score, and HHS were 2 (range 0 to 6), 23 (range 15 to 27), and 82 (range 44 to 93) points, respectively. No deep infection, dislocation, endoprosthetic breakage, aseptic loosening, or local recurrence occurred. Two patients experienced delayed wound healing; the wounds healed after débridement. Using digital tomography, we found that all implants were well-osseointegrated at the final follow-up examination.
Conclusions
A 3D-printed, custom-made, integrative hemipelvic endoprosthesis provides acceptable early outcomes in patients undergoing pelvic reconstruction. Osseointegration is possible, and we anticipate this will lead to biologic stability with a longer follow-up interval. The custom-made integrative design ensured precise implantation. Although a few patients in this study had only a short follow-up duration, the functional results were reasonable. We have observed no major complications so far, but this was a very small series and we caution that these are large reconstructions that will certainly result in complications for some patients. Our method uses a precise preoperative simulation and endoprosthesis design to aid the surgeon in performing challenging operations. If our early results are confirmed with more patients and longer follow-up and are replicated at other centers, this may be a reconstruction option for patients with periacetabular malignancies.
Level of Evidence
Level IV, therapeutic study.
Introduction
Limb salvage surgery is widely used to treat primary pelvic malignancies of the acetabulum, but functional reconstruction of hemipelvic bone defects is frequently associated with complications [5, 24, 28, 55]. Numerous biologic reconstruction methods have been proposed, including iliofemoral arthrodesis or pseudarthrosis [42], massive allografts [4], femoral-neck autografts [6], and autoclaved autografts [48]; however, various disadvantages have been reported for each of those approaches, including major complications, poor functional outcomes, and no clear benefit compared with not performing reconstruction [13, 43, 44, 46, 47]. Recently, prosthetic reconstruction has been proposed because of its initial stability, acceptable cosmesis, and relatively rapid restoration of function [21].
Several metal endoprostheses have been developed to substitute for the native acetabulum; these include the saddle endoprosthesis [11, 28], ice-cream cone endoprosthesis [8, 26], modular endoprosthesis [21, 40], and custom-made endoprosthesis [2, 27, 41]. However, the saddle endoprosthesis and ice-cream cone endoprosthesis require sufficient iliac bone during reconstruction. The modular endoprosthesis and custom-made endoprosthesis commonly compromise long-term survival because of loosening. The major factor associated with loosening of modular endoprostheses is sacrificing the fit at the anchor part in order to obtain a satisfactory acetabular location and orientation [19]. However, loosening of custom-made endoprostheses is often associated with incomplete osseointegration [2, 34, 41, 49, 51]. Therefore, alternative endoprostheses, some of which have an osteoconductive structure and anatomy-imitating shape, have been proposed [34, 36, 49]. Three-dimensionally (3D) printed, custom-made, endoprostheses with a porous structure might be an option for complicated and irregular hemipelvic bone defects. They have been reported to result in relatively good function and a low short-term complication rate, but shortcomings such as incomplete pelvic ring reconstruction or component endoprosthesis design still exist and may lead to degeneration of the sacroiliac joint or endoprosthetic breakage [25, 33, 49]. Currently, to our knowledge, the application of a 3D-printed, custom-made, endoprosthesis with an integrative design is rare in reconstructing massive hemipelvic bone defects of the acetabulum [54]. Recently, we designed a series of 3D-printed, custom-made, integrative hemipelvic endoprostheses and used them to treat patients with primary malignancies of the acetabulum. We wish to determine, at a minimum of 2 years, the early performance of this new device.
The purposes of this study were to (1) evaluate, in a small group of patients, whether the new endoprosthesis restores short-term lower-limb function; (2) identify short-term complications associated with the use of this endoprosthesis; and (3) assess osseointegration between the host bone and the 3D-printed integrative hemipelvic endoprosthesis with a porous structure.
Patients and Methods
Patients
Between October 2016 and May 2017, our center treated 26 patients with malignancies involving the acetabulum. Thirteen of these patients received hemipelvic replacement with a 3D-printed, custom-made, integrative endoprosthesis, six received hemipelvic replacement with a modular endoprosthesis, four received radiotherapy, and three received external hemipelvectomy. Resection and reconstruction with this 3D-printed, custom-made, integrative endoprosthesis were indicated if the planned resection margin would be the same as that achieved in hemipelvectomy, if reconstruction would preserve reasonable function after resection, if the patient had a good physical status and life expectancy of longer than 6 months, and if the patient was willing to accept the potential risks of the 3D-printed, custom-made, endoprosthesis. The exclusion criteria were an inability to achieve a satisfactory surgical margin with limb salvage, inability to preserve a functional limb because of tumor involvement of the sacral nerve or sciatic nerve, and patients who had unresectable and/or widely metastatic disease on presentation. There were six men and seven women with a median age of 46 years (range 31 to 66 years). The median BMI was 27 kg/m2 (range 18 to 34 kg/m2). Three patients had Type I + II resection, while 10 had Type I + II + III resection (Table 1) [15].
Table 1.
Demographics of the 13 patients treated with 3D-printed, custom-made, integrative hemipelvic endoprostheses
| Patient | Age (years) | Gender | Resection classificationa | Diagnosis | Follow-up (months) | Enneking stage | Neoadjuvant chemotherapy | Oncologic status | Complications | Intraoperative time (minutes) | Blood loss (ml) |
| 1 | 46 | F | Type I + II + III | Dedifferentiated parosteal osteosarcoma | 31 | III | No | AWD | DWH | 420 | 8200 |
| 2 | 37 | F | Type I + II + III | Chondrosarcoma | 30 | IIB | No | NED | 260 | 2500 | |
| 3 | 48 | M | Type I + II + III | Chondrosarcoma | 29 | IIB | No | NED | 230 | 4200 | |
| 4 | 65 | F | Type I + II + III | Fibrosarcoma | 25 | IIB | Two cycles | NED | DWH | 255 | 2100 |
| 5 | 31 | F | Type I + II + III | Ewing sarcoma | 29 | III | Two cycles | AWD | 390 | 5600 | |
| 6 | 61 | M | Type I + II | Solitary plasmacytoma | 28 | IIB | Two cycles | NED | 220 | 900 | |
| 7 | 40 | M | Type I + II | Chondrosarcoma | 27 | IIB | No | NED | 270 | 2600 | |
| 8 | 40 | M | Type I + II + III | Chondrosarcoma | 27 | IIB | No | NED | 180 | 2300 | |
| 9 | 46 | F | Type I + II | Chondrosarcoma | 26 | IIB | No | NED | 170 | 1700 | |
| 10 | 66 | M | Type I + II + III | Fibrosarcoma | 24 | IIB | One cycle | NED | 390 | 5100 | |
| 11 | 53 | F | Type I + II + III | Osteosarcoma | 28 | III | Two cycles | NED | 210 | 1900 | |
| 12 | 35 | M | Type I + II + III | Angiosarcoma | 26 | IIB | Two cycles | NED | 270 | 2600 | |
| 13 | 65 | F | Type I + II + III | Chondrosarcoma | 25 | IIB | No | NED | 540 | 6300 | |
| Median | 46 | 27 | 260 | 2600 |
According to Enneking and Dunham [15]. AWD = alive with disease; DWH = delayed wound healing; NED = no evidence of disease.
