Abstract
Purpose of review
This review focuses on the development and progression of glioblastoma through the brain and glioma microenvironment. Specifically we highlight how the tumor microenvironment contributes to the hallmarks of cancer in hopes of offering novel therapeutic options and tools to target this microenvironment.
Recent findings
The hallmarks of cancer, which represent elements of cancers that contribute to the disease’s malignancy, yet elements within the brain tumor microenvironment, such as other cellular types as well as biochemical and biophysical cues that can each uniquely affect tumor cells, have not been well-described in this context and serve as potential targets for modulation.
Summary:
Here, we highlight how the brain tumor microenvironment contributes to the progression and therapeutic response of tumor cells. Specifically, we examine these contributions through the lens of Hanahan & Weinberg’s Hallmarks of Cancer in order to identify potential novel targets within the brain that may offer a means to treat brain cancers, including the deadliest brain cancer, glioblastoma.
Keywords: Glioblastoma, tumor microenvironment, cancer hallmarks, brain microenvironment
Introduction
Glioblastoma (GBM), the most aggressive form of brain cancer, presents with unique challenges compared to other forms of disease in regards to treatment. Due to the complex and sensitive architecture of the brain, efficacy and cytotoxicity are major concerns for developing ameliorative therapeutics that can cross the blood brain barrier and avoid damage to the intricate structures that are important for nervous system function [1]. Course of treatment for GBM normally includes initial resection of the tumor; however, diffuse invasion of cells throughout the brain create a termed “moving target” for therapeutic access due to ineffective complete resection of tumor cells left behind. To overcome this limitation and treat residual tumor cells, radiation and/or chemotherapeutics are usually a follow-up treatment which comes with its own shortcomings. For instance, aberrant and compressed blood vasculature limits access of therapeutics to the tumor to begin with as well as tumor cells themselves have resistances that we have yet to define or target. Thus, recurrence is inevitable for patients with the tumor, resulting in a dismal survival outlook of 14–16 months [2]. Thus, we need new ideas for the treatment of all brain cancers, but especially GBM as the deadliest form.
The hallmarks of cancer as defined by Hanahan & Weinberg are characteristics of pro-tumorigenic re-programming that elicits tumor retention and progression [3–5]. They are common among many forms of cancer and are updated seemingly every ten years. There are myriad key players that aid cancer hallmarks residing within the tumor microenvironment where tumor cells reprogram functionally different cell populations and cues, the extracellular matrix (ECM), and surrounding stroma in order to sustain growth and progression. These components all interact with tumor cells, but also each other, to create a dynamic and evolving ecosystem in which tumor cells thrive. To this end, many researchers are investigating and targeting the tumor microenvironment in many cancers, but the scope of this review is to highlight cancer hallmarks through the lens of the GBM tumor microenvironment (GBM-TME) and recent advances in understanding its unique and complex TME and opportunities in potential therapeutics to target it.
Biological architecture of the GBM tumor microenvironment
The tumor microenvironment plays a significant role in all solid tumors, primarily through biochemical and biophysical cues as a result of cancer reprogramming of cell-cell and cell-ECM interactions to support the tumor [6]. Although the TME in many cancers share similarities, beyond the blood brain barrier, there are key characteristics that make the GBM-TME unique compared to other tissues. For instance, the ECM within the healthy brain possesses high levels of glycosaminoglycans, proteoglycans, and glycoproteins such as hyaluronan, lecticans, and tenascin, respectively, but low levels of fibrous matrix proteins, such as collagen in contrast with other tissues [7–9]. With a generally lower physiological stiffness compared to other tissues, the mechanical properties of the brain are tightly tuned to facilitate a vast range of important processes that have been reviewed in detail for brain development and homeostasis [9]. In the GBM-TME, the ECM is stiffer in general as compared to that of lower-grade gliomas, which as commonly observed in other cancers, mediates interactions and phenotypes promoting fates within the TME toward tumor progression and sustainability. Another distinguishing characteristic of the GBM-TME are the specialized cell types found exclusively in the brain, such as astrocytes, oligodendrocytes, microglia, and neurons (Table 1). These cells are essential for nervous system function [10], but cancer development often leads to co-option for the purpose of encouraging tumor growth and invasion. Specifically, GBM is a grade IV astrocytoma, indicating transformation of astrocytes. Microglia serve as brain-resident immune cells that function similarly to peripheral innate immune cells, and therefore are components of the GBM-TME as well [11]. Normally, these cells should inhibit tumor development and/or provide neuroinflammatory relief in the tissue; however, in glioma, many are repurposed for tumor promotion and anti-tumorigenic immune evasion [6]. Table 1 lists the cellular components of the brain microenvironment and how they are involved in GBM.
Table 1.
Glioblastoma Tumor Microenvironment Cellular Components.
| Brain Tumor Microenvironment Cellular Components | Physiological Function | Implications in Cancer |
|---|---|---|
 Microglia Microglia |
• Remove debris from CNS from injury & cell turnover • Modulate local inflammation |
• Coordinates astrocyte-mediated immunosuppression |
 Astrocytes Astrocytes |
• Regulates electrochemical signaling to protect neurons • Regulate blood flow and interact with BBB • Metabolic modulator |
• Tumor-associated astrocytes • Secretes IL-6 and promotes invasion through MMP activation |
 Glial stem cells Glial stem cells |
• Multipotent cells that can differentiate into multiple glial cell types | • Contributes to heterogeneity of tumor-derived endothelial cells, pericytes, and |
| Immune cells | • Cells that help aid in defense against pathogens, disease, and foreign bodies by releasing cytokines | • Coopted to promote tumor-promoting inflammation |
 Natural killer cells Natural killer cells | ||
 T cells T cells |
 Tumor associated macrophage Tumor associated macrophage |
|
 Macrophage Macrophage | ||
 Neurons Neurons |
• Fundamental nerve cells responsible for signal transmission in the body | • Contributes to glioma growth through secretion of necroligin-3 • Can aid in glioma growth |
 Endothelial cells Endothelial cells |
• Essential cell type for the generation of vasculature networks in the brain | • Recruited for enhanced angiogenesis |
 Pericytes Pericytes |
• Contractile cells that surround blood vessel walls • Stabilize blood vascularization properties and flow • BBB maintenance |
• Modulate immune cells within the TME |
 Myeloid derived suppressor cells (MDSCs) Myeloid derived suppressor cells (MDSCs) |
• Suppresses inflammation in a local area physiologically | • Enhances tumor-promoting inflammation |
Diffuse infiltration, meaning that tumor cells invade into normal brain parenchyma, is a defining characteristic of GBM and often leads to difficulty during resection and treatment because it leaves vast room for tumor cells to be left behind to continue to allow the cancer to exist and propagate [12]. Moreover, the GBM-TME presents unique manifestations that are specific to GBM dissemination, such as pseudopalisading necroses, which is defined by a region of necrotic tissue due to hypoxia and apoptosis surrounded by aligned nuclei of continually proliferative cells that is a classical histopathological indicator for GBM [13].
Tumor-associated angiogenesis allows the tumor to sequester nutrients in a low-nutrient and hypoxic environment, however, aberrant fenestrations within the endothelium lead to leaky vasculature, impacting intratumoral transport [14]. These abnormal vascular conditions can lead to heightened interstitial pressure within the tumor bulk compared with the lower pressure in the healthy stromal tissue, which leads to interstitial fluid flow [15]. As a result, draining vessels and pathways surrounding the cortex play a pivotal role in modulating fluid balance, solute and ion trafficking to exchange with the blood circulation, and immune cell trafficking [16, 17]. In the brain, lymphatic vessels surround the tissue in the meninges [16–18], and their role in GBM is, as yet, unknown. In the perivascular space, surrounding blood endothelial cells, pericytes are also essential in the healthy brain microenvironment for functions ranging from maintenance of the BBB to stabilizing endothelial cell structure and blood flow and implicated in glioma progression [19, 20]
Lastly, glioma stem cells are the subset of tumor cells that have self-renewal and proliferative capabilities to either differentiate to specific glial cell sub-types or remain in a stem-like state for turnover. They are typically the most aggressive and resistant to treatment due to their propensity to acquire mutations during the differentiation process. For instance, in the GBM-TME, pericytes and endothelial cells can be derived from “tumor-initiating” stem cells and propagate pro-tumorigenic reprogramming [20]. With a multitude of interactions and outcomes within the tumor microenvironment, there are many ways that GBM can manifest (Fig. 1), therefore how these different components influence pro-tumorigenesis mechanistically with recent advances is important to understand in order to gain a more informed perspective for therapeutic targeting and treatment of GBM.
