Abstract
Upper-respiratory tract infections (URTI) are the leading causes of childhood morbidities. This study investigated etiologies and patterns of URTI among children in Mwanza, Tanzania. A cross-sectional study involving 339 children was conducted between October-2017 and February-2018. Children with features suggestive of URTI such as nasal congestion, dry cough, painful swallowing and nasal discharge with/without fever were enrolled. Pathogens were detected from nasopharyngeal and ear-swabs by multiplex-PCR and culture respectively. Full blood count and C-reactive protein analysis were also done. The median age was 16 (IQR: 8–34) months. Majority (82.3%) had fever and nasal-congestion (65.5%). Rhinitis (55.9%) was the commonest diagnosis followed by pharyngitis (19.5%). Viruses were isolated in 46% of children, the commonest being Rhinoviruses (23.9%). Nineteen percent of children had more than 2 viruses; Rhinovirus and Enterovirus being the commonest combination. The commonest bacteria isolated from ears were Staphylococcus aureus and Pseudomonas aeruginosa. Children with viral pathogens had significantly right shift of lymphocytes (73%—sensitivity). Majority (257/339) of children were symptoms free on eighth day. Viruses are the commonest cause of URTI with Rhinitis being the common diagnosis. Rapid diagnostic assays for URTI pathogens are urgently needed in low-income countries to reduce unnecessary antibiotic prescriptions which is associated with antibiotic resistance.
Subject terms: Infectious diseases, Respiratory tract diseases
Introduction
Respiratory tract infections (RTIs) account for an estimated 3.9 million deaths annually among children worldwide with 42% of these deaths occurring in Africa1–3. In high income countries, up to 25% of children under 1 year of age and up to 18% of children aged 1 to 4 years develop RTIs4,5. These illnesses range from mild to severe and life-threatening illness accounting for over two million childhood deaths annually worldwide2,6,7. Respiratory tract infections involving just the upper respiratory tract usually are self-limited disease requiring only supportive management. Despite it being a less severe illness, it has emerged to be a major cause of childhood morbidity with a high cost to the society, and occasionally associated with serious sequelae. In high income countries, these infections are caused by several families of virus including the newly discovered Bocavirus1,2. Common viral agents which have been linked to RTIs include Rhinoviruses which accounts for 30%, Respiratory syncytial virus, influenza virus, Parainfluenza viruses, Human metapneumovirus and Adenoviruses accounting for 35% of RTI with about 10% being due to Coronaviruses8. Bacterial upper respiratory infections are mainly due to Streptococcus pneumoniae9, Haemophilus influenzae and Moraxella catarrhalis accounting to 90% of bacterial causes10.
Epidemiology of URTIs, particularly in Africa, remains poorly understood and consequently underappreciated. Despite being the commonest cause of preschool absenteeism, frequent visits to health care facilities and major reason for irrational antibiotics prescriptions there is paucity of data on the magnitude of these infections in many low- and middle-income countries. In Tanzania, there is only one study that has documented the viral etiologies of RTI3. Therefore, there is a paramount need to establish information on the common etiologies of RTIs in Tanzania, the information that can stimulate further studies and possible control interventions including introduction of cheap and reliable methods to detect these pathogens in clinical settings.
In addition due to increased use of antibiotic without a support of a diagnostic test in the treatment of URTI as observed in number of previous studies11–13, make the availability of epidemiological data on the patterns of etiology of URTI of paramount important. Overuse of antibiotics without prescriptions for URTI is widespread in developing countries14, this is partially contributed by lacking of data on the etiologies of URTIs. Therefore, these data are relevant to clinicians in developing countries and policy makers in order to invest on the improved diagnostic facilities and reduce antibiotic prescriptions for URTIs.
Methods
Study area, design and study population
A cross sectional hospital based study involving 339 children aged 1–59 months presenting with RTI symptoms was conducted from October 2017 to February 2018 in the city of Mwanza, Tanzania. The study was conducted in two health facilities namely: Buzuruga Health Center (BHC), and Nyamagana District Hospital (NDH). These are public health facilities providing free services to children below 5 years of age.
Selection criteria
The study included all children presented with nasal congestion or runny nose, hoarseness of voice with dry cough, painful swallowing with tender cervical lymph nodes and enlarged tonsils on examination, ear pain or ear discharge, nasal discharge with or without fever (axilla body temp of 37.5 °C and above).
