Abstract
Background
Aseptic loosening is one of the most common causes of revision of distal femoral endoprostheses and is considered a mid- to long-term complication. There are not many reports of 10-year survivorship free from aseptic loosening and all-cause survivorship in cemented stems. To our knowledge, there are no reports on radiographic features that are associated with aseptic loosening of these implants.
Questions/purposes
(1) What is the 5- and 10-year survivorship free from aseptic loosening in patients undergoing reconstruction with a cemented distal femoral endoprosthesis after a tumor resection? (2) What is the all-cause 5- and 10-year survivorship at in these patients? (3) What radiographic features are associated with aseptic loosening at long-term follow-up?
Methods
We performed a multicenter retrospective study reviewing aseptic loosening in cemented prostheses to determine radiographic features associated with long-term implant survivorship. Patients who underwent a cemented distal femoral reconstruction with a modular endoprosthesis after resection of a musculoskeletal tumor between 1997 and 2017 were reviewed. A total of 246 patients were identified from five institutions and met initial inclusion criteria. Of those, 21% (51) were lost to follow-up before 2 years, leaving 195 patients available for us to evaluate and analyze the survivorship and radiologic features associated with long-term implant survival. The mean (range) follow-up was 78 months (22 to 257). At the time of this analysis, 69% (135 of 195) of the patients were alive. Osteosarcoma was the most common diagnosis in 43% of patients (83 of 195), followed by metastatic carcinoma 13% (25 of 195). Fifty-six percent (110 of 195) of patients received chemotherapy; 15% (30 of 195) had radiation therapy. Aseptic loosening was diagnosed radiographically and was defined as a circumferential radiolucent line on all views, or subsidence around the stem in the absence of infection. We present 5- and 10-year Kaplan-Meier survivorship free from aseptic loosening, 5- and 10-year all-cause survivorship, and a qualitative assessment of radiographic features potentially associated with aseptic loosening (including the junctional radiolucent area, and cortical expansion remodeling). The junctional radiolucent area was defined as a radiolucent area of the bone starting at the bone-endoprosthesis junction to the tip of the femoral stem, and cortical expansion remodeling was defined as an increased cortical thickness at the stem tip. Although we wished to statistically analyze radiographic factors potentially associated with aseptic loosening, we did not have enough clinical material to do so (only nine patients developed loosening). Instead, we will report a few preliminary qualitative observations, which necessarily are preliminary, and which will need to be confirmed or refuted by future studies. We urge caution in interpreting these findings because of the very small numbers involved.
Results
Kaplan-Meier survivorship free from aseptic loosening of the femoral component at 5 and 10 years were 95% (95% CI 89 to 98) and 93% (95% CI 86 to 97), respectively. Kaplan-Meier survivorship free from revision for any cause at 5 and 10 years were 74% (95% CI 65 to 79) and 64% (95% CI 49 to 70), respectively. Although the numbers were too small to analyze statistically, all patients with aseptic loosening had a junctional radiolucent area more than 20% of the total length of the stem without cortical expansion remodeling at the stem tip. No aseptic loosening was observed if there was cortical expansion remodeling, a junctional radiolucent area less than 20%, or curved stems that were 13 mm or greater in diameter. The numbers of patients with aseptic loosening in this series were too small to analyze statistically.
Conclusions
Cemented distal femoral endoprostheses have a relatively low rate of aseptic loosening and acceptable projected first-decade survivorship. The presence of a radiolucent area more than 20% without cortical expansion remodeling at the stem tip may lead to aseptic loosening in patients with these implants. Close radiographic surveillance and revision surgery may be considered for progressive lucencies and clinical symptoms of pain. If revision is contemplated, we recommend using larger diameter curved cemented stems. These are preliminary and provisional observations based on a low number of patients with aseptic loosening; future studies with greater numbers of patients are needed to validate or refute these findings.
Level of Evidence
Level III, therapeutic study.
