Abstract
Marginal bone loss (MBL) is one of the leading causes of dental implant failure. This study aimed to investigate the feasibility of machine learning (ML) algorithms based on trabeculae microstructure parameters to predict the occurrence of severe MBL. Eighty-one patients (41 severe MBL cases and 40 normal controls) were involved in the current study. Four ML models, including support vector machine (SVM), artificial neural network (ANN), logistic regression (LR), and random forest (RF), were employed to predict severe MBL. The area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, and specificity were used to evaluate the performance of these models. At the early stage of functional loading, severe MBL cases showed a significant increase of structure model index and trabecular pattern factor in peri-implant alveolar bone. The SVM model exhibited the best outcome in predicting MBL (AUC = 0.967, sensitivity = 91.67%, specificity = 100.00%), followed by ANN (AUC = 0.928, sensitivity = 91.67%, specificity = 93.33%), LR (AUC = 0.906, sensitivity = 91.67%, specificity = 93.33%), RF (AUC = 0.842, sensitivity = 75.00%, specificity = 86.67%). Together, ML algorithms based on the morphological variation of trabecular bone can be used to predict severe MBL.
Subject terms: Computational biology and bioinformatics, Risk factors, Dental diseases, Oral diseases
Introduction
Peri-implant bone tissue is fundamental for the initial stability and long-term survival of dental implants, and marginal bone resorption around the implant could result in implant failure1,2. Currently, acceptable standards of implant success are defined as marginal bone loss (MBL) less than 1.5–2.0 mm after the first year of functional loading and subsequently less than 0.2 mm per year3–5. Although radiographic assessment of MBL has been considered as an authoritative criterion to evaluate implant success3,6, how to predict MBL remains unclear. Therefore, it is urgent to find an effective method to predict MBL and the survival rates of implants.
It has been widely acknowledged that MBL is a multicausal condition with different risk factors playing their roles simultaneously7,8. At the early post-loading stage, occlusal force transmits through the implant to the alveolar bone and promotes bone remolding. Plenty of morphology parameters can exhibit the morphological and mechanical properties of trabecular bone in this process9–11. Other factors that might impact bone remolding or MBL include the configuration of the dental implant9,12, cortical bone thickness10, periodontal disease susceptibility13, and smoking14. Some researchers have employed linear model to predict MBL based on bone structure parameters with sensitivity of 62.1% and specificity of 67.5%15. Hence, the establishment of a precise model to predict MBL is still a challenging exploration.
Machine learning (ML) can utilize statistical and optimization techniques to learn and detect in-depth relationships from complex and large data sets16. ML has already been applied in many aspects of the medical domain, including disease detection, diagnosis, and treatment17–19. Notably, prognosis prediction of dental implant which based on ML model has been applied in several clinical research20,21. These results prompt us to investigate whether ML models can predict MBL more accurately than conventional statistical methods.
This study aimed to find an effective way to predict the occurrence of MBL. We hypothesized that ML algorithms combined with clinical and CBCT data could predict MBL more accurately than conventional methods. Four ML models, including Support Vector Machine (SVM), Artificial Neural Network (ANN), Logistic Regression (LR), and Random Forest (RF), were employed to predict MBL. ML models showed superior performance compared to the single predictor in predicting MBL of mandibular implant.
