Supplemental Digital Content is available in the text.
Keywords: Air pollution, Gestational age, Inverse probability weighting, Quantile regression, Health disparity, Temperature, Air pollution, Gestational age, Inverse probability weighting, Quantile regression, Health disparity, Temperature
Background:
There is a lack of evidence on causal effects of air pollution on gestational age (GA) at delivery.
Methods:
Inverse probability weighting (IPW) quantile regression was applied to derive causal marginal population-level GA reduction for GA percentiles associated with increased ambient particulate matter with diameter <2.5 μm (PM2.5) levels at maternal residential address for each trimester and the month preceding delivery using Massachusetts birth registry 2001 to 2015. Stratified analyses were conducted for neonatal sex, maternal age/race/education, and extreme ambient temperature conditions.
Results:
For neonates at 2.5th, 10th, 25th, 50th, 75th, and 97.5th percentiles of GA at delivery, we estimated an adjusted GA reduction of 4.2 days (95% confidence interval [CI] = 3.4, 5.0), 1.9 days (1.6, 2.1), 1.2 days (1.0, 1.4), 0.82 days (0.72, 0.92), 0.74 days (0.54, 0.94), and 0.54 days (0.15, 0.93) for each 5 μg/m3 increment in third trimester average PM2.5 levels. Final gestational month average exposure yielded a similar effect with greater magnitude. Male neonates and neonates of younger (younger than 35 years) and African American mothers as well as with high/low extreme temperature exposure in third trimester were more affected. Estimates were consistently higher at lower GA percentiles, indicating preterm/early-term births being more affected. Low-exposure analyses yielded similar results, restricting to areas with PM2.5 levels under US ambient annual standard of 12 μg/m3.
Conclusions:
Prenatal exposure to PM2.5 in late pregnancy reduced GA at delivery among Massachusetts neonates, especially among preterm/early-term births, male neonates, and neonates of younger and African American mothers. Exposure to extremely high/low temperature amplifies the effect of PM2.5 on GA.
What this study adds
Population-level clinical interpretable marginal effect estimates were identified for the differences in percentiles of gestational age (GA) associated with increased levels of prenatal exposure to air pollution. Several important health disparities were uncovered comparing births across GA distribution and between different subpopulations. Exposure to extremely high or low temperature amplifies the effect of air pollution on GA. Counterfactual predictions of GA distribution under higher/lower pollution situations, as well as low-exposure analyses restricting to areas with particulate matter with diameter < 2.5 μm levels under current US annual ambient regulation standard still indicated a space for air pollution regulation to further improve maternal and child health.
Introduction
Gestational age at delivery (referred to as GA) is a clinical measure of the length of pregnancy starting from the date of a woman’s first day of her last menstrual period (LMP) to the date of delivery.1 It is a key reproductive and infant health measure because survival and overall health status of newborns depend on their maturity at birth, which is largely determined by GA. Evidence suggests that changes of GA at delivery could have a long-lasting influence on various health outcomes, including mortality, cognitive health, diabetes, respiratory health, and psychological and behavioral problems during all life stages.2–8
A growing body of literature explored the association between air pollution and birth outcomes,9–11 with only two focused directly on GA as a continuous outcome of interest.12,13 However, none of them applied causal modeling methods or examined whether the effect of exposure to air pollution varies within different levels of the population GA distribution. Most studies used preterm birth (<37 weeks) as a proxy outcome. Such a dichotomized outcome does not provide guidance on how much reduction in GA is associated with a given risk factor, whereas understanding the amount of reduction allows more accurate risk assessments and recommendations. Although the effects of prematurity (preterm births) in neonates are well known, a recent study at JAMA Pediatrics demonstrated that important developmental processes could occur even between 37 and 39 weeks of gestation and early-term births (37–39 weeks) were associated with higher neonatal morbidity compared with full-term births (39–41 weeks).14 Therefore, there is a need to quantify the effect of modifiable risk factors in a broader range of the distribution of GA at delivery instead of using the conventional preterm or not indicator. Given that the majority of epidemiological studies in air pollution are observational and estimating associations rather than causation, there is also a need for causal evidence. In addition, very few studies have examined effect modification in the context of environmental and health disparities.15–19 More specifically, less is known about the role of ambient temperature on the effects of air pollution on various birth outcomes. Whether temperature is a confounder or modifier or both is uncertain and requires more evidence.20
Quantile regression models a continuous outcome and its predictors without assuming a shape of the distribution of the outcome variable (or the model residuals). Because each percentile of the distribution of the outcome is modeled separately (still using the total population), it allows examination of the differential effect of an exposure in different percentiles of an outcome. Unlike dichotomizing at several cutoff points, it provides clinical interpretable effect estimates expressed in the unit of the outcome.21 The goal of such an analysis is to quantify the associations between exposure and specific percentiles of the outcome distribution, thereby identifying whether certain outcome levels are more affected.21 Inverse probability weighting (IPW) utilizes propensity score (PS) models to predict the exposure from covariates and render exposure independent of them.22 If all important covariates are measured and adjusted, we could obtain marginal causal estimates. IPW is one of the most common approaches in causal inference epidemiology, which uses inverse probability weights derived from the PS models predicting the exposure from the confounders, and allows the exposure independent of the measured covariates. When the exposure is independent of covariates, its effect on the outcome cannot be confounded by them and resulting estimates will not depend on the distributions of the confounders. If all important covariates are measured, these models can provide causal estimates of the marginal effects of exposure.
