Abstract
Basosquamous carcinoma (BSC) is a rare non-melanoma skin cancer that shares the characteristic features of both basal and squamous cell carcinomas (BCC, SCC). Our research enables better characterization of BSC in comparison to high-risk subtypes of BCC and SCC. Paper includes a retrospective analysis of BSC cases regarding sex, age, number of tumors and anatomical distribution in comparison to BCC and SCC evaluating the differences and defining the implications. Histologically confirmed carcinomas recorded between 1999 and 2019 were studied. 181 diagnosed BSC cases were identified, making this study the largest cohorts of BSC patients reported worldwide. Most cases were reported on head and neck. Analysis of facial anatomic distribution shows that most commonly affected sites were the nose (43%) and the cheek (25%). The age at excision of metatypical BCC was higher than those of low-risk BCC (P < 0.05), however similar to high-risk BCC (P = 0.20). We revisited that the concept of BSC is the most similar to high-risk subtypes of BCC. Patients with diagnosed BSC have higher risk of second nonmelanoma skin cancer. Therefore, the frequency of follow-up examination should be adjusted to the individual risk of another skin cancer.
Subject terms: Cancer epidemiology, Head and neck cancer, Skin cancer
Introduction
Basal cell carcinoma (BCC) followed by squamous cell carcinoma (SCC) are the two most common subtypes of nonmelanoma skin cancer (NMSC) affecting white-skinned individuals. Recently a significant world-wide increase in incidence of both cancers has been observed1–4. Although BCC and SCC share many similarities, they have important etiological differences which cause the substantial variation between these skin cancers’ incidence rates. Generally, NMSC is curable and BCC has minimal potential risk of metastasis, with an estimated incidence between 0.00281% and 0.5%5. Mortality rate for BCC is exceptionally low, while SCC has a higher metastatic rate estimated on 0.5–16% and higher related mortality6. Moreover, recent studies suggest that BCC is not a homogeneous entity and that specific histological subtypes show different clinical behavior, occur at certain body areas and might have different etiology and recurrence potential after standard excision7. Superficial BCCs are predominantly located on the trunk, whereas more common nodular type is mainly observed on the head and neck7, most likely due to more frequent intermittent UV exposure. This is confirmed by the fact that both intermittent and intense sun exposure are the leading risk factors of BCC8.
Morpheaform BCC is less common than superficial and nodular variants9 and this subtype is known to be more aggressive than other types, because of its tendency to infiltrate deep subcutis. Similarly, infiltrative BCC is considered a high-risk histopathological subtype because it is more likely to be incompletely excised or occurs especially on face where surgical margins might be conservative10–12.
Metatypical basal cell carcinoma synonymously labelled basosquamous carcinoma (BSC) is a rare cutaneous neoplasm that has caused considerable controversy concerning its classification and pathogenesis13. The diagnosis of BSC is confirmed by histopathological examination. Clinical differentiation from other BCC subtypes is difficult, although dermoscopic evaluation may provide some important clues14. The dermoscopic pattern of BSC combines characteristics of both BCC and SCC including unfocused arborizing vessels, white structureless areas, keratin masses, ulceration or blood crusts, white structures, blue-gray blotches and blood spots on keratin masses (Figs. 1, 2, 3). Detection of at least one overlapping dermoscopic criterion both of BCC and SCC is an alarming sign for BSC in dermoscopy15. This is crucial, especially because the non-specific clinical presentation of BSC may extend time to diagnosis and lead to underestimation of possible later consequences such as local recurrence and potential lymph node or distant metastasis. Basosquamous carcinoma is more aggressive and invasive than both BCC and probably also SCC, although the latter assumption is controversial16–20. Despite benign appearance, BSC is characterized by aggressive subclinical spread with higher rates of recurrence: 12–51% for surgical excision and 4% for Mohs micrographic surgery. Moreover, BSC has a relatively high metastatic potential, estimated on 5–10%21,22.