Introduction
For decades, cemented modular endoprostheses have been used for distal femoral reconstruction after tumor resection. The principal advantages are immediate full weightbearing and early restoration of function in most patients [1, 7, 12]. A systematic review by Haijie et al. [5] found that the overall implant survival rates of cemented distal femoral endoprostheses at 5 and 10 years were 82% and 70%, respectively. But prior studies have demonstrated that aseptic loosening is one of the most common reasons for revision of distal femoral endoprostheses [4, 7-9, 13-14, 16, 19]. Aseptic loosening rates have ranged from 4% to 9% at 4 to 12 years [3, 5, 13, 15-16, 19-20]. This is considered a mid-term to long-term complication, with reported times to revision ranging from 3 years to 12 years [2, 6, 11, 16, 18].
There are few reports of 10-year survivorship free from aseptic loosening [3] and all-cause survivorship in cemented stems [5, 8, 13, 17]. In addition, there are no reports, to our knowledge, of radiographic features that may help anticipate which endoprostheses are at risk for aseptic loosening, or which radiographic features are associated with durable cemented fixation. Identifying such factors may help identify implants at risk of future revision.
We therefore asked (1) What is the 5- and 10-year survivorship free from aseptic loosening in patients undergoing reconstruction with a cemented distal femoral endoprosthesis after a tumor resection? (2) What is the all-cause 5- and 10-year survivorship in these patients? (3) What radiographic features are associated with aseptic loosening at long-term follow-up?
Patients and Methods
We performed a retrospective study gathering data from five institutions. The study was approved by the institutional review board or ethics committee of the respective authors’ institutions. We identified 332 patients who underwent reconstruction with a cemented modular distal femoral endoprosthesis for a musculoskeletal tumor between 1997 and 2017. Twenty-six percent (86 of 332) of patients were excluded who either had an expandable or uncemented implant, had surgery for a nononcologic diagnosis or a revision procedure. Therefore, 246 patients met initial inclusion criteria. Of those, 21% (51) were lost to follow-up before 2 years, leaving 195 patients available for us to evaluate and analyze the survivorship and radiologic features associated with long-term implant survival (Fig. 1).
Fig. 1.
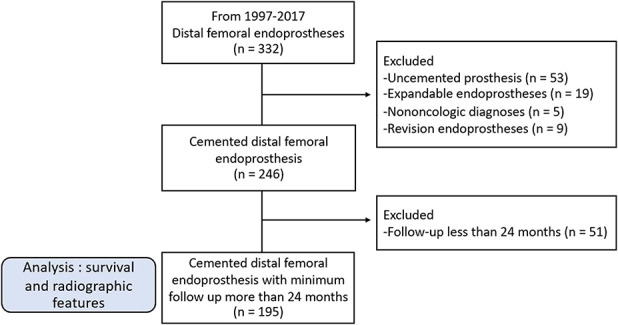
The STROBE study flow diagram is shown here.
In general, implant choice was based on individual surgeon experience. In four institutions, cemented stems were used for all patients, while one institution employed uncemented stems in young adult patients only. All surgeons used the Global Modular Replacement System (GMRS), manufactured by Stryker/Howmedica (Mahwah, NJ, USA). The knee mechanism was a rotating hinge platform in all implants. The shaft and intramedullary stem were composed of a titanium alloy (Ti 318 [Ti-6Al-4V]), and the rotating hinge cast was made of a cobalt-chromium-molybdenum alloy.
Data collected included: age, sex, BMI, Enneking stage, diagnosis, stem diameter and length, prosthesis length, and the use of chemotherapy and radiotherapy. Radiographs were reviewed and measurements were taken. The radiographic features we considered were the junctional radiolucent area, cortical expansion remodeling, as well as stem diameter and design (straight or curved). The decision to perform revision surgery was made individually by the treating surgeons, but generally revisions were performed for painful aseptic loosening, periprosthetic joint infection, a soft tissue problem, periprosthetic fracture, implant breakage or tumor recurrence.
We evaluated radiographs at 2 to 3 weeks postoperatively and then every 3 to 4 months for the first 2 years, every 6 to 12 months for the next 3 years, and annually thereafter. Each follow-up evaluation included a clinical examination and standard non-weightbearing AP and lateral radiographs of the affected extremity.
