Summary
Introduction
In Italy, three hexavalent pediatric vaccines are available: two are ready-to-use (RTU) as pre-filled syringes, while the third must be reconstituted (need-for-reconstitution [NFR]). The formulation is related to the vaccination timing, safety of preparation and administration, and possible errors in immunization. We surveyed Italian healthcare professionals (HCPs) experienced with RTU and NFR vaccines in order to investigate their opinions on key aspects of the vaccines.
Methods
In Q1 2018, a qualitative study, ethnographic observations and in-depth interviews were performed in public vaccination settings of three Italian Regions. Data on how the vaccination process was managed and perceptions about the value of the RTU formulation were collected. In Q2 2018, face-to-face interviews were carried out to explore the attitude and preferences of Italian HCPs from nine Regions, assessing advantages and disadvantages of the two formulations from a quantitative point of view. In Q3-Q4 data analysis was carried out, using both qualitative and quantitative methodologies.
Results
The first phase demonstrated the following advantages of the RTU versus the NFR formulation: time-saving, lower probability of needle contamination and needle stick incidents, better handling, simpler procedure, easier disposal of waste. For the survey, 149 HCPs were interviewed; 80% and 40%, respectively, were very satisfied with the RTU and NFR vaccine.
Conclusions
Our study demonstrated that HCPs prefer the RTU formulation, as it simplifies vaccinations, reduces preparation time and minimizes the risk of errors. This formulation also saves time that can be spent on more in-depth counseling.
Keywords: Healthcare professionals, Hexavalent vaccine, Pediatric vaccination, Pre-filled syringe, Ready-to-use
Introduction
The development of combination vaccines can undoubtedly be considered an important innovation for the prevention of infectious disease that has led to enormous improvements on health, and has also brought economic benefits to healthcare systems [1]. Indeed, combined vaccines have played a central role in prophylaxis of the pediatric population from infectious diseases over the past decades. The availability of combination vaccines represents an important means of achieving successful protection against numerous pathogens simultaneously, and is associated with several advantages. By reducing the number of injections, a better compliance to the vaccination schedule and higher rates of coverage can be achieved, and a safer profile assured, since most adverse events reported after vaccination are related to the act of injection [2, 3].
Furthermore, in terms of healthcare service organizations, combination vaccines have been proven to improve the efficiency of the vaccination service, both for the healthcare professionals (HCPs) involved, namely physicians, nurses, and pediatricians, and for the organization itself. In fact, combination vaccines save HCPs time during vaccine preparation [4], reduce administration costs, minimize storage space needed and reduce waste [3, 5]. Depending on the practice of vaccination in terms of the number and role of HCPs involved, the impact of using combination vaccines can be very relevant, especially in situations of personnel constrains, which are common nowadays, as well as in crowded pediatric vaccination schedules, as already implemented in many high-income countries [6, 7].
Currently, several pediatric combination vaccines are available. Among these, hexavalent vaccines represent the most innovative formulation to protect babies against six diseases: diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, and infection from Haemophilus influenzae type b. In the European Region, three hexavalent vaccines are authorized by the European Medicines Agency: Infanrix Hexa®, available since 2000 [8]; Hexyon®, available since 2013 [9]; and Vaxelis®, available since 2017 [10]. These three hexavalent vaccines have the same indication of use, including immunization against the six diseases and age of utilization, as described in their Summary of Product Characteristics (SmPC) [8-10]. Although a maximum age limit of use is not indicated for any of them, the fact that they contain a “pediatric” dose of antigens, make them recommended up to 7 years of age by health authorities and scientific societies in several countries [1]. Safety, immunogenicity and effectiveness of hexavalent vaccines is described in each SmPC and confirmed in several studies and clinical trials [1, 11-13]. Beyond indications, the main difference among the hexavalent vaccines is in regards to the preparation that is required for their administration: both Hexyon® and Vaxelis® are ready-to-use (RTU) in a pre-filled syringe, whereas for Infanrix Hexa® there is a need-for-reconstitution (NFR) of the Hib antigen with a syringe containing the five other components.
