Abstract
Epidemiological evidence has indicated that inflammatory markers and obesity are strongly correlated with insulin resistance (IR). However, there is a paucity of studies assessing the complex interaction between elevated hs-CRP and body mass index (BMI), particularly among Asians. This study investigated the additive interaction between hs-CRP and BMI on IR, using cross-sectional data from the 7th Korea National Health and Nutrition Examination Survey (2016–2018). A total of 5706 men and 6707 women aged 20 years or older were evaluated, and a multiple logistic regression analysis was used to assess the association of serum hs-CRP and BMI with IR, as measured by the triglyceride-glucose index (TyG index). Sex-specific median values were used to dichotomise the continuous TyG index variable into insulin-sensitive and IR categories. Biological interaction was evaluated using the Relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and synergy index (SI). The joint effects of high hs-CRP and overweight/obesity on IR were greater than would be expected from the effects of the individual exposures alone. Relative to those with low hs-CRP and BMI < 23, having both exposures was related to increased IR with an adjusted OR of 2.97 (95% CI 2.50–3.52) in men and 3.08 (95% CI 2.67–3.56) in women with significant additive interactions. These findings demonstrate that IR prevention strategies that reduce both systematic inflammation and BMI may exceed the expected benefits based on targeting these risk factors separately.
Subject terms: Endocrine system and metabolic diseases, Endocrinology, Risk factors, Epidemiology
Introduction
Insulin resistance (IR) is a condition characterised by the inability of the peripheral tissues to properly utilize endogenous insulin to maintain glucose homoeostasis. Several lines of evidence suggest that IR is an underlying cause of a range of health outcomes, including cardiovascular diseases1,2, cognitive dysfunction3, frailty4, and certain cancers5,6. IR has also been established as a major feature of the development and pathophysiology of type 2 diabetes (T2DM)7,8. It represents one of the leading causes of premature mortality in the adult population globally, with an estimated 1.6 million deaths caused by diabetes9. Further, the International Diabetes Federation (IDF) projected that by 2045, the number of people diagnosed with diabetes will increase by 51%, to 700 million10. Consequently, the disease burden associated with diabetes will continue to escalate over the next decades.
In light of the high morbidity and mortality rates associated with T2DM, prominent scientific efforts have been directed towards identifying possible risk factors for insulin resistance. Accumulating experimental and epidemiologic evidence has shown that inflammatory processes are linked to impaired glucose metabolism. In particular, the systemic inflammatory biomarker C-reactive protein (CRP), when measured in the blood with high sensitivity assay, has been reported to highly correlate with insulin resistance in diabetic11 and non-diabetic individuals12,13. IR is known to be associated with chronic inflammation, which is induced by various pro-inflammatory cytokines and oxidative stress biomarkers, notably interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and adipocytokines14,15. Chronic exposure to elevated levels of pro-inflammatory biomarkers stimulates the activation of cytokine signalling pathways, which prevent the activation of insulin signalling receptors in β-cells of pancreatic islets. In addition, obesity is closely related to insulin resistance. Furthermore, dysfunctional lipid metabolism accompanies obesity and can negatively regulate insulin action. Few studies, however, have assessed the combined association of increased hs-CRP and body mass index (BMI) on insulin resistance, particularly in Asian populations. Therefore, we sought to investigate this relationship in a large representative sample of non-diabetic adults from the 2016–2018 Korea National Health and Nutrition Examination Survey.
Results
Participant characteristics
The characteristics of 5706 men and 6707 women, according to insulin resistance status, who were included in this study are shown in Table 1. Among all participants, 45.97% were men, and the prevalence of insulin resistance was 50.04% and 50.02% in men and women, respectively. In both men and women, the insulin-resistant group had a significantly higher proportion of overweight/obese individuals, smokers, and drinkers than the insulin-sensitive group. Furthermore, a lower prevalence of hypertension and dyslipidemia was noted in the insulin-sensitive group. Table 2 outlines the general characteristics of participants with serum hs-CRP levels above and below the median value. Overall, individuals with high hs-CRP were more likely to be older, rural residents, of lower household incomes, less educated, overweight/obese, current smokers, and drinkers than those with hs-CRP values below the median. Moreover, individuals with high hs-CRP levels had a higher prevalence of hypertension and dyslipidemia and a lower physical activity level than their counterparts with lower hs-CRP levels.
Table 1.