In this study we defined: Pharyngitis as painful swallowing dry cough, plus or minus hoarseness of the voice (sore throat), Tonsillitis as painful swallowing with tender cervical lymph nodes and enlarged tonsils(primarily tonsillar inflammation) and Rhinitis as presence of one or more symptoms including sneezing, itching, nasal congestion, and rhinorrhea.
Sample size estimation and sampling procedures
Sample size was calculated using Yamane Taro (1967) with precision level of 5%. The minimum sample size estimated was 270 children. However, a total of 339 children were enrolled. All children who met the inclusion criteria were serially enrolled until the desired sample size was attained.
Data collection and sample collection
Sociodemographic and clinical information were collected using pretested structured data collection tool. Nasopharyngeal swabs (COPAN DIAGNOSTICS INC. USA, Canada) were obtained as previously described15 by inserting the swab into one nostril straight back along the floor of the nasal passage until reaching the nasopharynx. The swabs were rotated gently for 5–10 s to loosen the epithelial cells and collect the sample. The swabs were then inserted into viral transport medium and stored at − 80 °C until processing. In children presenting with ear discharge; ear swabs were collected using flexible shaft swab via an auditory speculum in case of inner ear while a sample from outer ear was obtained by firmly rotating swab in outer canal. Swabs were immediately taken to the laboratory for bacterial culture and sensitivity. For each consenting participant, about 4 mls of blood was also collected for blood cell counts and quantitative C reactive protein analysis. A thorough general and physical examination was performed to all enrolled children to establish clinical features.
All children were managed as per local hospitals protocol. Patients were followed two times; the first follow up was on the 4th day, where these patients were fully examined and their full blood count, CRP and ear swab culture results were revealed. In case of positive ear swab culture treatment was changed based on susceptibility patterns. The second follow up was done on the 8th day as clinical review to evaluate for disease progression.
Laboratory procedures
Ear swab specimens were inoculated onto Chocolate agar, blood agar (BA) and MacConkey agar (MCA) plates and incubated aerobically at 37 °C for 24–48 h. In-house biochemical identification tests were used to identify isolated bacteria to their species level16. The identified isolates were tested for antimicrobial susceptibility following CLSI guidelines, The tested antibiotic discs included: amikacin (30 µg), gentamicin (10 µg), erythromycin (15 µg), vancomycin (30 µg), clindamycin (2 µg), ciprofloxacin (5 µg) which were used for gram positive bacteria and ampicillin (10 µg), ceftazidime (30 µg), meropenem (10 µg), amikacin (30 µg), piperacillin-tazobactam (100/10 µg) and ciprofloxacin (5 µg) for gram negative bacteria17. Bacterial isolates obtained were inoculated into Brain Heart Infusion broth with 20% Glycerine and stored at – 80 °C freezer. E. coli ATCC 25992 and Staphylococcus aureus ATCC 25923 were used as control strains.
Nasopharyngeal swabs were transported to Mainz University Germany and were tested to detect Enterovirus (EV), Influenza virus type A (IVA), Influenza virus type B (IVB), Respiratory syncytial virus (RSV), Parainfluenza virus type 1 (PIV1), Parainfluenza virus type2 (PIV2), Parainfluenza virus type 3 (PIV3), Parainfluenza virus type 4 (PIV4), Adenovirus (AV), Rhinovirus (RV), Human metapneumovirus (MPV), Coronavirus (CV), Bocavirus, Mycoplasma pneumoniae (Mpn), Chlamydophila pneumoniae (Cpn), Bordetella pertussis (Bp), Bordetella parapertussis (Bpp) and Legionella pneumophila (Lpn) using multiplex PCR as previously described18.
Blood samples were quantitatively tested for C-reactive protein following manufacturer instructions (Medical Instruments Co., Ltd, Shanghai, China). Blood in EDTA container (BD Vacutainer, Nairobi, Kenya) was used to estimate complete blood count (FBC) using hematological analyzer (Beckman coulter (UK) LTD)19.
Statistical data analysis
Data entry was done using Microsoft excel then exported to STATA version 13 for analysis. Continuous variables (age and temperature) were summarized using median with interquartile ranges. Categorical variables (sex and level of education) were summarized using frequency and proportions. To determine the utility of FBC, and CRP in the determination of causative agents among children below 5 years of age, a 2 by 2 table and receiver operating curve (ROC) characteristic analysis were used to determine the sensitivity, specificity, positive and negative predictive values. Children presenting with symptoms for more than 7 days were classified as having chronic illness. A child who had no any symptom on day eight of follow was declared cure.