Preference for an RTU or NFR vaccine may be related to several factors, such as the preparation time required, the possibility to reduce mishandlings and dosage errors, cost, vaccination waste, the organization of the vaccination services in terms of time set for each vaccination, and to the characteristics of packaging that render the vaccine easier to integrate within existing databases. Moreover, individual experience and preferences of HCPs for a specific hexavalent vaccine may also dictate the selection of an RTU or NFR vaccine. Notably, it has been demonstrated that both physicians and nurses tend to prefer vaccines that require less time to prepare and manage [14]. As a consequence, the time saved may be spent on streamlining the vaccination session and providing parents with a more detailed vaccination counselling [15]. In addition, it has been reported that the higher acquisition costs of RTU vaccines are counterbalanced by lower administrative costs and increased safety compared with single-dose and multi-dose vial vaccines [16, 17].
In Italy, pediatric vaccinations are delivered by the public health sector, either in vaccination centers or in family pediatricians’ medical offices. In vaccination centers, public health physicians (also defined as hygienists) are those medical doctor specialists who are in charge of vaccines in vaccination centers, from the organizational and practical point of view.
Within Italy, each Region runs independent tenders that are driven by price and/or scientific criteria, while product technical criteria are usually not taken into account in the assessment. To date, there remains limited data on the opinion of HCPs regarding technical aspects related to vaccination. To gain more insight into the opinions of HCPs on key aspects of the vaccination process, as well as on preferences for hexavalent vaccines, we carried out a survey of HCPs experienced in pediatric vaccinations, working in nine Italian Regions that differ by the organizational models of the vaccination services. Our survey investigated preferences and critical issues reported by the HCPs, in order to obtain information that may be useful for optimizing pediatric vaccinations in the public setting.
Methods
QUALITATIVE PHASE
In Q1 2018 an experienced researcher performed ethnographic observations followed by in-depth interviews in public vaccination settings (vaccination centers and family pediatricians’ offices) of three Italian Regions: in Liguria, with 6 HCPs (3 hygienists and 3 nurses) where the NFR hexavalent vaccine is used; in Apulia with 3 nurses and in Tuscany with 3 primary care pediatricians, where the RTU hexavalent vaccine is used. In general, all HCPs were experienced with both NFR and RTU formulations that are commonly available in Italy. The main purpose of the ethnographic observation was to understand how the vaccination process was managed in different Regions, in terms of HCPs involved and their role in the vaccination process.
The purpose of the subsequent interviews was to highlight and discuss critical issues emerging from the daily routine vaccination process, investigating the overall image of the hexavalent vaccine (safety and tolerability), and the value of the RTU formulation.
QUANTITATIVE PHASE: SURVEY TARGET
In Q2 2018, personal in-depth interviews were carried out by inviting 265 HCPs (hygienists, nurses, and family pediatricians) from nine Italian Regions covering the north, center, and south of the country (Liguria, Lombardy, Piemonte, Emilia Romagna, Tuscany, Calabria, Campania, Apulia and Sicily). In these Regions, three hexavalent vaccines are used, including both RTU and NFR vaccines.
Invited participants were selected through a purposive sampling methodology among those professionals that are in charge of the hexavalent pediatric vaccination at regional vaccination centers or as family pediatricians. The inclusion criteria for the HCPs to be interviewed were: a minimum of 10 years of experience in pediatric vaccinations and a minimum of 200 children under 2 years of age vaccinated monthly in vaccination centers or around 50 children under 2 years of age vaccinated monthly for family pediatricians.
QUANTITATIVE PHASE: SURVEY CHARACTERISTICS
The survey consisted of 46 questions, requiring approximately 20 minutes for its completion (questionnaire in Annex 1). Computer-assisted interviews were conducted in person by an experienced interviewer and the anonymity of the results were assured before starting the interview. The overall objective was to identify the attributes of vaccination devices that may be valuable for HCPs and to evaluate advantages and disadvantages of the RTU formulation compared with the NFR formulation.
Firstly, demographic and professional data were collected including: region where HCPs work, gender, age, profession, years of experience in administering vaccination, number of children under 2 years of age vaccinated in a typical month (either in vaccination centers or with family pediatricians), number of children under 2 years of age vaccinated with hexavalent vaccines, and typology of the hexavalent vaccine used.
In order to investigate the daily practice of HCPs working in vaccination centers, where hygienists and nurses work together, the following data were collected: time and number of HCPs dedicated to vaccinations and activities that each of the two professional categories mostly deal with.
With the aim of assessing perceptions and satisfaction towards hexavalent vaccines, participants were asked to describe: their individual experience while preparing and administering hexavalent vaccines to children, the attributes they consider more valuable for a hexavalent device, and the time dedicated to the various phases of the vaccination session (counselling, vaccine preparation, vaccine administration).