Therefore, to address the current research gaps, we adopted a combination of quantile regression and causal modeling. We aimed to examine whether increased time window-specific particulate matter with diameter <2.5 μm (PM2.5) levels were associated with significant GA reduction at delivery among newborns using 2001–2015 Massachusetts birth registry based on causal inference method of IPW. Population-level marginal effects for each newborn GA percentile were computed. Sensitive exposure windows and effect modification by neonatal sex, maternal age, race and education, as well as ambient temperature were examined.
Methods
Study population
We obtained data for all live births in Massachusetts from the Massachusetts Registry of Vital Records and Statistics for the years 2001–2015. The residential address of each newborn’s mother at the time of delivery was recorded and later geocoded by Massachusetts Department of Public Health against TomTom Multinet (American Digital Cartography, Appleton, WI) using AccuMail address and zip code as the input address field and zone. We restricted to those women with GA at delivery of 20–42 weeks, gravidity and parity less than 10, and live singleton births to avoid possible bias from miscarriages, extremely high parity, and the effect of twins/triplets on gestation length. In addition, we excluded cesarean section (C-section) as a mode of delivery because these neonates were more likely to have a mother with other medical issues or other unrecorded medical indications, which made it hard to discern the effect of air pollution on their natural gestation length. The final study population was 652,167 births. This study was approved by the Harvard T.H. Chan School of Public Health Institutional Review Boards (IRBs).
Environmental data
Daily fine particulate matter (PM2.5) levels at 1-km2 grids were estimated from a validated, ensemble-based model, which integrated three machine learning algorithms and predictor variables derived from satellite remote sensing, chemical transport models, land used data, and meteorology.23 Each birth was assigned daily PM2.5 values during the pregnancy period and based on the 1-km2 grid into which their home address fell. We averaged the exposure by pregnancy trimester (first: gestational weeks 1–12; second: gestational weeks 13–28; third: gestational weeks 29–42)24 for trimester-specific modeling and also by the preceding month before delivery. Ambient temperature levels at 1-km2 grid for the study population were estimated from prediction models using the Moderate Resolution Imaging Spectroradiometer (MODIS) satellite (by Santa Barbara Remote Sensing) and was available for 2004–2015 only.25 Temperature data were assigned to each birth (2004–2015) in the same way.
Outcome of interest
GA at delivery in days calculated based on LMP.
Statistical analyses
We selected covariates a priori based on expert knowledge, literature review, and the data availability within this cohort. We adjusted for major individual covariates, community-level contextual variables, season of conception (spring: March to May; summer: June to August; fall: September to November; winter: January, February, and December), and calendar year in our analyses. The individual covariates included neonatal sex, maternal marital status, age, cigarette smoked per day before pregnancy, lung disease, cardiac disease, preexisting diabetes, chronic hypertension, incomplete cervix, previous infant with birth defects, previous infant over 4,000 g, previous small for gestational age infants, renal disease, rhesus isoimmunization sensitization, sickle cell, prenatal care paid by government, maternal race, maternal education level, and adequacy of prenatal care utilization (Kotelchuck index26). Contextual variables controlled included population density, median household income, and percentage of African American population at census tract level.
