Abstract
The impact of reperfusion therapies on cognition has been poorly explored and little knowledge exists. We explored the influence of endovascular treatment (EVT) on cognitive outcome in patients with anterior circulation ischemic stroke. Patients presenting with ischemic stroke due to anterior large vessel occlusion who underwent intravenous thrombolysis (IVT) alone or EVT plus IVT were recruited. Cognitive abilities were evaluated at 6 months from stroke through a neuropsychological test battery. A total of 88 patients with a mean age of 66.3 ± 12.9 years were included, of which 38 treated with IVT alone and 50 with IVT plus EVT. Compared to patients treated with IVT alone, patients who received EVT plus IVT performed significantly better at the neuropsychological tests exploring executive functions, attention, abstract reasoning, visuospatial ability, visual and verbal and memory. At multivariable regression analysis, the EVT was independently associated with the 6-month cognitive performance after the adjustment for age, sex, admission National Institutes of Health Stroke Scale score, systolic blood pressure, glucose level, Alberta Stroke Program Early CT score, side of stroke, site of occlusion, and Back Depression Inventory score [Stroop Test Word Reading: adjβ = 13.99, 95% confidence interval (CI) 8.47–19.50, p < 0.001; Stroop Test Colour Naming: adjβ = 6.63, 95% CI 2.46–10.81, p = 0.002; Trail Making Test-A: adjβ = − 92.98, 95% CI − 153.76 to − 32.20, p = 0.003; Trail Making Test-B: adjβ = − 181.12, 95% CI − 266.09 to − 96.15; p < 0.001; Digit Span Test Forward: adjβ = 1.44, 95% CI 0.77–2.10, p < 0.001; Digit Span Test Backward: adjβ = 1.10, 95% CI 0.42–1.77, p = 0.002; Coloured Progressive Matrices: adjβ = 5.82, 95% CI 2.71–8.93, p < 0.001; Rey Complex Figure Test-Copy: adjβ = 6.02, 95% CI 2.74–9.30, p < 0.001; Rey Complex Figure Test-Immediate recall: adjβ = 6.00, 95% CI 2.34–9.66, p = 0.002; Rey Complex Figure Test-Delayed recall: adjβ = 5.73, 95% CI 1.95–9.51, p = 0.003; Rey Auditory Verbal Learning Test-Immediate recall: adjβ = 12.60, 95% CI 6.69–18.52, p < 0.001; Rey Auditory Verbal Learning Test-Delayed recall: adjβ = 1.85, 95% CI 0.24–3.45, p = 0.025]. Patients treated with EVT plus IVT had better cognitive performance than patients treated with IVT alone at 6 months from anterior circulation ischemic stroke.
Subject terms: Medical research, Neurology
Introduction
Cognitive impairment is a common consequence after stroke1,2. It is closely related to disability, dependency and institutionalization, and it is a major determinant of poor quality of life in stroke survivors3–5. So far, the impact of reperfusion therapies on cognition has been poorly explored and little knowledge exists. Indeed, physical recovery represents the main endpoint in stroke trials, whereas cognitive outcome is generally overlooked6,7.
The aim of this study was to investigate the effect of the endovascular treatment (EVT) on cognitive functioning in patients with ischemic stroke due to proximal arterial occlusion of the anterior circulation by comparing the 6-month neuropsychological performance in patients treated with intravenous thrombolysis (IVT) alone and IVT plus EVT.