Lastly, the survey asked the participants to indicate which one of the two hexavalent formulations, RTU and NFR, had certain characteristics related to the ease and safety in the preparation, administration, and disposal of the vaccine.
The satisfaction and agreement of HCPs with the proposed statements were measured on a 1-10 scale (8-10 indicating high satisfaction/agreement).
Descriptive statistics were used to analyze and present results.
Results
QUALITATIVE PHASE
In the Liguria region, the observed vaccination staff included 2 HCPs: one hygienist and one nurse (dedicated or working mainly in other specialties). It was observed that when the nurse was dedicated, the role of the hygienist and of the nurse were interchangeable, while when the nurse was “rented” temporarily from another unit, the nurse prepared the vaccine but vaccine administration and family counselling were managed by the hygienist.
In Apulia, the vaccination staff included 2 or 3 HCPs: one hygienist and one to two nurses (one in small towns, two in the cities). It was observed that in this setting the nurse played a major role in the vaccination process, being involved in all phases from ordering to administration to disposal of the vaccine. The hygienist was in charge of checking the child’s record on the database, their vaccination history, their clinical history (filled in by the parents), and scheduling the following vaccination appointment.
Considering the time and the professional figures dedicated to vaccinations in vaccination centers, the respondents working in this setting declared that approximately 4 hours for 4 days were dedicated to the vaccination of children under 2 years of age, with 2 hygienists and 3 nurses dedicated to vaccination activities only.
In Tuscany, following a recent agreement with the Regional Health Authority, pediatric vaccinations have been shifted to family paediatricians, who also provide hexavalent vaccination in their practice.
As a result of the interviews, 6 HCPs (3 hygienists and 3 nurses) were interviewed in Liguria, 3 nurses in Apulia and 3 family pediatricians in Tuscany (Tab. I).
Tab. I.
Qualitative phase: methodology used.
| Region (hexavalent vaccine in use) | Ethnographic observation | Interviews |
|---|---|---|
| Liguria (NFR) | Vaccination center (2 days observation) | 6 HCPs (3 Hygienists + 3 Nurses) |
| Apulia (RTU) | Vaccination center (2 days observation) | 3 HCPs (3 Nurses) |
| Tuscany (RTU) | - | 3 HCPs (3 Pediatricians) |
Abbreviations: HCPs, healthcare professionals; NFR, need-for-reconstitution; RTU, ready-to-use.
The hexavalent vaccine showed a positive image across the board: it was perceived as safe and with a good level of tolerability. Moreover, although on a practical point of view vaccination is considered easy and simple to manage for the HCP, on a more emotional level, vaccine administration often becomes a potentially anxious moment for the family. As a consequence, the need for family counselling when administering the first dose of hexavalent vaccination emerged strongly and was across all Regions. The value of the RTU formulation emerged clearly, across both target and geographic areas: its value was spontaneously recognized, by users of both RTU and NFR vaccines. The advantages of the RTU formulation that emerged compared with the NFR formulation can be ranked as follows (from more relevant to less relevant): time-saving, better safety profile, better handling, simpler procedure, easier disposal of waste, more convenient set of needles.
These results were considered as preliminary and were further tested during the survey phase.
QUANTITATIVE PHASE
In the quantitative phase, face-to-face computer-assisted personal interviews were carried out with 149 out of the 265 (56.2%) invited HCPs from the nine selected Italian Regions. Among the respondents, 60 were hygienists, 59 were nurses working in vaccination centers, and 30 were family pediatricians; 66% were female and the overall mean age was 55 years (58 years for hygienists, 51 years for nurses, and 63 years for pediatricians). The overall average number of years spent in vaccination activities was 15 years (18, 13, and 12 years, respectively, for hygienists, nurses and pediatricians). The sociodemographic and professional data of the survey participants are described in Table II.
Tab. II.
