Abstract
Silk fibroin is a novel biomaterial for enhancing transplanted islet cell function and survival. This study investigated whether silk fibroin may have unique properties that improve islet function in the face of inflammatory-mediated stress during transplantation. Murine islet function was tested in vitro with either silk fibroin or alginate and challenged with inflammatory cytokines. The glucose-stimulated insulin secretion index for all conditions decreased with inflammatory cytokines, but was better preserved for islets exposed to silk compared to those exposed to alginate or medium. GLUT2 transporter expression on the cell surface of islets exposed to silk was increased compared to alginate or medium alone. Upon cytokine stress, a greater percentage of islet cells exposed to silk expressed GLUT2 on their surface. We conclude that preconditioning islets with silk fibroin stimulates islet cell surface GLUT2 expression, an increase, which persists under inflammatory stress, and may improve islet engraftment and function after transplantation.
Keywords: silk fibroin, islet transplantation, GLUT2 expression, insulin secretion, inflammatory stress
1. INTRODUCTION
The ultimate goal of islet cell transplantation as an effective treatment for type 1 diabetes (T1D) is restoration of a functional islet cell mass without the need for exogenous insulin. In current clinical practice, islets are isolated from a cadaver pancreas, perfused into the portal vein, and lodged in the liver of a patient with T1D[1, 2]. However, studies have shown that shortly after transplantation, an immediate intravascular inflammatory cytokine release of TNFα, IL-1β, and IFNγ may cause islet cell death and harm islet graft function[3]. A new possible therapeutic approach consists of using biomaterials in the form of hydrogels to encapsulate islets for delivery in vivo to overcome this obstacle. Alginate, a polysaccharide, has been the most commonly investigated hydrogel-based biomaterial for islet delivery in vivo[4, 5].
Our group developed a novel biomaterial system based on silk fibroin to safely deliver islets for transplantation, thus enhancing islet survival, vascularization, and function while minimizing inflammation-mediated cell damage[6, 7]. Our previous studies demonstrated that the islets encapsulated in silk hydrogels showed improved insulin response to high glucose in vitro and in vivo during the engraftment period, implying the existence of specific properties of islet-silk interaction that may be harnessed to improve engraftment success[6, 7].
Silk fibroin offers biocompatibility, controllable degradation rates, and strong mechanical properties[8-10]. The specific silk fibroin used in the current study is formulated for sonicated-induced gelation so that the aqueous silk solution may be mixed with cells, and gelation may occur in a reasonable timeframe for cell encapsulation[11]. The aqueous silk solution may also be tested in tissue culture medium in vitro. In the current study, we hypothesized that silk fibroin in different modalities (e.g. hydrogel or in solution) has unique properties that improve islet function during inflammatory-mediated stress. To evaluate whether the silk fibroin (in hydrogel form or in soluble solution) has inherent properties that enhance islet function when challenged by inflammatory stress, we analyzed in vitro the activation and expression of molecular pathway proteins that regulate insulin secretion in comparison to their levels when islets were exposed to alginate. A series of in vitro analyses were performed to investigate the activation of the mitogen activated protein kinase (MAPK)/extracellular receptor kinase (ERK) pathway, which regulates insulin gene expression. Additionally, we investigated the expression patterns of GLUT2, the transporter responsible for beta cell detection of glucose and secretion of insulin.
2. METHODS
2.1. Animals
C57BL/6 male mice, aged 8–14 weeks, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and from the University of Maryland Animal Resources (Baltimore, MD, USA), and housed in the University of Maryland animal facility. All mouse manipulations were conducted using protocols approved by the Institutional Animal Care and Use Committee (IACUC).
2.2. Islet isolation
Animals were euthanized, and the abdomen was subsequently incised. The pancreas was exposed and infused through the main bile duct with collagenase type V (SigmaAldrich, St. Louis, MO) suspended at 0.7 mg/mL in a buffer solution (Perfusion Solution, Cellgro-Mediatech, Herndon, VA). The distended pancreas was removed and digested at 37°C for 12 minutes, followed by vigorous agitation for 10 seconds. After washing, islet purification was obtained through centrifugation on a Euro-Ficoll (Corning, Manassas, VA) gradient. Islets were hand-picked under an inverted microscope, plated in supplemented cRPMI medium 1640 [10% fetal bovine serum (FBS; Gemini BioProducts, West Sacramento, CA, USA), 2 mM L-glutamine (Sigma-Aldrich), 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA) in RPMI 1640 (Lonza, Walkersville, MD, USA)], and incubated for 24 h at 37°C in 5% CO2 for recovery.
2.3. Experimental Design (Outlined in Fig. 1)
Figure 1.