Figure 1. Tumor microenvironment contributors to cancer hallmarks in glioblastoma.
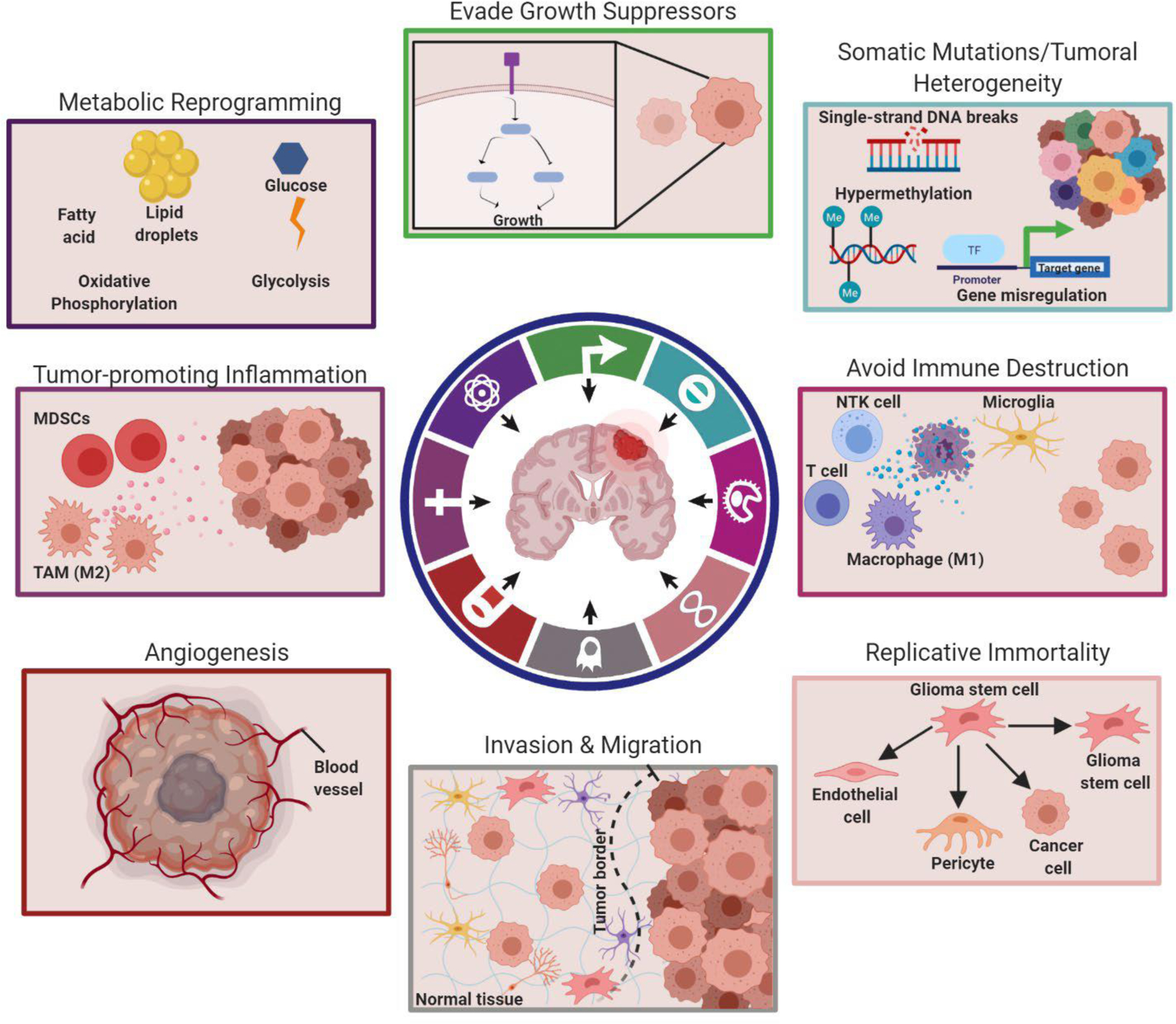
Each boxed schematic corresponds to a specific cancer hallmark that occurs and the key players that are involved in GBM. The top left box represents metabolic reprogramming where oxidative phosphorylation and glycolysis can be utilized for metabolism based on GBM-TME influenced phenotype. The top middle image corresponds to the evasion of growth suppressors, which can be promoted through mutations and mitogens based on conditions within the GBM-TME. The top right box represents common genetic reprogramming that promotes mutations and leads to both sustained proliferative signaling and tumoral heterogeneity. The middle right image represents tumor cells avoiding immune destruction. The bottom right image represents how GSCs possess plasticity to self-replicate or acquire other fates, such as tumor cells, endothelial cells, and pericytes, often influenced by the GBM-TME, and support tumor progression. The bottom middle image represents invasion and migration, which can be induced through autologous chemotaxis in glioblastoma as well as tumor-associated astrocytes and microglia among other mechanisms. The bottom left image represents angiogenesis, which occurs based on the tumor microenvironment influencing the sprouting of blood vessels. The middle left image represents tumor-promoting inflammation which can be induced by myeloid derived suppressor cells or tumor-associated macrophages. Boxed items correspond respectively with the colors within the cancer hallmark graphic circle. The figure was created with Biorender.com (The circular hallmark graphic was modified under the Copyright Clearance Center’s RightsLink service and reprinted from Cancer Cell, Volume 21(3), Douglas Hanahan & Lisa M. Coussens, Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment, Pages 309–322, 2012 with permission from Elsevier.) TAM=Tumor-associated macrophage; MDSC=Myeloid derived suppressor cell.
The tumor microenvironment and hallmarks of cancer in glioblastoma: Targets and tools
Tumor heterogeneity & Replicative Immortality
As with many cancers, the identification of molecular markers allowed identification of interpatient heterogeneity. As such, scientists and pathologists have determined classification systems based on molecular subtyping of GBM across patient populations. In 2016, the World Health Organization (WHO) revised their classification for GBM, primarily based on isocitrate dehydrogenase (IDH) status and enhanced opportunities in understanding molecular mechanisms that lead to specific classifications for incorporation into studies for consideration [21–23]. A still common classification system of GBM is based on specific gene expression levels of EGFR, NF1, and PDGFRA/IDH1, which corresponded to classical, mesenchymal, and proneural, respectively, and the neural sub-type corresponding to genes associated with neurons [21]. The efficacy of treatment in GBM as a function of subtype shows high variability, presenting barriers to success, specifically in the proneural subtype.
A major contributor to this difficulty in classifying and relating molecular classifications to therapeutic response is the vast amount of tumoral heterogeneity in GBM. Glioma stem cells (GSCs), a population of cells that have self-renewal capabilities and are typically associated with increased aggressiveness and resistance to treatment. Cancer stem cells are common to all cancers; however, what is most interesting in glioma is that characteristic diffuse infiltration allow GSCs to invade into the normal parenchyma processes and differentiate into cell types other than glioma cells even after exposure to treatment [24], such as tumor-associated endothelial cells, pericytes, and co-opting other cells within the GBM-TME. As a result, the GBM-TME can influence the plasticity of these cells and allow GSCs to escape treatment and tumor progression [25]. The mechanisms involved are still not clearly established. Yet historically, glycocalyx biopolymers have been implicated in mesenchymal stem-cell like phenotype maintenance, indicated by CD44 being a primary marker of GSCs. CD44 serves as one of the primary receptors of HA, suggesting some correlation of HA with stem-like maintenance or response [26–28]. Another glycocalyx biopolymer, α-dystroglycan, has been proposed to play a role in GSC phenotype aiding in the maintenance of GSC phenotype, particularly within the perivascular niche [29]. Moreover, development of a 3D nanofiber scaffold to ascertain biophysical mechanocoupling, migration modes, and plasticity of GSCs, which seemed to be dependent on galectin-3 and integrin-β1 overexpression further indicate the importance of the glycocalyx in GSC phenotype [30]. Since the glycocalyx is important for interactions with the extracellular environment, these phenotypic changes that contribute to the plasticity of GSCs can be modulated by the GBM-TME. Recently, in silico and in vitro studies have shown that GSC fate can result from the surrounding tumor microenvironment and not simply as a function of tumorigenic status [31]. Thus, the GBM-TME is important to continually be considered in understanding how it impacts GSC plasticity, differentiation, and elements, such as the extracellular matrix, offer as an appealing target for therapeutic intervention.