Diagnoses were chondrosarcoma in six patients, fibrosarcoma in two, osteosarcoma in one, dedifferentiated parosteal osteosarcoma in one, Ewing sarcoma in one, solitary plasmacytoma in one, and angiosarcoma in one. According to the Enneking staging system [16], 10 patients with chondrosarcoma, fibrosarcoma, solitary plasmacytoma, or angiosarcoma had Stage IIB disease; three patients with dedifferentiated parosteal osteosarcoma, Ewing sarcoma, or osteosarcoma resulting in a pulmonary metastasis had Stage III disease. Among the 13 patients in the study, two cycles of neoadjuvant chemotherapy were administered in one patient with an osteosarcoma (doxorubicin and cisplatin), one with Ewing sarcoma (vincristine, doxorubicin, cyclophosphamide/ifosfamide, and etoposide), one with fibrosarcoma (mesna, ifosfamide, doxorubicin, and dacarbazine), one with solitary plasmacytoma (melphalan and prednisone), and one with angiosarcoma (mesna, ifosfamide, doxorubicin, and dacarbazine). One patient with fibrosarcoma received one cycle of neoadjuvant chemotherapy (mesna, ifosfamide, doxorubicin, and dacarbazine) (Table 1). Preoperatively, all patients underwent plain radiography, 3D CT, and MRI of their lesions (Fig. 1). Single-photon emission CT or positron emission tomography/CT with biopsy was performed. The scores of the 10-cm VAS, the 1993 version of the Musculoskeletal Tumor Society (MSTS-93) scale, and the Harris hip score (HHS) were evaluated [14, 22, 23].
Fig. 1.
(A) A preoperative plain radiograph of a 35-year-old man with an angiosarcoma in the right ilium is shown (Patient 12). (B) A preoperative plain radiograph of 53-year-old woman with an osteosarcoma of the left pelvis is shown (Patient 11). (C) A preoperative plain radiograph of 46-year-old woman with a chondrosarcoma of the left ilium is shown (Patient 9).
This study was approved by the ethical committee of our institution. Written informed consent to participate in this study was obtained from all patients.
Endoprosthesis Design and Fabrication
All endoprostheses were designed by our clinical team and fabricated by Chunli Co., Ltd. (Tongzhou, Beijing, China). CT data were used to build virtual 3D pelvic models in Mimics V20.0 software (Materialise Corp., Leuven, Belgium). Afterwards, the image fusion technique integrated MRI data to build a virtual tumor model (Fig. 2). The tumor-free bone resection margin was set as 10 mm for chondrosarcoma and 30 mm for high-grade malignancies such as osteosarcoma and Ewing sarcoma. We based these margins on reports by others who reported a median tumor-free bone resection margin of 10 mm (range 5 to 15 mm) for chondrosarcoma [17, 36, 39, 54], whereas for patients with high-grade sarcomas receiving no or ineffective preoperative treatment, a 30-mm tumor-free bone resection margin was considered adequate. If the preoperative modality was effective, a 20-mm margin was permissible [30]. The intraoperative position of the patients and surgical approach were determined according to the tumor’s location and tumor-free bone resection margin. Thereafter, to facilitate the operation, we modified the osteotomy plane in accordance with the surgical approach and tumor-free bone resection margin. Subsequently, a preliminary endoprosthesis was generated and modified to a more streamlined shape, removing unnecessary anatomic prominences to help with soft-tissue closure. Next, patient-specific instruments were designed to fit on and fix to the tumor side of the osteotomy plane, with 2-mm holes for inserting K-wires along the midline. Thereafter, we designed the screw’s orientation while considering the surgical approach and natural body weight transmission to obtain easy and durable fixation. After the above procedures, the endoprosthesis design, including the acetabular location and orientation (natural 15° anteversion and 45° inclination), the endoprosthesis surface was modified and the structure was reinforced to reconstruct the intact pelvic ring. The time for simulation was approximately 4 hours. The sacroiliac joints were sacrificed in 10 patients and preserved in three. The pubic rami were partially preserved in 10 patients and left intact in three. Five ischia were totally resected and five were partially resected; three were left intact.
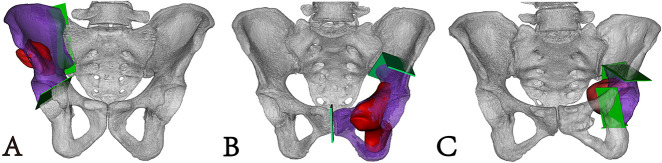
Fig. 2.
Preoperative simulation was performed; the pelvic model (white), osteotomy plane (light gray), resected specimen (dark gray), and tumor (black) are shown. (A) This image shows involvement of the right sacroiliac joint and no involvement of the right pubic ramus. (B) This image shows involvement of the left pubic ramus and no involvement of the sacroiliac joint. (C) This image shows no involvement of the left sacroiliac joint and left pubic ramus A color image accompanies the online version of this article.
Four types of endoprosthesis were designed (Fig. 3). The endoprosthesis connected the residual ilium or sacrum to a flat porous surface and was fixed with cancellous screws. Meanwhile, the endoprosthesis connected the residual acetabulum with a semi-porous acetabulum (in three patients) and the residual pubic ramus was connected with the stem or a “cap-like” structure (in 10). Reconstruction of the ischial ramus was determined based on whether the ischial tubercle was preserved. Features of the preliminarily designed endoprosthesis, including minimization of the iliac wing and removal of the ischial spine and posterior iliac spines, were simplified. The endoprosthesis was composed of solid and porous structures. A continuous “arc-like” supporting structure extended along an arcuate line connecting other solid structures such as screw holes, the acetabulum, and pubic ramus. The porous structure had a pore size of 600 µm and porosity of 70%. Suture holes for muscle reconstruction were made along the endoprosthesis crest.
Fig. 3.
