Abstract
Calcium is an important mineral that plays an integral role in human health, especially bone health. Marine biological calcium is an abundant resource that is generally accepted and has a complex active structure. This review evaluates research progress on marine biological calcium with regards to its sources, use of calcium supplements, calcium bioavailability, and novel applications of marine calcium. The potential for future development and the use of products incorporating marine biological calcium in biomedical research and the pharmaceutical, health care, and food industries are also reviewed. The goal of this review is to provide a comprehensive documentation on resource utilization and product development from marine organisms.
Subject terms: Nutrition, Natural products
Introduction
Calcium is an important micronutrient widely believed to affect bone health and human metabolism. Calcium deficiency can cause conditions like osteoporosis, rickets, epilepsy, and anemia. Calcium enters the circulation through food or calcium supplements, and a dynamic balance is maintained between blood and bone calcium1. The primary source of calcium is dairy products, including milk and its by-products like cheese and condensed milk, followed by other sources like cereals and tofu2. However, an inappropriate diet can decrease the bioavailability of calcium. For example, the presence of phytic acid in cereals and oxalic acid in green leafy vegetables can cause calcium to precipitate as calcium phytate and calcium oxalate, which are insoluble compounds3. In America, a study found that approximately 38% of adults who rely solely on food for mineral and vitamin intake consume inadequate levels of calcium, and approximately 93% consume inadequate levels of vitamin D, which plays a key role in calcium absorption rate, bone homeostasis, and bone repair4,5. Calcium deficiency becomes gradually debilitating with age6. Chronic calcium deficiency has caused osteoporosis to become an epidemic7. An increasing number of people continue to face calcium deficiency and diseases associated with calcium deficiency8–10. As a result, more people have increased their calcium intake through supplements based on the advice of doctors or the media11.
The calcium sources for these supplements include calcium carbonate ores, calcium-rich animal skeletons, marine shells, and crustaceans12. However, natural calcium carbonate ores may contain harmful elements, such as heavy metals13. Animal bones may carry the risk of prion transmission14,15. In recent years, calcium supplements from marine sources have gained attention due to their abundant reserves, high safety, and biological activity16,17. With the development and utilization of marine resources, more than 50% of fishery products, including bones, fins, heads, and internal organs, which are discarded as waste annually, can be used. Marine mineral supplements have the potential to increase bone turnover and may aid in preventing injuries and repairing damaged bone in humans18. As an abundant source of calcium, the use of marine biological calcium is an important way to improve the utilization rate of biological resources. This review comprehensively evaluates the marine calcium sources, the technology used for the preparation of calcium supplements, and the biological activity and bioavailability of marine calcium to provide references for the effective development of supplements using marine calcium.
Marine source of calcium
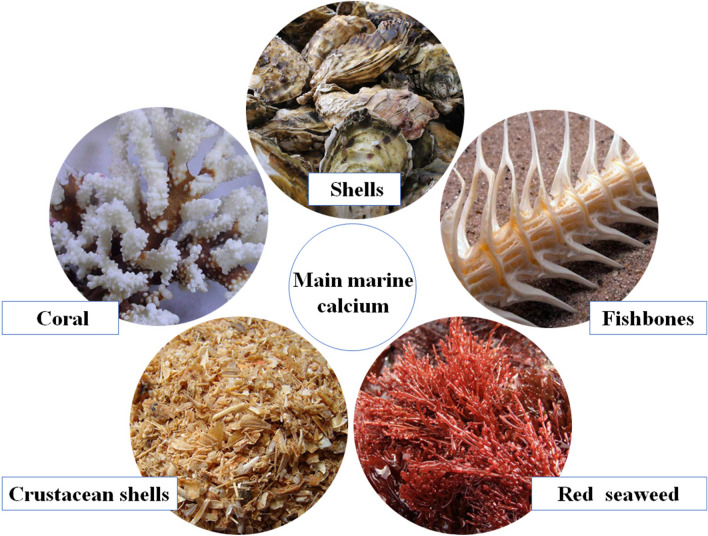
Oceans are rich in biological resources and calcium is an important mineral constituent of marine life. The major sources of calcium for humans from the oceans include fishbones, shellfish and crustacean shells, and coral and seaweed (Fig. 1).
Figure 1.
Main calcium source from Marine organism.
Calcium from fishbones
Fishbone is the general term encompassing the axial, appendage, and fishbone in the fish body, accounting for approximately 10–15% of the total body weight19. Fishbone tissue consists mainly of an organic extracellular matrix covered with hydroxyapatite [Ca5(PO4)3OH] and the calcium content found to be lowest in the salmonid species when compared to eight different species of fish was as high as 135–147 g/kg in the lipid-free dry matter20. Shark cartilage is another important source of calcium. For example, the calcium from the jaw cartilage of gummy shark is mainly in the form of hydroxy calcium phosphate crystal [Ca10(PO4)6(OH)2], and its calcium phosphate content is among the highest with 67% on a dry weight basis, which ranges between 124 and 258 g/kg21. Fishbones from large fishes need to be processed using chemical and biological methods to destroy organic material or bonded with collagen to increase the calcium dissolution rate because calcium in the form of hydroxyapatite is not suitable for absorption in humans22. Small fishes with soft bones, such as anchovies and lizardfish, can be processed into ready-to-eat food to be consumed with their bones23. Generally, calcium preparation from fishbones includes the removal of protein and fat by cooking, treating with alkali and organic solvents or enzymatic hydrolysis, and superfine crushing to obtain a fishbone powder.