Ethical approval and consent to participate
The approval for conducting the research was sought from the Joint CUHAS/BMC research ethics and review with ethical clearance number: CREC/255/2017. Permission to conduct the study was also sought from the Pediatrics departments at BHC and NDH. The aim and importance of the study was explained to parents/caretakers before enrollment of children to the study, followed by a signed informed consent by the parent/caretaker. All information regarding the patient remained confidential. Patient’s records were kept such that the identity of the patient was not disclosed. For those who refused to participate, were provided with services similar to the participants and had equal chance to treatment regardless of their inclusion status. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Socio-demographic characteristics of study participants
The median age of the enrolled children was 16 (IQR: 8–34) months. The slightly majority 200 (59%) of children were seen at BHC and the slightly majority 198 (58.4) were below two years of age (Table 1). All except one child had received at least one dose of pneumococcal and Hib vaccine.
Table 1.
Socio-demographic distribution for under five children with URTI in Mwanza city.
| Characteristics | Number (n) | Percent (%) |
|---|---|---|
| Gender | ||
| Male | 200 | 59 |
| Female | 139 | 41 |
| Age (months) | ||
| 1–23 | 198 | 58.4 |
| 24–59 | 141 | 41.6 |
| Caregiver’s education level | ||
| None | 28 | 8.3 |
| Primary | 222 | 65.5 |
| Secondary and above | 89 | 26.2 |
| Caregiver occupation | ||
| Employed | 22 | 6.5 |
| Traders | 255 | 75.2 |
| Unemployed | 62 | 18.3 |
| Family size* | ||
| Small | 209 | 61.7 |
| Large | 129 | 38.3 |
| Duration of illness prior to presentation | ||
| 1–7 days | 295 | 87.0 |
| > 7 days | 44 | 13.0 |
| Exposure to smoke (indoor cooking using charcoal/wood) | ||
| Yes | 314 | 92.6 |
| No | 25 | 7.4 |
| Animal keeping | ||
| Yes | 14 | 4.8 |
| No | 325 | 95.2 |
| History of antibiotic use during this illness | ||
| Yes | 156 | 46.0 |
| No | 183 | 54.0 |
Clinical findings and patterns of URTI among enrolled children with URTI symptoms
Among the enrolled children with URTI, the majority presented with fever 279 (82.3%) and 222 (65.5%) presented with nasal discharge. Few children 47 (13.9%) presented with abnormal chest findings (features suggestive of pneumonia) on physical examination. About 295 (87%) of the total population presented with history of illness ranging from 1 to 7 days and were classified as acute illness in this study (Table 2). The slightly majority 184 (55.9%) of children presented with rhinitis (Fig. 1).
Table 2.
Clinical presentations of upper respiratory tract infections.
| Clinical presentation | Number (n) | Percent (%) |
|---|---|---|
| Fever | ||
| Yes | 279 | 82.3 |
| No | 60 | 17.7 |
| Cough | ||
| Dry | 150 | 44.3 |
| Wet | 39 | 11.5 |
| Nasal discharge | ||
| Yes | 222 | 65.5 |
| No | 117 | 34.5 |
| Nasal congestion | ||
| Yes | 214 | 63.1 |
| No | 125 | 36.9 |
| Ear pain | ||
| Yes | 12 | 3.5 |
| No | 327 | 96.5 |
| Ear discharge | ||
| Yes | 14 | 4.1 |
| No | 325 | 95.8 |
| Rapid breathing | ||
| Yes | 36 | 89.4 |
| No | 303 | 10.6 |
| Chest findings | ||
| Normal | 292 | 86.1 |
| Abnormal | 47 | 13.9 |
| Tonsils examination | ||
| Normal size | 286 | 84.4 |
| Enlarged | 53 | 15.6 |
Figure 1.