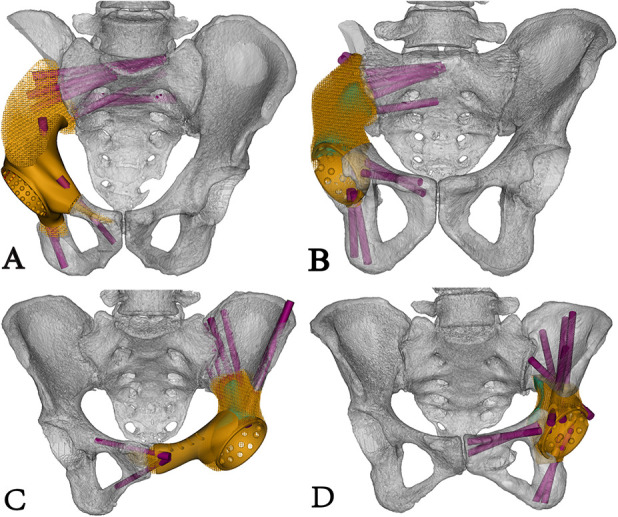
Four types of endoprosthesis designs for different hemipelvic bone defects are shown. (A) This image shows the Type 1 endoprosthesis design for a bone defect in which the sacroiliac joint was sacrificed and the pubis was partially preserved. (B) This image shows the Type 2 endoprosthesis design for a bone defect in which the sacroiliac joint was sacrificed and the pubis was totally preserved. (C) This image shows the Type 3 endoprosthesis design for a bone defect in which the preserved sacroiliac joint was preserved and the pubis was partially preserved. (D) This image shows the Type 4 endoprosthesis design for a bone defect with a preserved sacroiliac joint and totally preserved pubis. A color image accompanies the online version of this article.
The endoprosthesis was fabricated using the electron beam melting technique (ARCAM Q10plus, Mölndal, Sweden); meanwhile, the patient-specific instruments and plastic endoprosthesis models were fabricated using the stereolithography appearance technique (UnionTech Lite 450HD, Shanghai, China) (Fig. 4A). The design procedures took 2 days. 3D-printing fabrication, post-processing, and delivery took 3, 2, and 3 days, respectively. Additionally, the design and manufacture of the endoprosthesis and patient-specific instruments, including the femoral endoprosthesis, cost approximately USD 7000.
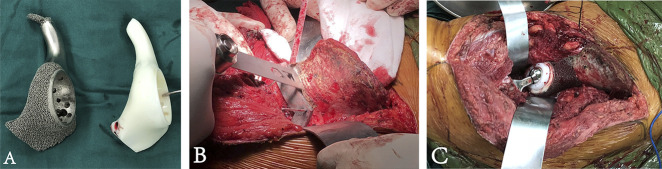
Fig. 4.
(A) The endoprosthesis model and prosthesis are exhibited, and the pubic stem part of the model was removed. (B) Intraoperative osteotomy was performed with the aid of patient-specific instruments. (C) The endoprosthesis was implanted precisely; the constrained acetabular liner can be observed. A color image accompanies the online version of this article.
Surgical Techniques
All operations were performed by the same senior surgeon (CT). The lateral position and combined posterior iliac and Smith-Petersen approaches, with or without an ilioinguinal approach, were mostly used. All of the tumors were resected en bloc. Intraoperatively, the origins of the muscles surrounding the hip joint were preserved, whenever possible. Soft tissue was removed according to a preoperative simulation to expose enough of the bone surface to seat the patient-specific instruments. Because of the preoperative simulation and careful surgical planning, this exposure for placing the cutting guides did not extend the amount of bone or soft-tissue dissection. Osteotomies were performed after the patient-specific instruments were stabilized with K-wires (Fig. 4B). A plastic implant trial was used to confirm the perfect fit before pulsatile lavage was performed. Thereafter, the wound was soaked in 10% povidone-iodine for 3 minutes and another pulsatile lavage was performed with 3 L of isotonic sodium chloride solution. Although we do not have evidence that this is effective or beneficial, we prefer to use this approach to possibly reduce the likelihood of a wound infection.
During reconstruction, trabecular bone of the sacrum was completely exposed with a rongeur. The definitive endoprosthesis was integrally inserted, and the fixation sequence was different in various situations. Sacral or iliac fixation was usually performed first; thereafter, the entire pelvis was reduced, and the endoprosthesis was fixed to the residual pelvis by inserting a screw into the pubic ramus. However, if there was sufficient pubic ramus remaining, a stem on the endoprosthesis was designed and inserted first, followed by sacral or iliac fixation. The endoprosthesis was finally affixed to the ischium (if it was preserved). After establishing rigid fixation, we cemented a constrained acetabular liner (5° to 10° over natural anteversion) into the prosthetic acetabulum, and the corresponding proximal femoral head and neck components were implanted with proper periacetabular muscle tension (Fig. 4C). No soft-tissue restraints were used in our series. Pulsatile lavage with 10% povidone-iodine and another pulsatile lavage with 3 L of isotonic sodium chloride solution were undertaken again, followed by autografting with bone chips in a slurry from the femoral head at the bone-implant interfaces. Then, preserved muscles and their origins such as the rectus femoris, sartorius, iliopsoas, and gluteus were reconstructed and sutured to the endoprosthesis with a nonabsorbable suture (EthibondTM Size 2; Johnson & Johnson, Ltd., New Brunswick, NJ, USA). In patients in whom most of the muscles were resected, soft tissue was reconstructed tightly with a nonabsorbable suture. Because the endoprosthesis had a low profile by design, we did not use rotational or free flaps in these patients. There was sufficient tissue to close the wound tightly and reduce dead space without them.
During surgery, R0 resection and planned reconstruction were achieved in all patients. The median intraoperative time and blood loss were 260 minutes (range 170 to 540 minutes) and 2600 ml (range 900 to 8200 ml), respectively.
Postoperative Management
Postoperatively, the limb was positioned in neutral rotation, 15° to 25° of abduction, 15° of hip flexion, and 15° of knee flexion, and maintained with an anti-rotation orthosis device. Within 3 days, overall hip muscle strength was evaluated with two tests. The first test was active hip stability by moving the hip into 15° to 25° of abduction, 60° of hip flexion, and 90° of knee flexion. The second test was active knee extension with the hip in 15° to 25° of abduction, 20° to 30° of hip flexion, and 30° to 45° of knee flexion.
When the patients were able to perform both tests easily, they were believed to have satisfactory hip joint stability and good strength of the knee muscle in extension, and they were qualified for early rehabilitation. During the first week postoperatively, the training included these two tests to reinforce the strength and balance of the hip muscles in internal and external rotation. During the next week, standing hip flexion with no weight-bearing of the affected limb was encouraged. At 2 weeks postoperatively, patients were encouraged to gradually increase weight-bearing on the affected limb from 10 kg until weight-bearing was equal to that of the contralateral side, and this process usually lasted for 2 weeks. Four weeks postoperatively, the patients progressed to hip abduction, adduction and extension training, and ambulation with walking aids.