Results
Trabecular microarchitecture changes in severe MBL cases
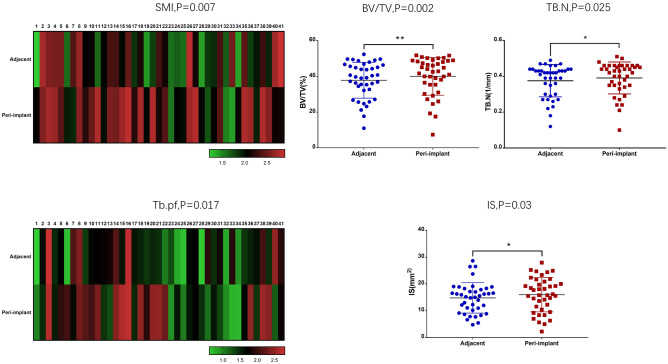
Eighty-one subjects were included in this study, and 41 were identified severe MBL of dental implants. Gender (P = 0.007), cortical bone thickness (P = 0.007), and smoking (P = 0.008) showed a significant difference between the severe MBL cases and the normal controls (Supplementary Table S1). Morphological variables of the peri-implant and the normal adjacent alveolar bone were also compared between the severe MBL cases and the normal controls. Structure model index (SMI) (P = 0.007) and trabecular pattern factor (Tb.Pf) (P = 0.017) significantly increased in peri-implant alveolar bone of severe MBL cases (Fig. 1). Percent bone volume (BV/TV) (P = 0.002), trabecular number (Tb.N) (P = 0.025), and intersection surface (I.S) (P = 0.030) significantly increased in peri-implant alveolar bone of normal controls (Fig. 1). Additionally, we analyzed preoperative trabecular microarchitecture parameters of all subjects at T0, and there was no difference between the two groups. The results of trabecular microarchitecture variables at T1 and T0 was exhibited in Supplementary Tables S2 and S3.
Figure 1.
Comparison of morphological parameters among the peri-implant and normal adjacent alveolar bone in cases and controls. In severe MBL cases, SMI and Tb.Pf showed the visible difference between the peri-implant and normal adjacent alveolar bone. BV/TV, Tb.N, and i.S exhibited a significant difference between the peri-implant and normal adjacent alveolar bone in normal controls.
Morphological variables and their role in predicting MBL
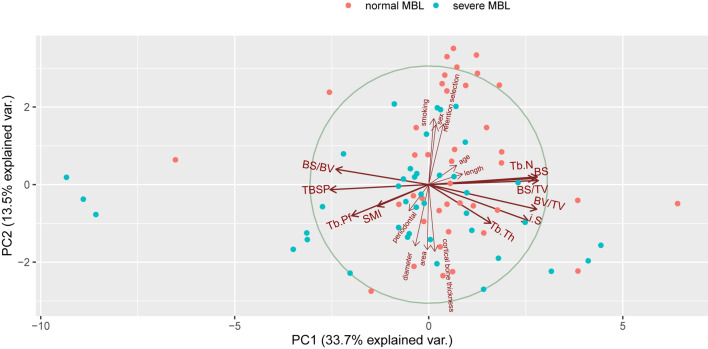
Inspired by the above findings, we analyzed all variables using principal component analysis and correlation covariance matrices. All results relevant to morphological variables were confirmed with a significant difference and reasonable collinearity. SMI, Tb.Pf, Tb.N, bone surface volume ratio (BS/BV), and BV/TV manifested a higher correlation with MBL, while other morphological variables could not bring a noteworthy contribution. Figure 2 reflected the ordination and contribution of all variables, along the first two “Multiple Factor Analysis” (MFA) components. The components explained 47.2% of the total variance in the data. Morphological parameters located in component 1 made significant contributions to the principal component, and all clinical parameters distributed in component 2.
Figure 2.
Plots of all variables in MFA. Closely clustered variables were positively correlated, while variables in opposing directions were negatively correlated. The length of the vector represented the importance of the variable in the MFA. Variables close to the midpoint of the circle plot had low contribution and weightage in the projection.
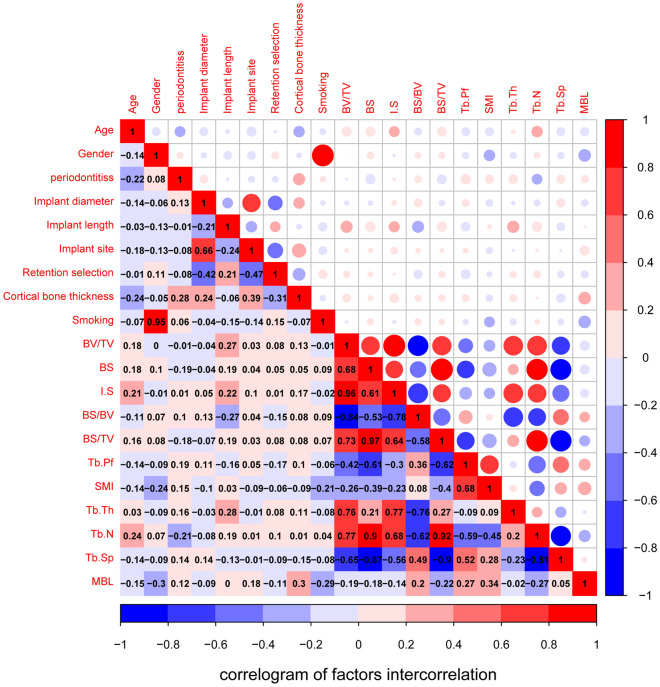
The logically obvious correlation between morphological parameters was shown in Fig. 3, and almost all correlation coefficients reached remarkably significant levels. Meanwhile, the linearity and credibility of trabecular bone microparameters were verified. SMI (P = 0.002) and Tb.Pf (P = 0.0165) exhibited a significantly high positive correlation with MBL. However, BV/TV and BS/BV manifested a negative correlation with MBL. Gender (P = 0.007), cortical bone thickness (P = 0.0072), and smoking (P = 0.0079) were powerfully correlated with MBL.