General characteristics of the study population by insulin resistance status, as estimated by TyG index, Korea National Health and Nutrition Examination Survey 2016–2018.
| Insulin resistance (TyG indexa) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 5706) | P value* | Women (n = 6707) | P value* | |||||||||||
| Total | No (< 8.706) | Yes (≥ 8.706) | Total | No (< 8.355) | Yes (≥ 8.355) | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| Serum hs-CRP level | < 0.0001 | < 0.0001 | ||||||||||||
| Low | 2715 | 47.58 | 1564 | 57.61 | 1151 | 42.39 | 3343 | 49.84 | 2046 | 61.20 | 1297 | 38.80 | ||
| High | 2991 | 52.42 | 1287 | 43.03 | 1704 | 56.97 | 3364 | 50.16 | 1306 | 38.82 | 2058 | 61.18 | ||
| Age in years | < 0.0001 | < 0.0001 | ||||||||||||
| 20–29 | 781 | 13.69 | 518 | 66.33 | 263 | 33.67 | 880 | 13.12 | 656 | 74.55 | 224 | 25.45 | ||
| 30—39 | 1101 | 19.30 | 537 | 48.77 | 564 | 51.23 | 1315 | 19.61 | 835 | 63.50 | 480 | 36.50 | ||
| 40—49 | 1153 | 20.21 | 465 | 40.33 | 688 | 59.67 | 1525 | 22.74 | 809 | 53.05 | 716 | 46.95 | ||
| 50—59 | 1037 | 18.17 | 445 | 42.91 | 592 | 57.09 | 1367 | 20.38 | 562 | 41.11 | 805 | 58.89 | ||
| ≥ 60 | 1634 | 28.64 | 886 | 54.22 | 748 | 45.78 | 1620 | 24.15 | 490 | 30.25 | 1130 | 69.75 | ||
| Monthly household income | 0.070 | < 0.0001 | ||||||||||||
| Low | 788 | 13.81 | 421 | 53.43 | 367 | 46.57 | 989 | 14.75 | 349 | 35.29 | 640 | 64.71 | ||
| Mid-low | 1325 | 23.22 | 661 | 49.89 | 664 | 50.11 | 1638 | 24.42 | 740 | 45.18 | 898 | 54.82 | ||
| Mid-high | 1677 | 29.39 | 849 | 50.63 | 828 | 49.37 | 1963 | 29.27 | 1021 | 52.01 | 942 | 47.99 | ||
| High | 1916 | 33.58 | 920 | 48.02 | 996 | 51.98 | 2117 | 31.56 | 1242 | 58.67 | 875 | 41.33 | ||
| Educational level | 0.001 | < 0.0001 | ||||||||||||
| High school diploma or below | 3093 | 54.21 | 1594 | 51.54 | 1499 | 48.46 | 3960 | 59.04 | 1665 | 42.05 | 2295 | 57.95 | ||
| College graduate or above | 2613 | 45.79 | 1257 | 48.11 | 1356 | 51.89 | 2747 | 40.96 | 1687 | 61.41 | 1060 | 38.59 | ||
| Region | 0.974 | 0.0002 | ||||||||||||
| Urban | 4732 | 82.93 | 2361 | 49.89 | 2371 | 50.11 | 5675 | 84.61 | 2892 | 50.96 | 2783 | 49.04 | ||
| Rural | 974 | 17.07 | 490 | 50.31 | 484 | 49.69 | 1032 | 15.39 | 460 | 44.57 | 572 | 55.43 | ||
| BMI (kg/m2) | < 0.0001 | < 0.0001 | ||||||||||||
| Underweight or normal (< 23.0) | 1899 | 33.28 | 1260 | 66.35 | 639 | 33.65 | 3566 | 53.17 | 2265 | 63.52 | 1301 | 36.48 | ||
| Overweight or obese (≥ 23.0) | 3807 | 66.72 | 1591 | 41.79 | 2216 | 58.21 | 3141 | 46.83 | 1087 | 34.61 | 2054 | 65.39 | ||
| Smoking status | < 0.0001 | < 0.0001 | ||||||||||||
| Non-smoker | 3637 | 63.74 | 1990 | 54.72 | 1647 | 45.28 | 6303 | 9398 | 3196 | 50.71 | 3107 | 49.29 | ||
| Current smoker | 2069 | 36.26 | 861 | 41.61 | 1208 | 58.39 | 404 | 6.02 | 156 | 38.61 | 248 | 61.39 | ||
| Drinking status | < 0.0001 | <0.0001 | ||||||||||||
| Non-drinker | 677 | 11.86 | 394 | 58.20 | 283 | 41.80 | 1405 | 20.95 | 595 | 42.35 | 810 | 57.65 | ||
| Drinker | 5029 | 88.14 | 2457 | 48.86 | 2572 | 51.14 | 5302 | 79.05 | 2757 | 52.00 | 2545 | 48.00 | ||
| Physical activity | 0.001 | < 0.0001 | ||||||||||||
| Active | 2740 | 48.02 | 1430 | 52.19 | 1310 | 47.81 | 2939 | 43.82 | 1598 | 54.37 | 1341 | 45.63 | ||