Patients who had difficulty finishing one or two of the tests were believed to have poor hip stability or lack muscle strength. Their hips were immobilized in a pelvic-thigh brace (15° to 25° of abduction, 15° of hip flexion, and 15° of knee flexion). The two tests were performed on a bed as daily trainings in the first 2 to 3 weeks. Thereafter, standing hip flexion with no weight-bearing on the affected limb was permitted. Four to 6 weeks postoperatively, gradually increased weight-bearing from 10 kg to full weight-bearing was applied. Hip abduction, adduction, and extension exercises were implemented, and ambulation with walking aids was undertaken from 6 to 8 weeks postoperatively.
In the first 3 months, the patient’s sleeping position was restricted to the supine position, with pillows placed between the legs. Thereafter, all patients were encouraged to walk without crutches and told to start cross-leg and squat training, which lasted for 1 week.
All patients underwent evaluations including a physical examination and plain radiography of the pelvis before discharge and monthly during the first 3 months postoperatively and every 3 months thereafter (Fig. 5A-C). CT of the pelvis was performed before discharge and yearly postoperatively. The same chemotherapy regimens were administered to patients who received preoperative chemotherapy. No patient in this series underwent postoperative radiotherapy. At a median follow-up interval of 27 months (range 24 to 31 months), no patient was lost to follow-up, 11 patients had no evidence of disease, and two patients were alive with disease. Of the two patients who are currently alive with disease, one patient with Ewing sarcoma is receiving targeted therapy with Apatinib (500 mg, qd) owing to ineffective chemotherapy and currently has stable disease. One patient with a dedifferentiated parosteal osteosarcoma is alive with a pulmonary metastasis but refused further treatment.
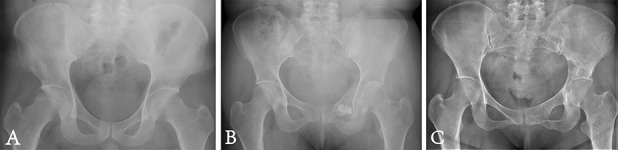
Fig. 5.
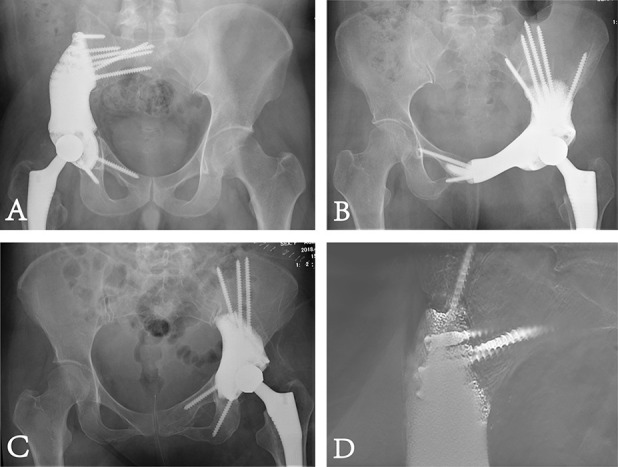
Postoperative radiographic examinations are shown. (A) This postoperative plain radiograph shows precise reconstruction with a Type 2 endoprosthesis. (B) This postoperative plain radiograph shows precise reconstruction with a Type 3 endoprosthesis. (C) This postoperative plain radiograph shows precise reconstruction with a Type 4 endoprosthesis. (D) This digital tomosynthesis image shows good osseointegration.
Primary and Secondary Study Endpoints
Our primary endpoint of interest was pain and function. Pain at rest was assessed according to the 10-cm VAS score (a lower score is desirable); the MSTS-93 score and HHS were assessed through a review of patient records performed by a surgeon (YW) who was not involved in the patient’s care [14, 22, 23]. The MSTS-93 is a limb-specific assessment based on six categories (pain, function, emotional acceptance, supports, walking ability, and gait) specific to the entire lower limb. Each category is scored from 0 to 5, with a total score from 0 to 30 (a higher score is desirable). The HHS is scored from 0 to 100 (a higher score indicates better function). The VAS, MSTS-93, and HHS were administered preoperatively and at the most-recent follow-up examination. The duration of continuous walking was recorded. Gait and the patient’s ability to cross their legs and squat were evaluated.
Our secondary endpoint was complications. Complications including infection, local recurrence, dislocation, aseptic loosening, endoprosthetic breakage, and delayed wound healing were assessed by a surgeon who was not involved in the patient’s care through reviewing the patient’s records.
Our third endpoint was osseointegration. Digital tomosynthesis (Sonialvision Safire II, Shimadzu, Kyoto, Japan) was performed every 3 months postoperatively (Fig. 5D). Two senior surgeons (LM and CT) independently evaluated digital tomosynthesis scans of the pelvis at the most-recent follow-up evaluation. We observed the trabecular structures connected to the implant surface to assess whether there was good osseointegration.
Statistical Analysis
The statistical analyses were performed using IBM SPSS Statistics software, version 25 (IBM SPSS, Armonk, NY, USA). Non-parametric preoperative and postoperative data including the VAS score, MSTS-93 score, and HHS were compared using Wilcoxon’s signed-rank test. A p value < 0.05 was considered statistically significant.
Results
Pain and Function
The VAS score improved from a median of 5 points (range 2 to 8) preoperatively to 2 points (range 0 to 6, difference of medians, 3 points; p = 0.002) at the most-recent follow-up examination. The MSTS-93 score improved from a median of 14 points (range 3 to 18) preoperatively to 23 points (range 15 to 27, difference of medians, 9 points; p = 0.001) at the most-recent follow-up examination. The median scores of the six categories of the MSTS-93 scale (pain, function, emotional acceptance, supports, walking ability, and gait) at the latest follow-up examination were 4 (range 2 to 5), 4 (range 2 to 4), 4 (range 3 to 5), 4 (range 2 to 5), 4 (range 3 to 5), and 4 (range 2 to 4) points, respectively. The HHS improved from a median of 64 points (range 20 to 76) preoperatively to 82 points (range 45 to 93, difference of medians, 18 points; p = 0.001) at the most-recent follow-up. Patients in our series could walk continuously for a median duration of 1 hour (range 0.5 to 2 hours). Nine of the 13 patients did not use support and four used a cane when walking. An obvious limp was observed in four of the 13 patients, while there was a slight limp in two. All patients could sit cross-legged and had good squatting function.
Complications
We did not observe any surgical complications, infection, local recurrence, dislocation, nerve palsy, and vascular incidents in this small series; two of the 13 patients experienced delayed wound healing. Both patients underwent two débridement and closure procedures; after 1 month, their wounds healed.