Figure 3.
The visualization of correlation and covariance matrices between all variables. Red and blue represented positive and negative correlations, respectively. Darker colors indicated a more significant correlation.
Performance of ML models
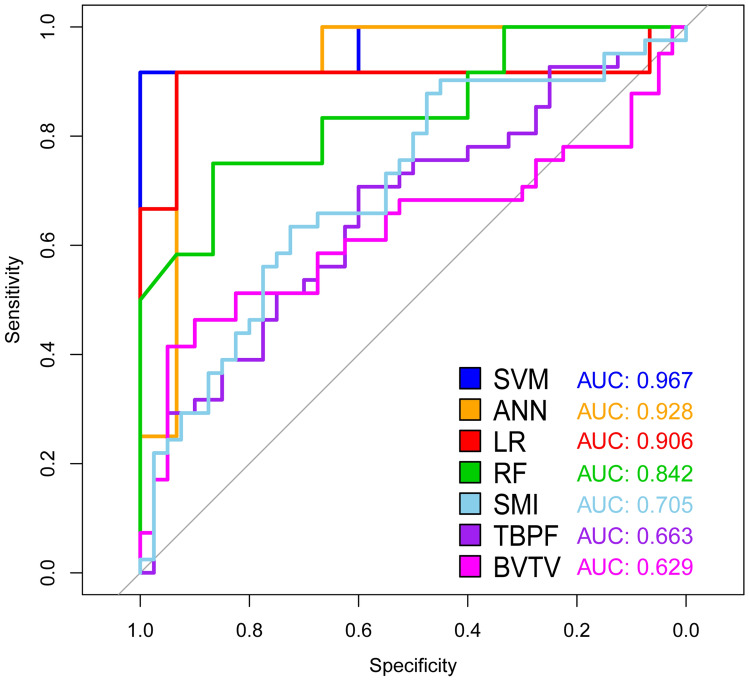
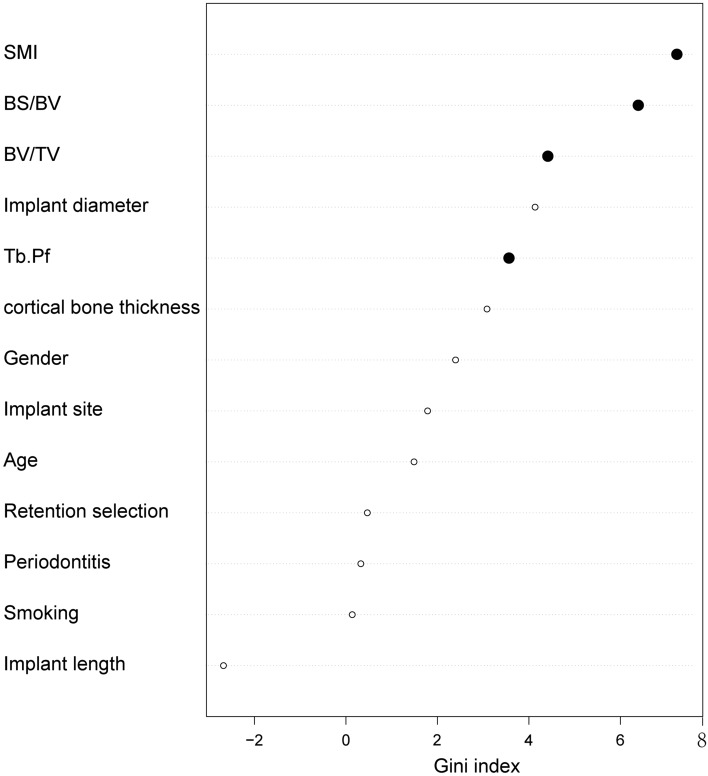
Based on the consequence of correlation analysis, we eliminated some meaningless variables to build ML models. Each model was superior to a single factor predictor. The SVM model performed the best (AUC = 0.967), followed by ANN (AUC = 0.928), LR (AUC = 0.906), RF (AUC = 0.842), SMI alone (AUC = 0.705), Tb.Pf alone (AUC = 0.663), and BV/TV alone (AUC = 0.629) (Fig. 4, Table 1). As the best model, the SVM’s sensitivity and specificity were 91.67% and 100.00% at its optimal cutoff, respectively. SVM also presented a perfect optimal criterion (0.917), satisfied positive (1.000) and negative (0.938) predictive values, the maximum positive diagnose-likelihood-ratio (Infinite), and the minimum negative diagnose-likelihood-ratio (0.083). Moreover, the SVM model had the smallest false positivity and false negativity. The cutoff value of SMI was 1.027, while the corresponding sensitivity and specificity were 65.85% and 67.50%, respectively. The cutoff value of Tb.Pf was 0.968, and the sensitivity and specificity were 63.41% and 62.50%, respectively. We listed the performance of each model in Table 2. Based on the RF model, we rearranged the variables according to the importance of predicting MBL measurement through the Gini index (Fig. 5).
Figure 4.
ROC & AUC of prediction models. The sensitivity and specificity of SVM, the best performing model, were 91.67% and 100.00%, respectively, at its optimal cutoff.
Table 1.