Calcium from shells
Shells account for approximately 60% of the mass of a shellfish, and calcium carbonate content in a shell can reach 95%. Shells are a rich source of high-quality marine calcium. Shellfish culture offers humans a low-impact source of sustainable protein24. In 2016, farmed shellfish reached 17.139 million tons globally, accounting for 21.42% of the total farm output25. Additionally, as the proportion of calcium in shells is higher than that in fishbones, the output from shells is greater26,27. The effects of calcium supplementation with Ezo giant scallop shell powder and fossil shellfish powder have been studied; the results indicated good solubility and bioavailability of calcium from these natural sources of calcium28. The shell calcium supplement was marketed in several countries worldwide; however, the utilization of shell resources remains low, and the comprehensive utilization and development of shell calcium require further support.
Calcium from crustacean shells
People can directly ingest calcium by eating small dried shrimp or crabs. Crustacean processing and consumption generate 30–40% of marine resource waste29. Crustacean shells mainly comprise calcium carbonate (CaCO3), chitin, and protein30. Research on shrimp and crab shells have mainly focused on the utilization of chitin and protein resources, while calcium is sometimes recycled as a by-product, such as calcium hydrogen phosphate, calcium lactate, and calcium31.
Calcium from coral
Coral calcium is formed from the exoskeleton of living organisms of many species32. Coral calcium is a natural source of marine calcium, containing 24% calcium, 12% magnesium, and more than 70 minerals; it has recently become a new international trend of calcium supplementation. Coral calcium is often used as a calcium supplement to treat bone metabolism disorders, osteoporosis, and other bone diseases33,34.
Calcium from seaweed
Seaweed from the ocean, especially green algae, is rich in minerals such as calcium35. For example, Aquamin, a typical calcium-rich supplement derived from the calcified skeletal remains of the red seaweed species Lithothamnion, has calcium concentrations of up to 31%/weight36. A previous study has indicated that concerning calcium sources for horses, marine algae is better than calcium carbonate supplements37. Calcium extracted from marine algae was also found to show a beneficial anabolic effect on bone skeletal calcification in animal models of osteoporosis38. Algal calcium prepared from oyster shell powder and seaweed has a higher bioavailability than calcium carbonate39.
Calcium supplements and bioavailability
Direct ingestion of marine-derived calcium
The most common direct calcium supplements are small dried shrimp, shell powder, and small fishes. Several marine calcium supplements, such as oyster shells and coral calcium, have been commercialized in different countries. However, the main components derived from these marine sources are calcium carbonate and calcium polyhydroxy phosphate, which are difficult to absorb and increase gastric burden40. To improve the calcium absorption rate, marine sources are typically crushed or vacuum heated first41,42. Studies have found that marine-derived calcium has certain advantages over calcium carbonate supplements or other calcium-rich food. For example, Aquamin has better bioavailability and potential to slow down bone loss compared to calcium carbonate36. Hake fishbone (HBF) was a good source of calcium, with comparable efficacy to Lithotame (L), a calcium supplement derived from Lithothamnion calcareum17. A fishbone powder (Phoscalim) and a ray cartilage hydrolysate (Glycollagene) were comparable to milk for both short-term calcium absorption and bone resorption16. Tablets made with calcium from haddock bones were adequate for calcium supplementation and osteoporosis prevention43. Currently, the international recommended daily intake of calcium for general population is 700–1200 mg per day. However, teenagers (9–18 years old) need approximately 1300 mg calcium per day, and pregnant women with low dietary calcium intake need 1500–2000 mg calcium per day44,45. Studies have shown that more than 50% of the calcium deficient population include men and women older than 70 years, women aged 51–70 years, boys and girls aged 9–13 years, and girls aged 14–18 years46. Taking a conscious supplement of marine calcium is very effective in preventing calcium deficiency. Direct calcium ingestion from marine organisms is very suitable for daily calcium supplementation; however, it is insufficient for treating calcium deficiency diseases. In the treatment of diseases such as calcium deficiency, there is also a need to choose higher doses of calcium supplements or drugs5.
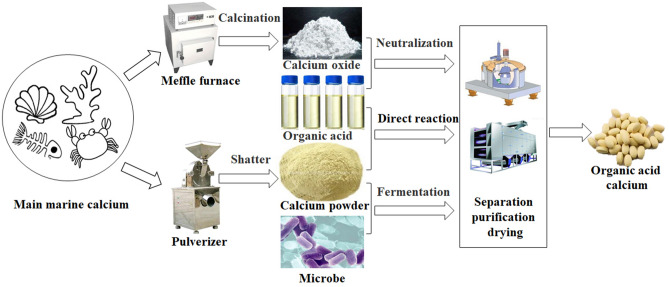
Organic acid calcium
Organic acid calcium, such as calcium citrate, l-calcium lactate, calcium gluconate, calcium acetate, calcium formate, and calcium propionate, have higher bioavailability, solubility, and absorption rates, regardless of gastric contents, because they are less sensitive to gastric pH than calcium carbonate11,40,47. It is mainly prepared by neutralization or fermentation of calcium compounds (Fig. 2). As a dietary calcium supplement, calcium formate has been found to exhibit significant advantages over both calcium carbonate and calcium citrate48. Calcium glucoheptonate has exhibited a high relative bioavailability of calcium and is well-tolerated in humans than calcium carbonate49. However, calcium gluconate and calcium lactate are less concentrated forms of calcium, making them impractical oral supplements. Calcium acetate and calcium propionate are not widely used either50. Calcium organic acids alone are not good for absorption because they can bind to oxalic acid or phytic acid present in food. Calcium combined two or more organic acids, such as calcium citrate malate (CCM)10, which combines bovine collagen peptides with calcium citrate51,52. The combined use of polycan and calcium lactate–gluconate53,54 was found to have beneficial synergistic effects compared with the use of calcium organic acids alone.
