To the Editor,
An individualized diagnostic approach determining molecular sensitization patterns of house dust mite (HDM) allergic patients may help to identify best eligible patients for allergen immunotherapy, as modern HDM immunotherapy preparations are usually standardized to the major allergens Der p 1, Der f 1, Der p 2 and Der f 2. 1 However, data on the reliability of molecular HDM allergy diagnosis using commercially available assays are limited.
We aimed to investigate the overall sensitivity of molecular HDM allergy diagnosis compared to extract‐based IgE testing using the singleplex assay ImmunoCAP (detecting Der p 1, 2, 10 and 23), the multiplex assay ImmunoCAP ISAC (detecting Der p 1, 2 and 10) and the newly available multiplex platform, Allergy Explorer (ALEX) versions 1 and 2 (version 2 detecting Der p 1, 2, 5, 7, 10, 11, 20, 21 and 23).
Initially, we searched our database for patients with positive skin prick tests to HDM. Data between January 1, 2005, and December 31, 2018, were analysed to determine sensitization rates to the two major species of house dust mite, Dermatophagoides pteronyssinus (D.p.) and Dermatophagoides farinae (D.f.), in Austria. In total, 28 572 patients had positive skin tests to D.p. and/or D.f. Of these, 23 930 (83.8%) had positive skin prick tests to both, and 3,212 (11.2%) and 1430 (5.0%) were mono‐sensitized to D.p. and D.f., respectively. To analyse the different diagnostic methods, sera of 215 HDM allergic patients with unequivocal history of HDM allergy, a positive skin prick test and detectable (≥0.35 kU/L) sIgE to D.p. extract were investigated. Patients were solely sensitized to HDM (defined by sIgE determination and skin prick testing with 7 and 14 inhalant allergens, respectively). For detailed explanation of methods and statistical analysis, see File S1. For demographic and clinical data of the study population, see Table S1.
Overall sensitivity of molecular allergy diagnosis (defined by a positive test reaction to at least one molecular allergen) was lower compared to singleplex extract‐based testing, and it usually increased the more house dust mite allergens were available. Overall sensitivity of ISAC, molecular‐based ImmunoCAP, ALEX and ALEX2 was 88.8%, 93.0%, 93.5% and 94.9%, respectively. Results of the molecular‐based ImmunoCAP, ALEX and ALEX2 did not differ significantly, whereas sensitivity of the ISAC was lower compared to ALEX and ALEX2 (P = .006 and P < .001) as well as to the molecular‐based ImmunoCAP (P = .022). This was mainly due to the unavailability of Der p 23: omission of Der p 23 using ImmunoCAP, ALEX and ALEX2 resulted in a lower overall sensitivity of 87.9%, 88.4% and 90.2%, respectively, which were all similar to the 88.8% of the ISAC (P = .392).
Overall sensitivity of the molecular test systems was clearly correlated with sIgE levels to D.p.: the higher the levels, the better the sensitivity of molecular testing (Table 1).
TABLE 1.
Overall sensitivity of molecular test methods increased with higher sIgE to D.p. extract
| sIgE to D.p. extract | n | ISAC (%) | P‐value | ImmunoCAP molecular (%) | P‐value | ALEX | P‐value | ALEX2 | P‐value |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 215 | 88.8 | <.001 | 93.0 | <.001 | 93.5 | <.001 | 94.9 | .001 |
| ≥0.7 kU/L | 204 | 89.7 | <.001 | 94.6 | .001 | 94.6 | .001 | 95.6 | .004 |
| ≥1.0 kU/L | 186 | 93.6 | <.001 | 96.2 | .016 | 96.8 | .031 | 97.3 | .063 |
| ≥3.5 kU/L | 141 | 97.9 | .250 | 99.3 | 1.000 | 99.3 | 1.000 | 99.3 | 1.000 |
Sensitivity of the four molecular assays tested increased with sIgE levels to D.p. extract. In the case of sIgE levels ≥ 1.0 kU/L, ALEX2 and in the case of sIgE levels ≥ 3.5 kU/L, all molecular assays performed statistically equal to extract‐based diagnosis. All P‐values listed are direct comparisons to extract‐based singleplex diagnosis using ImmunoCAP.