Quantitative phase: demographic and professional characteristics of healthcare professionals.
| Hygienists in vaccination centers (n = 60) |
Nurses (n = 59) |
Pediatricians (n = 30) |
Total (n = 149) |
|
|---|---|---|---|---|
| Female, n (%) | 28 | 55 | 15 | 98 (66%) |
| Male, n (%) | 32 | 4 | 15 | 51 (34%) |
| Age, mean (yrs) | 58 | 51 | 56 | 55 |
| Region | ||||
| Calabria | 5 | 5 | - | 10 |
| Campania | 8 | 8 | - | 16 |
| Emilia Romagna | 6 | 6 | - | 12 |
| Liguria | 5 | 5 | - | 10 |
| Lombardia | 8 | 8 | - | 16 |
| Piemonte | 7 | 7 | - | 14 |
| Apulia | 11 | 10 | - | 21 |
| Sicily | 10 | 10 | - | 20 |
| Tuscany | - | - | 30 | 30 |
| Experience with vaccinations, mean (yrs) | 18 | 13 | 12 | 15 |
| Approximate number of children < 2 yrs vaccinated monthly, n | 229 | 210 | 48 | 185 |
| Approximate number of children < 2 yrs vaccinated monthly with the hexavalent vaccine, n (% of total vaccinations) | 165 (72%) | 126 (60%) | 28 (58%) | 123 (66%) |
N: number; yrs: years.
Among the HCPs, 84 (56%) used the RTU hexavalent vaccine and 65 (44%) used the NFR one.
The activities in which HCPs reported being mostly involved varied amongst the professional category: talking to parents and collecting the medical history of the child were activities that hygienists mostly deal with, while nurses were in charge of preparing the vaccines and the room, taking inventory and orders, managing the stock, scheduling appointments and disposing of the waste materials. Pediatricians spent more time counselling (an average of 11 minutes) compared with hygienists (10 minutes) and nurses (8 minutes).
ASSESSMENT OF HEXAVALENT VACCINES
As for the time spent during vaccination, HCPs answered that out of an average of 17 minutes requested for each vaccination, more than half (approximately 10 minutes) was spent explaining the hexavalent vaccine and vaccination process to the parents. Vaccine preparation required an average of 3 minutes, 2 minutes were spent administering the vaccine, and 2 minutes for disposal of waste materials.
Regarding hexavalent vaccination sessions, most HCPs (83.2% of the target pediatricians, 90.2% of the hygienists, and 97.2% of the nurses) expressed an 8-10 rate of agreement (very or mostly) with the declaration that giving information regarding vaccination/vaccines to parents was very demanding and time-consuming. As for managing and administrating the vaccine, 27.4% of hygienists, 29.4% of nurses, and 47.4% of pediatricians expressed an 8-10 rate of agreement (very or mostly) with the possibility of making errors during the vaccine preparation; 20.5% of hygienists, 22.5% of nurses, and 40.5% of pediatricians expressed a high rate of agreement (very or mostly) with the possibility of making errors during the vaccine administration; 18.6% of hygienists, 20.6% of nurses, and 33.6% of pediatricians very/mostly agreed that it could be possible to forget the reconstitution of the vaccine.
Key aspects of the hexavalent vaccines rated as “very important” were: minimizing the risk of needle contamination (80% of all respondent HCPs) and of needle stick injuries (79% of HCPs), being stable in case of problems of the cold chain (78% of HCPs), having low risk of errors in the reconstitution (78% of HCPs), being easy to prepare and to manage (74% of HCPs), and being ready to use (66% of HCPs). These last two aspects were particularly important for pediatricians.
RTU vs NFR vaccines
As for the overall comparison between RTU and NFR hexavalent formulations, 80% of HCPs declared their satisfaction with the advantages of RTU hexavalent vaccines was “very good”: easy preparation and administration, no risk to reconstitute, low risk of needle contamination and stick injuries. On the other hand, only 40% of HCPs declared they were satisfied by the NFR formulation to a level of “very good”, due to more manipulations, higher risk of needle contamination and stick injuries (Fig. 1). Figures 2 and 3 describe in detail the assessment of the two formulations, as rated by HCPs.
Fig. 1.
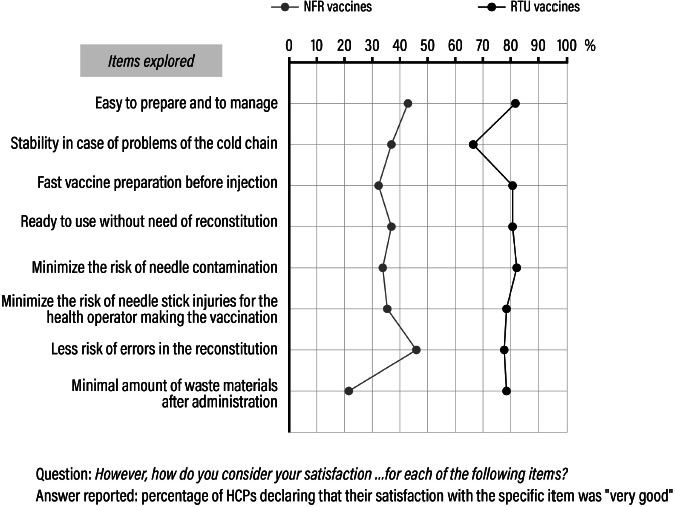
Perceived advantages of ready-to-use (RTU) vaccines over need-for-reconstitution (NFR) vaccines.