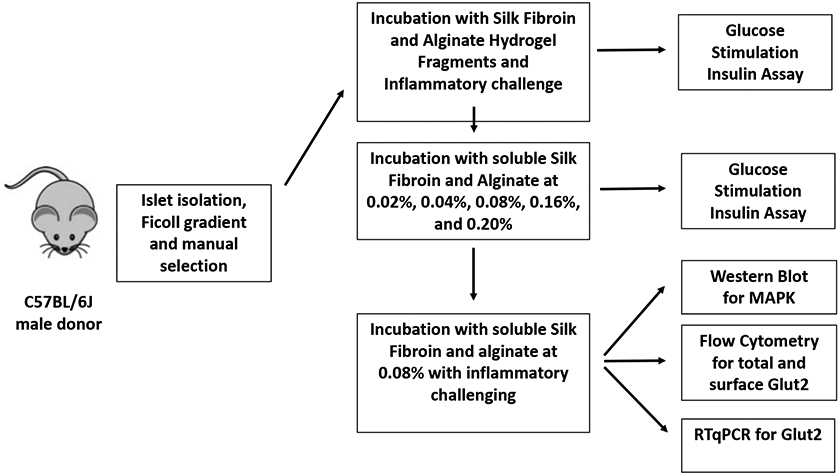
Experimental design.
To determine whether silk fibroin has inherent properties that improve islet function, both at baseline and against an inflammatory cytokine-mediated challenge, islets were treated in vitro as follows: 1) in contact with silk hydrogel fragments; 2) in contact with alginate hydrogel fragments; or 3) pelleted in medium alone. Increasing concentrations (0%−0.16%) of either silk or alginate in solution were evaluated as well. Islets were incubated in these conditions to determine optimal concentrations for further mechanistic studies of the silk-mediated effect on islet function. The silk fibroin effect was analyzed both via Western blot, to measure the activation of ERK1, as well as via flow cytometry, to measure the expression patterns of the GLUT2 transporter (Fig. 1).
2.4. Glucose-stimulated insulin secretion (GSIS)
Islet function was performed using a glucose-stimulated insulin secretion (GSIS) assay as follows. Following preparation of each experimental condition, islet samples (30 islet equivalents, or IEQs, per sample) were incubated consecutively in basal glucose (2.5 mM) followed by high glucose (16.7 mM) solutions for 60 min each. Following each incubation, supernatants were collected and analyzed via an insulin Rat/Mouse Insulin ELISA (Millipore, Burlington MA). Insulin secretion results for each sample are presented as the ratio of the insulin concentration in the high glucose solution relative to the concentration in the low glucose solution; this ratio is referred to as the stimulation index (SI).
2.5. Islets exposed to silk and alginate hydrogel fragments in vitro
Silk fibroin solutions were prepared as previously described[12]. Silk self-assembly and gelation was induced by sonication of 375 μL of 2 wt. % silk fibroin mixed with 625 μL of sterile water in a glass vial and vortexed for 7 min at 3200 rpm (VWR International, Radnor, PA). After phase separation, the white solid-like material was removed, and the remaining silk solution was diluted two-fold with media and allowed to gel for 2 h at 37°C. Subsequently, a sterile pipette tip was used to disperse the silk gel into fragments.
Alginic acid sodium salt from brown algae was obtained commercially (Sigma, Cream Ridge, NJ) and dissolved to 2% w/v in purified water. Alginate gel fragments were formed using 30 μL 2% w/v alginate dripped into 100 mM CaCl2 to crosslink and create gel pellets. These gel pellets were incubated at 37°C for 1 h, placed on an 80 μm strainer, and washed with PBS to remove residual CaCl2. Alginate pellets were then re-suspended in 600 μL medium and sonicated until broken up homogeneously in the medium. Islet samples (30 IEQs) were then plated with either silk or alginate fragments for 24 h (with medium alone serving as a control), followed by treatment with an inflammatory cytokine cocktail (10 ng/mL tumor necrosis factor (TNF)α, 5 ng/mL IL-1β, and 100 ng/mL IFNγ) for 4 h. Islet function was assessed by GSIS assay before and after cytokine exposure.
2.6. Islets exposed to silk and alginate in solution in vitro
In order to pursue mechanistic studies on the effect of silk on islet function, islets were incubated in silk or alginate soluble solution. Islet samples were treated with increasing concentrations of silk or alginate at 0.02, 0.04, 0.08, 0.16, and 0.20% w/v, diluted in RPMI in a 24-well plate (30 IEQs per well) for 24 h. Islet function was then assessed by GSIS assay and compared to the function of islets cultured in RPMI media alone.
2.7. Western blot analysis of MAPK/ERK phosphorylation
Islets (50 IEQs per sample) were incubated first in serum-free RPMI medium for 2 h at 37°C, and then incubated in 0.08% soluble solution of either silk or alginate for 30 min. Islets were extracted in Western lysis buffer, subjected to SDS-PAGE, and immunoblotted and quantitated using a Fluorochem Q imager and ImageQuant software (ProteinSimple, San Jose, CA) as previously described (Souza et al, 2007). Antibodies specific for p44/42 MAPK/ERK and phospho-p44/42 MAPK/ERK (Thr202/Tyr204) were purchased from Cell Signaling Technologies (Danvers, MA). ERK activation was determined using the average ratio of the phospho-ERK to ERK signal in two independent experiments.