Results
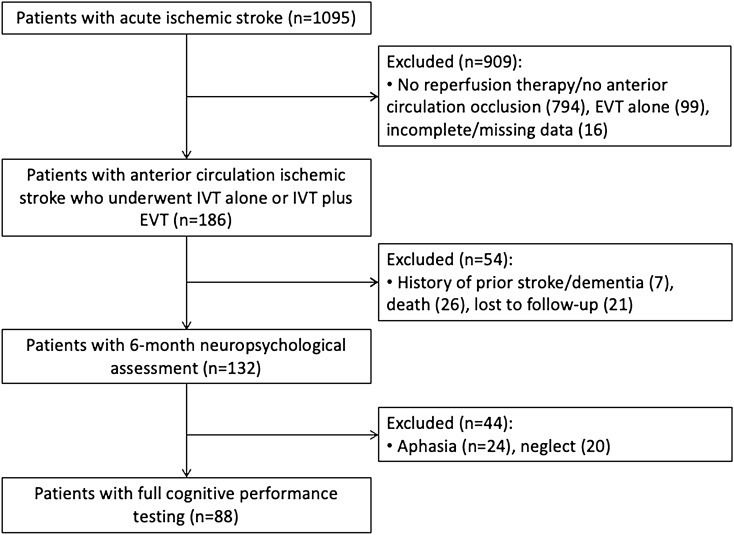
A total of 186 out of 1095 patients admitted to our Stroke Unit for ischemic stroke underwent IVT alone or IVT plus EVT for a proximal arterial occlusion of the anterior circulation (Fig. 1). Fifty-four patients were excluded due to history of prior stroke/dementia (n = 7), death (n = 26), and unavailability of neuropsychological assessment as lost to follow-up (n = 21). The comparison of baseline characteristics between the patients with 6-month neuropsychological assessment and those who were excluded due to lack of follow-up did not show significant differences (Supplementary Table S1). Among the patients (n = 132) who underwent neuropsychological evaluation at 6 months from stroke, 44 patients were further excluded from the full cognitive performance testing since they presented with aphasia (n = 24) and neglect (n = 20). The characteristics of the patients excluded due to the impairment in language and visuo-spatial inattention domains are shown in Supplementary Table S2.
Figure 1.
Patient selection flow diagram. EVT endovascular treatment, IVT intravenous thrombolysis.
Accordingly, 88 patients were included in the analysis, of which 38 treated with IVT alone and 50 with IVT plus EVT. Patients did not receive EVT due to stroke occurrence before full implementation of EVT delivery at the site (n = 21), mild neurologic deficit at onset (n = 8), successful opening of occlusion/marked improvement of neurological deficit by IVT (n = 8), and very elderly (n = 1).
The mean age of the patients was 66.3 ± 12.9 years and 31 (35.2%) were females; 45 (51.1%) patients had right and 43 (48.9%) left hemisphere stroke. Baseline demographic and clinical characteristics of the study cohort according to the treatment group are shown in Table 1. No statistically significant differences in the prevalence of vascular risk factors and stroke severity emerged among the two groups, with the exception of serum glucose levels and NIHSS score at admission, which were higher among the patients treated with IVT plus EVT.
Table 1.
Baseline characteristics of patients.
| IVT (n = 38) | IVT plus EVT (n = 50) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 67.2 (11.4) | 65.6 (14.1) | 0.562a |
| Male sex | 29 (76.3) | 28 (56.0) | 0.048b |
| Education (years) | 8 [5–13] | 8 [8–13] | 0.104c |
| Clinical history | |||
| Current smoking | 8 (21.1) | 11 (22.0) | 0.915b |
| Hypertension | 25 (65.8) | 31 (62.0) | 0.714b |
| Diabetes mellitus | 3 (13.2) | 5 (10.0) | 0.644b |
| Dyslipidaemia | 23 (60.5) | 22 (44.1) | 0.124b |
| Coronary artery disease | 4 (10.5) | 10 (20.0) | 0.229b |
| Admission assessment | |||
| Systolic BP (mmHg) | 150 [140–160] | 150 [140–160] | 0.352c |
| Serum glucose (mg/dl) | 106 [89–120] | 129 [109–140] | < 0.001c |
| NIHSS score | 10.2 (5.7) | 15.8 (4.0) | < 0.001a |
| ASPECTS value | 9 [8–10] | 8 [7–10] | 0.158c |
| Location of intracranial occlusion | 0.122b | ||
| Internal carotid artery | 3 (7.9) | 3 (6.0) | |
| dInternal carotid artery terminus | – | 4 (8.0) | |
| Middle cerebral artery | |||
| First segment | 22 (57.9) | 34 (68.0) | |
| Second segment | 11 (29.0) | 9 (18.0) | |
| Anterior cerebral artery A1 | 2 (5.3) | – | |
Data are mean (SD) or median [IQR] for continuous variables, and n (%) for categorical variables.