Figure 2.
Flow chart of preparation of calcium organic acid from Marine calcium.
Marine sources of calcium organic acids are primarily fishbones, shrimp, crab shells, and other shells3. To facilitate easy calcium absorption, appropriate processes such as calcination, enzymatic hydrolysis and fermentation methods should be selected according to the nutritional composition and associated processing properties55,56. Subsequently, citric acid, gluconic acid, lactic acid, acetic acid, and/or propionic acid are added to prepare calcium organic acids. The solubility and bioavailability of calcium from natural sources of shellfish calcium with citrate and lactate were increased after decompression treatment26. Fishbones can be fermented with Leuconostoc mesenteroides to obtain high amounts of soluble calcium with free calcium, calcium amino acids, calcium acetate, small peptide calcium, and calcium lactate. The fermentation of grass fishbones can increase calcium bioavailability and also help avoid wastage of fishbone calcium and aquatic proteins57.
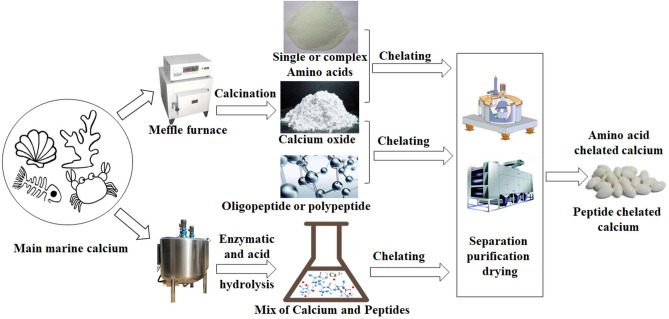
Calcium chelate
Calcium chelate refers to the metal complex formed by stable bonds between amino acids or peptides and metal calcium ions and includes two main products, calcium amino acid and calcium peptide chelate58–60. It is mainly prepared by chelating polypeptides or oligopeptides with calcium ions or when a single or complex amino acid chelates with calcium ions (Fig. 3). Amino acid chelated calcium is not dependent on vitamin D3 and can be absorbed by the human body through amino acid metabolism. For example, calcium lysinate, a new form of calcium preparation, may have better absorption, making it a better calcium supplement than calcium carbonate and CCM61. However, peptide chelated calcium has advantages over other calcium supplements62–64. A growing number of chelating peptides have been identified and have been shown to promote and improve mineral bioavailability65,66. The calcium peptide chelate produced by combining fishbone calcium and calcium-binding bone collagen peptide through enzymatic hydrolysis demonstrated improvement in calcium bioavailability67–69. The algae peptide-based calcium-chelating complex and calcium alginate nanoparticles have the potential to be utilized as a calcium supplement to improve bone health70–72. However, the production cost of peptide chelated calcium is high, and the yield is low. With the development of new preparation technology, peptide chelated calcium will likely become a good calcium supplement.
Figure 3.
Flow chart of preparation of chelate calcium from Marine calcium.
Other functions of marine source calcium
Biological activity
Marine biological calcium has biological functions other than improving calcium homeostasis and bone health. For example, coral calcium was shown to regulate blood pressure and prevent the metastasis of colon cancer30,73,74. Calcium spirulan, derived from Spirulina platensis (Arthrospira platensis), a filamentous blue-green microalga from rivers and lakes, was shown to inhibit herpes simplex virus 1 actively, and possibly, infections caused by other herpesviruses75. Coral calcium hydroxide can act as an antioxidant, slowing senescence in mice and preventing hepatic steatosis76–78. The calcium oxide made from scallop shells was shown to inhibit Pseudomonas aeruginosa, a spoilage bacterium for eggs with a strong resistance to chemical agents such as sanitizers and disinfectants79. Calcium derived from oysters exhibited good efficacy in suppressing the formation and proliferation of oral squamous cell carcinoma80.