Statistical significance of the difference between the areas under ROC curves.
| ANN | LR | RF | SMI alone | Tb.Pf alone | BV/TV alone | |
|---|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | P-value | P-value | |
| Support vector machine (SVM) | 0.268 | 0.243 | 0.078 | < 0.001*** | < 0.001*** | < 0.001*** |
| Artificial neural network(ANN) | 0.410 | 0.206 | 0.004** | < 0.001*** | < 0.001*** | |
| Logistic regression (LR) | 0.199 | 0.023* | 0.009** | 0.003** | ||
| Random forest (RF) | 0.088 | 0.040* | 0.018* | |||
| SMI alone | 0.169 | 0.164 | ||||
| Tb.Pf alone | 0.345 |
DeLong’s test and Bootstrap test were used.
*P < 0.05, **P < 0.01, ***P < 0.001.
Table 2.
Performance of each model at optimal cutoff point.
| Model | Sensitivity (%) | Specificity (%) | Optimal cutoff of probability | Positive predictive value | Negative predictive value | DLR positive | DLR negative | False positive | False negative | Optimal criterion |
|---|---|---|---|---|---|---|---|---|---|---|
| Support vector machine (SVM) | 91.67 | 100.00 | 0.547 | 1.000 | 0.938 | Infinite | 0.083 | 0 | 1 | 0.917 |
| Artificial neural network(ANN) | 91.67 | 93.33 | 0.998 | 0.917 | 0.933 | 13.750 | 0.089 | 1 | 1 | 0.917 |
| Logistic regression (LR) | 91.67 | 93.33 | 0.824 | 0.917 | 0.933 | 13.750 | 0.089 | 1 | 1 | 0.917 |
| Random forest (RF) | 75.00 | 86.67 | 0.560 | 0.818 | 0.813 | 5.625 | 0.288 | 2 | 3 | 0.750 |
| SMI alone | 65.90 | 67.50 | 1.027 | 0.675 | 0.659 | 2.026 | 0.506 | 13 | 14 | 0.659 |
| Tb.Pf alone | 63.40 | 62.50 | 0.968 | 0.634 | 0.625 | 1.691 | 0.585 | 15 | 15 | 0.625 |
| BV/TV alone | 39.00 | 45.00 | 1.049 | 0.421 | 0.419 | 0.710 | 1.355 | 22 | 25 | 0.390 |
The optimal cutoff was considered as the point maximizing the sum of sensitivity and specificity.