Genomic mutations and dysregulation of oncogenes and tumor suppressors
Biochemical cues within the TME can alter the genetic stability of cells and often are produced by the diverse parenchymal cells co-opted by the tumor [32–34]. Since its inception, The Cancer Genome Analysis (TCGA) Research Network has been a beneficial tool for providing access to a landscape of whole-genome sequenced data from tumor tissues and thus better insight to cellular and molecular players involved in GBM [35]. Thus, examination of changes to mutational burden has become commonplace. At the same time, co-culture models that incorporate multiple parenchymal cell types with tumor cells have grown in use. These models offer the ability to consider how treatments affect tumor cells along with nong-tumor cell types. Using an ex vivo system containing dorsal root ganglia axon-oligodendrocyte co-cultures with human GSCs, Zepecki et. al found that migration-specific RNA transcripts were activated in the pseudopodia, particularly Lck, a gene that is highly expressed in patients with GBM through RNAseq data and TCGA analysis [22]. Another critical factor in this study was the examination of multiple outcomes besides migration, including stemness and tumor growth, maximizing the information attained from one study and expanding our understanding of the impact of genetic changes on other hallmarks.
Other genetic events also play a substantial role in common re-programming of cells within the TME. Hypermethylation of the CpG island-associated gene promoters, which leads to silencing of tumor suppressor genes, is often hypothesized to result from aberrant microenvironment cues and hallmarks. Common hypermethylated tumor suppressors in GBM, such as DNA repair enzyme MGMT and invasion-related E-cadherin gene CDH1 among others that have been recently studied [34, 36]. For instance, Feng et al. examined hypoxia-activated tumor suppressor genes ankyrin repeat and death domain-containing 1A (ANKDD1A) both in vitro and in vivo revealing that GBM-related hypermethylation decreases its regulation of tumor cell metabolism, growth, and death [37]. Moreover, another study showed that hypoxia regulates PAX3, a common gene suggested to function as an oncogene, through inhibiting apoptosis by one of its functions to repress common tumor suppressor, p53 [38]. PAX3 binds to the promoter of p53 and represses its transcription, thus more p53 mutations were shown to be present in high PAX3 tumor tissues compared to low PAX3-tumors. Further, patient brain tissues showed that PAX3 levels positively correlated with GBM grade. Apart from its role in promoting growth and migration of GSCs, an interesting perspective in this study focused on differentiation of GSC where hypoxic conditions regulated the PAX3/p53 axis causing de-differentiation into GSC-like cells, supporting the hypothesis of hypoxia-mediated stemness and its potential for microenvironmental targeting. Considerations for therapeutic approaches to DNA methylation and other genetic aberrations have been recently reviewed [39], but overall, these studies highlight potential novel therapeutic targets for future consideration in GBM.
Cancer-metabolic reprogramming
Historically, our understanding is that tumor cells utilize glycolysis as opposed to oxidative phosphorylation, due to mitochondrial dysfunction, known as the Warburg effect [40, 41]. Therefore, many studies focus on glycolytic inhibition in various cancers with some centering around one of the main markers in GBM classification, IDH [42, 43]. In one such study, Abbadi et al. used primary brain tumor-initiating cells derived from human GBM samples treated with 2-deoxyglucose (2DG), a hexokinase inhibitor. This treatment enhanced aggressive phenotype, including stem-like properties, differentiation into astrocytes, migration/invasion, and overall desensitized cells to metabolic inhibition via upregulation of glucose-6-phosphatase (G6PC) [44]. Moreover, lentiviral shRNA knockdown of G6PC in cells injected in mice showed decreased invasion. Inhibition of lactate dehydrogenase-A (LDH-A), an enzyme that converts pyruvate to lactate during glycolysis, in GSCs derived from GBM cell lines induced apoptosis and differentiation, hypothesized through decreasing glycolytic rate [45]. These studies further stress that all components involved within the different steps of metabolic pathways can alter intratumoral heterogeneity. These alterations may also be occurring within the cells in the surrounding microenvironment as well.
An interesting paradigm shift through recent studies is that intratumoral heterogeneity is composed of both fast-cycling cells, which follow the Warburg effect, and slow cycling cells that utilize oxidative phosphorylation while leveraging other metabolites, such as fatty acid metabolic precursors, for survival [46, 47]. Slow cycling cells possess enhanced migratory potential, stemness, proliferation, and treatment resistance compared to fast cycling cells, especially at high densities [46, 48]. Similarly, another study examining metabolic stress in reprogramming of lipid metabolism, found that exogeneous loading of low-density lipoproteins (LDL) in hypoxic conditions in vitro resulted in a lipid-loaded phenotype similar to GBM patient tumors in hypoxic regions [49]. LDL-conditioned media from these cells prompted migration, proliferation, and infiltration of macrophages. In vivo studies in Apolipoprotein E knockout mice on a high fat diets implanted with lipid loaded GL261 glioma cells showed decreased survival compared to non-lipid loaded and healthy controls [49]. Overall, these studies underscored the need for future novel therapeutic treatments to consider disparate metabolites and profiles to maximize sensitivity of treatment to heterogeneous populations and exploration of how biochemical cues can cause a shift in other pathways that may be utilized for cells to continue to survive and escape treatment toxicity.
Migration and Invasion
GBM invasion and migration, as a defining feature of the disease, is one of the best-characterized interactions of tumor cells with the surrounding microenvironment. Characteristic diffusive infiltration of glioma involves cellular dissemination into healthy features of the brain parenchyma, such as the perivascular space. Recent studies highlight the molecular mediators of interactions between the tumor and parenchymal cells within these niches where infiltration normally occurs. Brain-resident cell types, such as astrocytes and microglia, and infiltrating macrophages alter glioma invasion and migration. For instance, reactive astrocytes aid in tumor survival through secretion of inflammatory cytokine interleukin-6 (IL-6), upregulation of MMP14 protein expression and subsequent activation of MMP2, thus increasing invasion of glioma invasion and migration [50]. Moreover, microglia and macrophages also have been recently shown to aid in tumor-promoting inflammation by serving as a source for chemokine C-C ligand 5 (CCL5) in the CCL5/CCR5 axis, which has been linked to glioma invasion [51]. Reactive astrocytes have also been implicated in promoting glioma invasion through expression of gap junction protein, Connexin43 (Cx43), which has been implicated in other cancers [52], and more recently, in coordination with activated microglia in contributing to glioma migration and invasion [53]. Moreover, three-dimensional (3D) models are rapidly replacing or supplementing traditional two-dimensional (2D) model because 3D model systems recapitulate a more physiologically representative tumor microenvironment. Tumor microenvironment cues can differentially affect invasion and migration in 3D versus 2D [54]. Such cues include matrix composition, stiffness, pH, interstitial pressure, and oxygen conditions and changes in these to outside of the physiological range can contribute to treatment resistance [15, 55]. Historically, little has been established in regards to pH in GBM; however, recent studies indicate that temozolomide (TMZ), an alkylating agent widely used in GBM treatment regimens, arrests growth of tumors in mice through regulation of extracellular pH [56, 57]. Molecular mechanisms of tumor volume and pH regulation have been recently reviewed [58], shifting the focus to Na+/H+ exchangers, specifically sodium-hydrogen exchanger isoform 1 (NHE1). NHE1, overexpressed in many tumors, regulates intracellular pH of tumor cells by extruding H+, making the extracellular pH acidic within a tumor, largely due to cancer-metabolic reprogramming for lactate production as the main energy source. In primary glioma cell lines and patient-derived xenografts, authors showed that NHE1-mediated extrusion of H+ maintained the alkaline pH which was countered by TMZ-induced intracellular acidosis leading instead, to glioma cell migration. Other studies suggest that acidic stress impacts cell motility and promotes a glioma stem cell phenotype, therefore consideration of how therapeutic treatments impact intracellular and extracellular signals should be fully explored [57, 59]. Moreover, NHE1 can be highly expressed not only by glioma cells, but also tumor-associated microglia and macrophages and, in turn, alter glioma growth, invasion, and migration [60].