2.8. Flow cytometry analysis of GLUT2 expression
The effect of the biomaterial exposure on GLUT2 expression was evaluated via flow cytometry. Islet samples (30 IEQs) were incubated for 24 h in 0.08% silk, 0.08% alginate, or medium alone, with or without an inflammatory cytokine cocktail (10 ng/mL TNFα, 5 ng/mL IL-1β, 100 ng/mL IFNγ). Islets were then hand-picked, washed with PBS once, re-suspended in 200 μL enzyme-free dissociation buffer (Thermofisher, Waltham, MA) at 37°C for 6 min, and disrupted through repeated pipetting. The dissociated single-cell suspension was spun down and moved to a v-bottom 96-well tissue culture plate. Cells were stained with 1:1000 Fixable Viability Dye eFluor™ 450 (eBioscience™, Waltham, MA) in MACS Buffer (PBS with 1% FBS, .5% EDTA) for 15 min at 4°C.
2.8.1. Surface GLUT2 Staining
Following viability staining, islet samples were incubated first with 250 μL rat anti-mouse GLUT2 primary antibody (R&D Systems, Minneapolis, MN) at 1:100 in MACS Buffer at 4°C for 30 min, and then with Alexa Fluor® 594 AffiniPure Goat Anti-Rat IgG (H+L) secondary antibody diluted at 1:200 (Jackson ImmunoResearch Laboratories, West Grove, PA) in MACS Buffer at 4°C for 30 min.
2.8.2. Total GLUT2 Staining
Following viability staining incubation, islet samples were first fixed using 4% PFA and then stained for GLUT2 using the same antibodies, resuspended in BD Perm/Wash Buffer instead of in MACS buffer for the staining incubation.
2.8.3. Flow Cytometry Analysis
Samples were analyzed using a BD LSRFortessa digital flow cytometer (BD Biosciences, San Jose CA). Sequential gating was performed as follows: 1) forward versus side scatter (FSc vs SSc) was used to exclude debris; 2) FSc-area versus FSc-height was used to select for single cells; 3) SSc-area versus the live/dead stain was used to select for viable cells. A sample stained with the isotype control (1:100 Rat IgG2B) was used to establish a GLUT2-positive threshold to include 1% of the cells in the isotype-stained sample. In experimental samples, events with a fluorescence intensity exceeding this positive threshold were defined as GLUT2-positive, and the percentage of these cells positive for either total or surface GLUT2 expression was calculated using FlowJo software. The median fluorescence intensity (MFI) was recorded for surface GLUT2 expression, and geometric mean fluorescence intensity (GMFI) was recorded for total GLUT2 expression. Both MFI and GMFI values represent overall expression of GLUT2 and are presented as output ratio MFI (rMFI) and ratio GMFI (rGMFI) values, calculated as the ratio of the experimental GMFI to the isotype GMFI.
2.9. RTqPCR for GLUT2 transcript
Islet samples (80 IEQs) were incubated for 24 h in either 0.08% silk fibroin, 0.08% alginate, or in medium alone as control. Islet RNA was then extracted using Trizol (Thermo Fisher Scientific) and converted to cDNA using the GoScript Reverse Transcriptase (Promega, Madison, WI) according to the manufacturer’s protocol. The cDNA was subjected to PCR on the ABI Prism 7900HT in triplicate using the LightCycler® 480 Probes Master kit (Roche Diagnostics, Indianapolis, IN) and Roche Universal Probe Library probes #106 and #80. The following primers were used: GTACTGAATGTGGATGAATAGCTG (Reverse GLUT2), CCCTGGGCCAGTAGTGTG (Forward GLUT2), AATGTGTCCGTCGTGGATCT (Forward GAPDH), and CCCAGCTCTCCCCATACATA (Reverse GAPDH). RTqPCR was run on an ABI Prism 7900HT and analyzed using the 2^(-Delta Delta Ct) method.
2.10. Statistical methods
One-Way Analysis of Variance (ANOVA) tests were run using Prism 7 with a Bonferroni multiple comparisons post-hoc test. A one-tailed, paired t-test was used to compare ERK phosphorylation, using islets in medium as the control.
3. RESULTS
3.1. Islet function improved in vitro following incubation with silk hydrogel fragments
Islet samples were incubated for 24 h in vitro with either silk hydrogel fragments, alginate fragments, or medium alone, and then analyzed via GSIS assay. Islets exposed to silk hydrogel fragments showed about a 2-fold increase in stimulation index (SI) compared to islets exposed to either alginate or to medium alone (p<0.05) (Fig. 2A).
Figure 2. Improvement of islet function following incubation with silk fragments, with and without inflammatory cytokine stress.