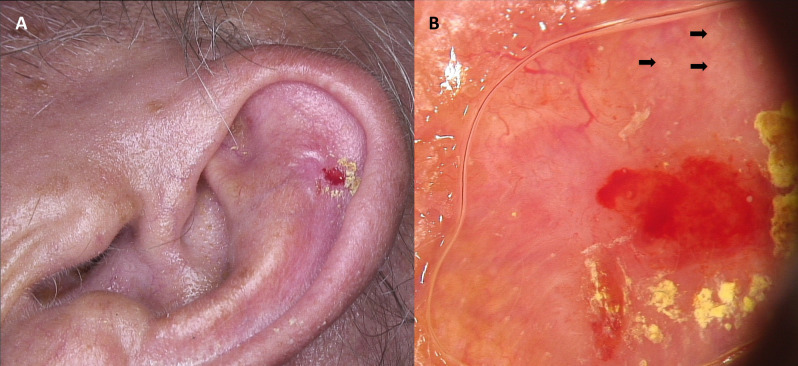
Figure 1.
Ulcerated basosquamous carcinoma of the left auricule (A). Dermoscopy shows central red and yellow structureless areas corresponding with the presence of ulceration and crust, respectively. On the periphery white circles (black arrows), arborizing vessels ad well as polymorphic vessels on the white-pinkish background may be observed (B).
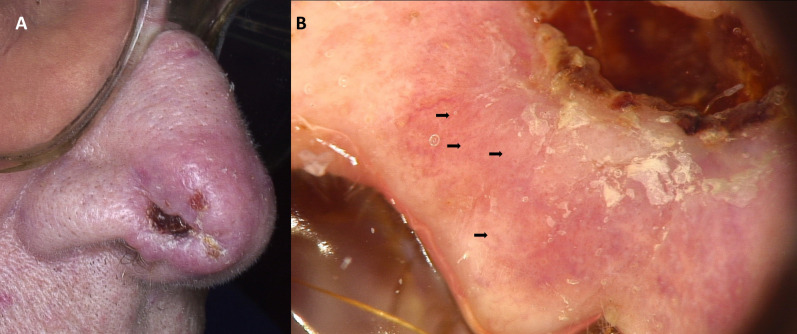
Figure 2.
Ulcerated basosquamous carcinoma of the nose (A). Dermoscopy shows central crust covering an ulceration. On the periphery white circles (black arrows), white scale, as well as unfocused serpentine vessels may be observed (B).
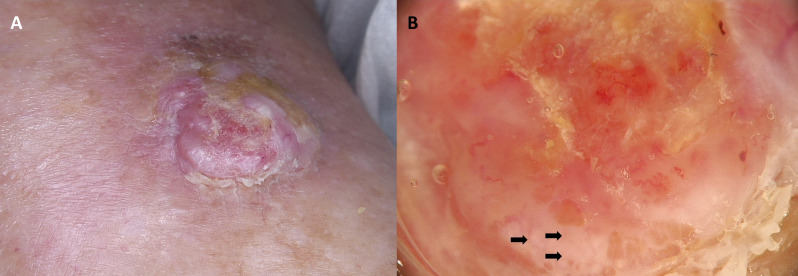
Figure 3.
Basosquamous carcinoma of the left lower leg (A). Dermoscopy shows white circles (black arrows), white-pinkish structureless areas, white-scale, whitish-yellowish crust as well as polymorphic vascular pattern (B).
Although from the first case description of a carcinoma with abutting basaloid and squamous features, without clear separation in 1894 is over hundred years, it remains unclear whether BSC should be classified as a BCC or SCC23,24. Initially, it was suggested that these lesions represent a collision of separate primary BCC and SCC which closely develop opposed to each other23,25. Summarization theory, which suggests that BSC is basal cell carcinoma that undergoes squamous differentiation21 is supported by the current broad definition provided by the World Health Organization (WHO) in Classification of Tumors, that have categorized BSCs as “basal cell carcinomas that are associated with squamous differentiation”. On the other hand, National Comprehensive Cancer Network (NCCN), states that some cases of basosquamous tumors may be prognostically similar to SCC, so that clinicopathologic correlation is recommended in these cases and these patients may require different approach with respect to treatment and follow-up compared to BCC patients. Due to an aggressive biological behavior and clinical course that distinguishes BSC from other forms of BCC, the use of term “metatypical basal cell carcinoma” may be misleading and is not recommended by some authors22.
This study was undertaken to learn more about characteristic epidemiological features of basosquamous carcinoma in patients treated surgically in a defined period. Moreover, we compared BSC with the general characteristics of BCC and SCC.
