Summary
Introduction
Ichthyoses include a heterogeneous group of skin diseases often characterized by persistent scaling and hyperkeratosis with variable erythema, pruritus, and sweating impairment. The aim of our review was to assess the quality of life in patients with ichthyosis.
Methods
In July 2018 we performed a systematic search in the electronic database PubMed (MEDLINE). The MESH term “quality of life” was combined, through the Boolean operator AND with the key word “ichthyosis”. We considered eligible for the systematic review studies written in English.
Results
The literature search yielded 63 publications, but 7 studies were included in the review. Studies were published in 2003-2014 and involved a minimum of 10 and a maximum of 235 patients. Authors used 5 types of tools: Dermatology Life Quality Index (DLQI), Dermatitis Family Impact Questionnaire (DFI), Nottingham Health Profile (NHP) questionnaire, Short Form Questionnaire 36 and 12 (SF-36, SF-12). Many patients reported worse scores than general population. Patients referred physical problems related to pain (which negatively influenced the mobility).
Conclusions
Ichthyosis considerably impaired the QoL, especially for paediatric patients. Further studies and efforts should be done to manage and treat the pain.
Keywords: Quality of life, Ichtyosis, Pain, Mental health
Introduction
The ichthyoses include a heterogeneous group of skin diseases linked by the common finding of abnormal barrier function, which leads to increased trans-epidermal water loss and compensatory hyperproliferation [1]. Ichthyosis vulgaris is caused by loss-of-function mutations in the filaggrin gene (FLG) [2]. Filaggrin is very important in the terminal differentiation of the skin and the formation of cornified envelope in the stratum corneum [3]. FLG mutations are observed in approximately 7.7% of Europeans and 3.0% of Asians, but appear to be infrequent in darker-skinned populations [2]. The inherited ichthyoses are classified as syndromic or non-syndromic, depending on the presence or absence of extracutaneous findings [4]. They are characterized by persistent scaling and hyperkeratosis with variable erythema, pruritus, and sweating impairment [5].
The new classification identifies 36 types of ichthyosis, which are subdivided according to their frequency, pattern of inheritance and extracutaneous involvement [6].
Among diseases that cause ichthyosis as one of the symptoms, there are some diseases that induce abnormalities in organs other than the skin. Of these, diseases with characteristic signs are regarded as syndromes. Although these syndromes are very rare, Netherton syndrome, Sjögren-Larsson syndrome, Conradi-Hünermann-Happle syndrome, Dorfman-Chanarin syndrome, ichthyosis follicularis, atrichia and photophobia (IFAP) syndrome, and Refsum syndrome have been described in texts as representative ones [7].
Quality of life may be adversely affected by the social and psychologic consequences of this disease [5].
Quality of life (QoL) is a concept used to indicate the general wellness of persons or societies, including wealth and employment elements, environment, physical and mental health, education, recreation and belonging to a social group [8]. For some authors it is a concept that could be compared to the paradigm of “happiness” [9].
Several authors studied the impact of ichthyosis on quality of life (QoL) [10, 11], and showed that congenital ichthyosis appears to affect several aspects of life negatively, and is responsible of lower scores in the used questionnaire. In literature there is not a review on this topic;the aim of our study was therefore to assess the quality of life in patients with ichtyosis, examining and summarizing all the peer reviewed literature published in PubMed on this field.
Methods
In July 2018 we performed a systematic search for original peer-reviewed papers in the electronic database PubMed (MEDLINE). The MESH term “quality of life” was combined, through the Boolean operator AND with the keyword “ichthyosis”. We searched for studies published without temporal limits, reporting information about the quality of life of patients suffering from ichthyosis.
We considered eligible for the review original articles that reported clear data on: i) number of involved patients; ii) tool (or tools) used to evaluate the quality of life; iii)setting. We considered eligible for the systematic review studies written in English.
Studies were selected in a 2-stage process. Titles and abstracts from electronic searches were first scrutinised. Then, full manuscripts and their citations list were analysed to retrieve missing articles and to select the eligible manuscripts according to the inclusion criteria.