New materials
Calcium from marine sources can serve as a raw material for the production of high-value-added compounds that can be used in biomedical research and pharmaceutical, healthcare, and food industries81. Previous studies have found a huge potential for producing porous scaffolds from oyster shells, clamshells, cuttlefish bones, and salmon bones82–85. The structural features of these scaffolds were found to be conducive to improve biological activities, including mechanical properties, and bone tissue growth and vascularization86. The production of natural hydroxyapatite (nHAP) from salmon bones and rainbow trout has a great potential as bone implant material substitutes in bone tissue engineering87. Marine biological calcium can also be used to prepare adsorption materials, demonstrating its potentially wide applications in water treatment. For example, calcium-rich biochar prepared from crab shells can be used to remove dyes and phosphorus from wastewater88,89. The acid-insoluble calcium silicate hydrates synthesized from oyster shells were also applicable in removing organic pollutants and heavy metal ions90. Single-phase hydroxyapatite (HA) and biphasic calcium phosphate (HA/β-TCP), which are derived from Atlantic cod bones, have no known cytotoxic effects and have demonstrated good bioactivity in simulated body fluid91. Consequently, calcium phosphate derived from marine organisms has a promising future in fabricating bacterial infection-resistant bone substitutes or bone defect healing. HA (Ca10(PO4)6(OH)2, HAp) derived from codfish bones is a calcium phosphate, which is a safer option for sunscreen formulation, indicating its potential across a wide range of applications in health care products and cosmetics92.
Food additives
Biological calcium from marine processing waste can still be used in food processing. For example, fish bones can be added to fish surimi to improve the gel performance of the product93. Oyster shell calcium powder can improve the chewiness and springiness of restructured ham94. Calcium-rich shrimp and crab shells can also be used to prepare food flocculants95. There are many food additives containing calcium, such as calcium carbonate, calcium silicate, calcium sulphate, and calcium lactate. The calcium additives from marine organisms may be safer because they have a natural origin.
Conclusions and future perspectives
Marine processing waste is often considered useless; however, it is an abundant and low-cost source of calcium. A study found that 55 brands of calcium supplements can be classified into seven categories based on the major ingredient in them and three or more categories were found to be derived from marine organisms mainly oyster/clamshells, algae, shark cartilage, and chelated calcium products (Table 1)10. In addition, calcium from marine organisms has good bioavailability and biological function. Reusing by-products from marine organisms can increase the added calcium value and reduce the risk of environmental pollution. For the development of calcium supplements, future work should focus on the comprehensive utilization of proteins, collagen, chitin, calcium, and other nutrients in marine organisms and the use of specific active ingredients to increase the bioavailability of calcium. In other applications, research must likely focus on the transformation of marine calcium into health foods, new materials, or food additives to expand to a commercial scale.
Table 1.
Common commercial calcium supplements from Marine sources.
| Name | Brand | Country | Calcium form | Source |
|---|---|---|---|---|
| Calcium carbonate | Life | Canada | Oyster shell powder, VD3 | Oyster shells |
| Calcian + D3 | Jamieson | Canada | Calcium citrate, calcium malate, fumaric acid calcium, calcium succinate, calcium carbonate, VD3 | Oyster shells |
| Natural calcium | FOR BECARED ONE | USA | VD3, calcium, collagen type II | Oyster shells |
| Shen Gu Pian | Duo Yuan Kang | China | Oyster shell powder | Oyster shells |
| MC calcium | MC | Japan | Oyster shell powder, ursodeoxycholic acid, lysine hydrochloride | Oyster shells |
| Coral calcium capsules | Catalo | USA | 100% pure coral powder | Coral |
| Coral calcium | Holland and Barrett | UK | 100% pure coral powder | Coral |
|
Haibrusk Shark cartilage |
Bjorge ocean | Norway | Shark cartilage powder | Shark cartilage |
| Bonecare Kids calcium complex chews | Clinicians | New Zealand | Calcified lithothamnion, alcarerum-red algae, trimagnesium citrate, boron citrate, zinc amino acid chelate, manganese amino acid chelate, VD3 | Red algae |
| Atomy tri-active calcium | Atomy | Korea | Seaweed meal, magnesium oxide, calcium citrate, serum calcium, VD | Red algae |
Acknowledgements
The research was supported by Public Projects of Zhejiang Province (2017C32098, LGN20C200003) and Wenzhou Science and Technology Project (ZD202003, N20180011, N20190017). We thank Editage (https://www.editage.cn/) for linguistic assistance during the preparation of this manuscript.