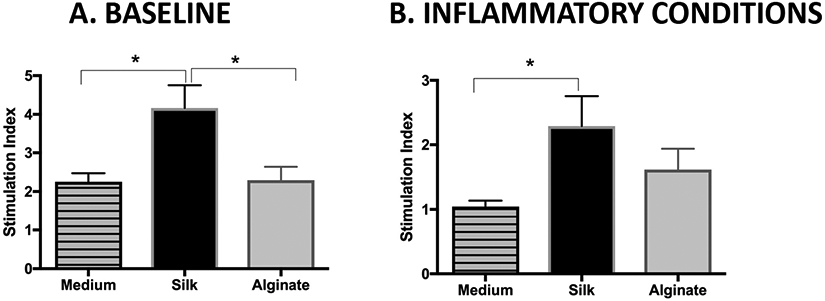
A glucose stimulated insulin secretion (GSIS) assay was performed on islet samples that had been pre-cultured in vitro in contact with biomaterial fragments for 12 h (A) and then challenged with an inflammatory cytokine cocktail (10 ng/mL TNFα, 5 ng/mL IL-1β, and 100 ng/mL IFNγ) for 4 h at 37°C (B). Islet insulin secretion is reported as a stimulation index (SI), or the ratio of insulin secreted in response to 16.7 mM glucose relative to insulin secreted in response to 2.5 mM glucose. Mean ± SEM using One-Way Analysis of Variance with a Bonferroni multiple comparisons post-hoc test; all conditions n ≥ 6. ** represents p<0.01; * represents p<0.05.
Islets exposed to inflammatory cytokines following a 24 h incubation with silk, alginate, or medium alone all showed decreased function, with the SI of each condition lower than at its baseline. However, the SI remained better preserved for islets exposed to silk, compared to those exposed to alginate (p=0.43) or medium alone (p<0.05) (Fig. 2B).
3.2. Islet function exposed to silk in aqueous solution concentration
In order to determine optimal silk concentrations for further mechanistic studies, islets were exposed to soluble silk or alginate solutions in vitro ranging from 0.02% to 0.20% w/v. (Fig. 3). Islets were incubated for 12 h in either soluble silk or alginate solution and then analyzed via GSIS assay. Islets treated with soluble silk showed a dose-dependent increase in SI from 0.02 to 0.04, 0.08, and 0.16% when compared to control medium, which was significant at 0.08% and at 0.16% (p<0.05) (Fig. 3). The SI for 0.08% soluble alginate-treated islets was slightly improved compared to control medium (p>0.05), but remained lower than that of the 0.08% silk-treated islets (p=0.487) (Fig. 3). A previous study by Tusi et al. determined that 0.08% was the optimal concentration of alginate for microencapsulation of pheochromocytoma cells on protecting these cells against apoptosis[13]. Islet cell function being well preserved at both 0.08% and 0.16% silk compared to control (no silk), subsequent mechanistic studies were performed using silk or alginate in solution at 0.08%.
Figure 3. Islet function following silk vs. alginate incubation in solution.
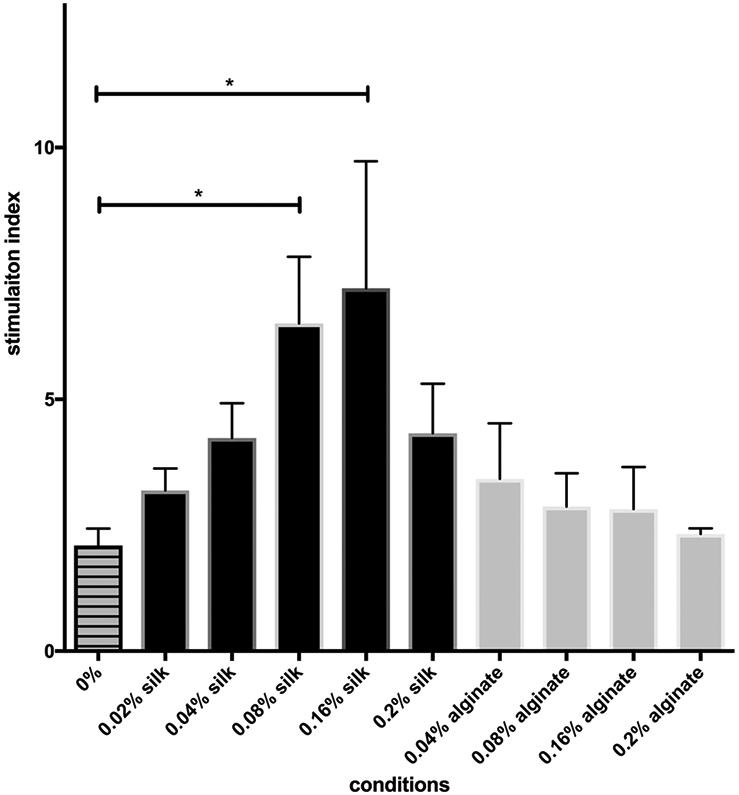
A glucose stimulated insulin secretion (GSIS) assay was performed on islet samples cultured in vitro for 12 h in increasing concentrations (0.02% - 0.2%) of either silk or alginate. Islet insulin secretion is reported as a stimulation index (SI), or the ratio of insulin secreted in response to 16.7 mM glucose relative to insulin secreted in response to 2.5 mM glucose. Mean ± SEM using One-Way Analysis of Variance with a Bonferroni multiple comparisons post-hoc test; all conditions n ≥ 3. * represents p<0.05.