Methods
All cases included in the study were histologically confirmed BCCs, SCCs or BSCs consecutively admitted and surgically excised at the seven sites in Poland: Gdansk, Gliwice, Lodz (2 sites), Rzeszow, Warsaw and Wroclaw, during a 21-year period (1999–2019).
All patients were Caucasians with skin phototype between I and III, diagnosed and treated either in a public hospital or in a private practice. Information recorded was the patient’s age, gender, anatomical localization of the cancer and histological diagnosis. Excision margins, the presence of lymphovascular or perineural invasion, AJCC pT stage, and the status of reexcision specimens were also documented when data was available.
Study approved by Human Research Ethics Committee of the Medical University of Lodz, Poland (RNN/209/18/KE) and informed consent was obtained from all organizations the subjects’ data is being sourced from. Written consent was not obtained from the study participants because the retrospective data used encrypted identification of the individuals. All methods and procedures were conducted in accordance with relevant guidelines and regulations as well as with the updated Declaration of Helsinki. This study was given a formal waiver for the need for consent by the Institutional Review Board of Medical University of Lodz.
The tumor excision was performed with standard excision or histographic surgery (Mohs micrographic surgery) in all operated cases. The tumors described by histopathologists as basosquamous carcinoma (BSC), basosquamous cell carcinoma, metatypical basal cell carcinoma, basal cell carcinoma with squamous differentiation, metatypical basal cell carcinoma as basosquamous have been analyzed. All histological specimens were excised and examined primarily by a dermatopathologist using haematoxylin and eosin (H + E) staining as a minimum. A small proportion of specimens were subsequently subjected to immunohistochemistry analysis using an anti-human epithelial antigen monoclonal antibody BerEP4, where the diagnosis was less clear. We used the following strict histologic criteria for the diagnosis of BSC: loss of BerEP4 staining in portions of the tumor, loss of palisading, increased nuclear atypia, significant squamous differentiation, and loss of basaloid cytology in portions of the tumor.
Recurrent tumors were excluded from the study. In tumors where multiple subtypes are noted, all subtypes are listed in the final diagnosis. Patients who had two or more tumors diagnosed were counted as two or more tumor cases. The data were compared with the same results obtained for BCCs and SCCs. The subtype classification was according to the National Comprehensive Cancer Network (NCCN) guidelines v1.2020 (low-risk BCC histologic subtypes include superficial, nodular, keratotic, infundibulocystic, and fibroepithelial BCC; high-risk BCC subtypes include infiltrative, sclerosing/morpheaform, micronodular, and BCC with carcinosarcomatous differentiation; high-risk SCC histologic subtypes include acantholytic, adenosquamous, desmoplastic, or carcinosarcomatous; low-risk SCC histologic subtypes include verrucous and keratoacanthomatous SCC).
All results were analyzed statistically with Statistica 13.0 (Statsoft, Kraków, Poland). The obtained results were analyzed statistically by the Student's t-test, analysis of variance (ANOVA) post hoc comparisons and χ2 test with Yates correction if needed. P-values less than 0.05 were considered as statistically significant.
Results
181 cases (women:men ratio: 0.83:1) reported in 168 patients (76 women and 92 men) with basosquamous carcinoma tumor were included in the study. In the selected cohort of patient in the analyzed period, SCC was found in 2065 cases (1020 men and 1045 women) including high-risk SCC in 162 cases (72 men and 90 women) and low-risk SCC in 814 cases (402 men 412 woman) and BCC in 11,848 cases, including high-risk BCC in 769 cases (373 men and 396 women) and low-risk BCC in 4032 cases (1958 men 2074 woman). BSC composed 2.1% of all NMSC.
The mean age of patients differed for various NMSC types. Patients with low-risk BCC were younger (68.7 ± 12.8 years) than patients with BSC (72.2 ± 11.5 years) and this difference was statistically significant (P < 0.05), while patients with SCC were significantly older than patients with BSC (P < 0.05). However, the average age of patients with high-risk BCC (71.1 ± 12.3 years) was similar to patients with BSC with no statistical significance (P = 0.20).
Women to men predominance was observed for low-risk SCC cases (1.02:1), high-risk SCC cases (1.25:1), low-risk BCC cases (1.06:1) and high-risk BCC cases (1.06:1), while opposite observation was noted for BSC cases (0.83:1) with the significant predominance of men (P < 0.05).