Another biophysical cue implicated in cancer is interstitial fluid flow (IFF). Physiologically, IFF is fluid that moves within the interstitial spaces of tissues. This is important for fluid balance and solute transport in tissues. In the tumor microenvironment, proliferation of tumor cells, leaky vasculature, and increased ECM accumulation leads to an increased tumoral interstitial pressure. This heightened pressure within tumor bulk adjacent to normal pressures in the surrounding tissues causes flow towards the healthy tissue [15, 61]. This heightened IFF leads to increased glioma invasion via multiple mechanisms, including CD44-mediated mechanotransduction and the CXCR4+/CXCL12+ signaling axis. CXCR4 and its ligand CXCL12 are expressed by gliomas and immune cells, including B and T lymphocytes, monocytes, microglia, macrophages, and vascular endothelial cells [15, 62, 63]. It is overexpressed in many cancers and is attributed to tumor resistance, growth, survival, and recruiting MDSCs and angiogenesis [64]. Convection-enhanced delivery (CED), a therapeutic technique to improve drug distribution, promotes tumor cell invasion through the CXCR4/CXCL12 signaling axis in an in vivo GBM mouse model [65]. Other biophysical forces, such as stiffening of the ECM and adhesion mechanosensing, also promote a pro-invasive phenotype [66], particularly through tenascin upregulation within the glycocalyx on glioma cells. Stem-like mesenchymal phenotype was primarily associated with increased molecular crowding of tenascin within the glycocalyx of GBM cells and altered mechanosignaling, potentially aiding maintenance of an aggressive and treatment resistant population of cells [67, 68]. Moreover, ionizing radiation, one of the most common therapeutic treatments of GBM, induces a similar tension-mediated mesenchymal shift. The contribution of radiation therapy to recurrence and invasion of glioma [69] along with changes within the GBM-TME has been recently reviewed [70]. One main mechanism that has been associated with the pro-invasive shift in the GBM-TME through radiation is its induction of HA abundance, thus increasing binding to its receptor CD44 and aiding in the signaling pathways that promote mesenchymal phenotype, survival, and invasion post-radiotherapy [71]. Ultimately, these studies highlight how treatments and a tip of the scale slightly away from physiological norms in the GBM-TME can really have dramatic impact on glioma progression and need to be taken into account with future treatments and consideration.
Angiogenesis
As the tumor grows, the demand for oxygen and nutrient supply increases. Historical dogma states that when supplies run low, tumor cells secrete factors to endothelial cells to stimulate new blood vessel formation; however, it is becoming apparent that the tumor microenvironment plays its own role in promoting angiogenesis. VEGFR2 blocking has been the primary focus in inhibiting angiogenesis in cancer with approved clinical therapeutics, such as bevacizumab (Avastin) [72]. However, resistance to antiangiogenic therapies and recurrence is still challenging in the clinic. VEGFR2 blocking using valatinib was performed on GBM mutation-specific modified cell lines for EGFR and p53 to consider mutational status and heterogeneity within GBM and treatment impact. Orthotopic transplantation of GBM tumor cells in mice and in vitro cell culture in the presence of valatinib resulted in translocation of VEGFR2 to the nucleus and promoted tumor cell proliferation, invasion, and evasion of apoptosis [23]. The authors stressed an important point that treatments should consider mutational status of patients to help guide proper treatment since their status could lead to these negative off-target effects that sustain the tumor. Though VEGFR2 has been the major target of anti-angiogenic therapies, other, GBM-TME factors are beginning to surface as potential novel targets. For instance, Molecule interacting with CasL (MICAL2), which catalyzes F-actin destabilization, has been recently proposed as a oncoprotein involved in cancer and promotes epithelial to mesenchymal transition and invasion [73]. However, it is upregulated in only tumor-associated neoangiogenic capillaries and not in normal endothelium within the tumor in GBM human tissue samples. The inhibition of MICAL2 abolished TNF-α activation and VEGF stimulation and tumor-associated endothelial cell function [74]. Thus, there are niche-specific effects on cancer hallmark activity that may be key as we seek to understand how these different hallmarks can propagate cancer despite targeted therapy and treatment [75] To highlight this, Talasila et al. recently proposed that angiogenesis and invasion of tumor cells occurs in different niches correspondent to different metabolic mechanisms. For instance, proneural xenografts displaying an invasive phenotype were exposed to long-term hypoxia in vivo as well as glioma spheroids derived from the xenografts for in vitro studies, led to a pro-angiogenic phenotype with a high glycolytic profile, indicating the need for models to incorporate the complex mixture of stromal interactions that occur in the tumor microenvironment to fully understand the complexity of pro-angiogenic processes [76].
Circumventing Immune Destruction and Resisting Cell Death
Another way that the tumor microenvironment can evade tumor destruction is through reprogramming immune cells. GBM can co-opt both resident and infiltrating immune cells through immunosuppression and promote tumor progression, rendering the tumor “cold” instead of “hot”, or eliciting normal pro-inflammatory responses even in the presence of targeted therapy [77]. A major immune cell population associated with this immunosuppression is myeloid derived suppressor cells (MDSCs). Currently, the largest challenge is the dismal outlook of current markers to detect and validate MDSCs in humans. In addition, the mechanisms behind on immune cell reprogramming in the GBM-TME is murky. Unfortunately, dexamethasone, an immunosuppressive drug used to reduce local inflammation and edema prior to resection, may aid immunosuppression complicating future tumor treatment. For instance, treatment with dexamethasone suppressed MHC II presentation on infiltrating macrophages and CD33, an associated MDSC marker, indicating how plastic immune cells in the GBM-TME are [78]. MDSCs have been recently reviewed in depth for cancer and other physiological and pathological states concluding that these cells are an important yet elusive component of the TME [79, 80]. MDSCs are not the only cells in the GBM-TME involved in immunosuppression, as other cell types promote immune reprogramming. For instance, astrocyte-microglia crosstalk drives glioma progression via signal transducer and activator of transcription 3 (STAT3) [81], thought to primarily attune the immune system towards a pro-inflammatory state [82]. Pericytes in healthy tissues can possess phagocytic activity along with other myeloid cell properties, so their ability to be transformed by GBM cells for immunosuppression is logical [83]. Tumor-associated pericytes modulated T cell response aiding in tumor growth both in vitro and in vivo [84]. Tumor-associated pericyte immunosuppressive ability may be attained through the chaperone-mediated autophagy pathway in GBM tumor cell growth and progression [85]. Outside of cancer-mediated processes, opportunistic infection of cytomegalovirus (CMV) has been historically linked to enhancement of tumor malignancy and hallmarks [86]. CMV has been recently found to infect pericytes and promote their migration and angiogenesis through the pericyte regulator, platelet derived growth factor D (PDGF-D) in an in vivo GBM mouse model [87].
Engineered and Therapeutic tools to study hallmarks and the GBM-TME
Many advances have been made to better understand how the tumor microenvironment contributes to the hallmarks of cancer in order to sustain tumor survival. Throughout this perspective, we see that these individual hallmarks intersect within the machinery of tumor progression and maintenance, and thus we need to utilize multifaceted models to better study potential synergies. To address this need, one recent study developed a glioma “tumor microenvironment array platform” (TMAP) to observe temporal, dynamic tumor characteristics within the tumor microenvironment niches, such as hypoxia, drug response heterogeneity within glioma spheroids and remodeling from the tumor [88]. One topic of interest within development of models is representing the physiological environment to accurately recapitulate the GBM-TME [54]. Specifically, one study to address this incorporated multicellular glioma spheroids in an in vitro setting in multiple conditions to mimic physiological representations of the perivascular space and an astrocyte-rich interstitium in 96-well format and compared to invasion in vivo [89]. Bioprinting-based models are also very interesting in the context of novel way to recapitulate functional 3D models for GBM, especially as potential high-throughput for point-of-care testing and drug screening [90, 91].