ASPECT Alberta Stroke Program Early CT, BP blood pressure, EVT endovascular treatment, IQR interquartile range, IVT intravenous thrombolysis, NIHSS National Institutes of Health Stroke Scale, SD standard deviation.
aTwo-sample t test.
bChi-squared test.
c Mann–Whitney test.
dAssociated internal carotid artery and middle cerebral artery occlusion (tandem occlusion).
The scores obtained by the patients in the neuropsychological tests performed at 6 months from stroke are summarized in Table 2. Patients treated with IVT alone obtained lower (worse) scores at the SCWT, DST, CPM, RCFT-C, RCFT-I, RCFT-D, RAVLT-I and RAVLT-D and higher (worse) scores at the TMT-A and TMT-B. Patients in the IVT group had also higher (worse) scores on the BDI in comparison to patients treated with IVT plus EVT [8.0 (2.0–11.0) versus 3.5 (0.0–9.0); p = 0.096] and higher (worse) scores on the mRS [3.0 (1.0–4.0) versus 1.0 (0.0–2.0); p < 0.001] in comparison to patients treated with IVT plus EVT.
Table 2.
Cognitive performance at 6 months from stroke.
| IVT (n = 38) | IVT plus EVT (n = 50) | p value | |
|---|---|---|---|
| Stroop test | |||
| Word reading | 33.4 (16.0) | 41.5 (11.1) | 0.006a |
| Colour naming | 18.4 (10.9) | 23.2 (7.8) | 0.018a |
| Trail making test | |||
| Part A | 90.5 [36.0–307.0] | 36.0 [27.0–87.0] | 0.005b |
| Part B | 233.5 [57.0–562.0] | 73.0 [41–227] | 0.003b |
| Digit span test | |||
| Forward | 4.8 [3.1–5.3] | 5.2 [4.3–5.8] | 0.018b |
| Backward | 3.2 [2.0–4.3] | 4.1 [3.3–5.0] | 0.005b |
| Coloured Progressive Matrices | 25.0 [18.5–31.0] | 28.8 [24.5–33.5] | 0.016b |
| Rey complex figure test | |||
| Copy | 28.0 [21.4–30.6] | 30.5 [27.0–31.5] | 0.007b |
| Immediate recall | 18.0 [12.6–24.6] | 24.3 [18.4–28.1] | 0.016b |
| Delayed recall | 16.9 [8.7–20.6] | 18.6 [13.7–26.5] | 0.022b |
| Rey auditory verbal learning test | 0.081a | ||
| Immediate recall | 22.9 (11.3) | 32.9 (13.7) | < 0.001a |
| Delayed recall | 7.0 (4.1) | 8.6 (3.0) | 0.038a |
Data are mean (SD) or median [IQR]. Higher values indicate worse performance for the Trail Making Tests and better performance for all the other cognitive tests.
EVT endovascular treatment, IVT intravenous thrombolysis.
aTwo-sample t test.
bMann–Whitney test.
The results of the regression analysis are shown in Table 3. The acute stroke treatment resulted a significant predictor of 6-month cognitive outcome being the EVT plus IVT associated with better cognitive performances, before and after the adjustment for potential confounding factors (Table 3). None of the multivariate models suffered from collinearity (variance inflation factors ranged from 1.10 to 2.05).
Table 3.
Associations between 6-month cognitive performance and stroke treatment.