In most cases lesions were pT1 [≤ 20 mm] (n = 102), followed by pT2 [> 20 mm to ≤ 40 mm] (n = 78) and pT3 [> 40 mm] (n = 3), with 3 lesions displaying lymphovascular invasion and 5 lesions with perineural invasion. Mean excision margins were 3.8 mm peripherally (SD ± 2.1 mm). The inadequate excision rate (defined as resection margins < 1 mm or involved margins) was observed for 43 cases (24%), mostly on facial area.
Single, excised and histologically confirmed BSC presented 100 patients. Multiple carcinomas were diagnosed in 68 patients with BSC (40%) and it is significantly higher rate in comparison to SCC (26%, P < 0.05) and BCC (23%, P < 0.05). Usually, BSCs were diagnosed 3 months after the diagnosis of high-risk BCC, while BSC occurred prior (3 months) to the first diagnosed low-risk BCC. In this context, no correlation has been observed for SCC.
In both sexes, anatomical distribution of all types of tumors was dominated by the face, with increased rate of BSC (86% for men, 89% for women) in comparison to other types of NMSC (P < 0.05). The second most frequent location was the trunk for BSC (6% for men, 8% for women). Table 1 presents anatomical distribution of NMSC according to gender.
Table 1.
Clinical characteristics of patients with NMSC.
| Low-risk SCC | High-risk SCC | BSC | Low-risk BCC | High-risk BCC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total number of lesions | 814 | 162 | 181 | 4032 | 769 | |||||
| mean age | 77.3a | 76.5a | 72.2a | 68.7a | 71.1a | |||||
| Sex ration (F/M) | 1.02 | 1.25 | 0.83b | 1.06 | 1.06 | |||||
| Men | ||||||||||
| Face | 225 | (61%) | 47 | (85%) | 81 | (86%) | 549 | (57%) | 269 | (76%) |
| Trunk | 46 | (13%) | 0 | (0%) | 6 | (6%) | 263 | (27%) | 31 | (9%) |
| Upper limb | 32 | (9%) | 2 | (4%) | 2 | (2%) | 62 | (6%) | 11 | (3%) |
| Lower limb | 41 | (11%) | 0 | (0%) | 0 | (0%) | 44 | (5%) | 4 | (1%) |
| Scalp | 11 | (3%) | 2 | (4%) | 2 | (2%) | 20 | (2%) | 25 | (7%) |
| Neck | 13 | (4%) | 4 | (7%) | 3 | (3%) | 51 | (5%) | 13 | (4%) |
| n/d | 34 | 17 | 5 | 969 | 20 | |||||
| all | 402 | 72 | 99 | 1958 | 373 | |||||
| Women | ||||||||||
| Face | 275 | (67%) | 82 | (92%) | 68 | (89%) | 549 | (57%) | 296 | (79%) |
| Trunk | 27 | (7%) | 0 | (0%) | 6 | (8%) | 248 | (26%) | 37 | (10%) |
| Upper limb | 23 | (6%) | 1 | (1%) | 0 | (0%) | 48 | (5%) | 7 | (2%) |
| Lower limb | 57 | (14%) | 2 | (2%) | 0 | (0%) | 70 | (7%) | 7 | (2%) |
| Scalp | 2 | (0%) | 1 | (1%) | 1 | (1%) | 25 | (3%) | 17 | (5%) |
| Neck | 12 | (3%) | 3 | (3%) | 1 | (1%) | 55 | (6%) | 11 | (3%) |
| n/d | 4 | 1 | 6 | 1079 | 21 | |||||
| All | 412 | 90 | 82 | 2074 | 396 | |||||
Data are presented as n (%). n/d: not defined.
BCC, basal cell carcinoma; SCC, squamous cell carcinoma; BSC, basosquamous cell carcinoma; F/M, female to male ratio.
aThe mean age of patients differs significantly between BSC and other subtypes (P < 0.05).
bMen to women predominance was statistically significant for BSC (P < 0.05).
In most cases (149) BSC was located on the face, especially on the nose (44% for men, 42% for women). Other locations on the face were as follows: the cheek (20% for men, 27% for women), the eyehole (15% for men, 21% for women), and the earlobe (16% for men, 6% for women). Body-site distribution of BSC was strongly dominated by the face in comparison to other types of NMSC (P < 0.05). Table 2 presents distribution of tumors on the face.