3.3. MAPK/ERK activated by both silk fibroin and alginate
To explore the mechanism of how silk fibroin may affect insulin production, ERK activation was evaluated. As shown in Fig. 4, a 30 min soluble silk treatment increased ERK phosphorylation by 50% (p=0.0354) and alginate treatment increased ERK phosphorylation by 20% (p=0.1307), each in comparison to control medium.
Figure 4. Measuring phosphorylated (p)ERK activity by western blotting.
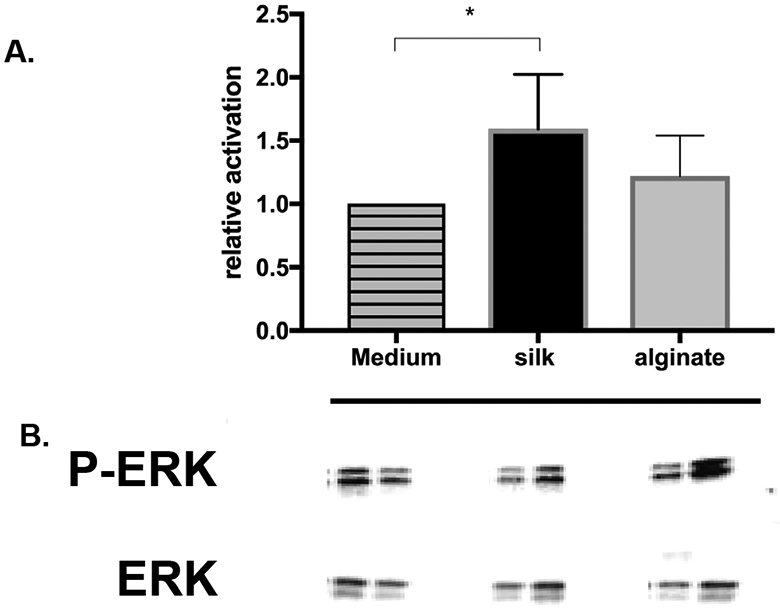
Islets were treated with 0.08% of either silk or alginate in vitro for 30 min. Islet protein was extracted, prepared for Western blotting, probed for expression of pERK(Thr202/Tyr204), and quantitated using a Fluorochem Q imager and ImageQuant software. ERK relative activation (A) in islets treated with either silk or alginate was determined using the average ratio of pERK to ERK signal on the immunoblot (B) in two independent experiments and compared to islets in medium as control using one tailed paired t test. * represents p<0.05.
3.4. GLUT2 islet expression following incubation with silk fibroin
3.4.1. Surface GLUT2 expression was increased
Islet cell surface GLUT2 expression (rMFI), measured following incubation with 0.08% soluble silk, increased by about 35% compared to islets incubated in either medium or in 0.08% soluble alginate (p<0.05). Under cytokine-induced inflammatory stress, surface GLUT2 expression remained significantly increased following incubation with silk (by 20%) compared to medium (p<0.05) (Fig. 5A). Moreover, the percentage of islet cells expressing GLUT2 on the surface was increased by about 30% following incubation with soluble silk compared to medium or soluble alginate incubation (p<0.05) (Fig. 5B). Under inflammatory conditions, the percentage of islet cells expressing GLUT2 on the surface remained increased (by 15%) following incubation with soluble silk compared to medium (0.05) or alginate (p<0.01) (Fig. 5B).
Figure 5. Flow cytometry analysis of islet cell surface GLUT2 expression.
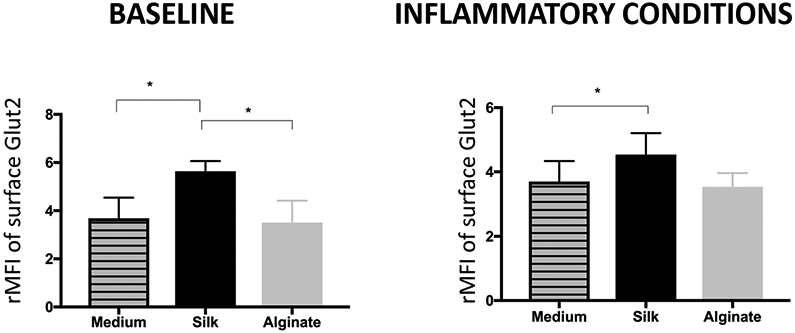
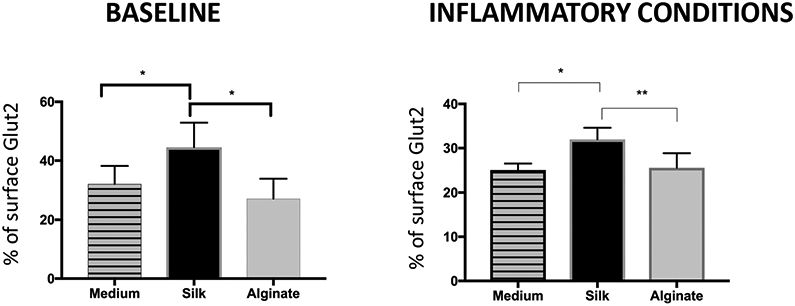
Following incubation in either silk or alginate at 0.08%, mean fluorescence intensity (MFI) was recorded for islet cell surface GLUT2 at baseline and in combination with an inflammatory cytokine cocktail, and presented as output ratio MFI (rMFI), the ratio between experimental and isotype MFI values for Mean ± SEM (A), and as percent of cells positive for the GLUT2 surface marker (B). All conditions n ≥ 5. * represents p<0.05.