From a therapeutic aspect, these tools are not simply useful for studying the TME, but also in identifying its potential for targeted modulation and thus, future potential treatments. In the past few years, studies have mostly focused on treatments against tumor-associated microglia and MDSCs as well as T cells in order to reprogram them in glioma, such as checkpoint inhibitor therapy and chimeric antigen receptor (CAR) T-cell therapy [51, 92–97]. Interestingly, exploration of both novel techniques and the repurposing of existing therapeutics have come to the forefront of GBM-TME modulation. For example, genetically-engineered isolated neural-like stem cells (NLSCs) from the periphery show promise as a potential therapeutic against tumor cells, primarily through their ability to not be co-opted by the tumor microenvironment nor transformed into tumor cells, but be engineered for potential enhanced efficacy of therapeutics [98]. Oncolytic HSV virus therapy inhibits tumor-promoting inflammation [99]. Aspirin can inhibit angiogenesis [100], peptide-guided magnetic nanoworms homed to tumor-associated vessels for passive tumor targeting [101], a selective peptide that serves as an antagonist for the CXCR4 receptor [102], and cell-type selective small molecule treatments that promotes apoptosis of GBM CSC tumor cells [103] to name a few advances that are thinking outside of the box to target a wide range of contributing factors within the GBM-TME.
Conclusions
Within this review, we see that undoubtedly, these cancer hallmarks do not occur in isolation and, in fact, are always involved in a dynamic dance contributing to and resulting from one another. This is particularly true within the context of the GBM-TME. As an environment full of resident and infiltrating cells that play an active role in tumor progression, until recently one of the major cellular components of the brain, neurons, had largely been ignored. For the first time ever, one study indicated that glioma cells form synapses with neurons and through electrochemical signaling can reciprocally cause glioma proliferation and neuron stimulation [104]. Studies have also shown that contributing mechanisms could include neuronal secretion of mitogens and oncogenic hijacking of transcription of neuronal factors can promote GBM progression [105, 106]. One proposed solution to neuronal involvement in glioma progression is differentiation into neurons, which previously was considered [107], and recently revisited [108, 109]. Looking forward, neuronal impact on the GBM-TME may lead to leveraging of novel and repurposed therapeutic targets for treatment. Thinking more broadly, the physiological context of the brain may be important for targeting of the GBM-TME. For example, melatonin, a hormone involved in sleep, yields multiple advantages shown across studies including inhibition of glioma cell invasion, promotion of antitumor immunity, particularly with monocytes, through modulation of the silent mating type information regulation 2 homolog (SIRT1) pathway leading to increases in glioma patient survival in combination with radiotherapy [110–113]. Similarly, the brain’s reward system may apply its positive reinforcement function to promote anti-tumor immune modulation of MDSCs in lung and melanoma models [114]. Leveraging the physiological balance of the brain and all of its processes at the cellular and behavioral level, may provide insight on the GBM-TME opening doors to new therapies as yet, unstudied.
When thinking of studying or identifying targets in the tumor microenvironment, we must note that the cancer field has broadly adopted patient-derived xenografts, as a means of testing. Though these models are useful, they are still a human tissue within a murine microenvironment, and we do not yet know how these interactions differ. Thus, for accurate treatment models, personalized models may be more effective in decoupling microenvironmental effects and signaling molecules. It is a lofty task indeed and even though it may be invasive, other native cellular components from the blood and or GBM-TME should be studied as much as possible from individual patients. With 3D bioprinting and cancer-on-a-chip technologies with patient-derived cells within the tumor microenvironment, these models offer a new potential gold standard. Thus, like with other players in the hallmarks of cancer, we should treat elements of the TME as patient-specific and hopefully with this personalized perspective, we can better identify and use treatments against GBM in a holistic context.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest Drs. Roberts and Munson declare no conflicts of interest
Human and Animal Rights and Informed Consent No human or animals were used in this manuscript
References
Papers published recently within past three years have been highlighted as:
• Of importance
•• Of major importance
- 1.Jain KK (2018) A critical overview of targeted therapies for glioblastoma. Front Oncol. 10.3389/fonc.2018.00419 [DOI] [PMC free article] [PubMed]
- 2.Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 10.1007/s11060-011-0738-7 [DOI] [PMC free article] [PubMed]
- 3.Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell. 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed]
- 4.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell. 10.1016/j.cell.2011.02.013 [DOI] [PubMed]
- 5.Hanahan D, Coussens LM (2012) Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 10.1016/j.ccr.2012.02.022 [DOI] [PubMed]
- 6.Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci. 10.1242/jcs.116392 [DOI] [PubMed]
- 7.Ruoslahti E (1996) Brain extracellular matrix. Glycobiology. 10.1093/glycob/6.5.489 [DOI] [PubMed]
- 8.Bonneh-Barkay D, Wiley CA (2009) Brain extracellular matrix in neurodegeneration. Brain Pathol. 10.1111/j.1750-3639.2008.00195.x [DOI] [PMC free article] [PubMed]
- 9.•.Barnes JM, Przybyla L, Weaver VM (2017) Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci. 10.1242/jcs.191742(This review describes in detail key biomechanical properties of interest within the brain, specifically in cancer)
- 10.Purves D, Augustine G, Fitzpatrick D, Hall W, LaMantia A, McNamara J, White L (2008) Neuroscience 4th edition. [Google Scholar]
- 11.•.Quail DF, Joyce JA (2017) The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 10.1016/j.ccell.2017.02.009(This review provides a perspective of the microenvironment in brain tumors)
- 12.Claes A, Idema AJ, Wesseling P (2007) Diffuse glioma growth: A guerilla war. Acta Neuropathol. 10.1007/s00401-007-0293-7 [DOI] [PMC free article] [PubMed]
- 13.Wippold FJ, Cairns N, Vo K, Holtzman DM, Morris JC (2008) Neuropathology for the neuroradiologist: Plaques and tangles. Am J Neuroradiol. 10.3174/ajnr.A0781 [DOI] [PMC free article] [PubMed]
- 14.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM (2000) Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 10.1016/S0002-9440(10)65006–7 [DOI] [PMC free article] [PubMed]
- 15.Munson JM, Bellamkonda RV., Swartz MA (2013) Interstitial flow in a 3d microenvironment increases glioma invasion by a cxcr4-dependent mechanism. Cancer Res. 10.1158/0008-5472.CAN-12-2838 [DOI] [PubMed]
- 16.Louveau A, Smirnov I, Keyes TJ, et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature. 10.1038/nature14432 [DOI] [PMC free article] [PubMed]
- 17.••.Raper D, Louveau A, Kipnis J (2016) How Do Meningeal Lymphatic Vessels Drain the CNS? Trends Neurosci. 10.1016/j.tins.2016.07.001 [DOI] [PMC free article] [PubMed]