Osseointegration
All implants were well-osseointegrated at the final follow-up examination.
Discussion
Endoprostheses are believed by some surgeons to be preferable to leaving the hip flail for reconstruction of defects after resections of hemipelvic tumors of bone involving the acetabulum [9, 12, 13, 18, 21, 32, 35, 42-44]. Patients have been reported to ambulate after endoprosthetic replacement with or without support and perform some activities of daily living; however, complications such as infection, dislocation, aseptic loosening, and endoprosthetic breakage were common, which limited its further application (Table 2) [2, 3, 7, 8, 10, 25, 27, 28, 38, 41, 45, 49, 51, 52]. A novel 3D-printed, custom-made, integrative hemipelvic endoprosthesis with a porous structure and integrative design that conforms to the patient’s anatomy might reduce the complication rate. We found function was acceptable and there was a low incidence of complications and good osseointegration with the use of such an endoprosthesis in our small series of patients.
Table 2.
Review of previous studies on hemipelvic reconstruction of the acetabulum, the proportion of patients with complications, and functional outcomes
| Study | Number of patients | Diagnosis (primary or metastatic tumor) | Reconstruction method | Follow-up (months, range) | Complications | Function |
| Current study | 13 | 13/0 | Custom-made endoprosthesis | Median 27 (24-31) | Delayed wound healing (two of 13 patients) | Median MSTS-93 score 22 of 30 |
| Liu et al. [36] | 38 | 38/0 | 19 custom-made endoprostheses 19 modular endoprostheses |
Mean 30 | Recurrence (nine of 38 patients), dislocation (four of 38 patients), loosening (four of 38 patients) | Mean MSTS-93 score 22 of 30 |
| Wang et al. [49] | 11 | 10/1 | Custom-made endoprosthesis | Mean 16 (6-24) | Dislocation (two of 11 patients) | Mean MSTS-93 score 19 of 30 |
| Holzapfel et al. [25] | 56 | 28/28 | Custom-made endoprosthesis | Mean 66 (1-270) | Deep infection (14 of 56 patients), recurrence (10 of 56 patients), dislocation (11 of 56 patients), loosening (three of 56 patients) | Mean MSTS-93 score 18 of 30 |
| Sun et al. [45] | 16 | 16/0 | Custom-made endoprosthesis | Mean 36 (23-62) | Deep infection (one of 16 patients), dislocation (three of 16 patients), recurrence (three of 16 patients), prosthesis breakage (four of 16 patients) | Mean MSTS-93 score 21 of 30 |
| Witte et al. [52] | 40 | 29/11 | Custom-made endoprosthesis | Mean 24 (1-60) | Infection (12 of 40 patients), recurrence (nine of 40 patients), loosening (eight of 40 patients), dislocation (one of 40 patients) | Mean MSTS-93 score 15 of 30 |
| Jaiswal et al. [27] | 98 | 96/0 | Custom-made endoprosthesis | Mean 91 (3-396) | Deep infection (17 of 98 patients), recurrence (29 of 98 patients), dislocation (19 of 98 patients), loosening (three of 98 patients) | Mean TESS 59.4% |
| Dai et al. [10] | 10 | 7/3 | Custom-made endoprosthesis | Mean 34 (21-48) | Deep infection (three of 10 patients), loosening (one of 10 patients), recurrence (three of 10 patients), dislocation (two of 10 patients) | Good function in seven of 10 patients |
| Ozaki et al. [41] | 12 | 12/0 | Custom-made endoprosthesis | Mean 57 (26-77) | Deep infection (three of 12 patients), loosening (three of 12 patients), recurrence (four of 12 patients), dislocation (one of 12 patients) | Mean MSTS-87 score 11 of 30 |
| Abudu et al. [2] | 35 | 32/3 | Custom-made endoprosthesis | Mean 84 (12-312) | Deep infection (nine of 35 patients), recurrence (eight of 35 patients), dislocation (six of 35 patients), loosening (two of 35 patients) | Mean MSTS-93 score 21 of 30 |
| Bruns et al. [7] | 15 | 11/4 | Custom-made endoprosthesis | Maximum 60 | Infection (two of 15 patients), recurrence (two of 15 patients) | Mean MSTS-87 score 15 of 30 |
| Windhager et al. [51] | 21 | 21/0 | Nine saddle endoprostheses, six custom-made endoprostheses | Mean 29 (15-48) | Infection (four of 21 patients), Dislocation (one of 21 patients) | 60% had good function |
| Campanacci et al. [9] | 33 | 32/1 | Allograft | Mean 33 (2-143) | Infection (five of 33 patients), dislocation (six of 33 patients), loosening (two of 33 patients) | Mean MSTS-93 score 18 of 30 |
| Donati et al. [12] | 35 | 35/0 | Alloprosthetic composite | Mean 120 (61-188) | Infection (eight of 35 patients), nonunion (seven of 35 patients), fracture (five of 35 patients) | Mean MSTS-93 score 21 of 30 |
| Gebert et al. [18] | 62 | 61/1 | Hip transposition | Mean 43 (5-185) | Local relapse (six of 62 patients), local recurrence (six of 62 patients), wound healing problems (14 of 62 patients), deep infection (20 of 62 patients) | Mean MSTS-93 score 19 of 30 |
| Lin et al. [35] | 11 | 11/0 | Autograft | Mean 37 (13-96) | Mechanical failure (two of 11 patients), nonunion (one of 11 patients), infection (two of 11 patients) | Median MSTS-93 score 21 of 30 points |
| Bus et al. [8] | 47 | 38/9 | Pedestal cup endoprosthesis | Minimum 24 months | Dislocation (10 of 47 patients); aseptic loosening (three of 47 patients), deep infection (13 of 47 patients), recurrence (six of 47 patients) | Mean MSTS-93 score 21 of 30 points |
| Jansen et al. [28] | 17 | 16/1 | Saddle endoprosthesis | Mean 145 (100-202) | Dislocation (seven of 17 patients), deep infection (three of 17 patients) | Mean MSTS-93 score 14 of 30 points |
| Guo et al. [21] | 28 | 24/4 | Modular endoprosthesis | Mean 30 (10-59) | Deep infection (four of 28 patients), wound complication (six of 28 patients), dislocation (one of 28 patients), local recurrence (seven of 28 patients) | Mean MSTS-93 score 18 of 30 points |
| Takami et al. [47] | 5 | 5/0 | Flail hip | Median 26 (14-98) | Deep infection (one of five patients) | Median MSTS-87 score 21 of 30 points |
| Schwartz et al. [43] | 8 | 8/0 | Flail hip | Mean 98 (13-272) | Mean MSTS-93 score 22 of 30 points |
MSTS = Musculoskeletal Tumor Society; TESS = Toronto Extremity Salvage Score.