3.4.2. Total GLUT2 islet cell content was not increased
To measure total cellular GLUT2 protein content, islets were permeabilized prior to GLUT2 staining and flow cytometry. Total cellular GLUT2 content (rGMFI) was increased following incubation with soluble silk compared to incubation with medium (by 40%) or soluble alginate (by 30%) (p>0.05) but did not change significantly across islet groups treated with soluble silk or alginate, or medium alone, following inflammatory conditions. (Fig. 6).
Figure 6. Flow cytometry analysis of total GLUT2 islet cell content.
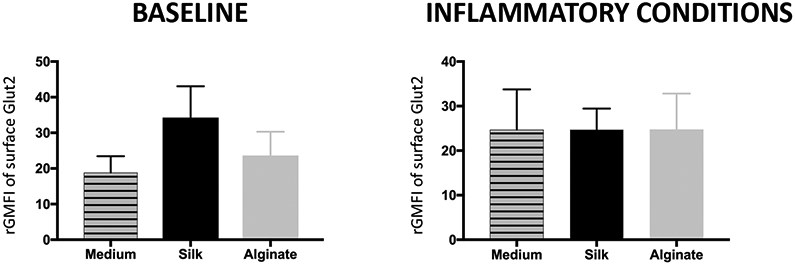
Following incubation with either silk or alginate at 0.08%, geometric mean fluorescence intensity (GMFI) was recorded for GLUT2 content in permeabilized islet cells at baseline and in combination with an inflammatory cytokine cocktail and presented as output ratio MFI (rGMFI), the ratio between experimental and isotype GMFI values for Mean ± SEM. All conditions n ≥ 5.
3.5. GLUT2 RTqPCR
There were no significant differences between 0.08% silk fibroin, 0.08% alginate, or medium alone in GLUT2 transcript quantities (data not shown).
4. DISCUSSION
In a previous study by our group using islets encapsulated in a silk hydrogel[7], we observed improved islet graft function recovery in vivo post transplantation. In that previous model, potentiation of islet function could be simply explained by the three-dimensional support provided by the silk hydrogel encapsulation scaffold[14]. The focus of the current study was to investigate whether a silk-mediated effect—improved islet response to high glucose conditions in vitro—is observed in the presence of cytokine-induced inflammatory stress. We hypothesized that silk-islet contact may in itself play a biochemical role in improving islet function. Indeed, a 24 h incubation with either silk fibroin hydrogel or soluble solution resulted in a significantly improved islet insulin response to high glucose stimulation. Our study is the first to explore the direct effect of the silk on the glucose-sensing machinery of islet beta cells (i.e. GLUT2 expression). Importantly, an increase in surface GLUT2 receptor expression was associated with this observation. Following silk exposure, GLUT2 gene expression and total GLUT2 protein content remained unchanged, suggesting that the silk-mediated increase in surface GLUT2 expression resulted from increased GLUT2 translocation from the cytoplasm to the cell surface. The impact of the current study in advancing the field of hydrogel-based islet encapsulation is significant. Silk, a novel biomaterial being tested for use in islet encapsulation, has been shown in this study to significantly enhance islet function at baseline and mitigate its decline under inflammatory conditions. Furthermore, these results support previous findings showing improved beta cell mass and function in diabetic mice that received an oral solution of silk fibroin[15].
GLUT2, an important protein for beta cell sensing of glucose, is primarily expressed in murine pancreatic beta cells and is less abundant in human beta cells. With its low affinity for glucose but high transport capacity[16], GLUT2 allows establishment of rapid glucose homeostasis across the beta cell membrane. GLUT2 expression on the beta cell surface is dependent on GlcNAcT-IVa glycosyltransferase, which constructs a glycoprotein-specific N-glycan ligand for pancreatic lectin receptors. A defect in GlcNAcT-IVa results in decreased GLUT2 cell surface expression, associated with its endocytosis and redistribution into endosomes and lysosomes[17]. Interestingly, murine diabetic models of hyperglycemia due to a high fat diet present with down-regulated expression of GLUT2 on the surface of beta cells[17],[18]; Wang et al. hypothesized that GLUT2 internalization and intracellular retention protected beta cells from hyperglycemia[19]. Nevertheless, a better understanding of the signaling pathways that lead to GLUT2 translocation is needed to determine the specific role that silk contact may play in regulating beta cell response to stressful conditions including hyperglycemic and inflammatory environments
In addition to regulation of GLUT2 expression, we explored the silk-mediated effect on the molecular pathways regulating insulin health. As part of the mitogen activated protein kinase (MAPK) pathway, the ERK cascade plays a key role in beta cell intracellular signaling through phosphorylation of MafA and up-regulation of insulin transcription[20]. The significant increase in ERK phosphorylation observed in islet cells incubated with silk for 30 min suggests that the MAPK/ERK pathway may mediate the silk-protein effect on beta cell function.