- 18.Da Mesquita S, Fu Z, Kipnis J (2018) The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron. 10.1016/j.neuron.2018.09.022 [DOI] [PMC free article] [PubMed]
- 19.•.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, Birbrair A (2018) Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis. 10.1007/s10456-018-9621-x(This review highlights the role of pericytes within the tumor microenvironment and their reprogramming in glioblastoma)
- 20.Cheng L, Huang Z, Zhou W, et al. (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 10.1016/j.cell.2013.02.021 [DOI] [PMC free article] [PubMed]
- 21.Verhaak RGW, Hoadley KA, Purdom E, et al. (2010) Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed]
- 22.Zepecki JP, Snyder KM, Moreno MM, Fajardo E, Fiser A, Ness J, Sarkar A, Toms SA, Tapinos N (2019) Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor. Oncogene. 10.1038/s41388-018-0546-z [DOI] [PMC free article] [PubMed]
- 23.Shankar A, Jain M (2016) Anti-VEGFR2 Driven Nuclear Translocation of VEGFR2 and Acquired Malignant Hallmarks are Mutation Dependent in Glioblastoma. J Cancer Sci Ther. 10.4172/1948-5956.1000410 [DOI] [PMC free article] [PubMed]
- 24.•.Minata M, Audia A, Shi J, et al. (2019) Phenotypic Plasticity of Invasive Edge Glioma Stem-like Cells in Response to Ionizing Radiation. Cell Rep. 10.1016/j.celrep.2019.01.076(This article highlights the link between ionizing radiation and its contribution to glioma stem cell plasticity, which leads to phenotypes that drive recurrence and resistance)
- 25.Audia A, Conroy S, Glass R, Bhat KPL (2017) The impact of the tumor microenvironment on the properties of glioma stem-like cells. Front Oncol. 10.3389/fonc.2017.00143 [DOI] [PMC free article] [PubMed]
- 26.•.Chanmee T, Ontong P, Izumikawa T, et al. (2016) Hyaluronan production regulates metabolic and cancer stem-like properties of breast cancer cells via hexosamine biosynthetic pathway-coupled HIF-1 signaling. J Biol Chem 291:24105–24120(This article describes how hyaluronan is directly linked to cancer hallmarks)
- 27.Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S (2011) Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 278:1429–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skandalis SS, Karalis TT, Chatzopoulos A, Karamanos NK (2019) Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 10.1016/j.cellsig.2019.109377 [DOI] [PubMed]
- 29.•.Day BW, Lathia JD, Bruce ZC, et al. (2019) The dystroglycan receptor maintains glioma stem cells in the vascular niche. Acta Neuropathol. 10.1007/s00401-019-02069-x(This article highlights another glycocalyx biopolymer other than hyaluronan involved in the maintenance of stem cell phenotype)
- 30.Saleh A, Marhuenda E, Fabre C, et al. (2019) A novel 3D nanofibre scaffold conserves the plasticity of glioblastoma stem cell invasion by regulating galectin-3 and integrin-β1 expression. Sci Rep. 10.1038/s41598-019-51108-w [DOI] [PMC free article] [PubMed]
- 31.Dirkse A, Golebiewska A, Buder T, et al. (2019) Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat Commun. 10.1038/s41467-019-09853-z [DOI] [PMC free article] [PubMed]
- 32.Murat A, Migliavacca E, Hussain SF, Heimberger AB, Desbaillets I, Hamou MF, Rüegg C, Stupp R, Delorenzi M, Hegi ME (2009) Modulation of angiogenic and inflammatory response in glioblastoma by hypoxia. PLoS One. 10.1371/journal.pone.0005947 [DOI] [PMC free article] [PubMed]
- 33.Gimple RC, Bhargava S, Dixit D, Rich JN (2019) Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 10.1101/gad.324301.119 [DOI] [PMC free article] [PubMed]
- 34.Carén H, Pollard SM, Beck S (2013) The good, the bad and the ugly: Epigenetic mechanisms in glioblastoma. Mol Aspects Med. 10.1016/j.mam.2012.06.007 [DOI] [PMC free article] [PubMed]
- 35.Brennan CW, Verhaak RGW, McKenna A, et al. (2013) The somatic genomic landscape of glioblastoma. Cell. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed]
- 36.Blanc JL, Wager M, Guilhot J, Kusy S, Bataille B, Chantereau T, Lapierre F, Larsen CJ, Karayan-Tapon L (2004) Correlation of clinical features and methylation status of MGMT gene promoter in glioblastomas. J Neurooncol. 10.1023/B:NEON.0000033385.37098.85 [DOI] [PubMed]
- 37.Feng J, Zhang Y, She X, et al. (2019) Hypermethylated gene ANKDD1A is a candidate tumor suppressor that interacts with FIH1 and decreases HIF1α stability to inhibit cell autophagy in the glioblastoma multiforme hypoxia microenvironment. Oncogene. 10.1038/s41388-018-0423-9 [DOI] [PMC free article] [PubMed]
- 38.Zhu H, Wang H, Huang Q, Liu Q, Guo Y, Lu J, Li X, Xue C, Han Q (2018) Transcriptional repression of p53 by PAX3 contributes to gliomagenesis and differentiation of glioma stem cells. Front Mol Neurosci. 10.3389/fnmol.2018.00187 [DOI] [PMC free article] [PubMed]
- 39.Zang L, Kondengaden SM, Che F, Wang L, Heng X (2018) Potential epigenetic-based therapeutic targets for glioma. Front Mol Neurosci. 10.3389/fnmol.2018.00408 [DOI] [PMC free article] [PubMed]
- 40.Warburg O (1956) Injuring of Respiration the Origin of Cancer Cells. Science (80-) 123:309–14 [DOI] [PubMed] [Google Scholar]
- 41.Ward PS, Thompson CB (2012) Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 10.1016/j.ccr.2012.02.014 [DOI] [PMC free article] [PubMed]
- 42.Akins NS, Nielson TC, Le HV. (2018) Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr Top Med Chem. 10.2174/1568026618666180523111351 [DOI] [PMC free article] [PubMed]
- 43.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 10.1007/s00401-016-1545-1 [DOI] [PubMed]
- 44.Abbadi S, Rodarte JJ, Abutaleb A, et al. (2014) Glucose-6-phosphatase is a Key Metabolic Regulator of Glioblastoma Invasion. Mol Cancer Res. 10.1158/1541-7786.MCR-14-0106-T [DOI] [PMC free article] [PubMed]
- 45.Daniele S, Giacomelli C, Zappelli E, Granchi C, Trincavelli ML, Minutolo F, Martini C (2015) Lactate dehydrogenase-A inhibition induces human glioblastoma multiforme stem cell differentiation and death. Sci Rep. 10.1038/srep15556 [DOI] [PMC free article] [PubMed]
- 46.••.Hoang-Minh LB, Siebzehnrubl FA, Yang C, et al. (2018) Infiltrative and drug-resistant slow-cycling cells support metabolic heterogeneity in glioblastoma. EMBO J. 10.15252/embj.201798772(This article highlights how cancer cells can utlitize oxidative phosphorylation through slow-cycling as opposed to the canonical thought of cancer cells mostly following glycolysis and being fast cycling)
- 47.Deleyrolle LP, Harding A, Cato K, et al. (2011) Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 10.1093/brain/awr081 [DOI] [PMC free article] [PubMed]
- 48.Sabelström H, Quigley DA, Fenster T, et al. (2019) High density is a property of slow-cycling and treatment-resistant human glioblastoma cells. Exp Cell Res. 10.1016/j.yexcr.2019.03.003 [DOI] [PubMed]
- 49.Offer S, Menard JA, Pérez JE, et al. (2019) Extracellular lipid loading augments hypoxic paracrine signaling and promotes glioma angiogenesis and macrophage infiltration. J Exp Clin Cancer Res. 10.1186/s13046-019-1228-6 [DOI] [PMC free article] [PubMed]
- 50.Chen W, Xia T, Wang D, Huang B, Zhao P, Wangc J, Qu X, Li X (2016) Human astrocytes secrete IL-6 to promote glioma migration and invasion through upregulation of cytomembrane MMP14. Oncotarget. 10.18632/oncotarget.11515 [DOI] [PMC free article] [PubMed]
- 51.Wu CY-J, Chen C-H, Lin C-Y, Feng L-Y, Lin Y-C, Wei K-C, Huang C-Y, Fang J-Y, Chen P-Y (2019) CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase-2. Neuro Oncol. 10.1093/neuonc/noz189 [DOI] [PMC free article] [PubMed]