Fig. 2.
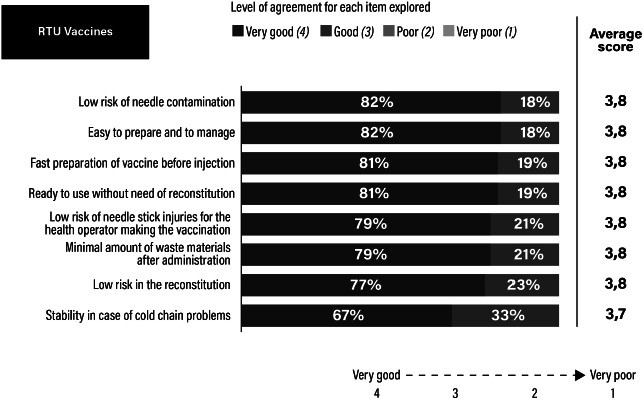
Assessment of the ready-to-use (RTU) formulation as rated by healthcare professionals.
Fig. 3.
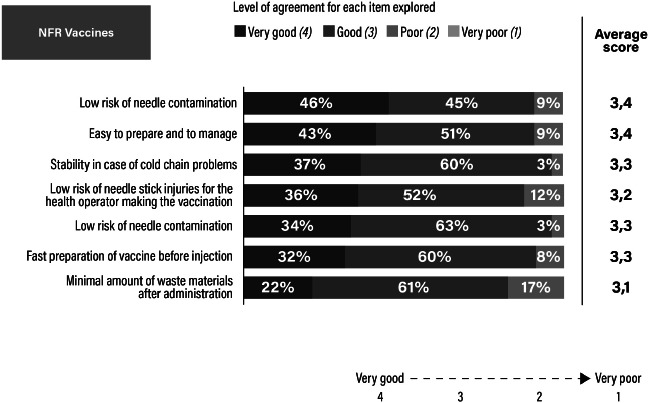
Assessment of the need-for-reconstitution (NFR) formulation as rated by healthcare professionals.
As for safety issues related to the different syringe formulations, HCPs declared to be overall satisfied with the safety of hexavalent vaccines (49% very satisfied and 40% mostly satisfied), but a difference appeared between the two formulations with 61% of HCPs very satisfied with RTU overall syringe safety compared with only 34% of HCPs being very satisfied with NFR overall syringe safety (Fig. 4).
Fig. 4.
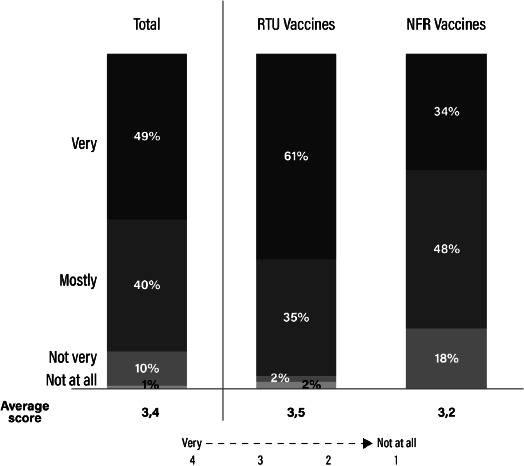
Ready-to-use (RTU) versus need-for-reconstitution (NFR) vaccines: assessment of safety.
Lastly, when asked how much the use of an RTU vaccine could facilitate when vaccinating children under the age of 2 years, 92% (from 90% of hygienists to 93% of both nurses and pediatricians) expressed a score of 8-10 (indicating high satisfaction/agreement). Moreover, HCPs declared that the time saved in preparation of RTU vaccines can be more effectively spent on vaccination counselling during the visit.
Discussion
This survey focused on relevant aspects of the hexavalent vaccines, such as handling, time needed for the different phases of vaccination sessions, errors and safety related to the formulation, with a comparison between RTU and NFR vaccines. Issues related to the safety or immunogenicity of hexavalent vaccines were not our objective because these aspects are already well documented and considered similar [18].