Author contributions
Conceptualization, L.S. and D.Z.; investigation, J.Y.; writing—original draft preparation, Y.X. and J.Y.; writing—review and editing, Y.X.; supervision, D.Z.; project administration, L.S.; funding acquisition, L.S. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shojaeian Z, Sadeghi R, Latifnejad RR. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ocvary syndrome: A systematic review and meta-analysis. Caspian J. Intern. Med. 2019;10:359–369. doi: 10.22088/cjim.10.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong AM, Kang K, Weiler HA, Morin SN. Fermented milk products and bone health in postmenopausal women: A systematic review of randomized controlled trials, prospective cohorts, and case–control studies. Adv. Nutr. 2020;11:251–265. doi: 10.1093/advances/nmz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SK, Ravichandran YD, Kong CS. Applications of calcium and its supplement derived from marine organisms. Crit. Rev. Food Sci. 2012;52:469–474. doi: 10.1080/10408391003753910. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg JB, Frei BB, Fulgoni VL, Weaver CM, Zeisel SH. Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in US adults. Nutrients. 2017;9:849. doi: 10.3390/nu9080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer V, Haffner-Luntzer M, Amling M, Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cells Mater. 2018;35:365–385. doi: 10.22203/eCM.v035a25. [DOI] [PubMed] [Google Scholar]
- 6.Lee YK, et al. Low calcium and vitamin D intake in Korean women over 50 years of age. J. Bone Miner. Metab. 2017;35:522–528. doi: 10.1007/s00774-016-0782-7. [DOI] [PubMed] [Google Scholar]
- 7.Weaver CM, Bischoff-Ferrari HA, Shanahan CJ. Cost-benefit analysis of calcium and vitamin D supplements. Arch. Osteoporos. 2019;14:50. doi: 10.1007/s11657-019-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RL, et al. Reduced dietary calcium and vitamin D results in preterm birth and altered placental morphogenesis in mice during pregnancy. Reprod. Sci. 2020;27:1330–1339. doi: 10.1007/s43032-019-00116-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim OH, et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. ELife. 2020;9:e52709. doi: 10.7554/eLife.52709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarosz M, Rychlik E. P-184-calcium and vitamin D intake and colorectal cancer morbidity rates in Poland. Ann. Oncol. 2019;30:v50. doi: 10.1093/annonc/mdz155.183. [DOI] [Google Scholar]
- 11.Reid IR, Bristow SM, Bolland MJ. Calcium supplements: Benefits and risks. J. Intern. Med. 2015;278:354–368. doi: 10.1111/joim.12394. [DOI] [PubMed] [Google Scholar]
- 12.Kim M. Mercury, cadmium and arsenic contents of calcium dietary supplements. Food Addit. Contam. 2004;21:763–767. doi: 10.1080/02652030410001713861. [DOI] [PubMed] [Google Scholar]
- 13.Ross EA, Szabo NJ, Tebbett IR. Lead content of calcium supplements. JAMA. 2000;284:1425–1429. doi: 10.1001/jama.284.11.1425. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Rodriguez AE, Nowzari H. The risk of prion infection through bovine grafting materials. Clin. Implant. Dent. R. 2016;18:1095–1102. doi: 10.1111/cid.12391. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Nowzari H, Rich SK. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant. Dent. R. 2013;15:645–653. doi: 10.1111/j.1708-8208.2011.00407.x. [DOI] [PubMed] [Google Scholar]
- 16.Lecerf JM, et al. Effects of two marine dietary supplements with high calcium content on calcium metabolism and biochemical marker of bone resorption. Eur. J. Clin. Nutr. 2008;62:879–884. doi: 10.1038/sj.ejcn.1602797. [DOI] [PubMed] [Google Scholar]
- 17.Flammini L, et al. Hake fish bone as a calcium source for efficient bone mineralization. Int. J. Food Sci. Nutr. 2016;67:265–273. doi: 10.3109/09637486.2016.1150434. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen BD, Cate RE, O Connor-Robison CI. A marine mineral supplement alters markers of bone metabolism in yearling arabians. J. Equine Vet. Sci. 2010;30:419–424. doi: 10.1016/j.jevs.2010.07.003. [DOI] [Google Scholar]
- 19.Pateiro M, et al. Nutritional profiling and the value of processing by-products from gilthead sea bream (Sparus Aurata) Mar. Drugs. 2020;18:101. doi: 10.3390/md18020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toppe J, Albrektsen S, Hope B, Aksnes A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:395–401. doi: 10.1016/j.cbpb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Patwardhan UN, Pahuja DN, Samuel AM. Calcium bioavailability: An in vivo assessment. Nutr. Res. 2001;21:667–675. doi: 10.1016/S0271-5317(01)00278-0. [DOI] [Google Scholar]
- 22.Edmonds JS, Shibata Y, Lenanton RCJ, Caputi N, Morita M. Elemental composition of jaw cartilage of gummy shark mustelus antarcticus Günther. Sci. Total Environ. 1996;192:151–161. doi: 10.1016/S0048-9697(96)05311-9. [DOI] [Google Scholar]
- 23.Chakraborty P, Sahoo S, Bhattacharyya DK, Ghosh M. Marine lizardfish (Harpadon nehereus) meal concentrate in preparation of ready-to-eat protein and calcium rich extruded snacks. J. Food Sci. Technol. 2020;57:338–349. doi: 10.1007/s13197-019-04066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart-Sinclair PJ, Last KS, Payne BL, Wilding TA. A global assessment of the vulnerability of shellfish aquaculture to climate change and ocean acidification. Ecol. Evol. 2020;10:3518–3534. doi: 10.1002/ece3.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FAO . The State of World Fisheries and Aquaculture 2018. Rome: FAO; 2018. [Google Scholar]
