Abstract
Chyluria, described as passage of milky white urine, has been recognized as a urological manifestation of the lymphatic system due to abnormal connection between the blocked and dilated lymphatics and the renal pelvicaliceal systems. We report the case of a 36-year-old female from an endemic area of filariasis in India who presented with chyluria. In addition to medical therapy, intermittent sclerotherapy of renal pelvis with 0.2% povidone iodine was carried out with a dose schedule different from the ones in practice earlier. The idea of intermittent sclerotherapy was to create pyelolymphatic and pyelosinus reflux so as to sclerose the communicating channel between the lymphatic and urinary systems. This method proved to be curative for the patient and she was relieved from her 8-year-old problem and subsequently remained asymptomatic.
Keywords: Chyluria, endoscopic sclerotherapy, milky urine, povidone iodine
Introduction
Chyluria is a condition of chronic lymphourinary reflux, through the fistulous communications, secondary to lymphatic stasis because of obstruction to the lymphatic flow. It is more common in the tropics and subtropics, predominantly due to filariasis.
Parasitic agents such as Wuchereria bancrofti, Echinococcus, Cysticercus cellulosae, Ascaris lumbricoides, Tinea nana, Cercorrenas hominis, and malaria can obstruct the lymphatics. Congenital lymphatic malformation, injury to kidney with lymphourinary fistulas and trauma, abscess, neoplasms, diabetes, pernicious anemia, pregnancy, and tuberculosis causing obstruction to the lymphatics are the nonparasitic causes.[1,2]
Several approaches have been reported for the treatment of chyluria. In the present study, endoscopic sclerotherapy with povidone iodine as a modified sclerotherapy regimen is reported. We successfully tried endoscopic sclerotherapy with povidone iodine in our patient to see the effectiveness of our dosage schedule, which was different from the existing ones.
Case presentation
A 36-year-old female presented with complaint of milky urine with an occasional episode of milky red urine for the last eight years. It was associated with passage of clots for the past six months. Patient also complained of weakness with a progressive loss of weight for the last 2 years. There was history of high-grade fever, associated with rigors and chills that lasted for one month. Patient took allopathic, ayurvedic, and indigenous medications from different practitioners for the same but without relief, before presenting to us in the urology department.
On examination, the patient was found malnourished. General and systemic examinations were unremarkable. Her blood investigation showed normal hemogram with a normal absolute eosinophil count (340/cu mm). Her renal function test was normal. Her urine was milky in appearance and settled down into three layers, i.e., an oily upper layer, a fibrinous middle layer, and a bottom layer consisting of debris and chylous clots. On urine analysis for albumin ++, 18–20 pus cells with numerous RBC/HPF were found. The turbidity of urine disappeared upon addition of ether. Ultrasonography revealed normal-sized kidneys with multiple internal septations with echoes suggestive of chylous and blood clots.
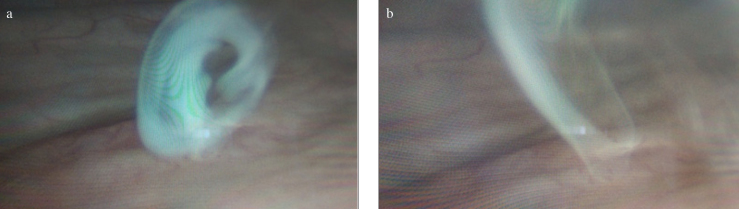
Cystourethroscopy revealed efflux of milky white urine from the left ureteric orifice (Figure 1a, b), whereas efflux from the right ureteric orifice was clear. Ureterorenoscopy was performed. Lymphourinary communication was seen in the left renal pelvis.
Figure 1. a, b.
Cystoscopy images showing efflux of milky urine
Patient was started with a fat-free, high-protein diet. Supplementation of coconut oil as medium chain triglycerides, hematinics, multivitamins, and green leafy vegetables was given. Diethylcarbazine at a dose of 6 mg/kg was given in three divided doses after food over a period of 14 days and a stat dose of albendazole 400 mg was given as the patient came from an endemic area of filariasis. Supportive treatment with abdominal binder and bed rest was given. After all this failed to relieve symptoms, considering the etiology as idiopathic, intermittent sclerotherapy with renal pelvic instillation therapy with povidone iodine was started. During the procedure, a retrograde ureteric catheter was passed up to the renal pelvis. A solution of 0.2% povidone iodine (10 mL of 5% povidone iodine in 40 ml of distilled water) was prepared and 5 mL was injected into the renal pelvis with a syringe in order to create pyelolymphatic reflux, kept for 1 min, and then allowed to drain out. The maneuver was repeated until whole of the solution was instilled into the pelvicaliceal system. Povidone iodine induces an intense aseptic sclerosing and obliterative inflammatory reaction in the lymphatic channels leading to immediate relief. The subsequent healing by fibrosis leads to permanent remission. The ureteric catheter was kept in situ. The instillations were repeated every alternate day for a total of three times under antibiotic coverage. Urine was transparent after the first instillation but then again changed to milky. However, after second and third instillation, urine remained clear/transparent without any chylous clots. During the whole course of her treatment, the patient was fully informed and formal consent was taken for all the procedures.
Discussion
Lymphatic drainage of the kidney occurs in a trilaminar fashion.[1] The intrarenal lymphatics emerge as 4–7 trunks at the renal hilum, to eventually converge along the renal vessels to the lateral aortic nodes.[3] Chyluria occurs after rupture of lymphatic varices into renal tubules because of the high intralymphatic pressure due to obliterative lymphangitis and lymphatic hypertension, subsequently forming collaterals. It may also occur along the ureter, bladder, prostate, or urethra.[3] Failure of the valvular system in dilated lymphatics adds to back flow and circulation.[1,2] The manifestation of chyluria is related to the site of involvement and the anastomotic variation of the lymphatic system that primarily occurs at the cisterna chyli. Here the lumbar trunks and the intestinal trunks join. The intestinal trunk in such cases drains in the lumbar trunks of one side or directly in the thoracic duct causing unilateral chylous edema of only one extremity or unilateral chyluria, more commonly on the left side.[1,4]
Chyluria is usually asymptomatic. Dysuria, hematuria, renal colics, backache, urinary tract infections, pedal lymphangitis, edema, hydroceles, hypoproteinemia, cachexia, weight loss, and malnutrition may be present in symptomatic patients. Nutritional deficiency, recurrent clot colic, urinary retention, hematuria/hematochyluria, and a state of compensated immunosuppression may be seen.[5]
Investigations in chyluria evaluate the presence of chyle in postprandial urine for chylomicrons and triglycerides. The intermittent passage of milky cloudy urine should be differentiated from phosphaturia (clears on adding 10% acetic acid), amorphous urates, severe pyuria, lipiduria secondary to fat embolism, pseudochylous urine, and caseousuria due to renal tuberculosis.[3] Location of the lymphourinary fistulae can be demonstrated by intravenous urography, retrograde pyelography, and lymphangiography which in some cases may be even therapeutic, due to the contrast-induced chemical pyelitis causing an obliterative sclerosis of the lymphatics leading to cessation of chyluria.[1] Lymphographic evidence of numerous perihilar lymphatics is the most pathognomonic sign of chyluria. Technetium-99m (metastable) diethylenetriamine pentaaceticacid radionuclide lymphoscintigraphy has now replaced traditional lymphography to precisely reveal the location of chyluria.[6] Routine radio imaging may not be necessary in filarial chyluria.[4] Computed tomography scan can demonstrate enlarged para-aortic lymphadenopathy. Detection of filarial antigens in serum and urine can be done routinely with ELISA sandwich assay but for rapid accurate diagnosis of Wuchereria bancrofti infection, immunochromatographic test was used, as it is 96.7% sensitive.[3] Unless complications are present, renal function is usually unaffected. Microfilaria may or may not be demonstrated in urine and/or blood. Eosinophilia may be present and leukocytosis may be present in acute filarial manifestations. Initially if chyluria is mild and stable and in the absence of microfilaria, no therapy may be necessary and up to 50% of cases may undergo spontaneous remission.[1]
The conservative measures for the treatment of chyluria include dietary manipulations with omission of long-chain triglycerides, use of coconut oil, drug therapy with diethyl carbamazine, bed rest, and use of abdominal binders (to decrease the lymphourinary reflex through higher intra-abdominal pressure). Medium-chain triglycerides have been advocated since these are directly absorbed via the portal vein bypassing the lacteals and lymphatic channels, unlike the long-chain fatty acids. Chyluria associated with other coexistent conditions may be controlled by treatment of the coexistent conditions.[1] Conservative management was tried initially in our patient, but the symptoms were not relieved.
Surgical management of chyluria is indicated in patients with refractory severe chyluria and failed medical therapy. This includes endoscopic sclerotherapy, surgical lymphatic disconnection done by an open surgical and minimally invasive approach.[1,3,6–10] In failed endoscopic sclerotherapy and surgical management, chyluria may be cured by transinguinal spermatic lymphangiovenous anastomosis or inguinal lymph node-saphenous vein anastomosis.[9] Due to the spontaneous emergence of collateral lymphatic channels, even these lymphatic shunts tend to fail over six months. Due to failure of conservative measures, we proceeded to endoscopic sclerotherapy in our patient. Instillation of sclerosing agents such as silver nitrate, (0.2%) povidone iodine, and radiographic contrast media (Urografin™)[11] in the renal pelvis[2] has been found to be a safe, effective, and minimally invasive procedure with an initial success rate of about 70–80%[3] and the long-term recurrence rate is 50%. The success rate with endoscopic sclerotherapy is known to fall with subsequent reinstallations. Sclerosing agents induce an intense aseptic sclerosing obliterative inflammatory reaction in the lymphatic channels leading to immediate relief. The subsequent healing by fibrosis leads to permanent remission. Flank pain, nausea, vomiting, and hematuria can occur after instillation but usually subsides by 24–28 hours. It is safer to manage bilateral chyluria one side at a time, and doing endoscopic sclerotherapy on the severe side first. Recently, localized chyluria (post-radical nephrectomy) has been cured using cyanoacrylate adhesives.[11] Subcutaneous octreotide has also been used to treat post-traumatic chyluria.[9] Lymphangioleiomyomatosis induced chyluria has been treated using sirolimus.[12] High-intensity focussed ultrasound ablation therapy is another noninvasive therapy used to treat chyluria.[13] Thus, the immediate results with endoscopic sclerotherapy are good but the long-term follow up and recurrence rates tend to be high.[14]
Intermittent sclerotherapy with povidone iodine was carried out in our patient. Povidone iodine is a water soluble, nonionic surfactant polymer (polyvinyl pyrrolidone), which releases iodine slowly.[14] Many dosage schedules of povidone iodine have been described in the literature for treating chyluria such as 8 hourly for 3 days or 12 hourly for 2 days or weekly for 6–8 weeks.[9] We followed a different dosage schedule which proved to be equally effective as no recurrence was seen in the follow-up. Thus, povidone iodine is an effective endoscopic sclerosing agent and 5 ml of 0.2% solution could be instilled on alternate days for three sessions for treating chyluria.