| Dependent variable | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Stroop test word reading | 8.16 | 2.40 to 13.91 | 0.006 | 13.99 | 8.47 to 19.50 | < 0.001 |
| Stroop test colour naming | 4.81 | 0.85 to 8.77 | 0.018 | 6.63 | 2.46 to 10.81 | 0.002 |
| Trail making test-A | − 113.49 | − 167.85 to − 59.14 | < 0.001 | − 92.98 | − 153.76 to − 32.20 | 0.003 |
| Trail making test-B | − 174.63 | − 254.64 to − 94.62 | < 0.001 | − 181.12 | − 266.09 to − 96.15 | < 0.001 |
| Digit span test forward | 0.79 | 0.19 to 1.39 | 0.011 | 1.44 | 0.77 to 2.10 | < 0.001 |
| Digit span test backward | 0.97 | 0.37 to 1.58 | 0.002 | 1.10 | 0.42 to 1.77 | 0.002 |
| Coloured progressive matrices | 3.81 | 0.75 to 6.87 | 0.015 | 5.82 | 2.71 to 8.93 | < 0.001 |
| Rey complex figure test-copy | 3.52 | 0.50 to 6.53 | 0.023 | 6.02 | 2.74 to 9.30 | < 0.001 |
| Rey complex figure test-immediate recall | 4.38 | 0.75 to 8.01 | 0.019 | 6.00 | 2.34 to 9.66 | 0.002 |
| Rey complex figure test-delayed recall | 4.44 | 1.18 to 7.69 | 0.008 | 5.73 | 1.95 to 9.51 | 0.003 |
| Rey auditory verbal learning test-immediate recall | 10.06 | 4.61 to 15.51 | < 0.001 | 12.60 | 6.69 to 18.52 | < 0.001 |
| Rey auditory verbal learning test-delayed recall | 1.60 | 0.09 to 3.11 | 0.038 | 1.85 | 0.24 to 3.45 | 0.025 |
Values are from linear regression models with cognitive scores as dependent variables.
ASPECT Alberta Stroke Program Early CT, BDI Back Depression Inventory, BP blood pressure, CI confidence interval, NIHSS National Institutes of Health.
aAdjustment for age, sex, admission NIHSS score, systolic BP, glucose level, ASPECT score, side of stroke, site of occlusion, BDI score.
Discussion
The main finding of this study was the better 6-month cognitive outcome observed in patients with stroke due to proximal large vessel occlusion who underwent IVT combined with EVT than IVT alone. At the follow-up visit, patients treated with IVT plus EVT performed better in the tests exploring executive functions and attention, abstract reasoning, constructive ability, and visuospatial and verbal memory.
The early recanalization of the occluded vessels is the main mechanism underlying the beneficial effects of the reperfusion therapies: it can restore flow to the ischemic penumbra and prevent its transformation into necrotic tissue8. Significant differences, however, exist between pharmacological intravenous fibrinolysis and mechanical endovascular clot removal as the responsiveness of large thrombi to enzymatic digestion is quite poor, whereas EVT can rapidly remove proximal clots9–12. The early recanalization through EVT has demonstrated to be more efficacious in lowering the risk of mortality, reduces the severity of disability and increases the rate of functional independence in comparison to standard medical care in patients with strokes due to large vessels occlusions13. The current study extends the findings of the recent randomized controlled trials and provides evidence that the EVT has the potentiality to favorably influence the post-stroke cognitive recovery. Indeed, the mRS—the most commonly used instrument to assess clinical outcome in stroke trials—relies mostly on physical functions and under-represents cognitive abilities. Detailed neuropsychological assessments can be beneficial both in stroke trials and clinical practice14–16. First, a broad spectrum of cognitive changes occurs after stroke and multiple domains and complex neuropsychological abilities are typically compromised. Second, cognitive deficits are prevalent also in patients with the most favorable clinical recovery and no apparent functional disability. Third, even milder cognitive deficits can have impact on independent functioning, occupational abilities and quality of life17,18. Additionally, items that are often affected by stroke, as processing speed, calculation, and praxis, are not included in current screening measures, and require a comprehensive investigation to be fully explored19.
In the prespecified secondary analysis of the REVASCAT (Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 h) trial, patients randomized to thrombectomy plus best medical treatment rather than best medical treatment alone performed better in the TMT-A and TMT-B at 3 months and 1 year after stroke due to anterior circulation proximal arterial occlusion20. It is however worth to notice that cognitive outcome was evaluated with one single test, which focused on executive functioning, and the lack of a comprehensive neuropsychological battery did not allow drawing conclusions on other cognitive domains. In addition, as motor dexterity is required to perform the TMT, the results and their interpretation could be biased by co-occurring impairment in motor function of the upper arm; the high proportion of participants who did not complete the task in the requested time and, hence, received the maximum time score, may have masked performance variability among the most severely impaired patients and allowed to identify significant differences between treatment groups only in functionally independent patients. Finally, symptoms of depression were collected indirectly and not included in multivariable analysis.