- 26.Petenuci ME, et al. Fatty acid concentration, proximate composition, and mineral composition in fishbone flour of Nile Tilapia. Arch. Latinoam. Nutr. 2008;58:87–90. [PubMed] [Google Scholar]
- 27.Fujita T, Fukase M, Miyamoto H, Matsumoto T, Ohue T. Increase of bone mineral density by calcium supplement with oyster shell electrolysate. Bone Miner. 1990;11:85–91. doi: 10.1016/0169-6009(90)90017-A. [DOI] [PubMed] [Google Scholar]
- 28.Miura T, Takayama Y, Nakano M. Effect of shellfish calcium on the apparent absorption of calcium and bone metabolism in ovariectomized rats. Biosci. Biotech. Bioch. 1999;63:40–45. doi: 10.1271/bbb.63.40. [DOI] [PubMed] [Google Scholar]
- 29.Kandra P, Challa MM, Jyothi HK. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biot. 2012;93:17–29. doi: 10.1007/s00253-011-3651-2. [DOI] [PubMed] [Google Scholar]
- 30.Gbenebor OP, Adeosun SO, Lawal GI, Jun S. Role of CaCO3 in the physicochemical properties of crustacean-sourced structural polysaccharides. Mater. Chem. Phys. 2016;184:203–209. doi: 10.1016/j.matchemphys.2016.09.043. [DOI] [Google Scholar]
- 31.Ding H, Lv L, Wang Z, Liu L. Study on the "glutamic acid-enzymolysis" process for extracting chitin from crab shell waste and its by-product recovery. Appl. Biochem. Biotech. 2020;190:1074–1091. doi: 10.1007/s12010-019-03139-2. [DOI] [PubMed] [Google Scholar]
- 32.Laine J, Labady M, Albornoz A, Yunes S. Porosities and pore sizes in coralline calcium carbonate. Mater. Charact. 2008;59:1522–1525. doi: 10.1016/j.matchar.2007.12.002. [DOI] [Google Scholar]
- 33.Reddy PN, Lakshmana M, Udupa UV. Effect of Praval bhasma (Coral Calx), a natural source of rich calcium on bone mineralization in rats. Pharmacol. Res. 2003;48:593–599. doi: 10.1016/S1043-6618(03)00224-X. [DOI] [PubMed] [Google Scholar]
- 34.Banu J, et al. Dietary coral calcium and zeolite protects bone in a mouse model for postmenopausal bone loss. Nutr. Res. 2012;32:965–975. doi: 10.1016/j.nutres.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Marsham S, Scott GW, Tobin ML. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 2007;100:1331–1336. doi: 10.1016/j.foodchem.2005.11.029. [DOI] [Google Scholar]
- 36.Brennan O, et al. A natural, calcium-rich marine multi-mineral complex preserves bone structure, composition and strength in an ovariectomised rat model of osteoporosis. Calcified Tissue Int. 2017;101:445–455. doi: 10.1007/s00223-017-0299-7. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs R, Gordon M, Jerina M. Feeding a seaweed-derived calcium source versus calcium carbonate on physiological parameters of horses. J. Equine Vet. Sci. 2019;76:83. doi: 10.1016/j.jevs.2019.03.108. [DOI] [Google Scholar]
- 38.Yamaguchi M, Hachiya S, Hiratuka S, Suzuki T. Effect of marine algae extract on bone calcification in the femoral-metaphyseal tissues of rats: Anabolic effect of sargassum horneri. J. Health Sci. 2001;47:533–538. doi: 10.1248/jhs.47.533. [DOI] [Google Scholar]
- 39.Uenishi K, et al. Fractional absorption of active absorbable algal calcium (AAACA) and calcium carbonate measured by a dual stable-isotope method. Nutrients. 2010;2:752–761. doi: 10.3390/nu2070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K, et al. The good, the bad, and the ugly of calcium supplementation: A review of calcium intake on human health. Clin. Interv. Aging. 2018;13:2443–2452. doi: 10.2147/CIA.S157523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita T, Ohue T, Fujii Y, Miyauchi A, Takagi Y. Heated oyster shell-seaweed calcium (AAACA) on osteoporosis. Calcif. Tissue Int. 1996;58:226–230. doi: 10.1007/BF02508640. [DOI] [PubMed] [Google Scholar]
- 42.Tsugawa N, et al. Bioavailability of calcium from calcium carbonate, dl-calcium lactate, l-calcium lactate and powdered oyster shell calcium in vitamin d-deficient or -replete rats. Biol. Pharm. Bull. 1995;18:677–682. doi: 10.1248/bpb.18.677. [DOI] [PubMed] [Google Scholar]
- 43.Huo J, Deng S, Xie C, Tong G. Preparation and biological efficacy of haddock bone calcium tablets. Chin. J. Oceanol. Limn. 2010;28:371–378. doi: 10.1007/s00343-010-9019-0. [DOI] [Google Scholar]
- 44.Vavrusova M, Skibsted LH. Calcium nutrition. Bioavailability and fortification. LWT Food Sci. Technol. 2014;59:1198–1204. doi: 10.1016/j.lwt.2014.04.034. [DOI] [Google Scholar]
- 45.Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. 2020;140:55–63. doi: 10.1016/j.maturitas.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Bailey RL, Picciano MF, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 2010;140:817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palermo A, et al. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metab. Dis. 2019;20:353–364. doi: 10.1007/s11154-019-09520-0. [DOI] [PubMed] [Google Scholar]
- 48.Hanzlik RP, Fowler SC, Fisher DH. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J. Pharmacol. Exp. Ther. 2005;313:1217–1222. doi: 10.1124/jpet.104.081893. [DOI] [PubMed] [Google Scholar]