Table 2.
NMSC subtypes localization on face based on sex.
| Low-risk SCC | High-risk SCC | BSC | Low-risk BCC | High-risk BCC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | ||||||||||
| Nose | 28 | (21%) | 12 | (32%) | 24 | (44%) | 204 | (49%) | 24 | (44%) |
| Eyehole | 6 | (4%) | 2 | (5%) | 8 | (15%) | 17 | (4%) | 7 | (13%) |
| Cheek | 41 | (30%) | 9 | (24%) | 11 | (20%) | 153 | (37%) | 9 | (17%) |
| Temple | 1 | (1%) | 1 | (3%) | 0 | (0%) | 9 | (2%) | 1 | (2%) |
| Forehead | 1 | (1%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Earlobe | 42 | (31%)a | 9 | (24%)a | 9 | (16%)a | 19 | (5%) | 9 | (17%)a |
| Lips | 15 | (11%) | 5 | (13%) | 2 | (4%) | 7 | (2%) | 2 | (4%) |
| Chin | 2 | (1%) | 0 | (0%) | 1 | (2%) | 8 | (2%) | 2 | (4%) |
| n/d | 89 | 9 | 26 | 133 | 215 | |||||
| All | 225 | 47 | 81 | 549 | 269 | |||||
| Women | ||||||||||
| Nose | 46 | (28%) | 20 | (36%) | 20 | (42%) | 263 | (53%) | 46 | (60%) |
| Eyehole | 3 | (2%) | 2 | (4%) | 10 | (21%) | 29 | (6%) | 5 | (6%) |
| Cheek | 94 | (58%) | 25 | (45%) | 13 | (27%) | 163 | (33%) | 13 | (17%) |
| Temple | 2 | (1%) | 1 | (2%) | 0 | (0%) | 10 | (2%) | 3 | (4%) |
| Forehead | 1 | (1%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Earlobe | 5 | (3%)a | 2 | (4%)a | 3 | (6%)a | 9 | (2%) | 4 | (5%)a |
| Lips | 10 | (6%) | 3 | (5%) | 2 | (4%) | 2 | (0%) | 0 | (0%) |
| Chin | 2 | (1%) | 2 | (4%) | 0 | (0%) | 20 | (4%) | 6 | (8%) |
| n/d | 112 | 27 | 20 | 53 | 219 | |||||
| All | 275 | 82 | 68 | 549 | 296 | |||||
Data are presented as n (%). n/d: not defined.
BCC, basal cell carcinoma; SCC, squamous cell carcinoma; BSC, basosquamous cell carcinoma.
aMore lesions on earlobe occurring in men than women (P < 0.05).
Discussion
Non-melanoma skin cancer represents the most common group of skin neoplasms in white population with an increasing incidence worldwide. Unfortunately, cases of BCC and SCC, unlike other cancers, are often not tracked or even excluded in cancer registries and statistical analyses. The most likely reasons for this are that NMSC are associated with lower mortality compared to other malignancies. However, they stand for social, cosmetic and economical issue, therefore, studying and understanding their current epidemiological trends is considered crucial in order to create a public health strategy to achieve early and adequate control of the disease.
BSC is a rare NMSC type, currently representing approximately 1.2% to 2.7% of all malignancies in this group13,16,22,24,25. However, it is also suspected to be underdiagnosed, partially due to non-representative biopsy specimens taken, as often characteristic histological features are present in deep layers only. In our study BSC was reported in 2.1% of all cases confirming the relative rarity of this tumor.
Recent studies showed that some variants of BCC and SCC are more aggressive than others26,27. It has been suggested that infiltrative and morpheaform BCC subtypes are generally regarded high-risk subtypes, either because of the higher risk of recurrence or due to their aggressive behavior28. In the opposite, superficial, nodular, and adenoid BCCs are considered less aggressive (low-risk) variants of BCC, with more indolent course. Well-differentiated histologic SCC subtypes with low metastatic potential include keratoacanthoma and verrucous carcinoma. In contrast, desmoplastic and adenosquamous SCCs are highly infiltrative, have a high risk of local recurrence, metastasis, and death29. Some authors believe that BSC is a variant of BCC, whereas others have suggested that BSC behaves more aggressively like SCC21.