| Inactive | 2966 | 51.98 | 1421 | 47.91 | 1545 | 52.09 | 3768 | 56.18 | 1754 | 46.55 | 2014 | 53.45 | ||
| Hypertension | < 0.0001 | < 0.0001 | ||||||||||||
| No | 3901 | 68.37 | 2115 | 54.22 | 1786 | 45.78 | 5280 | 78.72 | 2951 | 55.89 | 2329 | 44.11 | ||
| Yes | 1805 | 31.63 | 736 | 40.78 | 1069 | 59.22 | 1427 | 21.28 | 401 | 28.10 | 1026 | 71.90 | ||
| Dyslipidemia | < 0.0001 | < 0.0001 | ||||||||||||
| No | 3228 | 56.57 | 2189 | 67.81 | 1039 | 32.19 | 3479 | 51.87 | 2379 | 68.38 | 1100 | 31.62 | ||
| Yes | 2478 | 43.43 | 662 | 26.72 | 1816 | 73.28 | 3228 | 48.13 | 973 | 30.14 | 2255 | 69.86 | ||
| Cardiovascular diseases | 0.207 | < 0.0001 | ||||||||||||
| No | 5438 | 95.30 | 2707 | 49.78 | 2731 | 50.22 | 6552 | 97.69 | 3312 | 50.55 | 3240 | 49.45 | ||
| Yes | 268 | 4.70 | 144 | 53.73 | 124 | 46.27 | 155 | 2.31 | 40 | 25.81 | 115 | 74.19 | ||
| Total participants | 5706 | 100.0 | 2851 | 49.96 | 2855 | 50.04 | 6707 | 100.00 | 3352 | 49.98 | 3355 | 50.02 | ||
Boldface P-values indicate statistical significance.
ªTyG index was calculated by the formula ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2].
*P values were obtained by Chi-square test or Fisher’s exact test.
Table 2.
General characteristics of the study population according to sex-specific median hs-CRP levels.
| Serum hs-CRP level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 5706) | P value* | Women (n = 6707) | P value* | |||||||
| Low (< 0.60 mg/L) | High (≥ 0.60 mg/L) | Low (< 0.50 mg/L) | High (≥ 0.50 mg/L) | |||||||
| N | % | N | % | N | % | N | % | |||
| Age in years | < 0.0001 | < 0.0001 | ||||||||
| 20–29 | 454 | 16.72 | 327 | 10.93 | 534 | 15.97 | 346 | 10.29 | ||
| 30–39 | 527 | 19.41 | 574 | 19.19 | 704 | 21.06 | 611 | 18.16 | ||
| 40–49 | 575 | 21.18 | 578 | 19.32 | 840 | 25.13 | 685 | 20.36 | ||
| 50–59 | 451 | 16.61 | 586 | 19.60 | 632 | 18.91 | 735 | 21.85 | ||
| ≥ 60 | 708 | 26.08 | 926 | 30.96 | 633 | 18.94 | 987 | 29.34 | ||
| Monthly household income | < 0.0001 | < 0.0001 | ||||||||
| Low | 313 | 11.53 | 475 | 15.88 | 392 | 11.73 | 597 | 17.75 | ||
| Mid-low | 604 | 22.25 | 721 | 24.11 | 765 | 22.88 | 873 | 25.95 | ||
| Mid-high | 829 | 30.53 | 848 | 28.35 | 1007 | 30.12 | 956 | 28.42 | ||
| High | 969 | 35.69 | 947 | 31.66 | 1179 | 35.27 | 938 | 27.88 | ||
| Educational level | < 0.0001 | < 0.0001 | ||||||||
| High school diploma or below | 1385 | 51.01 | 1708 | 57.10 | 1850 | 55.34 | 2110 | 62.72 | ||
| College graduate or above | 1330 | 48.99 | 1283 | 42.90 | 1493 | 44.66 | 1254 | 37.28 | ||
| Region | 0.001 | 0.0004 | ||||||||
| Urban | 2297 | 84.60 | 2435 | 81.41 | 2881 | 86.18 | 2794 | 83.06 | ||
| Rural | 418 | 15.40 | 556 | 18.59 | 462 | 13.82 | 570 | 16.94 | ||
| BMI (kg/m2) | < 0.0001 | < 0.0001 | ||||||||
| Underweight or normal (< 23.0) | 1121 | 41.29 | 778 | 26.01 | 2264 | 67.72 | 1302 | 38.70 | ||
| Overweight or obese (≥ 23.0) | 1594 | 58.71 | 2213 | 73.99 | 1079 | 32.28 | 2062 | 61.30 | ||
| Smoking status | 0.003 | 0.243 | ||||||||
| Non-smoker | 1785 | 65.75 | 1852 | 61.92 | 3153 | 94.32 | 3150 | 93.64 | ||
| Current smoker | 930 | 34.25 | 1139 | 38.08 | 190 | 5.68 | 214 | 6.36 | ||