Figure 5.
Variable importance plot of random forest model. The plot indicated the relative importance of the variables in the random forest model. Trabecular microarchitecture variables were marked as solid black points.
Discussion
Due to the important role of MBL in dental implant failure, MBL have become an essential clinical examination in postoperative follow-up. The purpose of this study was to verify that ML algorithms combined with early-stage trabecular bone variables could predict MBL more effectively than conventional methods. Our results showed a great performance of ML methods for predicting MBL, which can be considered as the feasible early warning for severe MBL. It was noted that the other factors resulting in MBL should be taken into account in future studies.
Previous researches about MBL mainly concentrated on the cause and treatment6,9,12,13,22,23, but rarely on the prediction of MBL15. Factors such as trabecular bone microstructure parameters possibly affecting MBL during bone remolding have been well elucidated15, but the role of trabecular bone in this progression remains unclear. To our knowledge, this is the first study to establish and validate ML models based on trabecular microstructure parameters to predict the occurrence of MBL of the submerged dental implant in mandible.
It has been widely acknowledged that various factors, including cortical bone thickness, smoking, periodontitis, SMI, Tb.Pf, and BV/TV, function as a complex to cause MBL8. Previous studies have also demonstrated that the proportion of cancellous bone10, crown-to-implant ratio24, bone texture, and cortical width15, are risk factors of MBL. Therfore, single predictive factor cannot accurately predict MBL because MBL is a multifactorial outcome. One recent study attempted to employ Cox regression and mixed linear modeling to predict the occurrence of MBL, but they aimed to assess interventions and their consequences with regard to further bone loss at sites diagnosed with peri-implant inflammation25. Another study incorporated several radiographic features of cortical and cancellous bone texture, cortical width, and patient smoking habits to build a statistical model to predict MBL with a sensitivity of 62.1% and specificity of 67.5%15. Compared to conventional statistical methods, the current study verified that ML algorithms predicted MBL more accurately. Of note, the SVM model performed best with a sensitivity of 91.67% and specificity of 100.00%, which was significantly better than that of SMI, Tb.Pf or BV/TV alone.
To demonstrate the differences and correlations of morphological variables between the controls and severe MBL cases, we also analyzed morphological variables of trabecular bone in patients with MBL during bone remolding. At the early stage of functional loading, CBCT analysis exhibited a worse outcome of SMI and Tb.Pf in peri-implant alveolar bone of severe MBL cases. These findings revealed that severe MBL cases demonstrated the premonitory morphological variation in trabecular microarchitecture at the early stage. Consistent with our results, a previous study reported that the preservation and improvement of trabecular microarchitecture always brought about a better therapeutic benefit for osteoporosis at multiple skeletal sites26. SMI and Tb.Pf were the best determinants of the MBL level, which they reflected the structure quality of the trabecular bone in the peri-implant of severe MBL cases. However, in normal controls, Tb.N and BV/TV were superior to other morphological variables11.
Although the current study has demonstrated that ML could predict the occurrence of MBL more effectively than conventional methods, some limitations need to be acknowledged. Due to the proximity of the measurement point to the implant root, implant artifacts remain unavoidable. The variables in the current study lacked results of the periodontal examination, such as probing depth. Additionally, this study was limited to patients who received implant treatment in mandible. Further studies on larger sample sizes using more relative variables (e.g. periodontal or microbiological) might be better for the ML performance. At last, a mean 20.95 ± 2.67 months of follow-up after functional loading was also limited to predict MBL. Hence, we entitled this study as a preliminary one.
In conclusion, the current study verified that the severity of early bone resorption was closely related to trabecular microarchitecture during the early stage of functional loading. Change of trabecular microarchitecture can provide an early warning for severe MBL. ML models SVM, ANN, LR, and RF indicate superior performance compared to the single predictor in predicting MBL of mandibular implant.