In our study most cases of primary BSC excision of pT1 and pT2 tumors have good clinical outcome with none of the patients developing disease recurrence or metastasis. Positive margin is a significant risk factor for recurrence of a disease16,21. In most cases reported inadequate excision rate was associated with facial area. Tumors developed on face lead to challenges in resection and reconstruction, issues to achieve adequate surgical margin, and problems in providing a decent aesthetic outcome.
Another risk factor for recurrence of the disease is a perineural invasion16,25. However, only 5 patients in our study had histological evidence of perineural invasion, but no recurrence has been seen in the current study.
It is confirmed that UVR exposure is the most important risk factor of BCC, SCC and BSC30 and therefore, it is not surprising that the vast majority of these cancers occurs on the sun exposed areas such as the head compared to the other body sites31. Our research confirms that BSC and high-risk BCC and SCC tumors occur more frequently on the head and neck in comparison to low-risk BCC and SCC tumors, which are often located on the trunk and the limbs. However, the occurrence of low-risk BCC and SCC on the trunk was higher than aggressive subtypes of those tumors. There was a similarity in the anatomical localization between BSC and more aggressive subtypes of both BCC and SCC.
The results of other studies show that great majority of BSC cases are seen on the head and neck, mainly involving the central face16,24,25,32. In the series reported by Leibovitch et al.25 most tumors (170 out of 178, 95.6%) were located on the head and neck, with 59 patients (33.1%) having it on the nose. Other authors revealed the head and neck location in 82%16, 96%24, or even 97%32. Anatomical distribution in our cohort was similar, with 92% of tumors being located on the head and neck region. The most common primary tumor site on face was the nose that is similar to results provided by Wermker et al.33 that analyzed BSC exclusively on head and neck region.
It is also well established that BSC occurs more frequently in men21,25. Our study reflects similar results—male patients constituted 57%. However, when considering the occurrence of BSC on the ear only, we demonstrated that the gender disparity increased (Fig. 1). In our study, 79% of the BSCs occurring on the ear were diagnosed in men (P < 0.05). This finding is most likely attributable to the fact that it is more common for women to have hair covering the ears, thus shielding this anatomic site from ultraviolet radiation and making it relatively protected compared to the men’s ear30. What is more, great disparity of tumor type has been observed in term of lesions located on the earlobe in men. However, the great predilection for earlobe showed low-risk SCC, constituting 31% of the all low-risk SCC facial lesions, followed by the aggressive SCC subtypes (22%), aggressive BCC subtypes (17%) and BSC (16%). In contrast, only 5% of facial low-risk BCC were located on the ear.
Although patients usually presented in the six to eight decade of life17,21,32, the age range may be much wider, and there are reports of tumors occurring in the second decade21. We reported all patients at presentation between 18 and 102 years, while patients’ age with BSC lesions ranged from 46 to 92. The mean age at diagnosis for all BSC cases was 72 ± 11.5 years, which is similar to other high-risk BCC lesions, with mean age of 70.1 ± 12.3 years (P > 0.05). We have noticed an increasing proportion of SCC in older age groups (P < 0.05), and low-risk BCC in younger age groups in comparison to BSC (P < 0.05). The mean age of BSC was slightly higher compared to other studies17,32.
It is known that 30% to 50% of patients diagnosed with primary NMSC within the following 5 years will develop another similar outbreak of NMSC, which indicates that these patients are 10 times more likely to develop second BCC or SCC, compared to the general population. They are also more likely to develop melanoma. In our series 40% of patients diagnosed with BSC had associated another skin neoplasm, what is significantly higher in comparison to overall reported NMSC cases, where 23% represent patients with multiple lesions (P < 0.05). We reported the correlation between both low- and high-risk BCC and presentation of BSC lesions in single patients. Typically, diagnosis of low-risk BCC is followed by BSC after in average 2.5 years. On the other hand, usually BSC in average occurs 2.5 years before the presentation of high-risk BCC. No correlation has been observed between BSC and SCC presentation in single patients. These results confirm that BSC patients are more often than the general population diagnosed with new primary skin cancers. Therefore, the frequency of monitoring should be based on the risk of possible occurrence of another skin cancer. As we have shown, the period of the first two or three years of observation is the most critical, which is why patients at this time should undergo regular dermatological and dermoscopic examinations.