| Drinking status | 0.083 | < 0.0001 | ||||||||
| Non-drinker | 301 | 11.09 | 376 | 12.57 | 597 | 17.86 | 808 | 24.02 | ||
| Drinker | 2414 | 88.91 | 2615 | 87.43 | 2746 | 82.14 | 2554 | 75.98 | ||
| Physical activity | 0.022 | 0.001 | ||||||||
| Active | 1347 | 49.61 | 1393 | 46.57 | 1536 | 45.95 | 1403 | 41.71 | ||
| Inactive | 1368 | 50.39 | 1598 | 53.43 | 1807 | 54.05 | 1961 | 58.29 | ||
| Hypertension | < 0.0001 | < 0.0001 | ||||||||
| No | 1976 | 72.78 | 1925 | 64.36 | 2827 | 84.56 | 2453 | 72.92 | ||
| Yes | 739 | 27.22 | 1066 | 35.64 | 516 | 15.44 | 911 | 27.08 | ||
| Dyslipidemia | < 0.0001 | < 0.0001 | ||||||||
| No | 1707 | 62.87 | 1521 | 50.85 | 2097 | 62.73 | 1382 | 41.08 | ||
| Yes | 1008 | 37.13 | 1470 | 49.15 | 1246 | 37.27 | 1982 | 58.92 | ||
| Cardiovascular diseases | 0.346 | 0.008 | ||||||||
| No | 2595 | 95.58 | 2843 | 95.05 | 3286 | 98.29 | 3266 | 97.09 | ||
| Yes | 120 | 4.42 | 148 | 4.95 | 57 | 1.71 | 98 | 2.91 | ||
Boldface P-values indicate statistical significance.
*P values were obtained by Chi-square test or Fisher's exact test.
Association of insulin resistance with hs-CRP levels and BMI
The age- and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CI) for the associations of hs-CRP level or BMI with insulin resistance by sex are shown in Table 3. In the multivariable-adjusted analyses, serum hs-CRP and BMI were independently associated with insulin resistance, after controlling for age, region, monthly household income, educational level, smoking status, drinking status, physical activity, hypertension, dyslipidemia, and self-reported diagnosis of cardiovascular diseases. In the comparison of high versus low hs-CRP levels, elevated serum hs-CRP levels, above the median, were associated with a 1.57-fold (OR 1.57, 95%CI 1.39–1.76) and 1.84-fold (OR 1.84, 95% CI 1.65–2.05) increased OR of IR in men and women, respectively. Furthermore, in both men and women, higher BMI was associated with higher odds of being insulin resistant. Men with a BMI ≥ 23.0 had a 2.26-fold greater OR of becoming insulin resistant, compared to the reference group (OR: 2.26, 95% CI 1.98.04–2.57). The corresponding adjusted OR for IR in women was 2.14 (95% CI 1.91–2.39).
Table 3.
Age- and multivariable-adjusted ORs and 95% CIs for insulin resistance according to serum high-sensitivity CRP level and BMI in separate models, KNHANES 2016–2018.
| Age adjusteda | Multivariable adjustedb | ||
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Men | Serum hs-CRP | ||
| Low | 1.00 | 1.00 | |
| High | 1.78 (1.60–1.98) | 1.57 (1.39–1.76) | |
| BMI | |||
| Underweight or normal (< 23.0) | 1.00 | 1.00 | |
| Overweight or obese (≥ 23.0) | 2.65 (2.36–2.97) | 2.26 (1.98–2.57) | |
| Women | Serum hs-CRP | ||
| Low | 1.00 | 1.00 | |
| High | 2.26 (2.04–2.51) | 1.84 (1.65–2.05) | |
| BMI | |||
| Underweight or normal (< 23.0) | 1.00 | 1.00 | |
| Overweight or obese (≥ 23.0) | 2.74 (2.47–3.04) | 2.14 (1.91–2.39) | |
aAdjusted for age.
bAdjusted for age, region, household income, educational level, smoking status, drinking status, physical activity, hypertension, dyslipidemia, cardiovascular disease.
OR odds ratio, CI confidence interval, BMI body mass index.