Methods
Study design
To address the research purpose, we designed and implemented a cross-sectional study. All subjects receiving implant treatment between January 2016 and March 2019 in the Department of Oral and Maxillofacial Surgery of Affiliated Stomatological Hospital of Nanjing Medical University were screened. The inclusion criteria of subjects were as follows: (1) above 18 years of age with good health; (2) having received fixed prosthesis of the mandibular implant with at least 1 year of loading; (3) available integrated clinical data; (4) valid cone-beam computed tomography (CBCT) examination conducted at the following three time points: (T0) preoperative bone assessment, (T1) at the follow-up between 3 and 6 months after loading, and (T2) at the follow-up above one year of post-loading. The exclusion criteria of subjects were as follows: (1) patients diagnosed with clinical or absolute failure based on the current guideline4; (2) patients with an incomplete periodontal follow-up examination and treatment record. (3) Patients receiving bone augmentation. Patients with smoking cessation for more than three months before surgical implant placement were taken as non-smokers.
This study was approved by the ethics committee of the Affiliated Stomatological Hospital of Nanjing Medical University (Approval number: PJ2019-038-001, Approval data: March 15, 2019) in accordance with the Helsinki Declaration II. Written informed consents were obtained from all participants.
Measurement of MBL
All subjects were examined by the CBCT (NewTom 5G cone-beam computed tomography device, QR s.r.l, Verona, Italy) at a reconstruct voxel size of 150 μm at the three time points. Scanning voltage and current were 110 kV and 10 mA, while exposure and scanning times were 3.6 s and 18 s. We reconstructed the original radiographs using the center of the implant in the sagittal, coronal, and transverse plane. The implant length and diameter were used to test the accuracy of reconstructed images. MBL measurement was performed as follows: (1) the horizontal interface between implant and abutment was validated as the reference loci; (2) vertical distances from the loci to the most coronal level of bone to implant contact at the mesial and distal sites were measured at the preceding time points27,28; (3) analysis of radiographs was conducted by two investigators who did not participate in this study. We obtained the maximum MBL of the implant at T1 and T2 as the corresponding MBL level (Supplementary Fig. S1). According to the T2 MBL level, we divided all subjects into two groups:
Normal controls less than 2 mm MBL in the first year after fixed prosthesis, then less than 0.2 mm MBL per year
Severe MBL cases MBL level exceeding normal controls
Measurement of peri-implant bone morphological parameters
We imported the T1 radiographs to CT Analyzer (CTAn, SkyScan, Antwerpen, Belgium). The threshold value of binary selection was determined by completely distinguish cortical bone and trabecular bone (Supplementary Fig. S2a). We confirmed the region of interest (ROI) by the diameter of each implant. We selected five sequential ROI layers adjoining the implant root as the volume of interest (VOI) of peri-implant alveolar bone (Supplementary Fig. S2b). Another five sequential ROI layers away from the implant were chosen as VOI of the normal adjacent alveolar bone (Supplementary Fig. S2b). The trabecular bone morphological parameters, such as SMI, Tb.Pf, BV/TV, i.S, and Tb.N were extracted using three-dimensional analysis of each VOI. SMI, Tb.Pf, and i.S represent the shape and quality of trabecular bone. BV/TV and Tb.N usually mean quantity of trabecular bone. Finally, we calculated morphological variables by the ratio of peri-implant to normal adjacent alveolar bone.
PCA analysis
Including clinical and morphological parameters, all variables were utilized for ordination analysis and contribution degree evaluation of principle component using the “Multiple Factor Analysis” (MFA) function in the R package “FactoMineR”. As a dimensionality reduction method, MFA reduces the complexity of multivariate data and allows visual interpretation of significant patterns. It is suited to data that contains both continuous and categorical variables. MFA also allows grouping of variables where each group is normalized individually to balance their influence. An MFA correlation circle plot depicts the continuous variables, and the factors plot depicts the categorical variables.
Visualization of correlation and covariance matrices
Correlation and covariance matrices can visualize the patterns and relationships between the variables. We had twenty original variables, including object variable MBL. The visualization of matrices re-ordered the variables in a correlation matrix and displayed the value by sign and magnitude. All iconic encodings in the matrix displayed the pattern and significance level of correlations between variables. The R package “corrgram” and “Hmisc” were employed in this study.