The literature search yielded 63 publications. The titles and abstracts of these manuscripts were screened, resulting in 7 studies considered potentially eligible to be included in the review [10-16]: 17 were review, 2 studies were excluded because they were written in German, 1 study was excluded because written in Polish, 2 studies were excluded because they were written in French, and 34 because they were not in line with the aim of the study (Fig. 1).
Fig. 1.
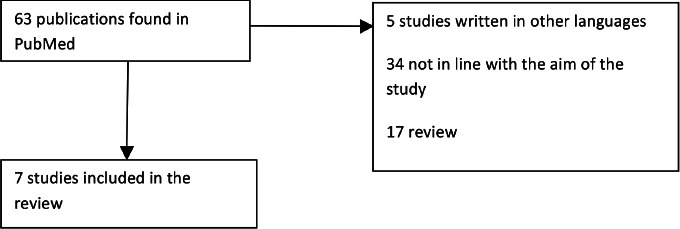
Flow diagram for identifying studies included in our review.
Studies were published in 2003-2014: 3 were settled in Sweden, 1 in USA, 3 in France. They involved a minimum of 10 and a maximum of 235 patients. One study was focused only on a paediatric population.
Authors used 5 types of tools: Dermatology Life Quality Index (DLQI), Dermatitis Family Impact
Questionnaire (DFI), Nottingham Health Profile (NHP) questionnaire, Short Form Questionnaire 36 and 12 (SF-36, SF-12).
The Dermatology Life Quality Index or DLQI, developed in 1994, was the first dermatology-specific Quality of Life instrument. It is a simple 10-question validated questionnaire that has been used in over 40 different skin conditions in over 80 countries and is available in over 90 languages. Its use has been described in over 1000 publications including many multinational studies [17].
The questionnaire DFI had 10 questions scoring 0-3, giving a maximum score of 30: the higher the score, the greater the impairment of QoL [18].
NHP I measures HRQoL and contains 39 questions in six areas (emotions, sleep, energy, pain, mobility and social isolation) [19].
NHP II contains seven yes/no questions about health-related problems in the following areas: paid employment, housework, social life, home life, sex life, hobbies and holidays [10].
The Short Form 36 (SF-36), is a validated questionnaire, which detects the health-related quality of life. It was developed in the ‘80s in the United States as a generic multi-dimensional questionnaire, with 36 questions that create 8 different scales: PA-physical activity, PR-role limitations due to physical health, ER -limitations due to emotional state, physical pain-BP, GH-perception of general health, vitality-VT, SA-social activities, MH mental health [20-22]. The shortened version is SF 12.
In addition, it should be mentioned that in 2013 Dreyfus et al. [14] created an innovative tool: the IQOL 32 specifically designed for ichthyosis. The questions are specifically dedicated to ichthyosis and explore all disease particularities such as skin pain/discomfort, ear- and eye-related problems, heat intolerance, skin odor, scalp involvement, restrictions related to the disease (dressings, sports, leisure), expenses, psychological aspects, and consequences of the treatment.
Results
The main results of the review are reported in Table I.
Tab. I.
Main characteristics of the studies included in the systematic review.