- 52.Bonacquisti EE, Nguyen J (2019) Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions. Cancer Lett. 10.1016/j.canlet.2018.10.043 [DOI] [PubMed]
- 53.Sin WC, Aftab Q, Bechberger JF, Leung JH, Chen H, Naus CC (2016) Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene. 10.1038/onc.2015.210 [DOI] [PubMed]
- 54.Nakod PS, Kim Y, Rao SS (2018) Biomimetic models to examine microenvironmental regulation of glioblastoma stem cells. Cancer Lett 429:41–53 [DOI] [PubMed] [Google Scholar]
- 55.Cong D, Zhu W, Shi Y, Pointer KB, Clark PA, Shen H, Kuo JS, Hu S, Sun D (2014) Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H+ extrusion, cell migration and survival. Carcinogenesis. 10.1093/carcin/bgu089 [DOI] [PMC free article] [PubMed]
- 56.•.Rao JU, Coman D, Walsh JJ, Ali MM, Huang Y, Hyder F (2017) Temozolomide arrests glioma growth and normalizes intratumoral extracellular pH. Sci Rep. 10.1038/s41598-017-07609-7(This article highlights pH, which is not largely included in hallmarks, but of importance, especially in the context of treatment)
- 57.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN (2011) Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ 18:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamtaji OR, Mirzaei H, Shamshirian A, Shamshirian D, Behnam M, Asemi Z (2020) New trends in glioma cancer therapy: Targeting Na+/H + exchangers. J Cell Physiol. 10.1002/jcp.29014 [DOI] [PubMed]
- 59.Riemann A, Schneider B, Gündel D, Stock C, Gekle M, Thews O (2016) Acidosis promotes metastasis formation by enhancing tumor cell motility. Adv Exp Med Biol. 10.1007/978-1-4939-3023-4_27 [DOI] [PubMed]
- 60.Zhu W, Carney KE, Pigott VM, Falgoust LM, Clark PA, Kuo JS, Sun D (2016) Glioma-mediated microglial activation promotes glioma proliferation and migration: Roles of Na + /H + exchanger isoform. Carcinogenesis. 10.1093/carcin/bgw068 [DOI] [PMC free article] [PubMed]
- 61.Munson JM, Shieh AC (2014) Interstitial fluid flow in cancer: Implications for disease progression and treatment. Cancer Manag Res. 10.2147/CMAR.S65444 [DOI] [PMC free article] [PubMed]
- 62.Wu A, Maxwell R, Xia Y, et al. (2019) Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J Neurooncol. 10.1007/s11060-019-03172-5 [DOI] [PubMed]
- 63.••.Kingsmore KM, Vaccari A, Abler D, Cui SX, Epstein FH, Rockne RC, Acton ST, Munson JM (2018) MRI analysis to map interstitial flow in the brain tumor microenvironment. APL Bioeng. 10.1063/1.5023503(This article highlights a new method to map interstitial fluid flow with MRI, which can be reconstructed and clinically translated)
- 64.Chatterjee S, Behnam Azad B, Nimmagadda S (2014) The intricate role of CXCR4 in cancer. Adv Cancer Res. 10.1016/B978-0-12-411638-2.00002-1 [DOI] [PMC free article] [PubMed]
- 65.••.Cornelison RC, Brennan CE, Kingsmore KM, Munson JM (2018) Convective forces increase CXCR4-dependent glioblastoma cell invasion in GL261 murine model. Sci Rep. 10.1038/s41598-018-35141-9(This article highlights how the recently novel technique of convective enhanced delivery (CED) mimics interstitial fluid flow and stimulates invasion through CXCR4/CXCL12 signaling axis both in vivo and in patient samples)
- 66.Gritsenko PG, Friedl P (2018) Adaptive adhesion systems mediate glioma cell invasion in complex environments. J Cell Sci. 10.1242/jcs.216382 [DOI] [PMC free article] [PubMed]
- 67.Paszek MJ, DuFort CC, Rossier O, et al. (2014) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511:319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.••.Barnes JM, Kaushik S, Bainer RO, et al. (2018) A tension-mediated glycocalyx–integrin feedback loop promotes mesenchymal-like glioblastoma. Nat Cell Biol. 10.1038/s41556-018-0183-3(This article discusses the link between the glycocalyx and its role in glioblastoma)
- 69.••.Kingsmore KM, Logsdon DK, Floyd DH, Peirce SM, Purow BW, Munson JM (2016) Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion: Via CXCR4, CXCL12, and CD44-mediated mechanisms. Integr Biol (United Kingdom). 10.1039/c6ib00167j(This article describes interstitial fluid flow and the link between radiation, autologous chemotaxis, and invasion in glioma)
- 70.Gupta K, Burns TC (2018) Radiation-induced alterations in the recurrent glioblastoma microenvironment: Therapeutic implications. Front Oncol. 10.3389/fonc.2018.00503 [DOI] [PMC free article] [PubMed]
- 71.•.Yoo KC, Suh Y, An Y, et al. (2018) Proinvasive extracellular matrix remodeling in tumor microenvironment in response to radiation. Oncogene. 10.1038/s41388-018-0199-y(This article highlights how radiation can cause tumor microenvironmental changes in glioma)
- 72.Falcon BL, Chintharlapalli S, Uhlik MT, Pytowski B (2016) Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther 164:204–225 [DOI] [PubMed] [Google Scholar]
- 73.Mariotti S, Barravecchia I, Vindigni C, et al. (2016) MICAL2 is a novel human cancer gene controlling mesenchymal to epithelial transition involved in cancer growth and invasion. Oncotarget 7:1808–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barravecchia I, Mariotti S, Pucci A, et al. (2019) MICAL2 is expressed in cancer associated neo-angiogenic capillary endothelia and it is required for endothelial cell viability, motility and VEGF response. Biochim Biophys Acta - Mol Basis Dis 1865:2111–2124 [DOI] [PubMed] [Google Scholar]
- 75.Schiffer D, Annovazzi L, Casalone C, Corona C, Mellai M (2019) Glioblastoma: Microenvironment and niche concept. Cancers (Basel). 10.3390/cancers11010005 [DOI] [PMC free article] [PubMed]
- 76.•.Talasila KM, Røsland GV, Hagland HR, et al. (2017) The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro Oncol. 10.1093/neuonc/now175(This article links angiogenesis and metabolism together in glioma)
- 77.Arlauckas SP, Garris CS, Kohler RH, et al. (2017) In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 10.1126/scitranslmed.aal3604 [DOI] [PMC free article] [PubMed]
- 78.•.Moyes KW, Davis A, Hoglund V, et al. (2018) Effects of tumor grade and dexamethasone on myeloid cells in patients with glioma. Oncoimmunology. 10.1080/2162402X.2018.1507668(This article explores how dexamethasone can impact immune cells in glioma outside of the tumor itself, which highlights the need for decoupling the impact of treatment versus the tumor on the tumor microenvironment need to make sure the treatment is effective at treating the tumor as opposed to disguising the effects)
- 79.Pawelec G, Verschoor CP, Ostrand-Rosenberg S (2019) Myeloid-derived suppressor cells: Not only in tumor immunity. Front Immunol. 10.3389/fimmu.2019.01099 [DOI] [PMC free article] [PubMed]
- 80.Tesi RJ (2019) MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 10.1016/j.tips.2018.10.008 [DOI] [PubMed]
- 81.Priego N, Zhu L, Monteiro C, et al. (2018) STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis article. Nat Med. 10.1038/s41591-018-0044-4 [DOI] [PubMed]
- 82.•.Henrik Heiland D, Ravi VM, Behringer SP, et al. (2019) Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 10.1038/s41467-019-10493-6(This article discusses the role of tumor associated astrocytes and their role in glioma, particularly through the STAT pathway).
- 83.•.Sena IFG, Paiva AE, Prazeres PHDM, Azevedo PO, Lousado L, Bhutia SK, Salmina AB, Mintz A, Birbrair A (2018) Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. 10.1002/cam4.1375(This review discusses the role of tumor associated pericytes and their role in glioma).