ML algorithms
Based on the R Programming Language (R Core Team, Vienna, Austria), four ML models, including SVM, ANN, LR model and RF, were constructed. The dataset was randomly split into two mutually exclusive sets, training (70%) and testing (30%), a method called holdout method29. LR model established with variable choice through backward elimination was implemented to assess risk factors and predict the diagnosis of diseases. The R package “e1071” was applied in the SVM model to accomplish regression and classification missions by constructing hyperplanes in a multidimensional space. SVM model could manage multiple continuous and categorical variables according to the decision plane. ANN model, a computerized encoding of artificial humanoid neuronal networks, included the input layer, hidden layers, and output layer. Neurons connected the adjacent layers as a medium for the delivery-feedback-correction-delivery cycle. This recursive process adjusted the weights for fewer errors and better accuracy. ANN model was implemented by R package “neural net”. RF model, a ML algorithm based on the decision tree, could combine the output of a single decision tree to improve the overall performance. RF model was superior to a single decision tree in eliminating overfitting. RF model also could display the relative importance of the variables by the Gini index. We utilized the R package “randomForest” in the establishment of the RF model.
Statistical analysis
The chi-square test and Fisher’s exact test were applied to compare the variables of severe MBL cases and normal controls. We utilized the Cochran-Armitage trend test for categorical variables, while continuous variables were assessed by Student’s t-test and the Mann–Whitney rank-sum test. R programming language was used for all statistical analyses, while P < 0.05 was regarded as statistically significant.
Supplementary information
Acknowledgments
We would like to thank our colleagues Shen Xin and Lin Jialing from Jiangsu Key Laboratory of Oral Diseases, Nanjing Medical University, for their support of radiographs analysis in this project.
Author contributions
The study was designed by H.Z. and H.J. The data was collected by H.Z. and analyzed by J.S. The ML models were built by H.Z., J.S. and X.C. The paper was written by H.Z. and revised by J.S., P.Z. and H.J. They all made great contributions to this study and should be listed as authors. All authors read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China Grant (81771092), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018-87), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1149).
Data availability
All CBCT files of patients and control subjects were stored in a non-public medical record database. CBCT data of the samples will not be shared.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hengguo Zhang and Jie Shan.
Supplementary information
is available for this paper at 10.1038/s41598-020-75563-y.
References
- 1.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998;106:527–551. doi: 10.1046/j.0909-8836..t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 2.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur. J. Oral Sci. 1998;106:721–764. doi: 10.1046/j.0909-8836..t01-6-.x. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants—A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 4.Misch CE, et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5–15. doi: 10.1097/ID.0b013e3181676059. [DOI] [PubMed] [Google Scholar]
- 5.Papaspyridakos P, Chen CJ, Singh M, Weber HP, Gallucci GO. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012;91:242–248. doi: 10.1177/0022034511431252. [DOI] [PubMed] [Google Scholar]
- 6.Duan XB, et al. Marginal bone loss around non-submerged implants is associated with salivary microbiome during bone healing. Int. J. Oral Sci. 2017;9:95–103. doi: 10.1038/ijos.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015;42(Suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 8.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- 9.Sener-Yamaner ID, Yamaner G, Sertgoz A, Canakci CF, Ozcan M. Marginal bone loss around early-loaded SLA and SLActive implants: Radiological follow-up evaluation up to 6.5 years. Implant Dent. 2017;26:592–599. doi: 10.1097/ID.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 10.Simons WF, De Smit M, Duyck J, Coucke W, Quirynen M. The proportion of cancellous bone as predictive factor for early marginal bone loss around implants in the posterior part of the mandible. Clin. Oral Implants Res. 2015;26:1051–1059. doi: 10.1111/clr.12398. [DOI] [PubMed] [Google Scholar]