Recently, Xu et al. found that patients with mild to moderate anterior circulation infarct who received mechanical thrombectomy at broadened therapeutic window had higher scores in Mini-Mental State Examination and Montreal Cognitive Assessment tests at the 90 days follow-up than those receiving standard therapy treatment21. Although both tests can assess multiple cognitive abilities, they represent global screening tools for detecting cognitive impairment rather than instruments to thoroughly evaluate the neuropsychological domains. Moreover, the very small differences in total scores observed between the treatment arms and the lack of data about the individual items of both tests make difficult the clinical interpretation of the findings.
The main strengths of the current study included the comparison of patients who underwent treatment with rt-PA alone versus rt-PA combined with mechanical thrombectomy, which allowed to minimize the heterogeneity in baseline patients’ characteristics and time onset-to-treatment and, hence, estimate the actual effect deriving from the EVT. The exclusion of patients presenting with aphasia or neglect from the comprehensive neuropsychological assessment allowed to obtain a more reliably evaluation of the neurocognitive performance as results in cognitive tests are significantly affected and confounded by the presence of deficits in the domains of language and visuo-spatial inattention22,23. Finally, the 6-month interval from stroke to follow-up can be considered a sufficiently long time for the acute stroke effects to subside24,25, and the real-world setting of the research increased the generalizability of the findings to routine clinical practice. Nonetheless, some study shortcomings need to be considered. The retrospective analysis of data collected at a single academic center could have led to selection bias and findings need to be validated in independent cohorts. The relatively small sample size prevented sub-group analyses and no data on health-related quality of life have been considered at the follow-up. Additionally, no specific information regarding treatment success, including reperfusion rates, follow-up infarct volumes or hemorrhage rates have been considered, and further studies designed to comprehensively assess these parameters as well as the relationship between infarct location and test scores are warranted.
Conclusion
The growing number of stroke survivors has increased the interest in long-term sequelae and prediction of cognitive outcome. In this regard, treatment with EVT plus IVT can result in better cognitive performance than IVT alone in patients with anterior circulation ischemic stroke.
Methods
Study participants
We retrospectively identified consecutive patients with anterior circulation ischemic stroke, admitted to the Stroke Unit of the Marche Polytechnic University (Ancona, Italy) from January 2012 to June 2019, who were treated with IVT alone and IVT plus EVT, and underwent neuropsychological assessment at 6 months from the index event as part of routine care. The site serves in the region as referral comprehensive stroke center (hub) for mechanical thrombectomy for large vessel occlusion according to a drip-and-ship organizational model of stroke care. Patients were included if they had intracranial proximal arterial occlusion in the anterior circulation [intracranial carotid artery (ICA) or middle cerebral artery (M1/M2) or anterior cerebral artery (A1/A2)] demonstrated by vascular imaging (computed tomographic angiography or magnetic resonance angiography or digital subtraction angiography), received IVT within 4.5 h and started EVT within 6.0 h after the onset of stroke. IVT consisted of the administration of recombinant tissue plasminogen activator (rt-PA) at the dose of 0.9 mg/kg (maximum 90 mg; 10% bolus followed by a 60-min infusion). EVT consisted of mechanical thrombectomy with aspiration catheters alone, stent-retrievers alone, or both, depending on occlusion type and interventionist’s choice.
Data about demographic, vascular risk factors, medical history, baseline stroke severity according to the National Institutes of Health Stroke Scale (NIHSS) score26, admission systolic blood pressure (BP) and serum glucose were collected, as previously detailed27–29. The ischemic lesion extension was estimated according to the Alberta Stroke Program Early CT Score (ASPECTS) on head computed tomography (CT) performed in emergency prior to IVT administration30. Patients with a neurological or psychiatric history, pre-stroke modified Rankin Scale (mRS)31 score > 2, patients who did not perform the neuropsychological evaluation at 6 months from stroke and those who presented aphasia or neglect at the 6-month evaluation according to the Aphasia Neuropsychological Exam (ANE) (language)32 and Apples Cancellation Test (ACT) (visuo-spatial inattention)33 were not included.