Synergistic interaction of elevated hs-CRP levels and BMI on insulin resistance
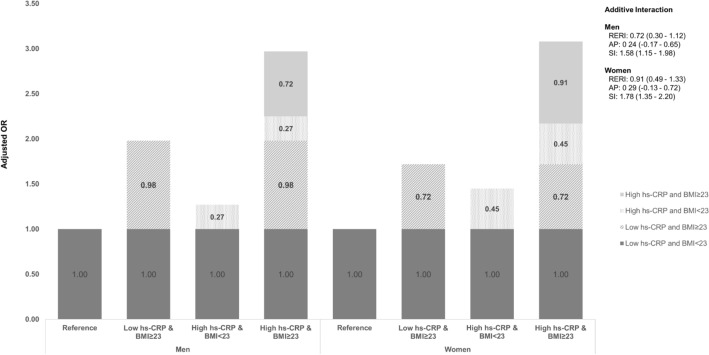
As shown in Fig. 1, we found a statistically significant synergistic interaction between hs-CRP and body mass index on insulin resistance. In the multivariable analysis and using men with low hs-CRP and BMI < 23 as the reference category, BMI ≥ 23 alone or high hs-CRP alone were associated with increased odds of insulin resistance (OR 1.98; 95% CI 1.66–2.37 and OR 1.27; 95% CI 1.03–1.58, respectively). Furthermore, having both risk factors greatly enhanced the OR of insulin resistance to 2.97 (95% CI 2.50–3.52). The estimated RERI was 0.72 (95% CI 0.30–1.12), indicating a synergistic interaction of hs-CRP and overweight/obesity on insulin resistance. In other words, the OR for the concurrence of obesity and elevated hs-CRP levels on insulin resistance was beyond the sum of the odds ratios associated with their individual effect. In addition, the attributable proportion (AP) revealed that 24% of the total odds of being insulin resistance was related to the interaction between elevated hs-CRP and BMI. Additionally, the synergy index (SI 1.58, 95% CI 1.15–1.98) also confirmed a synergistic interaction. Among women, the OR for the combination of high hs-CRP and BMI ≥ 23 (OR 3.08; 95% CI 2.67–3.56) was greater than the ORs for high hs-CRP and BMI < 23 (OR 1.45; 95% CI 1.24–1.69) and low hs-CRP and BMI ≥ 23 (OR 1.72; 95% CI 1.46–2.03). The RERI for insulin resistance was statistically significant in women (RERI 0.91; 95% CI 0.49–1.33). The AP was 0.29 (95% CI 0.13–0.72), and the SI was 1.78 (95% CI 1.35–2.20).
Figure 1.
Additive interaction of high hs-CRP and BMI on insulin resistance.
Sensitivity analysis
In order to evaluate the robustness of these associations, a sensitivity analysis was conducted, in which we excluded participants with hypertension, dyslipidemia, and self-reported diagnosis of cardiovascular diseases. As shown in Supplementary Table S1, the magnitude and direction of the association remained essentially unchanged in the sensitivity analysis. However, due to the smaller sample size the statistical power was limited for the interaction analyses, and additive interaction measures did not reach statistical significance16.
Discussion
In this population-based cross-sectional study of Korean non-diabetic participants aged 20 years or older, we found that individuals with elevated hs-CRP levels were at significantly higher odds of having insulin resistance, and that these associations persisted, even after adjusting for other relevant socio-demographic and health-related variables. Thus, our study confirms that systemic inflammation due to high hs-CRP levels has an independent effect on insulin resistance, as estimated by the TyG index.
The euglycemic hyperinsulinemic clamp method is considered the gold standard for estimating IR; however, because it is expensive and invasive, other surrogate IR indices have been developed based on anthropometric or biochemical parameters that are routinely collected in clinical practise. One such measure is the TyG index, which has been previously shown to be a simple, efficient, and clinically useful surrogate marker of IR. Two recent studies have shown that the TyG index is closely correlated with the homoeostasis model assessment of insulin resistance index (HOMA-IR)17,18. Moreover, other studies have reported that the TyG index value is superior for predicting IR to that of HOMA-IR18–20.
Elevated serum hs-CRP levels provide a sensitive marker of subclinical inflammation. Our finding of a positive association between serum hs-CRP levels and IR has been supported by several studies that have reported an association between CRP concentrations and surrogate measures of insulin resistance such as HOMA-IR and quantitative insulin sensitivity check index (QUICKI)21–23. The longitudinal cohort from the Bogalusa Heart Study of black and white adults showed that elevated hs-CRP at baseline was associated with future insulin resistance, as measured by HOMA-IR, after accounting for race, sex, age, BMI, smoking, alcohol drinking, and follow-up years24. This is also consistent with previous studies that reported associations between hs-CRP and insulin resistance among specific populations. In an occupational cohort of 10,308 British civil servants in the Whitehall II study, baseline subclinical inflammation biomarker levels were related to subsequent five-year changes in IR among non-diabetic individuals25. In contrast, a cross-sectional study of men aged 40–80 years did not find a significant association between CRP level and insulin resistance, after controlling for body composition parameters.