| Author, year | Setting | Sample size | Study design | Tool | Results |
|---|---|---|---|---|---|
| Ganemo, 2003 | Sweden | 10 (56-80 years old) | Cross sectional | Nottingham Health Profile (NHP) questionnaire | Individual overall NHP I scores varied from 0 to 427. Total scores for NHP II varied from 0% to 57% affirmative answers. |
| Ganemo, 2004 | Sweden | 122 (17-78 years old) | Cross sectional | DLQI and SF-36 (sent by email) | Median DLQI 5.0; SF-36 scores lower in 6 dimensions compared to normal population. |
| Ganemo, 2010 | Sweden | 15 (5-16 years old) | Cross sectional | Children’s DLQI and DFI | The median score was 9.0 (range 2-19) for CDLQI; 9.0 for DFI (range 3-21) |
| Kamalpour, 2011 | U.S.A. | 235 (mean age 27.3 years old) | Cross sectional | DLQI (online) | Mean DLQI scores were significantly higher for adults (>18) (9.5 SD 6.6) than for children (7.7 SD 5.7) (p = 0.04). Mean DLQI scores were significantly higher for women (9.7 SD 6.6) than for men (7.4 SD 5.6, p = 0.01), regardless of age. Alopecia severity had a much weaker but still significant correlation with QOL (r = 0.19, p < 0.01). |
| Mazereeuw-Hautier, 2012 | France | 25 (21-67 years old) | Qualitative | Qualitative approach using focus groups | Not reported numerical results |
| Dreyfus, 2013 | France | 59 (17-70 years old) | n.r. | DLQI and SF-12 (sent by email) | Mean IQOL-32 score: 74.5 SD 21.1 Mean SF 12 score: 46.6 SD 11.1 (physical component) and 36.6 SD 10.9 (health component) |
| Dreyfus, 2014 | France | 158 (16-88 years old) | Multicentre Prospective Study | DLQI | Mean score of DLQI was 8.3 ± 6.5 (0-27). |
Ganemo in his three studies reported that patients’ skin disease affected them negatively to varying degrees during their entire lives, and that the most problematic period was childhood.
Kamalpour reported that mean DLQI scores were significantly higher for adults than for children; the mean DLQI scores were significantly higher for women than for men, regardless of age. Alopecia severity had a much weaker but still significant correlation with QOL.
Mazereeuw-Hautier observed that the acceptance of the disease and support from families or friends were considered as positively influencing patients’ QOL. Difficulty in relationship with others was often reported by patients.
Dreyfus in his two studies showed that the clinical severity had strongest correlations with discomfort, pain, and social aspects. Females, patients who lived alone, patients suffering from cutaneous pain had the highest DLQI scores.
Discussion
The application of quality of life studies in dermatology is recent, but of great interest as skin diseases have a deep impact on psychological status and daily activities of patients [23].
Mazereeuw-Hautier et al. in 2012 [15] used an interesting approach. In their study they used in fact a qualitative approach, which let us better understand the impact of the disease on quality of life, without “measuring” it with a scale/score. Several patients referred physical problems related to pain (which negatively influenced the mobility). Daily cream application was considered to be time consuming and nasty and this practiced could negatively influence the possibility to travel. Although the patients didn’t consider themselves to be handicapped (even if they realized they have limitations), the relationship with others represented an important point negatively influenced by the disease. The problem of stigmatization about certain diseases has been already described in literature: Norman Sartorius [24] affirmed that there are a number of diseases that are stigmatized such as mental disorders, AIDS, venereal diseases, leprosy, and certain skin diseases. People living with these diseases are discriminated in several settings and tend not to receive enough social support with consequent difficulties in organizing their life.
The element that mostly affected the quality of life in all the studies that we examined was pain. Pain is one of the most common medical complaints [25] and a another study [26] focused on other diseases showed that pain affects most domains of QOL, primarily physical and emotional functioning. The effect depends on the extent, duration, acuteness, intensity, affectivity, and meaning of the pain as well as on the underlying disease and the individual’s characteristics. Many chronic pain sufferers reported that pain had deleterious effects on their mental health, employment status, sleep, and personal relationships [25].
Very interesting are the contrasting results deriving from the paediatric population. The study conducted by Ganemo et al in 2003 affirmed that the most problematic period for patients was childhood, and this evidence was confirmed by the study conducted in 2010 on children with congenital ichthyosis that demonstrated that ichthyosis affected both the children and the families and caused greater impairment of QoL than other skin diseases in children. However, Kalmapour et al. in 2011 showed that children tend to have a better quality of life than adults. The impact of skin diseases on children was investigated also by other authors: Catucci et al in 2016 [27] published a study on vitiligo that showed that the median DLQI scores in children and especially in adolescents was very high (almost 11) and that the disease influenced several aspects of children’s life.