- 84.Valdor R, García-Bernal D, Bueno C, Ródenas M, Moraleda JM, Macian F, Martínez S (2017) Glioblastoma progression is assisted by induction of immunosuppressive function of pericytes through interaction with tumor cells. Oncotarget. 10.18632/oncotarget.19804 [DOI] [PMC free article] [PubMed]
- 85.Valdor R, García-Bernal D, Riquelme D, Martinez CM, Moraleda JM, Cuervo AM, Macian F, Martinez S (2019) Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 10.1073/pnas.1903542116 [DOI] [PMC free article] [PubMed]
- 86.Michaelis M, Doerr HW, Cinatl J (2009) The story of human cytomegalovirus and cancer: Increasing evidence and open questions. Neoplasia. 10.1593/neo.81178 [DOI] [PMC free article] [PubMed]
- 87.Krenzlin H, Behera P, Lorenz V, et al. (2019) Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J Clin Invest. 10.1172/JCI123375 [DOI] [PMC free article] [PubMed]
- 88.•.Cha J, Kim P (2019) Time series assessment of the effects of hypoxic stress on glioma tumorsphere development within engineered microscale niches. Biomaterials. 10.1016/j.biomaterials.2018.12.018(This article highlights a model that can recapitulate cancer hallmarks simultaneously and provide high-throughput assessment of glioma)
- 89.Gritsenko P, Leenders W, Friedl P (2017) Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem Cell Biol. 10.1007/s00418-017-1604-2 [DOI] [PMC free article] [PubMed]
- 90.••.Lee C, Abelseth E, de la Vega L, Willerth SM (2019) Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening. Mater Today Chem 12:78–84(This article highlights a novel technique using bioprinting for recapitulating a 3D model in glioma)
- 91.••.Yi HG, Jeong YH, Kim Y, et al. (2019) A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng 3:509–519(This article highlights a novel technique using bioprinting for recapitulating a patient-specific 3D model in glioma)
- 92.Kim SS, Harford JB, Moghe M, Slaughter T, Doherty C, Chang EH (2019) A tumor-targeting nanomedicine carrying the p53 gene crosses the blood–brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int J Cancer. 10.1002/ijc.32531 [DOI] [PMC free article] [PubMed]
- 93.Brown CE, Badie B, Barish ME, et al. (2015) Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 10.1158/1078-0432.CCR-15-0428 [DOI] [PMC free article] [PubMed]
- 94.Kamran N, Kadiyala P, Saxena M, Candolfi M, Li Y, Moreno-Ayala MA, Raja N, Shah D, Lowenstein PR, Castro MG (2017) Immunosuppressive Myeloid Cells’ Blockade in the Glioma Microenvironment Enhances the Efficacy of Immune-Stimulatory Gene Therapy. Mol Ther. 10.1016/j.ymthe.2016.10.003 [DOI] [PMC free article] [PubMed]
- 95.Hoves S, Ooi CH, Wolter C, et al. (2018) Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 10.1084/jem.20171440 [DOI] [PMC free article] [PubMed]
- 96.Gao H, Zhang IY, Zhang L, et al. (2018) S100B suppression alters polarization of infiltrating myeloid-derived cells in gliomas and inhibits tumor growth. Cancer Lett. 10.1016/j.canlet.2018.07.034 [DOI] [PMC free article] [PubMed]
- 97.Hutter G, Theruvath J, Graef CM, et al. (2019) Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A. 10.1073/pnas.1721434116 [DOI] [PMC free article] [PubMed]
- 98.••.Birbrair A, Sattiraju A, Zhu D, et al. (2017) Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. Stem Cells Transl Med 6:471–481(This article discusses a novel technique with neural-like stem cells from the periphery can be genetically engineered to be therapeutic agents in glioma)
- 99.•.Hong B, Muili K, Bolyard C, et al. (2019) Suppression of HMGB1 Released in the Glioblastoma Tumor Microenvironment Reduces Tumoral Edema. Mol Ther - Oncolytics. 10.1016/j.omto.2018.11.005(This article highlights the use of oncolytic HSV virus therapy as a method to reduce inflammation in glioma tumor microenvironment)
- 100.•.Navone SE, Guarnaccia L, Cordiglieri C, et al. (2018) Aspirin Affects Tumor Angiogenesis and Sensitizes Human Glioblastoma Endothelial Cells to Temozolomide, Bevacizumab, and Sunitinib, Impairing Vascular Endothelial Growth Factor-Related Signaling. World Neurosurg. 10.1016/j.wneu.2018.08.080(This article highlights the repurposing of aspirin as a potential treatment to aid in overcoming drug resistance and angiogenesis in glioma)
- 101.•.Säälik P, Lingasamy P, Toome K, et al. (2019) Peptide-guided nanoparticles for glioblastoma targeting. J Control Release. 10.1016/j.jconrel.2019.06.018(This article highlights tumor-homing nanoparticles that are novel agents in therapeutics against glioblastoma)
- 102.•.Mercurio L, Ajmone-Cat MA, Cecchetti S, et al. (2016) Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J Exp Clin Cancer Res. 10.1186/s13046-016-0326-y(This article highlights a specific peptide inhibitor targeting CXCR4 in glioma)
- 103.•.Lucki NC, Villa GR, Vergani N, et al. (2019) A cell type-selective apoptosis-inducing small molecule for the treatment of brain cancer. Proc Natl Acad Sci U S A. 10.1073/pnas.1816626116(This article focuses on a therapeutic that is cell-type specific as potential treatment in glioma)
- 104.••.Venkatesh HS, Morishita W, Geraghty AC, et al. (2019) Electrical and synaptic integration of glioma into neural circuits. Nature. 10.1038/s41586-019-1563-y(This article is the first to link neuronal electrochemical signaling in glioma progression)
- 105.•.Venkatesh HS, Johung TB, Caretti V, et al. (2015) Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 10.1016/j.cell.2015.04.012(This article highlights how neurons can stimulate glioma growth through neuroligin-3, a mitogen secreted by neurons)
- 106.Agrawal R, Garg A, Benny Malgulwar P, Sharma V, Sarkar C, Kulshreshtha R (2018) p53 and miR-210 regulated NeuroD2, a neuronal basic helix–loop–helix transcription factor, is downregulated in glioblastoma patients and functions as a tumor suppressor under hypoxic microenvironment. Int J Cancer. 10.1002/ijc.31209 [DOI] [PubMed]
- 107.Zhao J, He H, Zhou K, Ren Y, Shi Z, Wu Z, Wang Y, Lu Y, Jiao J (2012) Neuronal transcription factors induce conversion of human glioma cells to neurons and inhibit tumorigenesis. PLoS One. 10.1371/journal.pone.0041506 [DOI] [PMC free article] [PubMed]
- 108.•.Cheng X, Tan Z, Huang X, Yuan Y, Qin S, Gu Y, Wang D, He C, Su Z (2019) Inhibition of Glioma Development by ASCL1-Mediated Direct Neuronal Reprogramming. Cells. 10.3390/cells8060571(This article explores reprogramming glioma cells by leveraging ASCL-1, a neuronal transcription factor, for terminal differentiation and inhibiting glioma progression)
- 109.•.Lee C, Robinson M, Willerth SM (2018) Direct Reprogramming of Glioblastoma Cells into Neurons Using Small Molecules. ACS Chem Neurosci 9:3175–3185(This article highlights the development of a chemically engineered small molecule that can reprogram glioma cells into neurons)
- 110.•.Lai SW, Liu YS, Lu DY, Tsai CF (2019) Melatonin modulates the microenvironment of glioblastoma multiforme by targeting sirtuin. Nutrients. 10.3390/nu11061343(This article highlights the impact of a native molecule in the brain, melatonin, on the tumor microenvironment in glioma)
- 111.Martín V, Herrera F, Carrera-Gonzalez P, García-Santos G, Antolín I, Rodriguez-Blanco J, Rodriguez C (2006) Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res. 10.1158/0008-5472.CAN-05-2354 [DOI] [PubMed]
- 112.Wang J, Hao H, Yao L, Zhang X, Zhao S, Ling EA, Hao A, Li G (2012) Melatonin suppresses migration and invasion via inhibition of oxidative stress pathway in glioma cells. J Pineal Res. 10.1111/j.1600-079X.2012.00985.x [DOI] [PubMed]
- 113.Lissoni P, Meregalli S, Nosetto L, Barni S, Tancini G, Fossati V, Maestroni G (1996) Increased survival time in brain glioblastomas by a radioneuroendocrine strategy with radiotherapy plus melatonin compared to radiotherapy alone. Oncol. 10.1159/000227533 [DOI] [PubMed]
- 114.•.Ben-Shaanan TL, Schiller M, Azulay-Debby H, et al. (2018) Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun. 10.1038/s41467-018-05283-5(This article discusses links a physiological process in the brain and how it can positively impact cancer)