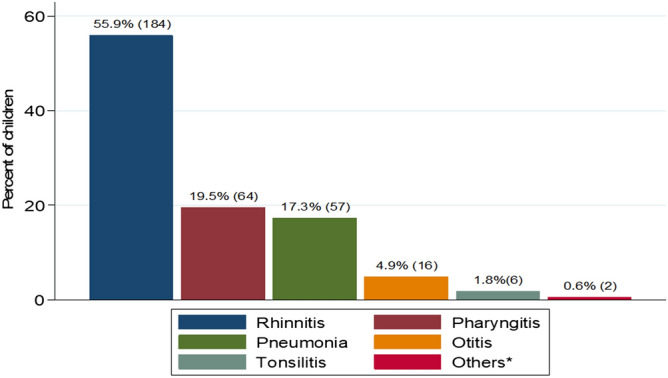
Patterns of UTRI among under five children in Mwanza city.
Etiologies of upper respiratory tract Infections among children below 5 years of age
Among 339 enrolled children, 159 (46.9%) children had viral pathogens detected. The commonly identified viruses were Rhinoviruses and Adenoviruses (Fig. 2). However, 40/339 (11.8%) had mixed viral infections. Only 3/339 (0.9%) children had both bacterial and viral infections. Out of 14 patients who underwent ear investigation, 11 (78.5%) yielded pathogenic bacteria. The commonest bacteria detected were S. aureus which was detected in 5 patients, P. aeruginosa in 2 patients. Other bacteria detected one each were S. pneumoniae, Providence spp. K. pneumoniae and S. marcescens. Among 159 children with viral pathogens, 136 (85.5%) presented with acute illness while 14.5% had chronic illness.
Figure 2.
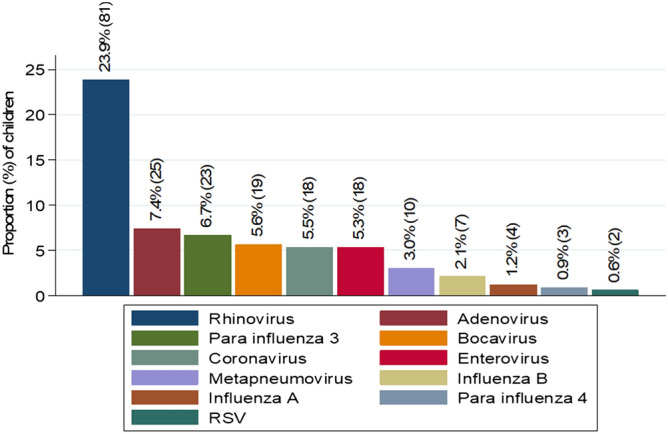
Viral pathogens isolated in the children under 5 years with URTI in Mwanza city.
Proportion of children with disappearance of symptoms on day eight of the illness
Out of 339, 289 were followed to determine disappearance of URTIs symptoms and 50 were loss to follow up. Majority of them 214/289 (74.0%), presented with less symptoms on the fourth day compared to the first day when they were seen at the hospital. During their second follow up visit the majority of them 257/289 (88.9%) were free from the initial symptoms and were declared cured while 32/289 (11.1%) had mild symptoms.
Utility of lymphocytes, neutrophils and CRP in determination of etiologies of URTI among under five children in Mwanza city
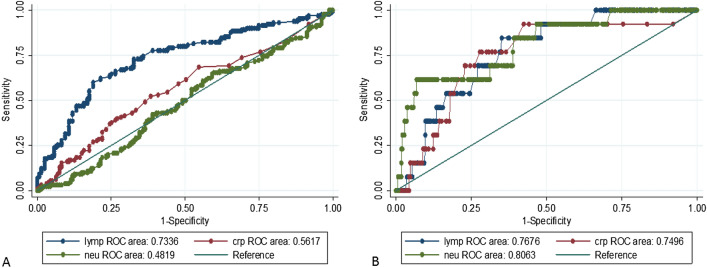
Lymphocytes, neutrophils and CRP were used to predict the possible causative agents. Children with viral pathogens had significantly elevated lymphocytes, with normal or elevated CRP. The sensitivity of elevated lymphocytes in detecting viral pathogens was 73.4% (Fig. 3A,B).
Figure 3.
(A) CRP, Lymphocytes and Neutrophils response in viral agents caused URTI, (B) Lymphocytes and Neutrophils response in bacterial agents caused URTI.