- 49.Wiria M, et al. Relative bioavailability and pharmacokinetic comparison of calcium glucoheptonate with calcium carbonate. Pharmacol. Res. Perspect. 2020;8:e589. doi: 10.1002/prp2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straub DA. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007;22:286–296. doi: 10.1177/0115426507022003286. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Wang J, Guo Y. Effect of collagen peptide, alone and in combination with calcium citrate, on bone loss in tail-suspended rats. Molecules. 2020;25:782. doi: 10.3390/molecules25040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, et al. Combined oral administration of bovine collagen peptides with calcium citrate inhibits bone loss in ovariectomized rats. PLoS One. 2015;10:e135019. doi: 10.1371/journal.pone.0135019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JS, et al. Effect of polycalcium, a mixture of polycan and calcium lactate-gluconate in a 1:9 weight ratio, on rats with surgery-induced osteoarthritis. Exp. Ther. Med. 2015;9:1780–1790. doi: 10.3892/etm.2015.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi JS, et al. Antiosteoporotic effects of polycan in combination with calcium lactate–gluconate in ovariectomized rats. Exp. Ther. Med. 2014;8:957–967. doi: 10.3892/etm.2014.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, et al. Preparation of cucumber seed peptide-calcium chelate by liquid state fermentation and its characterization. Food Chem. 2017;229:487–494. doi: 10.1016/j.foodchem.2017.02.121. [DOI] [PubMed] [Google Scholar]
- 56.Bajaj M, Freiberg A, Winter J, Xu Y, Gallert C. Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl. Microbiol. Biot. 2015;99:9835–9846. doi: 10.1007/s00253-015-6841-5. [DOI] [PubMed] [Google Scholar]
- 57.Tang S, et al. Preparation of a fermentation solution of grass fish bones and its calcium bioavailability in rats. Food Funct. 2018;9:4135–4142. doi: 10.1039/C8FO00674A. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, et al. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018;239:416–426. doi: 10.1016/j.foodchem.2017.06.090. [DOI] [PubMed] [Google Scholar]
- 59.Wu W, et al. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019;284:80–89. doi: 10.1016/j.foodchem.2019.01.103. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, et al. Isolation and identification of a whey protein-sourced calcium-binding tripeptide Tyr-Asp-Thr. Int. Dairy J. 2015;40:16–23. doi: 10.1016/j.idairyj.2014.08.013. [DOI] [Google Scholar]
- 61.Shankar KMS, Raizada P, Jain R. A randomized open-label clinical study comparing the efficacy, safety, and bioavailability of calcium lysinate with calcium carbonate and calcium citrate malate in osteopenia patients. J. Orthop. Case Rep. 2018;8:15–19. doi: 10.13107/jocr.2250-0685.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo L, et al. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci. Tech. 2014;37:92–105. doi: 10.1016/j.tifs.2014.02.007. [DOI] [Google Scholar]
- 63.Liu FR, Wang L, Wang R, Chen ZX. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide–calcium complex. J. Agric. Food Chem. 2013;61:7537–7544. doi: 10.1021/jf401868z. [DOI] [PubMed] [Google Scholar]
- 64.Zhao L, Huang S, Cai X, Hong J, Wang S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods. 2014;10:46–53. doi: 10.1016/j.jff.2014.05.013. [DOI] [Google Scholar]
- 65.Hou H, et al. A novel calcium-binding peptide from antarctic krill protein hydrolysates and identification of binding sites of calcium–peptide complex. Food Chem. 2018;243:389–395. doi: 10.1016/j.foodchem.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 66.Sun N, Jin Z, Li D, Yin H, Lin S. An exploration of the calcium-binding mode of egg white peptide, Asp-His-Thr-Lys-Glu, and in vitro calcium absorption studies of peptide-calcium complex. J. Agric. Food Chem. 2017;65:9782–9789. doi: 10.1021/acs.jafc.7b03705. [DOI] [PubMed] [Google Scholar]
- 67.Kim SK, Jung WK. Beneficial effect of teleost fish bone peptide as calcium supplements for bone mineralization. Adv. Food Nutr. Res. 2012;65:287–295. doi: 10.1016/B978-0-12-416003-3.00019-6. [DOI] [PubMed] [Google Scholar]
- 68.Peng Z, Hou H, Zhang K, Li B. Effect of calcium-binding peptide from pacific cod (Gadus Macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017;221:373–378. doi: 10.1016/j.foodchem.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 69.Jung WK, Lee BJ, Kim SK. Fish-bone peptide increases calcium solubility and bioavailability in ovariectomised rats. Br. J. Nutr. 2006;95:124–128. doi: 10.1079/BJN20051615. [DOI] [PubMed] [Google Scholar]
- 70.Lin J, Cai X, Tang M, Wang S. Preparation and evaluation of the chelating nanocomposite fabricated with marine algae Schizochytrium sp. protein hydrolysate and calcium. J. Agric. Food Chem. 2015;63:9704–9714. doi: 10.1021/acs.jafc.5b04001. [DOI] [PubMed] [Google Scholar]
- 71.Guo H, Hong Z, Yi R. Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J. Food Sci. 2015;80:N1595–N1601. doi: 10.1111/1750-3841.12912. [DOI] [PubMed] [Google Scholar]
- 72.Bae YJ, et al. Magnesium supplementation through seaweed calcium extract rather than synthetic magnesium oxide improves femur bone mineral density and strength in ovariectomized rats. Biol. Trace Elem. Res. 2011;144:992–1002. doi: 10.1007/s12011-011-9073-2. [DOI] [PubMed] [Google Scholar]