According to the inclusion criteria, vaccination centers and family pediatricians, respectively, had to vaccinate a minimum of 200 children and around 50 children under the age of 2 years each month. Of these, more than two-thirds were administered a hexavalent vaccine. Thus, the surveys respondents’ long-standing knowledge of the issues involved in vaccinations constitutes a reasonable guarantee of validity in the assessment of hexavalent vaccines.
For Italian family pediatricians, vaccination is not a routine activity in their daily practice, but we chose to include this category as the Tuscany region has recently stated that family pediatricians should administer hexavalent vaccines in their medical offices, and this practice could be soon adopted by the other Italian Regions as a measure to increase coverage rates. In this regard, it has been demonstrated that physicians’ recommendation is an important predictor of vaccine acceptance, constituting a major factor in receiving or intending to receive any vaccine [19]. For this reason, the involvement of all HCPs in our survey resulted essential to identify critical issues and thus highlight potential areas for additional intervention targeted at specific professional categories. Family pediatricians work autonomously in their office, thus being in charge of all the different phases of vaccine administration. As a consequence, as emerged in our study, they are able to perform only a limited number of vaccinations per month (i.e., 48 vaccinations to children < 2 years of age) and appeared more concerned about making errors during preparation, administration and reconstitution of the hexavalent vaccine compared with other HCPs. As is known in the literature, pediatricians can have a key role in increasing awareness about the benefits of pediatric vaccinations and educating parents [20]: in our study, pediatricians spent more time counselling than hygienists and nurses.
For all these reasons, an RTU formulation may be preferable, for all HCPs, and in particular for pediatricians, as it was demonstrated to render all processes not only easier and safer, but also more rapid. Similarly, our research demonstrated that RTU formulation of hexavalent vaccines was widely preferred to NFR vaccines among all HCPs because it simplified the preparation, minimized the number of manipulations and error risks: in fact, 80% of HCPs declared they were very satisfied with RTU vaccines compared with only 40% of HCPs who were very satisfied with NFR. The perceived benefits of an RTU vaccine included easier and quicker preparation with less risk of errors such as the risk of forgetting to reconstitute the Hib or not taking all the Hib antigen from the vial. It was also seen to minimize the risk of needle contamination and needle stick injury and to produce less waste material.
Although we should consider that previous published studies used different definitions in vaccine preparation time, as well as different methodologies for data collection and analysis, we can say that our results are in line with the existing literature. In fact, handling, dosage errors, and reduced preparation time were all highlighted as being important attributes of a fully-liquid RTU vaccine versus one that requires reconstitution in a previous survey of physicians and nurses conducted in Germany on hexavalent pediatric vaccines [14]. In particular, both the present and previous studies highlighted that HCPs are concerned about minimizing the risk of errors during vaccination, which may thus be reduced by using a fully-liquid hexavalent vaccine [4, 14, 21]. In fact, in a time and motion study, comparing RTU versus non-fully liquid vaccines showed that mishandlings were five times more common with a NFR hexavalent vaccine compared with the RTU vaccine [4]. In our study, 77% of HCPs rated as “very good” the low risk of errors in the reconstitution for RTU vaccines versus 46% for the NFR formulation. In addition to the reduced risk of error, it was reported that an RTU hexavalent vaccine can be prepared in less than half the time needed to prepare a NFR vaccine [4, 15]. Using the time difference of 35 seconds that was observed in the study of De Coster and colleagues for a HCP to prepare an RTU hexavalent vaccine versus a NFR vaccine, we can estimate the number of hours per year that are saved due to the simpler and quicker process of the RTU formulation. We applied these data to the Italian context, using hexavalent vaccination coverage (95%) of the birth cohort (440,000 newborns in 2018) and number of doses of hexavalent to be administered in the pediatric recommended schedule (3 doses, 2+1 schedule). We estimated approximately 12,000 hours saved/year, that correspond approximately to the workload of 7 HCPs working in public settings, that could therefore be re-allocated to other tasks or units, if a broader healthcare service perspective is used, with a potential saving for the public organization. Time saved is a significant aspect considering that the HCPs involved in our study devoted a substantial amount of time to vaccinations (approximately 17 minutes per vaccination), a large part of which was dedicated to informing and educating parents (around 10 minutes). Therefore, time saved in the act of preparing and administering the vaccine could be used in a more productive way with parents and the baby.