Additionally, we observed that the combined effects of systemic inflammation and BMI were greater than the sum of their individual impacts. Relative to individuals with low hs-CRP and BMI < 23, those with concomitant systemic inflammation and overweight/obese status had up to three-times greater odds of being insulin resistant. In our study, there was a relatively modest synergistic interaction between elevated serum hs-CRP levels and overweight/obese status on the OR of insulin resistance, and approximately 24% and 29% of effects were attributable to additive interactions in men and women, respectively. These results are in accordance with the findings of an earlier study that identified a significant interaction between body size and serum hs-CRP on insulin resistance in a cohort of Japanese adults aged 35–69 years23.
Mechanistically, systemic inflammation and obesity may affect insulin resistance via several pathways. Notably, fat accumulation in the liver or adipose tissues has been found to induce the production of pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNF-α) and IL-626,27. These adipocyte-produced cytokines stimulate hepatic CRP production and activation of the innate immune system28,29. An in vivo in mice found that chronic exposure to IL-6 impairs insulin receptor signalling in primary hepatocytes30. Therefore, increased TNF-α and/or IL-6 secretion may be responsible for the observed relationship between elevated serum hs-CRP and IR. Furthermore, inflammation may promote the development of IR by triggering the development of hypertension by influencing platelet adhesion, aggregation, and oxidant production31. Moreover, prior studies suggest that subclinical inflammation leads to diminished nitric oxide synthesis in endothelial cells and endothelial dysfunction, which promotes IR32–35. Additional studies are required to clarify the precise mechanisms and establish the causal effects of systemic inflammation and obesity on insulin sensitivity.
The present study has several strengths. First, this study features a nationally representative sample that surveyed a large sample of Korean adults using standardised questionnaires and laboratory procedures. Hence, the findings are likely to be generalizable to the overall Korean population. Furthermore, we attempted to minimise potential bias due to reverse causation by excluding participants with a diabetes and those who use anti-diabetic medication. However, our results should be considered in light of certain limitations. First, important questions that arises from these observations is whether adiposity-induced inflammation precedes IR or vice versa, and whether this relationship is bidirectional, particularly in the pathophysiology of T2DM. A cross-sectional study design with a single assessment of hs-CRP limits inferences regarding the temporal relationship between hs-CRP and IR. Therefore, prospective cohort studies may provide a better context for answering these questions. Second, the self-reporting of health-related factors and history of cardiovascular diseases could have led to recall bias. Lastly, although a wide spectrum of confounders was included in the adjustments, the presence of certain residual or undetected confounding factors cannot be completely ruled out.
In summary, our study provides further evidence that concomitant systemic inflammation and overweight/obesity may synergistically contribute to IR, such that prevention strategies for IR that aim to reduce both systematic inflammation and BMI may exceed the benefits that are expected from targeting these risk factors separately. These findings need to be confirmed in future longitudinal prospective studies to establish causality between systemic inflammation, obesity, and IR.
Material and methods
Study population
The current study is based on data from the 2016–2018 Korean National Health and Nutrition Examination Survey (KNHANES)36. In brief, the KNHANES is a population-based cross-sectional study designed to assess the health and nutritional status of people residing in South Korea. The survey included a stratified multistage probability sample that is representative of the non-institutionalized civilian population, aged one year or above. The three major components of the KNHANES are health interviews, health examinations, and nutrition surveys. The KNHANES interview includes detailed questions on demographic, socioeconomic, dietary, and health-related characteristics. The health examination component comprises medical, dental, and physiological measurements, as well as laboratory tests administered by trained medical personnel. The KNHANES survey protocols were approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (IRB No. 2018-01-03-P-A), and the study complied with the Declaration of Helsinki for medical research involving human subjects. Informed consent was obtained from all participants.
Of the 24,269 participants (men: 11,071; women: 13,198) who participated in the 2016–2018 survey, we restricted our analysis to include adults aged 20 years or older. Exclusion criteria included those with a previous diagnosis of diabetes, individuals who were using anti-diabetic medication, or pregnant women (N = 16,638, men: 7198; women: 9440). After excluding those with missing data on IR status or other covariates, 12,413 participants (men: 5706; women: 6707) were analysed.
Definition of insulin resistance
The main study outcome was IR, as measured by the triglyceride-glucose index (TyG index)37. The TyG index was calculated according to the following formula: log [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2]. The TyG index has been proposed as a simple and clinically useful surrogate for IR18,38,39. As no cut-off points have been suggested in the literature, sex-specific median values (men: 8.076, women: 8.355) were used to dichotomise the continuous TyG index variable into insulin-sensitive and insulin-resistant groups.