Patient Characteristics
Of the 195 patients, 106 were male and 89 were female. The mean (range) patient age was 43 years (12 to 87). A total of 69% (135 of 195) of the patients were alive at the last follow-up examination. The minimum follow-up for inclusion in the study was 24 months. The mean (range) total follow-up time was 78 months (22-257). Osteosarcoma was the most common diagnosis in 43% (83 of 195) of the patients, followed by metastatic carcinoma in 13% (25 of 195). Fifty-six percent (110 of 195) of patients received chemotherapy preoperatively, postoperatively, or both as part of their treatment. Fifteen percent (30 of 195) of patients had radiation therapy to the affected extremity. All patients received a unilateral implant; 100 were inserted on the right side and 95 on the left. The mean BMI was 26 kg/m2 (range 13 to 48 kg/m2). The mean (range) bone resection length was 15 cm (11 to 31). The mean length of the distal femoral prosthesis was 14.5 cm (10.5 to 30.6). The mean diameter of the stem was 13 mm (8 to 17) and the mean length of the stem was 12.7 cm (10.2 to 20.3). All patients underwent reconstruction with a cemented tibial component with or without metal backing and variable length stems.
The tumor resection was planned based on preoperative imaging that included radiographs, CT and MRI scans and the length of resection was carefully measured intraoperatively. The bone resection was made perpendicular to the anatomical axis of the femur in all patients. The bone canal was reamed 1 mm to 2 mm above the planned stem diameter. Third-generation cement technique, with the insertion of pressurized cement with a cement gun was used. All implants were in neutral alignment (7° of valgus was built into the implant) with a solid cement stem mantle (1 mm to 2 mm of cement thickness) and good contact between the stem and cortical femoral bone.
Radiographic Analysis
Radiographs were reviewed in all 195 patients. All films were evaluated by one reviewer (PP), who was only involved in a small number of the subjects in this study. Radiographs were evaluated for two radiographic features: junctional radiolucent area and cortical expansion remodeling (Fig. 2). All radiographs were reviewed in a blind fashion and then 2 weeks later all were re-reviewed. Intraobserver agreement (kappa statistic) was 92% for the junctional radiolucent area and 94% in assessing cortical expansion remodeling.
Fig. 2.
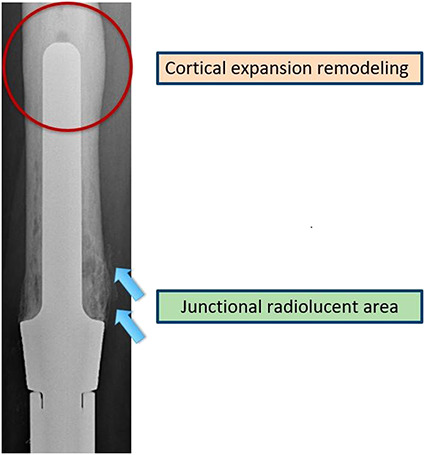
This image shows the junctional radiolucent area at the bone-endoprosthesis junction (arrows), with cortical expansion remodeling at the tip of the stem (circled).
Aseptic loosening was diagnosed radiographically and was defined as a circumferential radiolucent line on all views or subsidence around the stem in the absence of infection.
The junctional radiolucent area was defined as a radiolucent area of the bone starting at the bone-endoprosthesis junction of the femoral stem that can progress up to the stem tip. This was measured using the minimum length of the radiolucent area divided by the total length of the stem on AP and lateral radiographs (Fig. 3). We grouped junctional radiolucent area findings into four zones: Zone I: junctional radiolucent area 1% to 20%; Zone II: 21% to 40%; Zone III: 41% to 60%; and Zone IV: 61% to 100%. Junctional zone lucencies were observed in 48% of patients (94 of 195). Among the 94 patients, 52 had Zone I junctional radiolucent area, 29 had Zone II, four had Zone III, and nine had Zone IV (Fig. 4).
Fig. 3.