- 73.Hirota Y, Sugisaki T. Effects of the coral calcium as an inhibitory substance against colon cancer and its metastasis in the lungs. Nutr. Res. 2000;20:1557–1567. doi: 10.1016/S0271-5317(00)00240-2. [DOI] [Google Scholar]
- 74.Ripamonti U, Crooks J, Khoali L, Roden L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials. 2009;30:1428–1439. doi: 10.1016/j.biomaterials.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 75.Mader J, et al. Calcium spirulan derived from spirulina platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J. Allergy Clin. Immunol. 2016;137:197–203. doi: 10.1016/j.jaci.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 76.Hou C, et al. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem. Pharmacol. 2016;103:85–97. doi: 10.1016/j.bcp.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 77.Ueda Y, Kojima T, Oikawa T. Hippocampal gene network analysis suggests that coral calcium hydride may reduce accelerated senescence in mice. Nutr. Res. 2011;31:863–872. doi: 10.1016/j.nutres.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Ueda Y, Nakajima A, Oikawa T. Hydrogen-related enhancement of in vivo antioxidant ability in the brain of rats fed coral calcium hydride. Neurochem. Res. 2010;35:1510–1515. doi: 10.1007/s11064-010-0204-5. [DOI] [PubMed] [Google Scholar]
- 79.Jung SJ, et al. Bactericidal effect of calcium oxide (scallop-shell powder) against pseudomonas aeruginosa biofilm on quail egg shell, stainless steel, plastic, and rubber. J. Food Sci. 2017;82:1682–1687. doi: 10.1111/1750-3841.13753. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y, et al. Inhibition of 4NQO-induced oral carcinogenesis by dietary oyster shell calcium. Integr. Cancer. Ther. 2016;15:96–101. doi: 10.1177/1534735415596572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terzioglu P, Ogut H, Kalemtas A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;91:899–911. doi: 10.1016/j.msec.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Shen Y, et al. Engineering scaffolds integrated with calcium sulfate and oyster shell for enhanced bone tissue regeneration. ACS Appl. Mater. Inter. 2014;6:12177–12188. doi: 10.1021/am501448t. [DOI] [PubMed] [Google Scholar]
- 83.Naga SM, El-Maghraby HF, Mahmoud EM, Talaat MS, Ibrhim AM. Preparation and characterization of highly porous ceramic scaffolds based on thermally treated fish bone. Ceram. Int. 2015;41:15010–15016. doi: 10.1016/j.ceramint.2015.08.057. [DOI] [Google Scholar]
- 84.Rocha JHG, et al. Scaffolds for bone restoration from cuttlefish. Bone. 2005;37:850–857. doi: 10.1016/j.bone.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Bramhe S, Kim TN, Balakrishnan A, Chu MC. Conversion from biowaste venerupis clam shells to hydroxyapatite nanowires. Mater. Lett. 2014;135:195–198. doi: 10.1016/j.matlet.2014.07.137. [DOI] [Google Scholar]
- 86.Brennan O, Stenson B, Widaa A, O Gorman DM, O Brien FJ. Incorporation of the natural marine multi-mineral dietary supplement aquamin enhances osteogenesis and improves the mechanical properties of a collagen-based bone graft substitute. J. Mech. Behav. Biomed. 2015;47:114–123. doi: 10.1016/j.jmbbm.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Shi P, et al. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;90:706–712. doi: 10.1016/j.msec.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 88.Dai L, et al. Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal. Bioresour. Technol. 2018;267:510–516. doi: 10.1016/j.biortech.2018.07.090. [DOI] [PubMed] [Google Scholar]
- 89.Dai L, et al. Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J. Environ. Manag. 2017;198:70–74. doi: 10.1016/j.jenvman.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 90.You W, et al. Functionalized calcium silicate nanofibers with hierarchical structure derived from oyster shells and their application in heavy metal ions removal. Phys. Chem. Chem. Phys. 2016;18:15564–15573. doi: 10.1039/C6CP01199C. [DOI] [PubMed] [Google Scholar]
- 91.Piccirillo C, et al. Hydroxyapatite-based materials of marine origin: A bioactivity and sintering study. Mat. Sci. Eng. C. 2015;51:309–315. doi: 10.1016/j.msec.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 92.Teixeira CMA, et al. Effect of preparation and processing conditions on UV absorbing properties of hydroxyapatite-Fe2O3 sunscreen. Mat. Sci. Eng. C. 2017;71:141–149. doi: 10.1016/j.msec.2016.09.065. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Z, Lanier T, Farkas B, Li B. Transglutaminase and high pressure effects on heat-induced gelation of alaska pollock (Theragra Chalcogramma) surimi. J. Food Eng. 2014;131:154–160. doi: 10.1016/j.jfoodeng.2014.01.022. [DOI] [Google Scholar]
- 94.Choi JS, Lee HJ, Jin SK, Lee HJ, Choi YI. Effect of oyster shell calcium powder on the quality of restructured pork ham. Korean J. Food Sci. Technol. 2014;34:372–377. doi: 10.5851/kosfa.2014.34.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jun JY, et al. Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd) Food Sci. Nutr. 2019;7:547–553. doi: 10.1002/fsn3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]