Discussion
Etiologies of URTI among under five children in Mwanza city
This is the first study to establish etiologies of RTIs in the Lake Victoria zone Tanzania. Findings from this study shows that, number of viruses are responsible for RTIs among children below 5 years of age attending outpatient clinics in the city of Mwanza. The prevalence of 46.9% reported in the current study is low compared to a previous study conducted in Ifakara and Dar es Salaam, which reported prevalence of 70.5%3. The possible explanation for these differences could be criteria used in the enrollment of the study participants. In the previous study fever was the main inclusion criterion which was not the case in the current study. In addition, this study was conducted in different season of the year (October 2017 through February 2018) compared to the previous which was conducted from April to August and from June to December at two different sites, respectively. Viral infections have been found to be influenced by season variations20–24. Further studies to establish seasonality of these viruses are warranted in developing countries.
The findings in this study are comparable to the previous study in Kenya among children below 5 years of age, whereby viruses were isolated in 45% of children with RTIs25. In comparison to a previous study in refugee camp in Kenya the prevalence of viral infection reported in this study is low (46% vs. 66.6%)26. The possible explanation could be overcrowding conditions in refugee camp which has been found to facilitate transmission of RTIs viruses27,28.
Regarding distribution of viruses; Rhinovirus, Adenovirus and Parainfluenza 3 were the commonest viral pathogens in the current study which is contrary to a previous study in Kenya26 whereby Influenza A virus, Respiratory Syncytial virus and Influenza B were the commonest. The predominance of Rhinovirus, Influenza viruses was also reported in a previous study in Tanzania3. The distribution of respiratory viruses is associated with climatic changes; the peak of infection usually occurs in winter period in temperate regions which is equivalent to wet season in hot climates like Tanzania29,30.
As documented earlier, a significant proportion of children who had viral infection presented with acute illness27. However, it should be noted that some studies have shown that some viral diseases may last for several weeks27,28 which may account for the few children in the present study who presented with chronicity.
Regarding ear bacterial infections, Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Klebsiella pneumoniae, Providencia spp. and Streptococcus spp. were isolated. This is contrary to the previous study whereby Streptococcus pneumoniae was the commonest isolate3. The possible explanation could be wide coverage of pneumococcal vaccines. Decrease in Streptococcus pneumoniae infections has been observed after introduction of the vaccine. A previous study by Mushi et al.31 in similar settings established that Staphylococcus aureus and Pseudomonas aeruginosa were the commonest pathogens causing CSOM among adult patients.
In the current study, only one nasopharyngeal specimens yielded bacterial isolate (Bordetella parapertussis) which is contrary to a previous observation by Ndossa et al.32 that detected Streptococcus pneumoniae to be colonizing the nasopharynx of children in the city of Mwanza. Moreover, the bacterial isolates in the current study were highly resistant to the readily available and the over counter antibiotics like amoxicillin, trimethoprim/sulphamethoxazole and ampicillin with majority being susceptible to ciprofloxacin ear drops. This could be explained by the fact that, ciprofloxacin is not readily used in children below 12 years of age.
Patterns of URTI among under five children in the Mwanza city
In the current study, rhinitis was the commonest presenting disease, followed by pharyngitis and pneumonia. This is further supported by findings in the current study which reported Rhinovirus to be the commonest pathogen. A previous study33, documented Rhinovirus to cause up to 25–85% of the upper respiratory tract infections. Systemic responses of Th1 for Rhinovirus results into stimulation of specific clones of CD4 T cells and secretions of large amount of granulocytes macrophage colony stimulating factor (GM-CSF) which are also responsible for the URTI symptoms34. Moreover, Rhinovirus infection has also been associated with lower respiratory tract disease, asthma exacerbations and fatal pneumonia33–35. On the other hand, the spectrum of diseases in this study could be also explained by the commonly isolated viruses; Influenza viruses, RSV, Parainfluenza viruses and Adenoviruses which are the common viruses responsible for most of the upper respiratory diseases as previously reported36,37.
In the current study, children with viral pathogens had a right shift of lymphocytes with an increased sensitivity of 78.0%, with left deviation of the neutrophils or slightly raised CRP (not more than 10 mg/dl). These findings are important and could be used to predict these infections in resource limited setting and assist in decision making on the management of the patients which eventually might reduce unnecessary prescriptions of antibiotics. The observation is further supported by the fact that the majority of children were free from the initial symptoms on day eighth, underscoring that viral caused URTI have mild symptoms which tend to disappear with time38,39.
Study limitations
Diagnosis of patterns of URTI was done clinically with no imaging to support the diagnosis, this might have caused misclassification. However, efforts were made to minimize this by consulting senior pediatricians whenever overlap of symptoms occurred. Another potential limitation was failure to perform multiplex PCR for all ear swabs therefore viral pathogens might have been missed in these samples.