There are no clear guidelines concerning BSC management. Similarly to BCC and SCC, the first-line treatment option is surgical excision, but surgical margins should be wider than those for low-risk BCC due to the infiltrative growth pattern of this tumor. The aggressive behavior of BSC caused by high attention being paid to the role radiotherapy and Mohs' micrographic surgery in this neoplasm.
Conclusions
To the best of our knowledge, this is the largest analysis of BSC worldwide which presents for the first time the detailed anatomical distribution, patients’ sex ratio and age, based on 181 histopathologically confirmed BSC in comparison to high- and low-risk BCC and SCC. We revisited the concept of BSC that is the most similar to high-risk subtypes of BCC and concluded that BSC, like morpheaform and infiltrative BCC subtypes, occurs more frequently on the head and neck compared to other locations. The reporting of the histopathological subtype of the BCC, BSC and SCC together with host demographics and body-site information should enhance clinical information and ultimately improve treatment regimens.
In contrast to previous data, the present study has shown that BSCs seam less aggressive and less likely to recur or metastasize. However, BSC tends to develop subsequent primary skin cancer more often than other types of NMSC. These data underline the need for frequent follow up with whole body surface examination for all patients diagnosed with BSC.
Abbreviations
- BCC
Basal cell carcinoma
- BSC
Basosquamous cell carcinoma
- NMSC
Non-melanoma skin cancer
- SCC
Squamous cell carcinoma
Author contributions
M.C. planned the study design and the sample cohort. M.C., G.K.W., D.L., B.L., A.R., Martyna Sławińska, M.P., K.S., A.H., J.M., M.B.M., Z.M., J.P., R.B., A.Z., Michał Sobjanek, Monika Słowińska, K.W., A.B. and W.N. gathered the subjects’ data Martyna Sławińska prepared the figures. M.C. and K.C analyzed the data and wrote the manuscript. M.C. prepared the tables M.C., J.N. and A.L. reviewed the data analysis and interpretations. A.R., W.O. and Małgorzata Skibińska revised the manuscript. M.U., A.W., J.N. and A.L. supervised the project.
Funding
This work was supported by Medical University of Lodz [503/5‑064‑01/503‑1]; and the National Centre of Science [Grant No. 2017/27/B/NZ5/02011].
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dacosta Byfield S, Chen D, Yim YM, Reyes C. Age distribution of patients with advanced non-melanoma skin cancer in the United States. Arch. Dermatol. Res. 2013;305:845–850. doi: 10.1007/s00403-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katalinic A, Kunze U, Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br. J. Dermatol. 2003;149:1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 4.Ciazynska M, Nartbutt J, Wozniacka A, Lesiak A. Trends in basal cell carcinoma incidence rates: a 16-year retrospective study of a population in central Poland. Adv. Dermatol. Allergol. 2018;35:47–52. doi: 10.5114/ada.2018.73164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malone JP, Fedok FG, Belchis DA, Maloney ME. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J. 2000;79(511–515):518–519. [PubMed] [Google Scholar]
- 6.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol. Surg. 2002;28:268–273. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 7.Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002;147:41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 8.Kricker A, Amstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? A casecontrol study in Western Australia. Int. J. Cancer. 1995;60:489–494. doi: 10.1002/ijc.2910600411. [DOI] [PubMed] [Google Scholar]
- 9.Salasche SJ, Amonette RA. Morpheiform basal cell epitheliomas. A study of subclinical extensions in a series of 51 cases. J. Dermatol. Surg. Oncol. 1981;7:387–394. doi: 10.1111/j.1524-4725.1981.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 10.Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393–398. doi: 10.1046/j.1365-2559.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 11.Milroy CJ, Horlock N, Wilson GD, Sanders R. Aggressive basal cel carcinoma in young patients: fact or fiction? Br. J. Plast. Surg. 2000;53:393–396. doi: 10.1054/bjps.1999.3267. [DOI] [PubMed] [Google Scholar]