Laboratory measures
Blood samples were drawn from the antecubital vein in the morning of examinees who had fasted for at least eight hours. Serum hs-CRP levels were measured using an immunoturbidimetric assay on a Roche Cobas analyser. The subjects were dichotomised into “high” and “low” categories, based on sex-specific median hs-CRP values. The median hs-CRP cut-off values were 0.60 mg/L in men and 0.50 mg/L in women.
All other biochemical parameters, including triglycerides (TG), total cholesterol (TC), and fasting blood glucose (FBG) were measured immediately and detected enzymatically using an automatic chemistry analyser (Hitachi 7600, Hitachi Ltd., Tokyo, Japan). Low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were measured using homogeneous enzymatic colourimetric methods.
Other covariates
All potential confounding variables were selected based on prior knowledge of the existing literature40–42. Standardised questionnaires were administered by well-trained interviewers to collect information on age, sex, socioeconomic characteristics, and health-related risk factors. Participants, based on age, were categorised into the following groups: 20–29, 30–39, 40–49, 50–59, and ≥ 60 years. Participants were also categorised by educational attainment (High school diploma or below and college graduate or above) and monthly household income quartiles. Residential areas were divided into urban and rural areas.
Information on smoking status, drinking status, and physical activity was also obtained. Smoking status was categorised as either non-smoker (including never- and former-smokers) or current smoker. Participants were asked about alcohol consumption as follows: In the past year, how often did you drink any type of alcoholic beverage? Individuals were considered current drinkers if they consumed at least one drink in the past year. Physical activity was defined as engagement in 150 min of moderate-intensity aerobic activity or 75 min of vigorous-intensity aerobic activity every week.
In KNHANES, anthropometric data were collected by well-trained personnel according to a standardised protocol. Body mass index (BMI [kg/m2]) was calculated using measurement of height and weight. A BMI < 23.0 was considered normal weight and a BMI greater than or equal to 23.0 was considered overweight/obese, according to the Asia–Pacific obesity classification43. Following the 2015 Korean Guidelines, dyslipidemia (yes/no) was defined as having one or more of the following lipid abnormalities: hypercholesterolemia (total cholesterol ≥ 240 mg/dL or use of lipid-lowering drugs), hypertriglyceridemia (TG ≥ 200 mg/dL), hyper-low density lipoprotein (LDL) cholesterolemia (≥ 160 mg/dL), and hypo-high density lipoprotein (HDL)-cholesterolemia (< 40 mg/dL in men and < 50 mg/dL in women)44. Hypertension (yes/no) was defined as a systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher, or on antihypertensive treatment. Based on self-report, adults who answered ‘yes’ to the following questions were also ascertained as having cardiovascular diseases: ‘Has a doctor or other health professional told you have had a stroke, angina, or myocardial infarction?”.
Statistical analyses
Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). For descriptive purposes, variables were summarised in frequencies, and sample proportions of categorical variables were obtained. Chi-square or Fisher's exact tests were used to determine whether the distribution of categorical covariates of insulin-sensitive participants differed from the distributions of insulin-resistant individuals. To examine associations of hs-CRP and body mass index with IR, odds ratios (ORs) and 95% confidence intervals were estimated using logistic regression. Age, region, monthly household income, educational level, smoking status, drinking status, physical activity, hypertension, dyslipidemia, and self-reported diagnosis of cardiovascular diseases were adjusted, when appropriate.
Additionally, we investigated the presence of biological interactions on an additive scale, that is, whether the combination of high hs-CRP and BMI poses greater risk than the sum of their independent effects. This was evaluated using three indices of additive interaction: RERI (the relative excess risk due to interaction), AP (the proportion attributable to interaction), and the synergy index (SI)45,46. The RERI is defined as the additional risk due to interaction, which is calculated as the difference between the expected risk based on the addition of the ORs of the separate effects of the two risk factors under exposure and the observed risk in the doubly exposed group: RERI = OR11-OR10-OR01 + 1, where OR11 refers to the odds ratio of insulin resistance for high hs-CRP combined with BMI ≥ 23; OR10 is the OR of insulin resistance for elevated serum hs-CRP with BMI < 23; and OR01 represents exposure to BMI ≥ 23 only. The AP is interpreted as the proportion of disease that is due to interaction among individuals with both exposures: AP = RERI/OR11. The SI refers to the excess risk from both exposures when there is interaction, relative to the risk from exposure without interaction: SI = (OR11–1)/[(OR01–1) + (OR10–1)]). The resulting confidence intervals of RERI > 0 or AP > 0 or SI > 1 were indicative of positive departure from additivity, or synergistic interaction of the effects from two exposures on insulin resistance. In these analyses, adjustments were made for the same sets of potential confounders as described for the logistic regression models. All statistical test with a 2-tailed P < 0 0.05 were considered statistically significant.