Neuropsychological assessment
The neuropsychological assessment was administered by a trained examiner at a single session 6 months after stroke using standardized cognitive tests at the Clinic of Neurorehabilitation of the Marche Polytechnic University as part of clinical care. Scores obtained in the following neuropsychological tests were considered in the current analysis as representative of different cognitive domains: Stroop Colour and Word Test (SCWT)34, Trail Making Test parts A (TMT-A) and B (TMT-B)35, Digit Span Test (DST) (executive functions and attention)36, Coloured Progressive Matrices (CPM) (abstract reasoning)37, Rey Complex Figure Test Copy (RCFT-C) (visuospatial ability), Rey Complex Figure Test immediate (RCFT-I) and delayed recall (RCFT-D) (visual memory)38, Rey Auditory Verbal Learning Test immediate (RAVLT-I) and delayed recall (RAVLT-D) (verbal memory)39. All test scores were corrected according to normative values; the score ranges of the cognitive tests are summarized in Supplementary Appendix-SI. Post-stroke depressive symptoms and functional abilities were assessed with the Beck Depression Inventory (BDI)40 and mRS31.
Statistical analysis
Values were presented as mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables and as the number (%) of subjects for categorical variables. Univariate comparisons were made through the Student t test, Mann–Whitney test, or Chi-squared test, as appropriate. Linear regressions were performed to assess the influence of treatment (IVT alone versus IVT plus EVT) on scores obtained in each cognitive test, adjusting for pre-specified potential confounding factors (age, sex, admission NIHSS score, systolic BP, glucose level, ASPECT score, side of stroke, site of occlusion, BDI score). The collinearity between exposure variables was assessed with the variance inflation index. Results were considered significant for p values < 0.05 (two sided). Data analysis was performed using STATA/IC 13.1 statistical package (StataCorp LP, Texas, USA).
Standard protocol approvals, registrations, and patient consents
The study was approved by the Ethics Committee of the Marche Polytechnic University and conducted according to the Declaration of Helsinki. Informed consent was obtained from any patient or the legal representative.
Supplementary information
Author contributions
S.L.: study concept and design, analysis and interpretation of data, writing and critical revision of the manuscript for important intellectual content. M.C., A.P., C.C., F.L.G., L.V., S.C., M.D., G.P., M.G.C.: acquisition of data, analysis and interpretation. M.S.: study concept and design, critical revision of the manuscript for important intellectual content, study supervision. All authors reviewed the manuscript.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75609-1.
References
- 1.Leśniak M, Bak T, Czepiel W, Seniów J, Członkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement. Geriatr. Cogn. Disord. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- 2.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 3.Nys GM, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology. 2005;64:821–827. doi: 10.1212/01.WNL.0000152984.28420.5A. [DOI] [PubMed] [Google Scholar]
- 4.Nys GM, et al. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J. Neurol. Sci. 2006;247:149–156. doi: 10.1016/j.jns.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: Clinical determinants and its associations with long-term stroke outcomes. J. Am. Geriatr. Soc. 2002;50:700–706. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- 6.McKevitt C, et al. Self-reported long-term needs after stroke. Stroke. 2011;42:1398–1403. doi: 10.1161/STROKEAHA.110.598839. [DOI] [PubMed] [Google Scholar]
- 7.Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol. 2012;11:209. doi: 10.1016/S1474-4422(12)70029-7. [DOI] [PubMed] [Google Scholar]
- 8.Kumar G, Goyal MK, Sahota PK, Jain R. Penumbra, the basis of neuroimaging in acute stroke treatment: Current evidence. J. Neurol. Sci. 2010;288:13–24. doi: 10.1016/j.jns.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Saqqur M, CLOTBUST Investigators et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 10.De Silva DA, Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Stroke. 2010;41:295–299. doi: 10.1161/STROKEAHA.109.562827. [DOI] [PubMed] [Google Scholar]
- 11.Paciaroni M, et al. Systemic thrombolysis in patients with acute ischemic stroke and Internal Carotid ARtery Occlusion: The ICARO study. Stroke. 2012;43:125–130. doi: 10.1161/STROKEAHA.111.630624. [DOI] [PubMed] [Google Scholar]