Footnotes
Informed Consent: Written informed consent was obtained for all procedures from the patient.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.S., A.S., A.J.; Design - H.S., A.S., A.J.; Supervision - H.S., A.S.; Resources - H.S., A.S., A.J.; Materials - H.S., A.S., A.J.; Data Collection and/or Processing - H.S., A.S., A.J.; Analysis and/or Interpretation - H.S., A.S.; Literature Search - H.S., A.S., A.J.; Writing Manuscript - H.S., A.S., A.J.; Critical Review - H.S., A.S.; Other - H.S., A.S., A.J.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Saha M, Ray S, Goswami M, Kundu S, Saha P, Saha A, et al. An occult filarial infection presenting as chyluria with proteinuria: a case report and review of literature. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.01.2012.5635. bcr0120125635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalela D. Issues in etiology and diagnosis making of chyluria. Indian J Urol. 2005;21:18–23. doi: 10.4103/0970-1591.19545. [DOI] [Google Scholar]
- 3.Singh I, Dargan P, Sharma N. Chyluria - a clinical and diagnostic step ladder algorithm with review of literature. Indian J Urol. 2004;20:79–85. [Google Scholar]
- 4.Mahmood K, Ahmad A, Kumar K, Singh M, Pankaj S, Singh K. Chyluria in pregnancy- A decade of experience in a single tertiary care hospital. Nephrourol Mon. 2015;7:e26309. doi: 10.5812/numonthly.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gun T, Celik A, Yeldez S, Cavdar C, Coker C, Goktay Y, et al. Intermittent chyluria: case report. Turk J Nephrol. 2005;14:140–2. [Google Scholar]
- 6.Thet L, Takeda T, Kuramochi M, Sato M, Wu J, Myo-Min, et al. Tc-99m diethylenetriamine penta-acetic acid (DTPA)-human serum albumin (HAS) radionuclide lymphography for detecting the location of chyluria. Ann Nucl Med. 1998;12:205–7. doi: 10.1007/BF03164846. [DOI] [PubMed] [Google Scholar]
- 7.Hemal AK, Kumar M, Pawar RS, Gupta NP. The laparoscopic management of chyluria by retroperitoneal access. BJU Int. 2000;86:402. [Google Scholar]
- 8.Hemal AK, Gupta NP. Retroperitoneoscopic lymphatic management of intractable chyluria. J Urol. 2002;167:2473–6. doi: 10.1016/S0022-5347(05)65007-0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Hemal AK. Chyluria- An Overview. Int J Nephrol Urol. 2009;1:14–26. [Google Scholar]
- 10.Kumar M, Kumar R, Hemal AK, Gupta NP. Complications of retroperitoneoscopic surgery at one center. BJU Int. 2001;87:607–12. doi: 10.1046/j.1464-410x.2001.02137.x. [DOI] [PubMed] [Google Scholar]
- 11.Tada I. Pathogenesis and treatment of chronic symptoms with emphasis on chyluria and elephantiasis. Trop Med Health. 2011;39:47–50. doi: 10.2149/tmh.39-1-suppl_2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaue T, Tominaga M, Niizeki T, Zaizen Y, Matsukuma K, Koganemaru M, et al. Successful Treatment of a Patient with Chyluria due to Lymphangioleiomyomatosis Using Sirolimus. Respir Med Case Rep. 2018;23:86–9. doi: 10.1016/j.rmcr.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao J, Sun T, Zhang S, Ma M, Yang X, Zhou J, et al. HIFU, a noninvasive and effective treatment for chyluria: 15 years of experience. Surg Endosc. 2018;32:3064–9. doi: 10.1007/s00464-017-6017-8. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam TV, Parkash JV, Sivashankar G. Povidone iodine used as a sclerosing agent in the treatment of chyluria. Br J Urol. 1998;82:587–8. doi: 10.1046/j.1464-410X.1998.00861.x. [DOI] [PubMed] [Google Scholar]