The person with ichtyosis had been in hospital about 25 times during childhood and early adulthood, mainly for skin infections. All respondents described their skin as very problematic during childhood, with thick scaling, fissures, wounds and pain. The skin symptoms had improved in adulthood when medical treatments such as oral retinoids and new cream formulations containing salicylic acid, urea and alpha-hydroxy acids had became available [10].
In conclusion, this review highlighted that ichthyosis considerably impaired the QoL: the disease was responsible of negative effects on patients lives, and several studies showed that children had worse scores compared to adults. Several further factors (gender, acceptance of the disease, support from families or friends) could influence patients’ QOL. Pain is one of the most important factors, so further studies and efforts should be done to manage and treat the pain deeply involved in the reduction of quality of life of these patients.
Figures and tables
Acknowledgements
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflicts of interest statement
The authors declare no conflict of interest.
Authors’ contributions
GT and GL had the idea of the article, collected data and wrote the article.
References
- [1].Marukian NV, Choate KA. Recent advances in understanding ichthyosis pathogenesis. F1000Res 2016;5 https://doi.org/10.12688/f1000research.8584.1 10.12688/f1000research.8584.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: the filaggrin mutation disease. Br J Dermatol 2013;168:1155-66. https://doi.org/10.1111/bjd.12219 10.1111/bjd.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De D, Handa S. Filaggrin mutations and the skin. Indian J Dermatol Venereol Leprol, 2012;78:545-51. https://doi.org/10.4103/0378-6323.100518 10.4103/0378-6323.100518 [DOI] [PubMed] [Google Scholar]
- [4].Oji V, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E. Revised nomenclature and classification of inherited ichthyosis: results of the first ichthyosis consensus conference in Soreze 2009 J Am Acad Dermatol 2010;63:607-41. https://doi.org/10.1016/j.jaad.2009.11.020 10.1016/j.jaad.2009.11.020 [DOI] [PubMed] [Google Scholar]
- [5].Hernandez-Martin A. A systematic review of clinical trials of treatments for the congenital ichthyoses, excluding ichthyosis vulgaris. J Am Acad Dermatol 2013;69:544-549e8. https://doi.org/10.1016/j.jaad.2013.05.017 10.1016/j.jaad.2013.05.017 [DOI] [PubMed] [Google Scholar]
- [6].Vega Almendra N, Aranibar Duran L. Hereditary ichthyosis: A diagnostic and therapeutic challenge. Rev Chil Pediatr 2016;87:213-23. http://dx.doi.org/10.1016/j.rchipe.2015.07.025 10.1016/j.rchipe.2015.07.025 [DOI] [PubMed] [Google Scholar]
- [7].Yoneda K. Inherited ichthyosis: Syndromic forms. J Dermatol 2016; 43(3): 252-63. https://doi.org/10.1111/1346-8138.13284 10.1111/1346-8138.13284 [DOI] [PubMed] [Google Scholar]
- [8].Gregory D, Jr, Pratt G, Watts M, Whatmore S. The Quality of Life. the dictionary of human geography. Oxford: John Wiley & Sons; 2009. [Google Scholar]
- [9].Veenhoven R. Quality of life and happiness: not quite the same. Salute e Qualità della Vita 2001;67-95. [Google Scholar]
- [10].Ganemo A. Quality of life in adults with congenital ichthyosis. J Adv Nurs 2003;44:412-9. https://doi.org/10.1046/j.0309-2402.2003.02820.x 10.1046/j.0309-2402.2003.02820.x [DOI] [PubMed] [Google Scholar]
- [11].Ganemo A. Health-related quality of life among patients with ichthyosis. Eur J Dermatol 2004;14:61-6. [PubMed] [Google Scholar]