Limitations
This study had a number of limitations. First, the oncologic outcome was not evaluated because our cohort of 13 patients was too small and diverse with respect to diagnosis to assess this outcome. Second, a selection bias might have existed in this study. However, the patients were fully informed of the advantages and disadvantages of various reconstruction methods including no reconstruction, a modular hemipelvic endoprosthesis, and a 3D-printed hemipelvic endoprosthesis, and chose this option, so even if bias existed, these results are from a preliminary analysis of the endoprosthesis in the short term. Third, osseointegration was assessed by surgeons, which might result in assessment bias. We tried to mitigate this by having two surgeons assess the radiographies independently. Additionally, during the follow-up period, pain relief, improved walking ability, and stability of the endoprosthesis without migration were observed, indicating good osseointegration. Hence, the influence of assessment bias was not severe. Fourth, our follow-up period was short, and unknown drawbacks might occur in the long term. We will need to follow these patients over a longer period to see whether the generally good results we observed will endure over time. Finally, although our series is one of the largest studies on 3D-printed, custom-made, integrative hemipelvic prosthetic replacement, because there were so few patients, it is impossible to estimate how frequently complications will occur. Given the magnitude of these reconstructions, we expect complications will occur as more of these implants are used. Larger multicenter studies are needed to compare this approach with other types of reconstruction.
Pain and Function
The VAS score, MSTS-93 score, and HHS improved postoperatively compared with at the initial presentation in all our patients. Compared with previous studies that demonstrated a mean MSTS score with or without reconstruction ranging from 11 to 22, the function we observed was acceptable (Table 2) [2, 7, 9, 12, 21, 25, 35, 36, 41, 43-45, 49, 51, 52]. We believe the reasons are as follows: first, the patient-specific instruments that were fixed to the tumor side diminished the normal disruption of muscle and allowed early rehabilitation. Second, an intact pelvic ring reconstructed by the 3D-printed, custom-made, endoprosthesis offered 3D stability of the hip and pelvis, which is more reliable than only longitudinal weight-transmission reconstruction. Third, the origins of the muscles around the hip were carefully reconstructed with nonabsorbable sutures, providing durability for further rehabilitation. Fourth, the personalized rehabilitation plan was performed according to an early functional evaluation. In patients with good early restoration of function, early rehabilitation may facilitate functional recovery. For patients with poor early restoration of function, a longer bedrest period was adopted to allow scar tissue to adhere tightly and to stabilize the hip [3, 10, 50, 54]. Consequently, our patients’ function recovered. However, as might be expected from such large resections and reconstructions, some patients had important functional limitations. The most obvious of these were limp and restricted hip mobility; even limb-length discrepancy was diminished by reconstructing the acetabulum in situ. Obvious limp and restricted hip mobility occurred in four patients because of massive muscle removal, especially of the gluteus muscles, even though soft-tissue reconstruction and personalized rehabilitation were performed carefully.
Complications
We did not observe any surgical complications; two of our 13 patients experienced delayed wound healing. However, this was a very small series, and we know that as more of these large reconstructions are performed, complications will occur. The most commonly reported reasons for failure of endoprosthesis reconstruction were infection (0% to 30%), recurrence (0% to 33%), and hip dislocation (0% to 20%) [2, 8, 10, 21, 25, 27, 29, 38, 41, 45, 49, 52]. To reduce deep infection, four procedures were carefully performed during the design and perioperative periods. First, the endoprosthesis had a streamlined shape and porous surface, allowing close contact and ingrowth of soft tissue to reduce dead space. Second, on the premise of en bloc resection, patient-specific-instruments attached to the tumor side allowed for minimized surgical exposure to prevent infection. Further, preoperative simulation and intraoperative assistance from patient-specific instruments diminished the operation time. Additionally, the wound was flushed repeatedly with pulsed lavage and soaked in povidone-iodine, although we cannot prove this helped reduce infections in our patients. To minimize local recurrence, precise surgical planning, simulation, and implementation were performed. Additionally, determining the tumor-free bone resection margin is important for en bloc resection. Previous studies reported a median tumor-free bone resection margin of 10 mm (range 5 to 15 mm) in patients with chondrosarcoma [17, 36, 39, 54]. Meanwhile, for patients with high-grade sarcomas who received no or ineffective preoperative treatment, a 30-mm tumor-free bone resection margin is considered adequate, and if the preoperative modality is effective, a 20-mm margin is permissible [30]. Therefore, in our series, a 30-mm tumor-free bone resection margin was used for high-grade sarcomas and a 10-mm margin was used for chondrosarcomas. None of our patients had local recurrence; however, with a larger group of patients and a longer follow-up duration, this might change. To reduce the occurrence of hip dislocation, we adopted a carefully designed acetabular orientation, precise resection and reconstruction procedures, a constrained acetabular liner with increased anteversion during implantation, and proper muscle and soft-tissue tension. Endoprosthetic breakage usually results from a modular design, relatively low strength, and poor osseointegration [10, 41, 45]. The continuous solid structure inside the endoprosthesis including the arcuate-line support, acetabulum, screw holes, and pubis reinforce the supporting structure to ensure the overall strength of the endoprosthesis. Additionally, in terms of biomechanical transmission, osseointegration between the endoprosthesis and the host bone can disperse stress.
Osseointegration
All implants osseointegrated. Osseointegration is essential for the long-term survival of an endoprosthesis; otherwise, aseptic loosening is inevitable [10, 25, 27, 41, 52]. To avoid inadequate osseointegration, an osteoconductive structure, enhanced initial stability, and timely load bearing were used in our series. The endoprosthesis design should focus on the porous structure between the implant-bone interface, and the contact area should be enlarged in the pubic connection by the stem or a “cap-like” structure [1]. We believe trabecular bone should be exposed during surgery, and an autograft from the femoral head should be applied near the implant-bone connection. Adequate initial stability should be achieved with an anatomy-conforming endoprosthesis and well-positioned screws [53]. Suitable weight-bearing exercise may promote osteogenesis; nevertheless, early, excessive weight-bearing should be avoided. Digital tomosynthesis was used in this study to evaluate osseointegration after hemipelvic endoprosthesis reconstruction. This technique is known to provide good radiographic views of the bone-implant interface and periprosthetic cancellous bone [20, 31, 37]. However, sometimes, high-quality images cannot be obtained, considering the irregular shape of a hemipelvic endoprosthesis [20]. To solve this problem, patients were repositioned to ensure the bone-implant interfaces were vertical to the examination platform during radiography.