- 11.Maquer G, Musy SN, Wandel J, Gross T, Zysset PK. Bone volume fraction and fabric anisotropy are better determinants of trabecular bone stiffness than other morphological variables. J. Bone Miner. Res. 2015;30:1000–1008. doi: 10.1002/jbmr.2437. [DOI] [PubMed] [Google Scholar]
- 12.Zechner W, et al. Radiologic follow-up of peri-implant bone loss around machine-surfaced and rough-surfaced interforaminal implants in the mandible functionally loaded for 3 to 7 years. Int. J. Oral Maxillofac. Implants. 2004;19:216–221. [PubMed] [Google Scholar]
- 13.Corcuera-Flores JR, et al. Relationship between osteoporosis and marginal bone loss in osseointegrated implants: A 2-year retrospective study. J. Periodontol. 2016;87:14–20. doi: 10.1902/jop.2015.150229. [DOI] [PubMed] [Google Scholar]
- 14.Levin L, Hertzberg R, Har-Nes S, Schwartz-Arad D. Long-term marginal bone loss around single dental implants affected by current and past smoking habits. Implant Dent. 2008;17:422–429. doi: 10.1097/ID.0b013e31818c4a24. [DOI] [PubMed] [Google Scholar]
- 15.Merheb J, et al. Prediction of implant loss and marginal bone loss by analysis of dental panoramic radiographs. Int. J. Oral Maxillofac. Implants. 2015;30:372–377. doi: 10.11607/jomi.3604. [DOI] [PubMed] [Google Scholar]
- 16.Cruz JA, Wishart DS. Applications of machine learning in cancer prediction and prognosis. Cancer Inf. 2007;2:59–77. [PMC free article] [PubMed] [Google Scholar]
- 17.Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015;13:8–17. doi: 10.1016/j.csbj.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DW, Kim H, Nam W, Kim HJ, Cha IH. Machine learning to predict the occurrence of bisphosphonate-related osteonecrosis of the jaw associated with dental extraction: A preliminary report. Bone. 2018;116:207–214. doi: 10.1016/j.bone.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 19.McKinney SM, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577:89–94. doi: 10.1038/s41586-019-1799-6. [DOI] [PubMed] [Google Scholar]
- 20.Papantonopoulos G, Gogos C, Housos E, Bountis T, Loos BG. Prediction of individual implant bone levels and the existence of implant "phenotypes". Clin. Oral Implants Res. 2017;28:823–832. doi: 10.1111/clr.12887. [DOI] [PubMed] [Google Scholar]
- 21.Ha SR, et al. A pilot study using machine learning methods about factors influencing prognosis of dental implants. J. Adv. Prosthodont. 2018;10:395–400. doi: 10.4047/jap.2018.10.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo-Guirado JL, et al. Marginal bone loss evaluation around immediate non-occlusal microthreaded implants placed in fresh extraction sockets in the maxilla: A 3-year study. Clin. Oral Implants Res. 2015;26:761–767. doi: 10.1111/clr.12336. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura E, Stegaroiu R, Nomura S, Miyakawa O. Biomechanical aspects of marginal bone resorption around osseointegrated implants: Considerations based on a three-dimensional finite element analysis. Clin. Oral Implants Res. 2004;15:401–412. doi: 10.1111/j.1600-0501.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- 24.Di Fiore A, et al. Influence of crown-to-implant ratio on long-term marginal bone loss around short implants. Int. J. Oral Maxillofac. Implants. 2019;34:992–998. doi: 10.11607/jomi.7161. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson K, et al. Interventions for peri-implantitis and their effects on further bone loss: A retrospective analysis of a registry-based cohort. J. Clin. Periodontol. 2019;46:872–879. doi: 10.1111/jcpe.13129. [DOI] [PubMed] [Google Scholar]
- 26.Chesnut CH, 3rd, et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: Results from the QUEST study. J. Bone Miner. Res. 2005;20:1548–1561. doi: 10.1359/JBMR.050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XX, Shi JY, Gu YX, Lai HC. Long-term outcomes of early loading of straumann implant-supported fixed segmented bridgeworks in edentulous maxillae: A 10-year prospective study. Clin. Implant Dent. Relat. Res. 2016;18:1227–1237. doi: 10.1111/cid.12420. [DOI] [PubMed] [Google Scholar]
- 28.Kumar VV, Sagheb K, Kammerer PW, Al-Nawas B, Wagner W. Retrospective clinical study of marginal bone level changes with two different screw-implant types: Comparison between tissue level (TE) and bone level (BL) implant. J. Maxillofac. Oral Surg. 2014;13:259–266. doi: 10.1007/s12663-013-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J. Breast Cancer. 2012;15:230–238. doi: 10.4048/jbc.2012.15.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All CBCT files of patients and control subjects were stored in a non-public medical record database. CBCT data of the samples will not be shared.