- 12.Saldanha G, Fletcher A, Slater DN. Basal cell carcinoma: a dermatopathological and biological update. Br. J. Dermatol. 2003;148:195–202. doi: 10.1046/j.1365-2133.2003.05151.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan CZ, Rieger KE, Sarin KY. Basosquamous carcinoma: controversy, advances, and future directions. Dermatol. Surg. 2017;43(1):23–31. doi: 10.1097/DSS.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 14.Akay BN, Saral S, Heper AO, Erdem C, Rosendahl C. Basosquamous carcinoma: dermoscopic clues to diagnosis. J. Dermatol. 2017;44(2):127–134. doi: 10.1111/1346-8138.13563. [DOI] [PubMed] [Google Scholar]
- 15.Giacomel J, Lallas A, Argenziano G, et al. Dermoscopy of basosquamous carcinoma. Br. J. Dermatol. 2013;169:358–364. doi: 10.1111/bjd.12394. [DOI] [PubMed] [Google Scholar]
- 16.Martin RC, 2nd, Edwards MJ, Cawte TG, Sewell CL, McMasters KM. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer. 2000;88:1365–1369. doi: 10.1002/(SICI)1097-0142(20000315)88:6<1365::AID-CNCR13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Schuller DE, Berg JW, Sherman G, Krause CJ. Cutaneous basosquamous carcinoma of the head and neck: a comparative analysis. Otolaryngol. Head Neck. Surg. 1979;87:420–427. doi: 10.1177/019459987908700405. [DOI] [PubMed] [Google Scholar]
- 18.Jones MS, Helm KF, Maloney ME. The immunohistochemical characteristics of the basosquamous cell carcinoma. Dermatol. Surg. 1997;23:181–184. doi: 10.1111/j.1524-4725.1997.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 19.Maloney ML. What is basosquamous carcinoma? Dermatol. Surg. 2000;26:505–506. doi: 10.1046/j.1524-4725.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 20.Randle HW. Basal cell carcinoma. Identification and treatment of the high-risk patient. Dermatol. Surg. 1996;22:255–261. doi: 10.1111/j.1524-4725.1996.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 21.Oldbury JW, Wain RA, Abas S, Dobson CM, Iyer SS. Basosquamous carcinoma: a single centre clinicopathological evaluation and proposal of an evidence-based protocol. J. Skin Cancer. 2018 doi: 10.1155/2018/6061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J. Am. Acad. Dermatol. 2009;60(1):137–143. doi: 10.1016/j.jaad.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 23.De Stefano A, Dispenza F, Petrucci AG, Citraro L, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol. Pol. 2012;66:419–423. doi: 10.1016/j.otpol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Bowman PH, Ratz JL, Knoepp TG, Barnes CJ, Finley EM, Geisse J. Basosquamous carcinoma. Dermatol. Surg. 2003;29(8):830–833. doi: 10.1046/j.1524-4725.2003.29217.x. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basosquamous carcinoma: Treatment with Mohs micrographic surgery. Cancer. 2005;104(1):170–175. doi: 10.1002/cncr.21143. [DOI] [PubMed] [Google Scholar]
- 26.Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution, Epidemiology and health services research. Br. J. Dermatol. 2006;155:401–407. doi: 10.1111/j.1365-2133.2006.07234.x. [DOI] [PubMed] [Google Scholar]
- 27.Bichakjian C, Olencki T, Aasi S, Alam M, Andersen J, Blitzblau R, et al, Basal Cell Carcinoma, NCCN Clinical Practice Guidelines in Oncology. www.nccn.org (2018) [DOI] [PMC free article] [PubMed]
- 28.Jarell AD, Mully TW. Basal cell carcinoma on the ear is more likely to be of an aggressive phenotype in both men and women. J. Am. Acad. Dermatol. 2011;66(5):780–784. doi: 10.1016/j.jaad.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018;78(2):237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher RP, Hill GB, Bajdik CD, Fincham S, Coldman AJ, McLean DI, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer, I: basal cell carcinoma. Arch Dermatol. 1995;131:157–163. doi: 10.1001/archderm.1995.01690140041006. [DOI] [PubMed] [Google Scholar]
- 31.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N. Engl. J. Med. 2005;353:2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 32.Borel DM. Cutaneous basosquamous carcinoma. Arch. Pathol. Lab. Med. 1973;95:293–297. [PubMed] [Google Scholar]
- 33.Wermker K, Roknic N, Goessling K, Klein M, Schulze HJ, Hallermann C. Basosquamous carcinoma of the head and neck: clinical and histologic characteristics and their impact on disease progression. Neoplasia. 2015;17(3):301–305. doi: 10.1016/j.neo.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.