Conclusions
In our small group of 13 patients who had 3D-printed, custom-made, integrative hemipelvic endoprostheses, we observed acceptable early results using custom 3D-designed hemipelvic reconstruction with osteointegrative potential. We hope that over time, osseointegration between the endoprosthesis and host bone will result in long-term biologic stability of the implant. The custom-made integrative design facilitates precise implantation during surgery. Our functional results, assessed by MSTS-93 scores and HHS, were acceptable and we had a low incidence of complications, but this was a very small series. We caution that these are reconstructions are complex and will result in complications over time and with further experience. However, we think there are advantages to this approach to a difficult reconstructive problem. If similar results can be obtained by us and others with larger numbers of patients and longer follow-up, this may be a reasonable reconstruction option for tumors of the acetabulum.
Acknowledgments
We thank Xianliang Zhang MD, Department of Pathology, West China Hospital, for an evaluation of pathology.
Footnotes
The institution of one or more of the authors (LM, YZ, CT) has received, during the study period, funding from the Chengdu Science and Technology Project (2017-CY02-00032-GX), National Natural Science Foundation of China (81801852), and National Key Research and Development Program of China (2016YFC1102003; 2017YFB0702604). Each author certifies that neither he or she, nor any member of his or her immediate family, has any other funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
This work was performed at West China Hospital, Sichuan University, Chengdu, People’s Republic of China.
The first two authors contributed equally to this manuscript.
References
- 1.Abdel MP, von Roth P, Perry KI, Rose PS, Lewallen DG, Sim FH. Early results of acetabular reconstruction after wide periacetabular oncologic resection. J Bone Joint Surg Am. 2017;99:e9. [DOI] [PubMed] [Google Scholar]
- 2.Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg Br. 1997;79:773-779. [DOI] [PubMed] [Google Scholar]
- 3.Angelini A, Trovarelli G, Berizzi A, Pala E, Breda A, Ruggieri P. Three-dimension-printed custom-made prosthetic reconstructions: from revision surgery to oncologic reconstructions. Int Orthop. 2019;43:123-132. [DOI] [PubMed] [Google Scholar]
- 4.Ayvaz M, Bekmez S, Mermerkaya MU, Caglar O, Acaroglu E, Tokgozoglu AM. Long-term results of reconstruction with pelvic allografts after wide resection of pelvic sarcomas. ScientificWorldJournal. 2014;2014:605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos-Ruiz I, Ortiz-Cruz EJ, Peleteiro-Pensado M. Reconstruction after hemipelvectomy with the ice-cream cone prosthesis: what are the short-term clinical results? Clin Orthop Relat Res. 2017;475:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biau DJ, Thevenin F, Dumaine V, Babinet A, Tomeno B, Anract P. Ipsilateral femoral autograft reconstruction after resection of a pelvic tumor. J Bone Joint Surg Am. 2009;91:142-151. [DOI] [PubMed] [Google Scholar]
- 7.Bruns J, Luessenhop SL, Dahmen G, Sr Internal hemipelvectomy and endoprosthetic pelvic replacement: long-term follow-up results. Arch Orthop Trauma Surg. 1997;116:27-31. [DOI] [PubMed] [Google Scholar]
- 8.Bus MP, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JA, Fiocco M, Streitburger A, Kotrych D, van de Sande MA, Dijkstra PD. LUMiC((R)) Endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clin Orthop Relat Res. 2017;475:686-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campanacci D, Chacon S, Mondanelli N, Beltrami G, Scoccianti G, Caff G, Frenos F, Capanna R. Pelvic massive allograft reconstruction after bone tumour resection. Int Orthop. 2012;36:2529-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai KR, Yan MN, Zhu ZA, Sun YH. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty. 2007;22:981-986. [DOI] [PubMed] [Google Scholar]
- 11.Danisman M, Mermerkaya MU, Bekmez S, Ayvaz M, Atilla B, Tokgozoglu AM. Reconstruction of periacetabular tumours with saddle prosthesis or custom-made prosthesis, functional results and complications. Hip Int. 2016;26:e14-e18. [DOI] [PubMed] [Google Scholar]
- 12.Donati D, Di Bella C, Frisoni T, Cevolani L, DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop Relat Res. 2011;469:1450-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eilber FR, Grant TT, Sakai D, Morton DL. Internal hemipelvectomy--excision of the hemipelvis with limb preservation. An alternative to hemipelvectomy. Cancer. 1979;43:806-809. [DOI] [PubMed] [Google Scholar]
- 14.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993:241-246. [PubMed] [Google Scholar]
- 15.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731-746. [PubMed] [Google Scholar]
- 16.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980:106-120. [PubMed] [Google Scholar]
- 17.Evrard R, Schubert T, Paul L, Docquier PL. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: a case-control study. Orthop Traumatol Surg Res. 2019;105:781-787. [DOI] [PubMed] [Google Scholar]
- 18.Gebert C, Wessling M, Hoffmann C, Roedl R, Winkelmann W, Gosheger G, Hardes J. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol. 2011;103:269-275. [DOI] [PubMed] [Google Scholar]
- 19.Gradinger R, Rechl H, Hipp E. Pelvic osteosarcoma. Resection, reconstruction, local control, and survival statistics. Clin Orthop Relat Res. 1991:149-158. [PubMed] [Google Scholar]
- 20.Guo S, Tang H, Zhou Y, Huang Y, Shao H, Yang D. Accuracy of digital tomosynthesis with metal artifact reduction for detecting osteointegration in cementless hip arthroplasty. J Arthroplasty. 2018;33:1579-1587. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180-188. [DOI] [PubMed] [Google Scholar]
- 22.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737-755. [PubMed] [Google Scholar]
- 23.Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand J Pain. 2016;13:67-75. [DOI] [PubMed] [Google Scholar]
- 24.Hipfl C, Stihsen C, Puchner SE, Kaider A, Dominkus M, Funovics PT, Windhager R. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J. 2017;99:841-848. [DOI] [PubMed] [Google Scholar]