Our study is limited by the generalizability of our results. In fact, the purposive sampling methodology adopted to select the HCPs and the Regions involved in the two phases of the study may reduce the representativeness of our results. Moreover, our results may not generalize appropriately to other countries, due to potential differences in the organization of vaccination programs and cultural preferences for specific pharmaceutical forms. On the other hand, this study represents one of the very few evidences that support the switch from NFR to RTU vaccines, taking in consideration HCPs preferences, as well as time saved, simplification of vaccine preparation and management, as is already known in the literature. The extension of this work to a larger sample and to other contexts could confirm our findings.
Conclusions
The present study has highlighted aspects that are important for HCPs when considering a hexavalent vaccine. We observed that a vaccine that can reduce the time needed for preparation, while reducing the risk of errors as much as possible, is preferred by HCPs. Accordingly, easy-to-use, fully liquid vaccines are desirable, and fully liquid, hexavalent vaccines in pre-filled syringes have many characteristics that HCPs value as important. An RTU vaccine minimizes the risk of errors, and especially the risk of forgetting to reconstitute the powder in the main syringe or reconstituting all the powder. RTU vaccines also reduce the risk of needle contamination and needle stick injuries as only one needle is used. The advantages in terms of time saving are clear as less time is needed for vaccine preparation and administration, which allows more time for counselling by the single HCP or can allow re-allocation to other tasks or units if a broader healthcare service perspective is used. Therefore, in comparable contexts of immunogenicity, tolerability and safety, it would thus seem likely that RTU vaccines present satisfactory characteristics over NFR vaccines. We also envisage that these technical aspects will be taken into account by regional decision makers in deciding to adopt one or another typology of vaccine.
Figures and tables
Acknowledgements
Writing and editorial assistance was provided to the authors by Health Publishing & Services Srl, funded by Sanofi Pasteur. Data collection was provided by GfK Italy (Growth from Knowledge), including preliminary data analysis.
Funding sources: Sanofi Pasteur funded every phase of this research.
Footnotes
Conflict of interest statement
All Authors have participated in advisory boards or expert meetings or were speakers or organizers of congresses/conferences on hexavalent vaccines sponsored by GlaxoSmithKline Biologicals SA, Sanofi Pasteur-MSD, MSD or Sanofi Pasteur.
Authors’ contributions
All Authors made a substantial contribution to the interpretation of data, read and approved the final manuscript.











References
- [1].Orsi A, Azzari C, Bozzola E, Chiamenti G, Chirico G, Esposito S, Francia F, Lopalco P, Prato R, Russo R, Villani A, Franco E. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg 2018;59:E107-E19. [PMC free article] [PubMed] [Google Scholar]
- [2].Decker MD. Principles of pediatric combination vaccines and practical issues related to use in clinical practice. Pediatr Infect Dis J 2001;20:S10-8. https://doi.org/10.1097/00006454-200111001-00002 10.1097/00006454-200111001-00002 [DOI] [PubMed] [Google Scholar]
- [3].Skibinski DA, Baudner BC, Singh M, O’Hagan DT. Combination vaccines. J Glob Infect Dis 2011;3:63-72. https://doi.org/10.4103/0974-777X.77298 10.4103/0974-777X.77298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Coster I, Fournie X, Faure C, Ziani E, Nicolas L, Soubeyrand B, Van Damme P. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine 2015;33:3976-82. https://doi.org/10.1016/j.vaccine.2015.06.030 10.1016/j.vaccine.2015.06.030 [DOI] [PubMed] [Google Scholar]
- [5].Maman K, Zollner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: From beliefs to evidence. Hum Vaccin Immunother 2015;11:2132-41. https://doi.org/10.1080/21645515.2015.1044180 10.1080/21645515.2015.1044180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bozzola E, Spina G, Russo R, Bozzola M, Corsello G, Villani A. Mandatory vaccinations in European countries, undocumented information, false news and the impact on vaccination uptake: the position of the Italian pediatric society. Ital J Pediatr 2018;44:67 https://doi.org/10.1186/s13052-018-0504-y 10.1186/s13052-018-0504-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].World Health Organization (WHO). WHO vaccine-preventable diseases: monitoring system. 2019. global summary. Available at: http://apps.who.int/immunization_monitoring/globalsummary/schedules. Accessed on 23/03/2020. [Google Scholar]
- [8].European Medicines Agency. Infanrix Hexa. Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/infanrix-hexa. Accessed on 23/03/2020.