Conclusions and recommendations
URTI are common in children below 5 years of age and are predominantly caused by viruses. Elevated lymphocytes, normal neutrophils with elevation or normal CRP levels can predict viral causes of URTI while the raise in neutrophils and CRP are more likely to predict bacterial infections. Clinicians should suspect URTI caused by viral infections whenever the children present with runny nose with congestion, fever and dry cough. The use of antibiotics should be minimized in children with URTI symptoms since most of the symptoms disappears within a week. Further studies to determine etiologies of lower respiratory tract infections are warranted in this setting.
Acknowledgements
The authors acknowledge the assistance provided by department of pediatrics Mainz University, the department of Microbiology and Immunology-CUHAS-Bugando, and the department of pediatrics and child health-CUHAS-Bugando.
Author contributions
M.M.M., S.E.M., N.K. and S.G., participated in the designing the study. E.K., F.M. and N.K. participated in data/sample collection, clinical evaluation and management of the children, S.G., B.G., B.O. and M.M.M. did laboratory procedures, B.R.K. and S.E.M. participated in data analysis, M.M.M., M.M. and S.E.M. did data interpretation. M.M.M. wrote the first draft of the manuscript. S.E.M., M.M.M. and S.G. did the critical review of the manuscript. All authors approved the last version of the manuscript.
Funding
This study was supported by the departments of Pediatrics-University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, department of microbiology and immunology CUHAS-Bugando and the department of Pediatrics and child health of the CUAS-Bugando.
Data availability
All data are included in the manuscript. Raw data is available upon request and the request should be made to the Director of research and Innovation, Catholic University of Health and allied Sciences.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allander T. Human bocavirus. J. Clin. Virol. 2008;41:29–33. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Green RJ. Symptomatic treatment of upper respiratory tract symptoms in children. S. Afr. Fam. Pract. 2006;48:38–42. doi: 10.1080/20786204.2006.10873374. [DOI] [Google Scholar]
- 3.D’acremont V, et al. Beyond malaria—causes of fever in outpatient Tanzanian children. N. Engl. J. Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 4.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2002;2:25–32. doi: 10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 5.Zoorob R, Sidani MA, Fremont RD, Kihlberg C. Antibiotic use in acute upper respiratory tract infections. Am. Fam. Phys. 2012;86:817. [PubMed] [Google Scholar]
- 6.Sazawal S, Black R. Pneumonia case management trials group: Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A meta-analysis of community-based trials. Lancet Infect. Dis. 2003;3:547–556. doi: 10.1016/S1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 7.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull. World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant JH, Harrison PF. Disease Control Priorities in Developing Countries. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 9.Denny FW., Jr The clinical impact of human respiratory virus infections. Am. J. Respir. Crit. Care Med. 1995;152:S4. doi: 10.1164/ajrccm/152.4_Pt_2.S4. [DOI] [PubMed] [Google Scholar]
- 10.Jha P, et al. Disease Control Priorities in Developing Countries. New York: Oxford University Press; 2006. [PubMed] [Google Scholar]
- 11.Leblebicioglu H, Canbaz S, Peksen Y, Gunaydin M. Physicians' antibiotic prescribing habits for upper respiratory tract infections in Turkey. J. Chemother. 2002;14:181–184. doi: 10.1179/joc.2002.14.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Mainous AG, III, Hueston WJ, Davis MP, Pearson WS. Trends in antimicrobial prescribing for bronchitis and upper respiratory infections among adults and children. Am. J. Public Health. 2003;93:1910–1914. doi: 10.2105/AJPH.93.11.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alumran A, Hou X-Y, Hurst C. Assessing the overuse of antibiotics in children with URTIs in Saudi Arabia: Development of the parental perception on antibiotics scale (PAPA scale) J. Epidemiol. Glob. Health. 2013;3:3–10. doi: 10.1016/j.jegh.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horumpende PG, et al. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE. 2018;13:e0207465. doi: 10.1371/journal.pone.0207465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS ONE. 2011;6:e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulthess B, Bloemberg GV, Zbinden R, Böttger EC, Hombach M. Evaluation of the Bruker MALDI Biotyper for identification of Gram-positive rods—Development of a diagnostic algorithm for the clinical laboratory. J. of Clin. Microbiol. 