- 12.Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. Am. J. Neuroradiol. 1995;16:1977–1986. [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, HERMES Collaborators et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin EJ, American Heart Association Statistics Committee and Stroke Statistics Subcommittee et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattanzi S, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307–315. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]
- 16.Lattanzi S, et al. Predictors of cognitive functioning after carotid revascularization. J. Neurol. Sci. 2019;405:116435. doi: 10.1016/j.jns.2019.116435. [DOI] [PubMed] [Google Scholar]
- 17.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/S1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 18.Jokinen H, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur. J. Neurol. 2015;22:1288–1294. doi: 10.1111/ene.12743. [DOI] [PubMed] [Google Scholar]
- 19.Stolwyk RJ, O'Neill MH, McKay AJ, Wong DK. Are cognitive screening tools sensitive and specific enough for use after stroke? A systematic literature review. Stroke. 2014;45:3129–3134. doi: 10.1161/STROKEAHA.114.004232. [DOI] [PubMed] [Google Scholar]
- 20.López-Cancio E, et al. Endovascular treatment improves cognition after stroke: A secondary analysis of REVASCAT trial. Neurology. 2017;88:245–251. doi: 10.1212/WNL.0000000000003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu G, et al. Cognitive function and prognosis of multimodal neuroimage-guided thrombectomy on mild to moderate anterior circulation infarction patients with broadened therapeutic window: A prospective study. Eur. Neurol. 2017;78:257–263. doi: 10.1159/000479735. [DOI] [PubMed] [Google Scholar]
- 22.Wall KJ, Cumming TB, Copland DA. Determining the association between language and cognitive tests in poststroke aphasia. Front. Neurol. 2017;8:149. doi: 10.3389/fneur.2017.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeyere N, et al. Domain-specific versus generalized cognitive screening in acute stroke. J. Neurol. 2016;263:306–315. doi: 10.1007/s00415-015-7964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatemichi TK, et al. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25:1915–1919. doi: 10.1161/01.STR.25.10.1915. [DOI] [PubMed] [Google Scholar]
- 25.Woo J, Kay R, Yuen YK, Nicholls MG. Factors influencing long-term survival and disability among three-month stroke survivors. Neuroepidemiology. 1992;11:143–150. doi: 10.1159/000110924. [DOI] [PubMed] [Google Scholar]
- 26.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25:362–365. doi: 10.1161/01.STR.25.2.362. [DOI] [PubMed] [Google Scholar]
- 27.Lattanzi S, et al. The P-wave terminal force in embolic strokes of undetermined source. J. Neurol. Sci. 2017;375:175–178. doi: 10.1016/j.jns.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 28.Lattanzi S, et al. Prediction of outcome in embolic strokes of undetermined source. J. Stroke Cerebrovasc. Dis. 2020;29:104486. doi: 10.1016/j.jstrokecerebrovasdis.2019.104486. [DOI] [PubMed] [Google Scholar]
- 29.Lattanzi S, et al. Clinical phenotypes of embolic strokes of undetermined source. Neurol. Sci. 2020 doi: 10.1007/s10072-020-04700-2. [DOI] [PubMed] [Google Scholar]
- 30.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 31.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 32.Capasso R, Miceli G. Esame Neuropsicologico per l’Afasia (ENPA) Berlin: Springer; 2001. [Google Scholar]
- 33.Mancuso M, et al. Italian standardization of the Apples Cancellation Test. Neurol. Sci. 2015;36:1233–1240. doi: 10.1007/s10072-015-2088-2. [DOI] [PubMed] [Google Scholar]
- 34.Barbarotto R, et al. A normative study on visual reaction times and two Stroop colour-word tests. Ital. J. Neurol. Sci. 1998;19:161–170. doi: 10.1007/BF00831566. [DOI] [PubMed] [Google Scholar]
- 35.Giovagnoli AR, et al. Trail Making Test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996;17:305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 36.Monaco M, Costa A, Caltagirone C, Carlesimo GA. Forward and Backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013;34:749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- 37.Basso A, Capitani E, Laiacona M. Raven's coloured progressive matrices: Normative values on 305 adult normal controls. Funct. Neurol. 1987;2:189–194. [PubMed] [Google Scholar]
- 38.Le Osterrieth PA. test de copie d’une figure complexe: Contribution a l’´etude de la perception et de la memoire. Arch. Psychol. 1944;30:286–350. [Google Scholar]
- 39.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 40.Ghisi M, Flebus GB, Montano A, Sanavio E, Sica C. Beck Depression Inventory—II (BDI-II) Manuale. Florence: Organizzazioni Speciali; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.