In our study population, three allergens, Der p 1, 2 and 23, constituted major allergens, with sensitization rates of 55.3%, 77.7% and 54.0%, respectively, whereas all other allergens were minor allergens. Mono‐sensitization to Der p 2 was most frequently observed as 21.4% of patients were solely sensitized to Der p 2, followed by 10.7% who were mono‐sensitized to Der p 1; 4.7% were solely sensitized to Der p 23. A mere 0.5% of patients were mono‐sensitized to Der p 10 and 20, respectively. Importantly, we did not observe mono‐sensitization to Der p 5, 7, 11 or 21, indicating that these allergens do not increase sensitivity of the test panel. Using the ImmunoCAP, sensitization rates to Der p 1, 2 and 23 were similar with 58.1%, 77.2% and 46.5%, respectively. Mono‐sensitization to Der p 23 was observed in 4.7%, which was identical to the observed rate with the ALEX2. The sensitization pattern to nine molecular allergens tested with ALEX2 is depicted in Figure S1. Interestingly, all molecular test systems correlated strongly (Figure 1).
FIGURE 1.
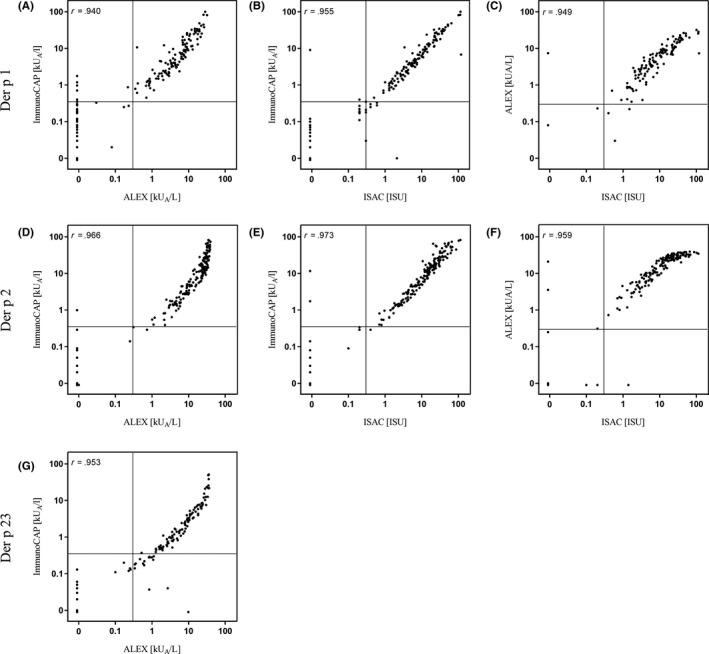
High correlation of the molecular allergy test systems. Molecular test systems correlated strongly with Spearman's rho ranging between 0.940 and 0.955 (Der p 1), between 0.959 and 0.973 (Der p 2), and 0.953 (Der p 23), (P < .001). Due to the low number of Der p 10 sensitizations, correlations were not calculated for Der p 10
Overall sensitivity of the molecular test platforms investigated was good, ranging from 88.8% to 94.9%. However, even with the best method, 11 out of 215 (5.1%) sera were negative for the nine molecular allergens investigated. Following reasons may explain the lower sensitivity: although nine molecular allergens have been tested, this could still be insufficient, as 30 D.p. molecular allergens have been described so far (retrieved from www.allergen.org, January 26, 2020). Several years ago, it was reported that using a combination of Der p 1 and 2 could detect at least 97% of D.p. allergic patients in Europe, 2 whereas more recent data do not support this observation. 3 , 4 Besides Der p 1 and 2, Der p 23 is the third major HDM allergen with (mono‐) sensitization rates in our study population of 4.7% and 54.0%, respectively, which is similar to previously reported rates between 4.2% and 5.3% for mono‐sensitization and between 46.5% and 75.8% of HDM patients sensitized to Der p 23. 5 , 6 , 7 This makes Der p 23 indispensable for diagnosis and explains why all molecular test systems including Der p 23 had a higher sensitivity. In our study, additional testing with Der p 10 and 20 at least slightly increased sensitivity, whereas Der p 5, 7, 11 and 21 did not. Therefore, it would be crucial to add only clinically relevant molecular allergens to a multiplex test panel in the future.
Technical issues could be another reason why modern molecular allergy diagnosis cannot detect all HDM allergic patients. Compared to singleplex assays, sensitivity of multiplex test systems can be decreased in patients with low sIgE levels due to higher limits of detection, higher coefficients of variation and a potential inhibition by antigen‐specific IgG. 8 We could clearly show that sensitivity of molecular assays was impaired in patients with low sIgE levels. It should be mentioned that our study population reflected an unbiased random sample out of daily practice, with low (≤1.0 kU/L) sIgE to D.p. in 13.5% of patients. Under optimal conditions, namely in patients with sIgE levels <3.5 kU/L, sensitivities of the molecular test systems were very high, ranging from 97.9% to 99.3%. The newest multiplex assay, ALEX2, performed statistically equal to extract‐based diagnosis in patients with sIgE levels >1.0 kU/L with a sensitivity of 97.3%.
Taken together, modern multiplex testing is an individualized diagnostic approach determining sensitization patterns of HDM allergic patients, which may help to identify best eligible patients for allergen immunotherapy. Sensitivity of up‐to‐date multiplex systems is now comparable to extract‐based testing. In patients with low sIgE levels, however, additional singleplex extract‐based testing or prick testing may be necessary.
CONFLICTS OF INTEREST
GJ Sturm reports consulting and lecture fees from Novartis, Bencard, Stallergenes, HAL, Allergopharma and Mylan outside of the submitted work. U Cerpes reports fees from Mylan outside of the submitted work.
Supporting information
Fig S1
Supplementary Material
REFERENCES
- 1. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine‐Tebbe J. Effective treatment of house dust mite‐induced allergic rhinitis with 2 doses of the SQ HDM SLIT‐tablet: results from a randomized, double‐blind, placebo‐controlled phase III trial. J Allergy Clin Immunol. 2016;137(2):444‐451.e8. [DOI] [PubMed] [Google Scholar]
- 2. Weghofer M, Thomas WR, Kronqvist M, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;38(12):959‐965. [DOI] [PubMed] [Google Scholar]
- 3. Becker S, Schlederer T, Kramer MF, et al. Real‐life study for the diagnosis of house dust mite allergy – the value of recombinant allergen‐based IgE serology. Int Arch Allergy Immunol. 2016;170(2):132‐137. [DOI] [PubMed] [Google Scholar]
- 4. Barber D, Arias J, Boquete M, et al. Analysis of mite allergic patients in a diverse territory by improved diagnostic tools. Clin Exp Allergy. 2012;42(7):1129‐1138. [DOI] [PubMed] [Google Scholar]
- 5. Jimenez‐Feijoo R, Pascal M, Moya R, et al. Molecular diagnosis in house dust mite allergic patients suggests clinical relevance of Der p 23 in asthmatic children. J Investig Allergol Clin Immunol. 2019;30(4). 10.18176/jiaci.0431. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Matos Semedo F, Dorofeeva Y, Pires AP, et al. Der p 23: clinical relevance of molecular monosensitization in house dust mite allergy. J Investig Allergol Clin Immunol. 2019;29(4):314‐316. [DOI] [PubMed] [Google Scholar]
- 7. Batard T, Baron‐Bodo V, Martelet A, et al. Patterns of IgE sensitization in house dust mite‐allergic patients: implications for allergen immunotherapy. Allergy. 2016;71(2):220‐229. [DOI] [PubMed] [Google Scholar]
- 8. Jakob T, Forstenlechner P, Matricardi P, Kleine‐Tebbe J. Molecular allergy diagnostics using multiplex assays: methodological and practical considerations for use in research and clinical routine: part 21 of the series molecular allergology. Allergo J Int. 2015;24:320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