- 25.Holzapfel BM, Pilge H, Prodinger PM, Toepfer A, Mayer-Wagner S, Hutmacher DW, von Eisenhart-Rothe R, Rudert M, Gradinger R, Rechl H. Customised osteotomy guides and endoprosthetic reconstruction for periacetabular tumours. Int Orthop. 2014;38:1435-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issa SP, Biau D, Babinet A, Dumaine V, Le Hanneur M, Anract P. Pelvic reconstructions following peri-acetabular bone tumour resections using a cementless ice-cream cone prosthesis with dual mobility cup. Int Orthop. 2018;42:1987-1997. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal PK, Aston WJ, Grimer RJ, Abudu A, Carter S, Blunn G, Briggs TW, Cannon S. Peri-acetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Joint Surg Br. 2008;90:1222-1227. [DOI] [PubMed] [Google Scholar]
- 28.Jansen JA, van de Sande MA, Dijkstra PD. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin Orthop Relat Res. 2013;471:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji T, Guo W, Yang RL, Tang XD, Wang YF. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol. 2013;39:53-60. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004:165-172. [DOI] [PubMed] [Google Scholar]
- 31.Kim W, Oravec D, Nekkanty S, Yerramshetty J, Sander EA, Divine GW, Flynn MJ, Yeni YN. Digital tomosynthesis (DTS) for quantitative assessment of trabecular microstructure in human vertebral bone. Med Eng Phys. 2015;37:109-120. [DOI] [PubMed] [Google Scholar]
- 32.Kunisada T, Fujiwara T, Hasei J, Nakata E, Senda M, Ozaki T. Temporary external fixation can stabilize hip transposition arthroplasty after resection of malignant periacetabular bone tumors. Clin Orthop Relat Res. 2019;477:1892-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Li X, Hou W, Nune KC, Misra RDK, Correa-Rodriguez VL, Guo Z, Hao Y, Yang R, Murr LE. Fabrication of open-cellular (porous) titanium alloy implants: osseointegration, vascularization and preliminary human trials. Science China Materials. 2017;61:525-536. [Google Scholar]
- 34.Liang H, Ji T, Zhang Y, Wang Y, Guo W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J. 2017;99:267-275. [DOI] [PubMed] [Google Scholar]
- 35.Lin N, Li H, Li W, Huang X, Liu M, Yan X, Pan W, Yang D, Ye Z. Upshifting the ipsilateral proximal femur may provide satisfactory reconstruction of periacetabular pelvic bone defects after tumor resection. Clin Orthop Relat Res. 2018;476:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Liu Y, Lu W, Liao S, Du Q, Deng Z, Lu W. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J Arthroplasty. 2019;34:338-345.e1. [DOI] [PubMed] [Google Scholar]
- 37.Minoda Y, Yoshida T, Sugimoto K, Baba S, Ikebuchi M, Nakamura H. Detection of small periprosthetic bone defects after total knee arthroplasty. J Arthroplasty. 2014;29:2280-2284. [DOI] [PubMed] [Google Scholar]
- 38.Muller PE, Durr HR, Wegener B, Pellengahr C, Refior HJ, Jansson V. Internal hemipelvectomy and reconstruction with a megaprosthesis. Int Orthop. 2002;26:76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nandra R, Matharu G, Stevenson J, Parry M, Grimer R, Jeys L. Long-term outcomes after an initial experience of computer-navigated resection of primary pelvic and sacral bone tumours: soft-tissue margins must be adequate to reduce local recurrences. Bone Joint J. 2019;101:484-490. [DOI] [PubMed] [Google Scholar]
- 40.Ogura K, Susa M, Morioka H, Matsumine A, Ishii T, Hamada K, Ueda T, Kawai A. Reconstruction using a constrained-type hip tumor prosthesis after resection of malignant periacetabular tumors: a study by the Japanese Musculoskeletal Oncology Group (JMOG). J Surg Oncol. 2018;117:1455-1463. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki T, Hoffmann C, Hillmann A, Gosheger G, Lindner N, Winkelmann W. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res. 2002:197-205. [DOI] [PubMed] [Google Scholar]
- 42.Puri A, Gulia A, Jambhekar NA, Laskar S. Results of surgical resection in pelvic Ewing's sarcoma. J Surg Oncol. 2012;106:417-422. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz AJ, Kiatisevi P, Eilber FC, Eilber FR, Eckardt JJ. The Friedman-Eilber resection arthroplasty of the pelvis. Clin Orthop Relat Res. 2009;467:2825-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steel HH. Partial or complete resection of the hemipelvis. An alternative to hindquarter amputation for periacetabular chondrosarcoma of the pelvis. J Bone Joint Surg Am. 1978;60:719-730. [PubMed] [Google Scholar]
- 45.Sun W, Li J, Li Q, Li G, Cai Z. Clinical effectiveness of hemipelvic reconstruction using computer-aided custom-made prostheses after resection of malignant pelvic tumors. J Arthroplasty. 2011;26:1508-1513. [DOI] [PubMed] [Google Scholar]
- 46.Takami M, Ieguchi M, Aono M, Hoshi M, Takada J, Oebisu N, Iwai T. Flail hip joint following periacetabular tumor resection of the pelvis using upper surface of the femoral neck as a saddle: A case report. Oncol Lett. 2015;10:3529-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takami M, Ieguchi M, Takamatsu K, Kitano T, Aono M, Ishida T, Yamano Y. Functional evaluation of flail hip joint after periacetabular resection of the pelvis. Osaka City Med J. 1997;43:173-183. [PubMed] [Google Scholar]
- 48.Wafa H, Grimer RJ, Jeys L, Abudu AT, Carter SR, Tillman RM. The use of extracorporeally irradiated autografts in pelvic reconstruction following tumour resection. Bone Joint J. 2014;96:1404-1410. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Hao Y, Pu F, Jiang W, Shao Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int Orthop. 2018;42:687-694. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Xie X, Yin J, Zou C, Wang J, Huang G, Wang Y, Shen J. Reconstruction with modular hemipelvic endoprosthesis after pelvic tumor resection: a report of 50 consecutive cases. PLoS One. 2015;10:e0127263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Windhager R, Karner J, Kutschera HP, Polterauer P, Salzer-Kuntschik M, Kotz R. Limb salvage in periacetabular sarcomas: review of 21 consecutive cases. Clin Orthop Relat Res. 1996:265-276. [PubMed] [Google Scholar]
- 52.Witte D, Bernd L, Bruns J, Gosheger G, Hardes J, Hartwig E, Lehner B, Melcher I, Mutschler W, Schulte M, Tunn PU, Wozniak W, Zahlten-Hinguranage A, Zeifang F. Limb-salvage reconstruction with MUTARS hemipelvic endoprosthesis: a prospective multicenter study. Eur J Surg Oncol. 2009;35:1318-1325. [DOI] [PubMed] [Google Scholar]
- 53.Wong KC, Kumta SM, Chiu KH, Cheung KW, Leung KS, Unwin P, Wong MC. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg. 2007;12:225-232. [DOI] [PubMed] [Google Scholar]
- 54.Wong KC, Kumta SM, Geel NV, Demol J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput Aided Surg. 2015;20:14-23. [DOI] [PubMed] [Google Scholar]
- 55.Zang J, Guo W, Yang Y, Xie L. Reconstruction of the hemipelvis with a modular prosthesis after resection of a primary malignant peri-acetabular tumour involving the sacroiliac joint. Bone Joint J. 2014;96:399-405. [DOI] [PubMed] [Google Scholar]