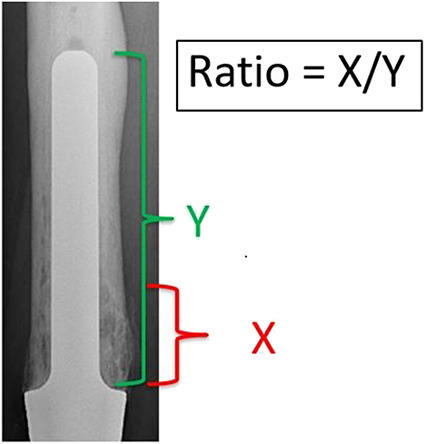
The junctional radiolucent area was measured as the minimum percentage of the length of the radiolucent area divided by the total length of the stem on AP and lateral radiographic views.
Fig. 4.
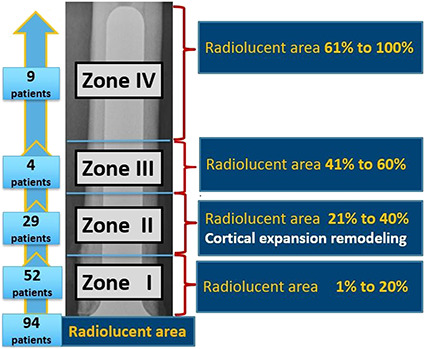
The four zones of the junctional radiolucent area are shown in this figure. We grouped junctional radiolucent area findings into four zones: Zone I: junctional radiolucent area 1% to 20%; Zone II: 21% to 40%; Zone III: 41% to 60%; and Zone IV: 61% to 100%. Junctional zone lucencies were observed in 48% of the patients (94 of 195). Among the 94 patients, 52 had Zone I junctional radiolucent area, 29 had Zone II, four had Zone III, and nine had Zone IV.
Cortical expansion remodeling was defined as an increased cortical thickness at the stem tip. This sign was measured using the ratio of the maximum bone diameter at the stem tip, divided by the cortical diameter 2 cm above the stem tip on AP and lateral views (Fig. 5). This sign was observed in 15% (29 of 195) of patients. In these 29 patients, all cortical expansion remodeling was observed exclusively in junctional radiolucent area Zone II (21% to 40%) and the average increase diameter of the cortical expansion remodeling at the tip of the stem was 6% (range 3% to 15%).
Fig. 5.
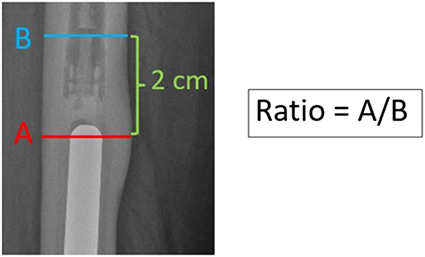
The cortical expansion remodeling was measured as the ratio between the maximum thickness at the tip of the stem divided by the cortical diameter 2 cm above the tip of the stem on AP and lateral radiographic views.
Statistical Analysis
The 5- and 10-year aseptic loosening free survivorship, and 5- and 10-year revision-free survivorship for any reason, were analyzed using a Kaplan-Meier survival analysis [10], performed on STATA software version 16.1 (StataCorp, College Station, Texas, USA).
Although we wished to analyze radiographic factors potentially associated with aseptic loosening statistically, we did not have enough clinical material to do so (only nine patients developed loosening). Instead, we will report a few early qualitative observations, which necessarily are preliminary, and that will need to be confirmed or refuted by future studies. We urge caution in interpreting these findings because of the very small numbers involved.
Results
Survivorship Free from Aseptic Loosening
Kaplan-Meier survivorship free from aseptic loosening of the femoral component at 5 and 10 years were 95% (95% CI 89 to 98) and 93% (95% CI 86 to 97), respectively (Fig. 6). Aseptic loosening involving the femoral stem occurred in 4.6% of the patients (9 of 195) and aseptic loosening involving the tibial component occurred in 2.6% (5 of 195). The mean (range) time to revision for aseptic loosening of the femoral stem was 59 months (22 to 178).
Fig. 6.