Importantly, when challenged by an inflammatory cytokine cocktail, islet function was better preserved following silk incubation compared to incubation with alginate or in medium alone. Furthermore, islet cell surface GLUT2 expression, which was decreased in all groups by the inflammatory stress, remained better preserved following silk incubation. GLUT2 gene expression in islets exposed to inflammatory stress was not explored in this study, but has previously been shown to be decreased by inflammatory cytokine exposure (i.e. TNFα, IL-1β, and IFNγ), which similarly decreased expression of crucial functional beta cell genes such as those encoding insulin, pancreatic and duodenal homeobox 1 (PDX1)[21].
The composition of silk may explain the upregulation of GLUT2 observed in the current study. Silk is a polypeptide, whose primary component, fibroin, consists of approximately 44% glycine and 30% alanine as the major amino acids[9]. In contrast, alginates are polysaccharides composed of repeating sequences of d-mannuronic acid or l-glucuronic acid[22]. A Chinese hamster ovary (CHO)-derived epithelial cell line exposed in vitro to a mixture containing these amino acids, glycine and alanine, showed increased translocation of another glucose transporter, GLUT4[23]. Similarly, in a study by Hyun et al, the murine 3T3-L1 adipocyte cell line, when exposed in vitro to a soluble solution of silk fibroin, rich in glycine and alanine, showed upregulated glucose transporter GLUT1 translocation to the cell surface[24]. In light of these results, we could hypothesize that specific peptide epitopes rich in glycine and alanine, as in silk, might be responsible for the activation of the ERK pathway and ultimately lead to GLUT2 translocation in the murine islets from our studies.
Future studies will evaluate other silk-mediated mechanisms on beta cell signaling pathways that may potentiate glucose-stimulated insulin secretion. These same amino acids, glycine and alanine, are known to trigger the secretion of insulin in a similar manner to glucose and to be carried through the beta cell membrane through pairing with Na+ using the same transporter, resulting in a Na+ influx and depolarization of the beta cell. This depolarization is sufficient to open Ca2+ channels, and through Ca2+ uptake trigger insulin release[25]. Thus, we will also explore the effects of silk on calcium signaling, as insulin secretion is directly tied to changes in cytoplasmic calcium.
Consideration of biomaterials (i.e. alginate, polyethylene glycol (PEG), poly (lactic-co-glycolic) acid (PLGA), and silk) for islet encapsulation and delivery in vivo focuses on three major objectives: 1) restoration of islet vascularization; 2) restoration of islet response to high glucose conditions; and 3) protection of islet survival against inflammatory-mediated stress and allo- and auto-immune rejection[26]. This study demonstrates the potential of the silk hydrogel to address two of these objectives: restoration of islet function and protection against inflammatory stress. In the configuration presented here, the silk hydrogel will not provide immune protection, which could be considered a weakness of this method. However, we are considering engineering ligation of inhibitory peptides (i.e. Interleukin-1 receptor) on the surface of the silk hydrogel encapsulated islets. These peptide-modified hydrogel models may further improve islet cell immunoprotection[27, 28]. Silk hydrogels could also be considered as an adjunct to an islet macroencapsulation device such as the one manufactured by Viacyte Inc.[29]. Silk hydrogels could then be tested alongside alginate for islet encapsulation in combination with immuno-isolation chambers[4].
5. CONCLUSIONS
The current study demonstrates that the improved insulin response to high glucose in vitro and in vivo of islets encapsulated in silk hydrogel, as previously observed by our group, may be related to specific islet-silk interactions. These interactions were elucidated through in vitro studies using soluble silk solution, which showed increased ERK1 pathway activation and increased islet cell surface expression of GLUT2 proteins that regulate insulin secretion. These new observations may be capitalized upon to optimize the intracapsular milieu (i.e. improve islet function and resistance to inflammatory stress), which would advance the field of islet transplantation towards a cure for diabetes.
6. ACKNOWLEDGMENTS
We acknowledge the efforts of Amanda Jones in editing this manuscript.
7. FUNDINGS:
This work was supported by the Living Legacy Foundation, the National Institutes of Health (P41EB002520), and the Department of Pathology from the University of Maryland School of Medicine.