2014;52:1089. doi: 10.1128/JCM.02399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 474 M100–S27. Wayne: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 18.Puppe W, et al. Validation of a multiplex reverse transcriptase PCR ELISA for the detection of 19 respiratory tract pathogens. Infection. 2013;41:77–91. doi: 10.1007/s15010-012-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puppe W, et al. Evaluation of a multiplex reverse transcriptase PCR ELISA for the detection of nine respiratory tract pathogens. J. Clin. Virol. 2004;30:165–174. doi: 10.1016/j.jcv.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Heymann PW, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisman D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012;18:946–954. doi: 10.1111/j.1469-0691.2012.03968.x. [DOI] [PubMed] [Google Scholar]
- 22.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002;122:183–191. doi: 10.1080/00016480252814207. [DOI] [PubMed] [Google Scholar]
- 23.Monto AS. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin. Ther. 2002;24:1987–1997. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khor C-S, Sam I-C, Hooi P-S, Quek K-F, Chan Y-F. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: A retrospective study of 27 years. BMC Pediatrics. 2012;12:32. doi: 10.1186/1471-2431-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matu M, Kikuvi G, Wanzala P, Karama M, Symekher S. Aetiology of acute respiratory infections in children under five years in Nakuru, Kenya. J. Microbiol. Exp. 2014;1:00021. [Google Scholar]
- 26.Munywoki PK, et al. Improved detection of respiratory viruses in pediatric outpatients with acute respiratory illness by real-time PCR using nasopharyngeal flocked swabs. J. Clin. Microbiol. 2011;49:3365–3367. doi: 10.1128/JCM.02231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ujunwa F, Ezeonu C. Risk factors for acute respiratory tract infections in under-five children in Enugu Southeast Nigeria. Ann. Med. Health Sci. Res. 2014;4:95–99. doi: 10.4103/2141-9248.126610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inglis TJ. Respiratory tract infections in the tropics. Primer Trop. Med. Austral. Coll. Trop. Med. Publ. 2005;215:740. [Google Scholar]
- 29.Albogami SS, Alotaibi MR, Alsahli SA, Masuadi E, Alshaalan M. Seasonal variations of respiratory viruses detected from children with respiratory tract infections in Riyadh, Saudi Arabia. J. Infect. Public Health. 2017;4:105. doi: 10.1016/j.jiph.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Shek LP-C, Lee B-W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003;4:105–111. doi: 10.1016/S1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 31.Mushi MF, et al. Predictors of disease complications and treatment outcome among patients with chronic suppurative otitis media attending a tertiary hospital, Mwanza Tanzania. BMC Ear Nose Throat Disord. 2016;16:1. doi: 10.1186/s12901-015-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndosa, A. et al. Factors associated with colonization of Streptococcus pneumoniae among under-fives attending clinic in Mwanza City, Tanzania. Tanzan. J. Health Res.17 (2015).
- 33.Turner RB. Rhinovirus: more than just a common cold virus. J. Infect. Dis. 2007;195:765–766. doi: 10.1086/511829. [DOI] [PubMed] [Google Scholar]
- 34.Winther B. Rhinovirus infections in the upper airway. Proc. Am. Thorac. Soc. 2011;8:79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 35.Van Kempen M, Bachert C. An update on the pathophysiology of rhinovirus upper respiratory tract infections. Nihon Bika Gakkai Kaishi (Jpn. J. Rhinol.) 1999;38:389–399. doi: 10.7248/jjrhi1982.38.4_389. [DOI] [PubMed] [Google Scholar]
- 36.Rocholl C, Gerber K, Daly J, Pavia AT, Byington CL. Adenoviral infections in children: The impact of rapid diagnosis. Pediatrics. 2004;113:e51–e56. doi: 10.1542/peds.113.1.e51. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Wei Q, Tan A, Wang L. Epidemiological analysis of respiratory viral etiology for influenza-like illness during 2010 in Zhuhai, China. Virol. J. 2013;10:143. doi: 10.1186/1743-422X-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessler J, et al. Incubation periods of acute respiratory viral infections: A systematic review. Lancet. Infect. Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reich NG, Lessler J, Cummings DA, Brookmeyer R. Estimating incubation period distributions with coarse data. Stat. Med. 2009;28:2769–2784. doi: 10.1002/sim.3659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript. Raw data is available upon request and the request should be made to the Director of research and Innovation, Catholic University of Health and allied Sciences.