- [12].Ganemo A. Quality of life in Swedish children with congenital ichthyosis. Dermatol Reports 2010;2:e7 https://doi.org/10.4081/dr.2010.e7 10.4081/dr.2010.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kamalpour L. Resource utilization and quality of life associated with congenital ichthyoses. Pediatr Dermatol 2011;28:512-8. https://doi.org/10.1111/j.1525-1470.2011.01432.x 10.1111/j.1525-1470.2011.01432.x [DOI] [PubMed] [Google Scholar]
- [14].Dreyfus I. IQoL-32: a new ichthyosis-specific measure of quality of life. J Am Acad Dermatol 2013;69: 82-7. https://doi.org/10.1016/j.jaad.2013.01.022 10.1016/j.jaad.2013.01.022 [DOI] [PubMed] [Google Scholar]
- [15].Mazereeuw-Hautier J. Factors influencing quality of life in patients with inherited ichthyosis: a qualitative study in adults using focus groups. Br J Dermatol 2012;166:646-8. https://doi.org/10.1111/j.1365-2133.2011.10701.x 10.1111/j.1365-2133.2011.10701.x [DOI] [PubMed] [Google Scholar]
- [16].Dreyfus I. Factors associated with impaired quality of life in adult patients suffering from ichthyosis. Acta Derm Venereol 2014;94:344-6. https://doi.org/10.2340/00015555-1710 10.2340/00015555-1710 [DOI] [PubMed] [Google Scholar]
- [17].Dermatology CU-Do. Dermatology Quality of Life Index (DLQI) 20 July 2018; Available from: http://sites.cardiff.ac.uk/dermatology/quality-of-life/dermatology-quality-of-life-index-dlqi/
- [18].Lawson VL-JM, Finlay AY. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107-13. https://doi.org/10.1046/J.1365-2133.1998.02034.X 10.1046/J.1365-2133.1998.02034.X [DOI] [PubMed] [Google Scholar]
- [19].NHP Manual IW. Svensk version av Nottingham Health Profile – ett fra°geformula¨r som ma¨ter ha¨lsorelaterad liv-skvalitet (Swedish version). Gothenburg: OFTA Grafiska; 1992. [Google Scholar]
- [20].Messina G. Italian medical students quality of life: years 2005-2015. Ann Ig 2016;28:245-51. https://doi.org/10.7416/ai.2016.2103 10.7416/ai.2016.2103 [DOI] [PubMed] [Google Scholar]
- [21].Nante N. Quality of life in refugees and asylum seekers in Italy: a pilot study. Ann Ist Super Sanita 2016;52:424-7. https://doi.org/10.4415/ANN_16_03_14 10.4415/ANN_16_03_14 [DOI] [PubMed] [Google Scholar]
- [22].Levorato S. Health status of homeless persons: a pilot study in the Padua municipal dorm. Ann Ig 2017;29:54-62. https://doi.org/10.7416/ai.2017.2132 10.7416/ai.2017.2132 [DOI] [PubMed] [Google Scholar]
- [23].Finlay AY. Quality of life assessments in dermatology. Semin Cutan Med Surg, 1998;17:291-6. https://doi.org/10.1016/s1085-5629(98)80026-6 10.1016/s1085-5629(98)80026-6 [DOI] [PubMed] [Google Scholar]
- [24].Sartorius N. Stigmatized illnesses and health care. Croat Med J 2007;48:396-7. [PMC free article] [PubMed] [Google Scholar]
- [25].McCarberg BH. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther 2008;15:312-20. https://doi.org/10.1097/MJT.0b013e31818164f2 10.1097/MJT.0b013e31818164f2 [DOI] [PubMed] [Google Scholar]
- [26].Niv D, Kreitler S. Pain and quality of life. Pain Pract 2001;1:150-61. https://doi.org/10.1046/j.1533-2500.2001.01016.x 10.1046/j.1533-2500.2001.01016.x [DOI] [PubMed] [Google Scholar]
- [27].Juliana Catucci Boza NG, Machado P, Horn R, Fabbrin A, Cestari T. Quality of life impairment in children and adults with vitiligo: a cross-sectional study based on dermatology-specific and disease-specific quality of life instruments. Dermatology 2016;232:619-25. https://doi.org/10.1159/000448656 10.1159/000448656 [DOI] [PubMed] [Google Scholar]