Footnotes
Declarations of interest: none
9. REFERENCES
- [1].Noguchi H, Pancreatic islet transplantation, World Journal of Gastrointestinal Surgery, 1 (2009) 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jamiolkowski RM, Guo LY, Li YR, Shaffer SM, Naji A, Islet transplantation in type I diabetes mellitus, The Yale journal of biology and medicine, 85 (2012) 37–43. [PMC free article] [PubMed] [Google Scholar]
- [3].Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV, Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production, The Journal of clinical endocrinology and metabolism, 79 (1994) 1058–1062. [DOI] [PubMed] [Google Scholar]
- [4].Korsgren O, Islet Encapsulation: Physiological Possibilities and Limitations, Diabetes, 66 (2017) 1748–1754. [DOI] [PubMed] [Google Scholar]
- [5].Vos P, Faas MM, Strand B, Alginate-based microcapsules for immunoisolation of pancreatic islets, Biomaterials, 27 (2006) 5603–5617. [DOI] [PubMed] [Google Scholar]
- [6].Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ, Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel, Biomaterials, 33 (2012) 6691–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hamilton DC, Shih HH, Schubert RA, Michie SA, Staats PN, Kaplan DL, Fontaine MJ, A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo, Journal of tissue engineering and regenerative medicine, (2015). [DOI] [PubMed] [Google Scholar]
- [8].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL, Silk-based biomaterials, Biomaterials, 24 (2003) 401–416. [DOI] [PubMed] [Google Scholar]
- [9].Vepari C, Kaplan DL, Silk as a Biomaterial, Prog Polym Sci, 32 (2007) 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Preda RC, Leisk G, Omenetto F, Kaplan DL, Bioengineered Silk Proteins to Control Cell and Tissue Functions, in: Gerrard JA (Ed.) Protein Nanotechnology, Humana Press, 2013, pp. 19–41. [DOI] [PubMed] [Google Scholar]
- [11].Yucel T, Cebe P, Kaplan DL, Vortex-induced injectable silk fibroin hydrogels, Biophys. J, 97 (2009) 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL, Materials fabrication from Bombyx mori silk fibroin, Nat. Protocols, 6 (2011) 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tusi SK, Khalaj L, Ashabi G, Kiaei M, Khodagholi F, Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress, Biomaterials, 32 (2011) 5438–5458. [DOI] [PubMed] [Google Scholar]
- [14].Liao SW, Rawson J, Omori K, Ishiyama K, Mozhdehi D, Oancea AR, Ito T, Guan Z, Mullen Y, Maintaining functional islets through encapsulation in an injectable saccharide-peptide hydrogel, Biomaterials, 34 (2013) 3984–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Do SG, Park JH, Nam H, Kim JB, Lee JY, Oh YS, Suh JG, Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice, Journal of veterinary science, 13 (2012) 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thorens B, GLUT2, glucose sensing and glucose homeostasis, Diabetologia, 58 (2015) 221–232. [DOI] [PubMed] [Google Scholar]
- [17].Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD, Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes, Cell, 123 (2005) 1307–1321. [DOI] [PubMed] [Google Scholar]
- [18].Reimer MK, Ahren B, Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice, Diabetes, 51 Suppl 1 (2002) S138–143. [DOI] [PubMed] [Google Scholar]
- [19].Wang ZV, Mu J, Schraw TD, Gautron L, Elmquist JK, Zhang BB, Brownlee M, Scherer PE, PANIC-ATTAC: a mouse model for inducible and reversible beta-cell ablation, Diabetes, 57 (2008) 2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puddu A, Sanguineti R, Mach F, Dallegri F, Viviani GL, Montecucco F, Update on the protective molecular pathways improving pancreatic beta-cell dysfunction, Mediators of inflammation, 2013 (2013) 750540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moore F, Naamane N, Colli ML, Bouckenooghe T, Ortis F, Gurzov EN, Igoillo-Esteve M, Mathieu C, Bontempi G, Thykjaer T, Orntoft TF, Eizirik DL, STAT1 is a master regulator of pancreatic {beta}-cell apoptosis and islet inflammation, The Journal of biological chemistry, 286 (2011) 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Formo K, Aarstad OA, Skjak-Braek G, Strand BL, Lyase-catalyzed degradation of alginate in the gelled state: effect of gelling ions and lyase specificity, Carbohydrate polymers, 110 (2014) 100–106. [DOI] [PubMed] [Google Scholar]
- [23].Selvi R, Bhuvanasundar R, Saijyothi AV, Sulochana KN, Angayarkanni N, Amino acids potentiate insulin signaling in CHO-K1 at high glucose conditions, Archives of medical research, 43 (2012) 173–182. [DOI] [PubMed] [Google Scholar]
- [24].Hyun CK, Kim IY, Frost SC, Soluble fibroin enhances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes, The Journal of nutrition, 134 (2004) 3257–3263. [DOI] [PubMed] [Google Scholar]
- [25].Ahmed M, Grapengiesser E, Hellman B, Amino acid transformation of oscillatory Ca2+ signals in mouse pancreatic beta-cells, The Journal of endocrinology, 160 (1999) 191–195. [DOI] [PubMed] [Google Scholar]
- [26].Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution, Advanced drug delivery reviews, 67–68 (2014) 35–73. [DOI] [PubMed] [Google Scholar]
- [27].Reeves AR, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL, Controlled release of cytokines using silk-biomaterials for macrophage polarization, Biomaterials, 73 (2015) 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kumar M, Coburn J, Kaplan DL, Mandal BB, Immuno-Informed 3D Silk Biomaterials for Tailoring Biological Responses, ACS applied materials & interfaces, 8 (2016) 29310–29322. [DOI] [PubMed] [Google Scholar]
- [29].Desai T, Shea LD, Advances in islet encapsulation technologies, Nature reviews. Drug discovery, 16 (2017) 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]