Supplementary information
Acknowledgements
The authors would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
G.R.K., D.W.C., E.C.P. were responsible for the conception and design of the study. G.R.K. performed the data analysis. All authors participated in interpretation of the findings. G.R.K. and D.W.C. drafted the manuscript. E.C.P. revised and commented on the draft, and all authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75390-1.
References
- 1.Kernan W, et al. Insulin resistance and risk for stroke. Neurology. 2002;59:809–815. doi: 10.1212/WNL.59.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Mcfarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J .Clin. Endocrinol. Metab. 2001;86:713–718. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- 3.Ekblad LL, et al. Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care. 2017;40:751–758. doi: 10.2337/dc16-2001. [DOI] [PubMed] [Google Scholar]
- 4.Barzilay JI, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch. Intern. Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez AV, et al. Insulin resistance and endometrial cancer risk: a systematic review and meta-analysis. Eur. J. Cancer. 2015;51:2747–2758. doi: 10.1016/j.ejca.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Stolzenberg-Solomon RZ, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 7.Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N. Engl. J. Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 8.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. World health organization diabetes fact sheet (Accessed 20 March 2020). https://www.who.int/news-room/fact-sheets/detail/diabetes (2018).
- 10.International Diabetes Federation . IDF diabetes Atlas. 9. Brussels: Belgium; 2019. [PubMed] [Google Scholar]
- 11.Anan F, et al. High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Metabolism. 2005;54:552–558. doi: 10.1016/j.metabol.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Fizelova M, et al. Differential associations of inflammatory markers with insulin sensitivity and secretion: the prospective METSIM study. J. Clin. Endocrinol. Metab. 2017;102:3600–3609. doi: 10.1210/jc.2017-01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, et al. Association between inflammation and insulin resistance in US nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–2965. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 14.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 15.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol. Metab. Clin. N. Am. 2008;37:685–711. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanderWeele TJ. Sample size and power calculations for additive interactions. Epidemiol. Methods. 2012;1:159–188. doi: 10.1515/2161-962X.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrero-Romero F, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch. Med. Res. 2016;47:382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Er L-K, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasques ACJ, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Kang B, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int. J. Obes. 2017;41:789–792. doi: 10.1038/ijo.2017.14. [DOI] [PubMed] [Google Scholar]
- 21.Meng Y-X, et al. Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic US adults: findings from national health and nutrition examination survey 1999–2002. Clin. Chem. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- 22.Lee W-Y, et al. C-reactive protein concentrations are related to insulin resistance and metabolic syndrome as defined by the ATP III report. Int. J. Cardiol. 2004;97:101–106. doi: 10.1016/j.ijcard.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Uemura H, et al. Relationships of serum high-sensitivity C-reactive protein and body size with insulin resistance in a Japanese cohort. PLoS ONE. 2017;12:e0178672. doi: 10.1371/journal.pone.0178672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y, et al. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: the Bogalusa Heart Study. Cardiovasc. Diabetol. 2019;18:109. doi: 10.1186/s12933-019-0913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herder C, et al. Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: the Whitehall II study. Eur. J. Endocrinol. 2016;175:367–377. doi: 10.1530/EJE-16-0528. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 27.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 28.Schäffler A, Schölmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31:228–235. doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. Int. Sch. Res. Not. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klover PJ, Zimmers TA, Koniaris LG, Mooney RAJD. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 31.Sesso HD, et al. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 32.Petrie JR, Ueda S, Webb DJ, Elliott HL, Connell JM. Endothelial nitric oxide production and insulin sensitivity: a physiological link with implications for pathogenesis of cardiovascular disease. Circulation. 1996;93:1331–1333. doi: 10.1161/01.CIR.93.7.1331. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62:4030–4042. doi: 10.2337/db13-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.CIR.0000029802.88087.5E. [DOI] [PubMed] [Google Scholar]
- 35.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.CIR.0000033116.22237.F9. [DOI] [PubMed] [Google Scholar]
- 36.Kweon S, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES) Int. J. Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 38.Du T, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrero-Romero F, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity Comparison with the euglycemic–hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 40.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. J. Intern. Med. 1993;233:327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 41.Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng S, et al. Associations of lipid profiles with insulin resistance and β cell function in adults with normal glucose tolerance and different categories of impaired glucose regulation. PLoS ONE. 2017;12:e0172221. doi: 10.1371/journal.pone.0172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization . The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 44.Kim K-I, et al. 2015 Korean guidelines for the management of dyslipidemia: executive summary (English translation) Korean Circ. J. 2016;46:275. doi: 10.4070/kcj.2016.46.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur. J. Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 46.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol. Methods. 2014;3:33–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.