- [9].European Medicines Agency. Hexyon. Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/hexyon. Accessed on 23/03/2020. [Google Scholar]
- [10].European Medicines Agency. Vaxelis. Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxelis. Accessed on 23/03/2020. [Google Scholar]
- [11].Petrolini C, Chiappini E, Caffarelli C, Calvani M, Cardinale F, Duse M, Licari A, Manti S, Martelli A, Minasi D, Del Giudice MM, Pajno GB, Pietrasanta C, Pugni L, Tosca M, Mosca F, Marseglia GL. Vaccinazione esavalente nei nati pretermine. Rivista di Immunologia e Allergologia Pediatrica 2019;3-2019:7-23. [Google Scholar]
- [12].Silfverdal SA, Icardi G, Vesikari T, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, Thomas S, Ziani E, Lee AW. A Phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at 2, 4, and 11-12 months. Vaccine 2016;34:3810-6. https://doi.org/10.1016/j.vaccine.2016.05.054 10.1016/j.vaccine.2016.05.054 [DOI] [PubMed] [Google Scholar]
- [13].Vesikari T, Silfverdal SA, Jordanov E, Feroldi E. A Randomized, Controlled Study of DTaP-IPV-HB-PRP-T, a Fully Liquid Hexavalent Vaccine, Administered in a 3-, 5- and 11- to 12-month Schedule. Pediatr Infect Dis J 2017;36:87-93. https://doi.org/10.1097/INF.0000000000001358 10.1097/INF.0000000000001358 [DOI] [PubMed] [Google Scholar]
- [14].Lloyd AJ, Nafees B, Ziani E, Nicolas L, Fordham BA, Soubeyrand B, Bornhoft C. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pedatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence 2015;9:1517-24. https://doi.org/10.2147/PPA.S87229 10.2147/PPA.S87229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wiedenmayer KA, Weiss S, Chattopadhyay C, Mukherjee A, Kundu R, Aye R, Tediosi F, Hetzel MW, Tanner M. Simplifying paediatric immunization with a fully liquid DTP-HepB-Hib combination vaccine: evidence from a comparative time-motion study in India. Vaccine 2009;27:655-9. https://doi.org/10.1016/j.vaccine.2008.11.045 10.1016/j.vaccine.2008.11.045 [DOI] [PubMed] [Google Scholar]
- [16].Nogier C, Hanlon P, Wiedenmayer K, Maire N. Can a Compact Pre-Filled Auto-Disable Injection System (cPAD) Save Costs for DTP-HepB-Hib Vaccine as Compared with Single-Dose (SDV) and Multi-Dose Vials (MDV)? Evidence from Cambodia, Ghana, and Peru. Drugs Real World Outcomes 2015;2:43-52. https://doi.org/10.1007/s40801-015-0010-0 10.1007/s40801-015-0010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pereira CC, Bishai D. Vaccine presentation in the USA: economics of prefilled syringes versus multidose vials for influenza vaccination. Expert Rev Vaccines 2010;9:1343-9. https://doi.org/10.1586/erv.10.129 10.1586/erv.10.129 [DOI] [PubMed] [Google Scholar]
- [18].McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexaxim(R)): a review of its use in primary and booster vaccination. Paediatr Drugs 2013;15:59-70. https://doi.org/10.1007/s40272-013-0007-7 10.1007/s40272-013-0007-7 [DOI] [PubMed] [Google Scholar]
- [19].Gargano LM, Herbert NL, Painter JE, Sales JM, Morfaw C, Rask K, Murray D, DiClemente RJ, Hughes JM. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother 2013;9:2627-33. https://doi.org/10.4161/hv.25823 10.4161/hv.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrara P, Zenzeri L, Fabrizio GC, Gatto A, Pio L, Gargiullo L, Ianniello F, Valentini P, Ranno O. Second-generation immigrant children: health prevention for a new population in terms of vaccination coverage and health assessment. Minerva Pediatr 2016;68:121-6. [PubMed] [Google Scholar]
- [21].Sharp B, Whyte P. New directions in the development of pre-filled syringes. Innov Pharm Technol 2010:64-8. Available at: http://iptonline.com/articles/public/p-8%20non-print.pdf. Accessed on 3/03/2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials