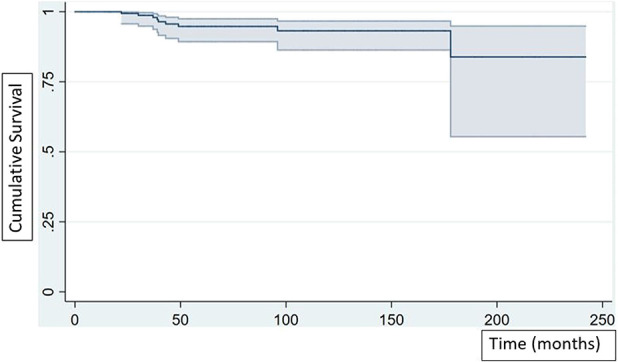
Kaplan-Meier survivorship free from aseptic loosening of the femoral component at 5 and 10 years was 95% (95% CI 89 to 98) and 93% (95% CI 86 to 97), respectively.
All-cause Survivorship
Kaplan-Meier survivorship free from revision for any cause at 5 and 10 years was 74% (95% CI 65 to 79) and 64% (95% CI 49 to 70), respectively (Fig. 7). Revision for any cause occurred in 27% of patients (53 of 195). Infection was the most common reason for revision in 10% of patients (19 of 195), followed by aseptic loosening in 7.2% (14 of 195 patients), aseptic loosening involving the femoral stem in 4.6% (9 of 195), and aseptic loosening of the tibial component in 2.6% (5 of 195).
Fig. 7.
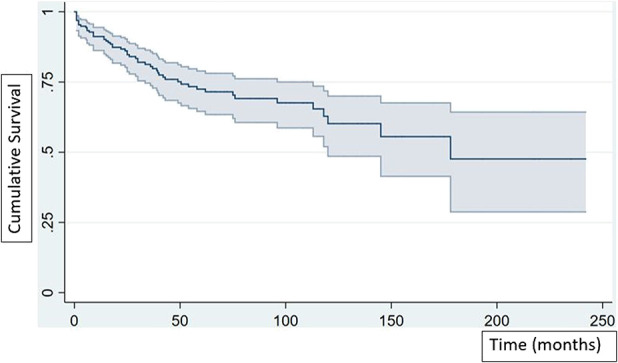
Kaplan-Meier survivorship free from revision for any cause at 5 and 10 years was 74% (95% CI 65 to 79) and 64% (95% CI 49 to 70), respectively.
Radiographic Features Associated with Loosening
Small patient numbers precluded statistical analysis; only nine patients developed loosening. These findings are qualitative and should be considered preliminary.
All nine patients who underwent revision surgery for aseptic loosening of the femoral stem had a junctional radiolucent area more than 20% of the total length of the stem and no cortical expansion remodeling at the tip of the stem. If the junctional radiolucent area was less than 20% or cortical expansion remodeling was present, no aseptic loosening was observed in this small series. No aseptic loosening was observed in patients with implants with curved stems that were 13 mm or greater in diameter.
None of the 52 patients with Zone I (junctional radiolucent area 1% to 20%) changes without cortical expansion remodeling progressed to revision due to aseptic loosening. All 29 patients with Zone II (21% to 40%) changes had cortical expansion remodeling at the stem tip and did not progress or undergo revision surgery for aseptic loosening. Four patients in Zone III (41% to 60%) had mechanical implant failure. Three of four patients had a broken stem and one patient had a periprosthetic fracture. All broken stems occurred in patients with a stem diameter less than or equal to 11 mm. The patient with a periprosthetic fracture had a stem diameter of 13 mm. All nine patients with Zone IV changes (61% to 100%) underwent revision for aseptic loosening (Fig. 8).
Fig. 8.
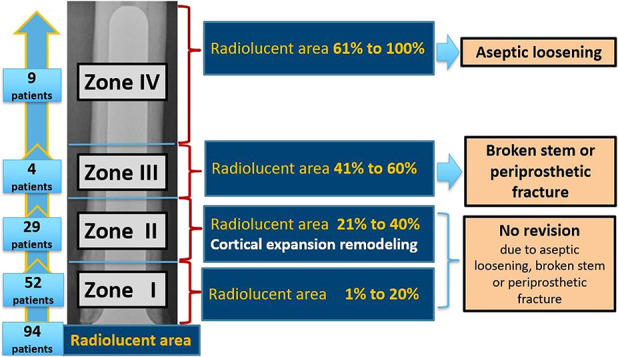
This figure shows the association of the four zones of junctional radiolucent area with cortical expansion remodeling and aseptic loosening.
Discussion
For patients who undergo distal femoral endoprosthetic reconstruction, aseptic loosening is one of the most common reasons for revision [4, 7-9, 13-14, 16, 19]. The reported incidence of aseptic loosening ranges from 4% to 9% at 4 years to 12 years [3, 5, 13, 15-16, 19-20] with time to revision ranging from 3 years to 12 years [2, 6, 11, 16, 18]. Relatively little is known about the 10-year survivorship free from aseptic loosening in cemented stems [3]. There are no reports to our knowledge of radiographic criteria that may help to anticipate which endoprostheses are at risk for aseptic loosening, or alternatively, which radiographic features might be associated with durable cemented fixation. We therefore sought to study these endpoints.
This study has several limitations. First, this is a retrospective, nonrandomized study with potential selection bias. However, we think the effect of this bias was relatively small, since indications for using this device were consistent at four of five study sites (four institutions preferred cemented implants in all patients, while another used uncemented implants only in young adult patients.). In addition, we raise a concern about generalizability; although the same distal femoral endoprosthesis (Stryker GMRS) was used exclusively after musculoskeletal tumor resection in this study, our findings may not apply to all cemented distal femur implants. We also remind readers that children in whom the limb could not be salvaged and who underwent amputation were also excluded from this review.
The second major limitation is transfer bias. Twenty-one percent of patients were lost to follow-up before 2 years (51 of 246); in addition, because the mean time to aseptic loosening was 59 months, 4% (8 of 195) of the patients were lost to follow-up at more than 5 years postoperatively. These patients were at risk of exhibiting aseptic loosening. Therefore, our results may be a best-case estimate. We note that Kaplan-Meier survival analysis addresses loss to follow-up to some degree, and the CIs are informative on this point.
Third, the radiographic measurements were performed by one author (PP), who assisted in a few of the procedures, therefore, assessment bias is a concern that could have led to underestimates of radiographic loosening. Fourth, this paper considered loosening and revision as the primary endpoints; however, it is possible that some patients experience stem-related pain or limited function that was not assessed because a rigorous clinical outcome analysis was not performed.
Finally, although we aimed to analyze the factors (both radiographic and clinical) associated with aseptic loosening, we did not have enough clinical material to do so (only nine patients developed loosening). We can report a few preliminary qualitative observations that will need to be confirmed or refuted by future studies. We urge caution in interpreting these findings.
Survivorship Free from Aseptic Loosening
Our study showed survivorship free from aseptic loosening of cemented stem at 5 and 10 years in 95% (95% CI 89 to 98) and 93% (95% CI 86 to 97), respectively, similar to cemented stem loosening reported by Coathup et al. [3] (5 and 10 years was 94% and 92%, respectively). Our study also showed that 4.6% of stems were aseptically loose at 6.5 years, similar to that reported by Schwartz et al. (3.5% at 8 years) [16] and lower than that reported by Coathup et al. [3] (8% at 8.5 years), Zhang et al. [20] (7.7% at 4.4 years), and Haijie et al. [5] (9.3% at 6.5 years) (Table 1). Taken together, these findings suggest that cemented stems in patients with distal femoral replacement after tumor resection will likely result in low rates of aseptic loosening in the first postoperative decade.
Table 1.
Review of the literature in cemented distal femoral endoprosthesis
| Study | Stem type | Number | Percentage of patients with aseptic loosening |
| Schwartz et al. [16] | Cemented (modular) | 85 | 3.5% (3 of 85) at a minimum follow-up 1 month (mean follow-up 8 years; range 0.08 to 28) |
| Coathup et al. [3] | Cemented (custom-made) | 61 | 8% (5 of 61) at a minimum follow-up 24 months (mean follow-up 8.5 years; range 2 to 8) |
| Zhang et al. [20] | Cemented (modular) | 78 | 7.7% (6 of 78) at a minimum follow-up 12 months (mean follow-up 4.4 years; range 1 to 10.4) |
| Haijie et al. [5] | Cemented | 1574 | 9.3% (147 of 1574) (mean follow-up 6.5 years) |
The mean (range) time to revision due to aseptic loosening was 59 months (22 to 178), which is consistent with other studies ranging from 3 years to 12 years [2, 6, 11, 16, 18]. We have observed in this study, however, that aseptic loosening can occur early, within the first 2 years after surgery. Therefore, surveillance for aseptic loosening should begin early because it can be a short-term complication that may or may not be related to technique.
All-cause Survivorship
Our study showed that all-cause survivorship of cemented stems at 5 and 10 years was 74% (95% CI 65 to 79) and 64% (95% CI 49 to 70), respectively, similar to the all-cause survivorship reported in a systematic review by Haijie et al. [5] (survivorship at 5 and 10 years was 82% and 70%, respectively), Houdek et al. [8] (survivorship at 5 and 10 years was 74% and 59%, respectively), Sharma et al. [17] (survivorship at 5 and 10 years was 84% and 79%, respectively), and Myers et al. [13] (survivorship at 5 and 10 years was 83% and 58%, respectively). Deep infection was the most common reason for revision followed by aseptic loosening.
Radiographic Features Associated with Loosening
We found no aseptic loosening in patients with radiographs that demonstrated cortical expansion remodeling or junctional radiolucent area less than 20% of the total length of the stem; we also saw no aseptic loosening in curved stems that were 13 mm or greater in diameter, but the numbers on this observation were quite small. We remind readers that these observations are preliminary: The numbers were too small to analyze statistically, and only nine patients had aseptic loosening in this study. These findings, therefore, will need to be substantiated or refuted in larger prospective studies. We speculate that these findings are consistent with stress shielding (Fig. 9). The weight of the limb is transferred through the stem to the proximal femoral bone, resulting in a radiolucent area at the bone-endoprosthesis junction. When the radiolucent area develops, contact at the bone-endoprosthesis area decreases and the load at the stem tip increases. The cortex at the stem tip thickens in response, resulting in what we have termed cortical expansion remodeling (Fig. 10). In patients with progressive junctional radiolucencies without cortical thickening for stabilization of the stem, a periprosthetic fracture, broken stem, or aseptic loosening might occur. Close radiographic surveillance is therefore very important over the course of the patient’s lifetime.
Fig. 9.
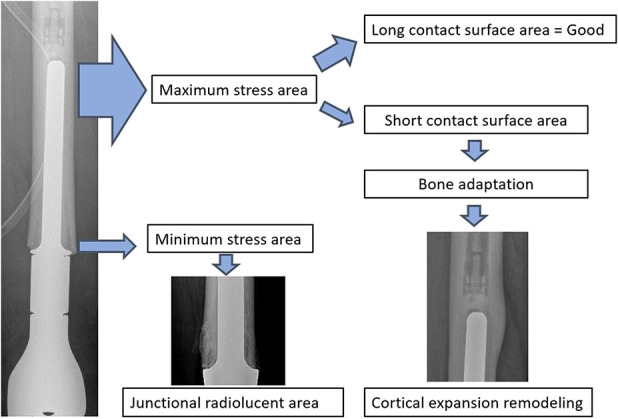
This image shows the stress shielding between the prosthesis and bone.
Fig. 10.
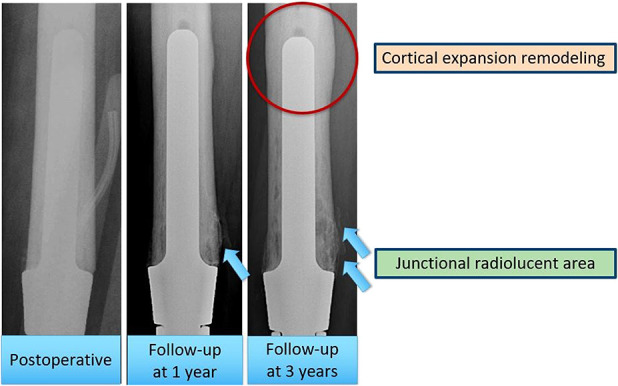
This image shows the radiographs that have junctional radiolucent area and cortical expansion remodeling after follow-up at 1 and 3 years.
Conclusions
We demonstrated that cemented distal femoral endoprostheses have a relatively low rate of aseptic loosening and acceptable projected first decade survivorship. Factors that may be associated with aseptic loosening are a junctional radiolucent area greater than 20%, and the absence of cortical expansion remodeling at the tip of the stem. Larger, curved stems (13 mm or greater in diameter) may be associated with more-durable fixation. However, we did not have sufficient numbers to analyze any of these putative associations statistically, and with only nine patients developing aseptic loosening, these findings must be considered preliminary. Future, larger studies are needed to confirm or refute them. Patients with distal femoral replacements using cemented stems should be monitored over their lifetime to assess stem stability and aseptic loosening.
Acknowledgments
We thank Piyabuth Kittithamvongs MD, MSc (Clin Epi), and Waranya Tasomboon MPH (Epidemiology) of the Institute of Orthopaedics, Lerdsin Hospital, Bangkok, Thailand for their assistance in statistical analysis.
Footnotes
Each author certifies that neither he nor she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The work was performed at H. Lee Moffitt Cancer and Research Institute, Tampa, Florida, USA.
References
- 1.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term follow-up study. Clin Orthop Relat Res. 2002;400:225-235. [DOI] [PubMed] [Google Scholar]
- 3.Coathup MJ, Batta V, Pollock RC, Aston WJ, Cannon SR, Skinner JA, Briggs TW, Unwin PS, Blunn GW. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. J Bone Joint Surg Am. 2013;95:1569-1575. [DOI] [PubMed] [Google Scholar]
- 4.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164-171. [DOI] [PubMed] [Google Scholar]
- 5.Haijie L, Dasen L, Tao J, Yi Y, Xiaodong T, Wei G. Implant survival and complication profiles of endoprostheses for treating tumor around the knee in adults: a systematic review of the literature over the past 30 years. J Arthroplasty. 2018;33:1275-1287. [DOI] [PubMed] [Google Scholar]
- 6.Hardes J, Henrichs MP, Gosheger G, Gebert C, Höll S, Dieckmann R, Hauschild G, Streitbürger A. Endoprosthetic replacement after extra-articular resection of bone and soft-tissue tumours around the knee. Bone Joint J. 2013;95:1425-1431. [DOI] [PubMed] [Google Scholar]
- 7.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418-429. [DOI] [PubMed] [Google Scholar]
- 8.Houdek MT, Wagner ER, Wilke BK, Wyles CC, Taunton MJ, Sim FH. Long term outcomes of cemented endoprosthetic reconstruction for periarticular tumors of the distal femur. Knee. 2016;23:167-172. [DOI] [PubMed] [Google Scholar]
- 9.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265-1271. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [Google Scholar]
- 11.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998;80:636-647. [DOI] [PubMed] [Google Scholar]
- 12.Kotz RI. Progress in musculoskeletal oncology from 1922-2012. Int Orthop. 2014;38:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521-526. [DOI] [PubMed] [Google Scholar]
- 14.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89:1632-1637. [DOI] [PubMed] [Google Scholar]
- 15.Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res. 2015;473:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28-32. [DOI] [PubMed] [Google Scholar]
- 18.Shih LY, Sim FH, Pritchard DJ, Rock MG, Chao EY. Segmental total knee arthroplasty after distal femoral resection for tumor. Clin Orthop Relat Res. 1993;292:269-281. [PubMed] [Google Scholar]
- 19.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5-13. [PubMed] [Google Scholar]
- 20.Zhang C, Hu J, Zhu K, Cai T, Ma X. Survival, complications and functional outcomes of cemented megaprostheses for high-grade osteosarcoma around the knee. Int Orthop. 2018;42:927-938. [DOI] [PubMed] [Google Scholar]