We fit quantile regressions incorporating IPW weights from propensity score models. The construction of weights followed the methods developed by Cole and Hernán,27 but extended to generalized propensity scores.28 For each averaged trimester level and the last month preceding delivery (abbreviated as last month in results) averaged PM2.5, we fitted a linear regression to predict the exposure as a function of included covariates. Stabilized weights were generated and truncated between 1st and 99th percentile. We excluded those that violated positivity where the predicted probability (given the covariates) of receiving a high (>90 percentile) or low (<10 percentile) was less than 0.001. Within the pseudorandomized population created using the weights, we further ran marginal structured weighted quantile regressions with GA regressed against the average trimester PM2.5 for each trimester and preceding month average exposure. Effect estimates were extracted from each percentile of GA, and robust Sandwich Huber standard errors were obtained. Stratified analyses for third trimester exposure were presented comparing male versus female neonates; neonates with younger (younger than 35 years) versus older mothers (35 years or older) at delivery; neonates with different maternal race, including African Americans, Caucasians, Asian/Pacific Islanders, Native Americans, and other nonmissing race; neonates with maternal education less than or equal to high school versus more than high school; and neonates with maternal address at delivery at high versus low extreme ambient temperature (using 90th and 10th as the cutoffs). The use of 35 years as the cutoff for grouping woman by age is with reference to the findings established by Mittendorf et al.,29 showing that women older than 34 years were associated with a significantly shorter gestation length. In a second effort to inform policy making, we did a low-exposure analysis by restricting analyses to population situated at locations with annual average PM2.5 exposure levels of <12 μg/m3 (US Environmental Protection Agency [EPA] long-term standard) at birth year. Under the assumptions of exchangeability, consistency, and positivity,22 the effect estimates generated from the marginal structured weighted quantile regression models can be interpreted as causal effects. To test the exchangeability assumption, we further ran sensitivity analyses by additionally adjusting for paternal race and age, previous time window exposures, and ambient temperature to see if there was significant deviation from the main estimates after including more covariates. Missing values for paternal factors were imputed via. R package Amelia II. Positivity was assured using positivity violation exclusion. Consistency was assumed.30 Quality control and assumption test was achieved by checking PS model residual distribution and creating covariate balance plot using standardized differences of adjusted covariates with reference to the methodology developed by Austin.31 Percentiles and counts of births within this cohort were computed and plotted. Counterfactual distribution of expected GA at delivery under increased (5 μg/m3 higher) and decreased (5 μg/m3 lower) particulate air pollution scenarios was also plotted based on predictions using percentile-specific effect estimates generated in the main analyses for third trimester. Distribution of average ambient PM2.5 levels at maternal residential address at delivery for various maternal racial groups was plotted using boxplot, especially for the third trimester, to examine potential air pollution exposure disparity. Temporal trends of annual PM2.5 exposures and population average GA at delivery for Massachusetts were computed for each year from 2001 to 2015 among the study population. Additional sensitivity analyses were conducted by adding paternal factors, previous time window exposures, and ambient temperature. All analyses were conducted in R software (version 3.5.1; by R Development Core Team).
Results
Demographic characteristics of the study population are presented in Table 1. The trimester-specific population average PM2.5 levels at maternal home addresses over the entire study period were similar (first: 9.2 μg/m3, SD: 2.4 μg/m3; second: 9.1 μg/m3, SD: 2.2 μg/m3; third: 9.1 μg/m3, SD: 2.5 μg/m3). Figure 1A demonstrates the births count distribution for percentiles of GA in both days and weeks + days. The 2.5th percentile corresponds to neonates with GA at delivery centered around 245 days (35 weeks and 0 days), the median percentile around 277 days (39 weeks and 4 days), and the 97.5th percentile around 292 days (41 weeks and 5 days). Other percentiles and their corresponding GA at delivery can also be seen. Figure 1B presents the counterfactual distributions of expected GA at delivery under increased (5 μg/m3 higher) and decreased (5 μg/m3 lower) PM2.5 levels during the third trimester. Although the distributions of full-term neonates under these two counterfactual circumstances were nearly identical, the difference of the distributions for nonfull-term neonates, especially with 220–270 days (approximately 31– 39 weeks) GA at delivery, is large. The boxplot (Figure 2) showed that there was not an obvious differential exposure distribution at maternal residential addresses at delivery during third trimester when comparing across various maternal racial groups, including Caucasians, African Americans, Asians and Pacific Islanders, Native Americans, and other races. Not all births entered into the third trimester based on our study population restriction. Although all the 652,167 births entered into second trimester, about 650,692 births entered into third trimester. Therefore, the third trimester estimates reported in the current study all corresponded to the 650,692 births.
Table 1.
Study population characteristics of Massachusetts birth cohort in the years of 2001 to 2015
| Overall | |
| Study population size, n | 652,167 |
| Neonatal characteristics | |
| Birth weight, g, mean (SD) | 3,367 (500) |
| Gestational age, d, mean (SD) | 275 (12) |
| Gestational age, wks, mean (SD) | 39.3 (1.8) |
| Female sex, N (%) | 323,592 (49.6) |
| Preterm, N (%) | 47,579 (7.3) |
| Parental characteristics | |
| Mother’s age, yr, mean (SD) | 29.7 (5.9) |
| Father’s age, yr, mean (SD) | 32.3 (6.6) |
| Cigarette per day before pregnancy, No., mean (SD) | 1.5 (4.8) |
| Married, N (%) | 442,726 (67.9) |
| Mother’s race, N (%) | |
| White | 468,919 (71.9) |
| African American | 58,408 (9.0) |
| Asian/Pacific Islander | 53,485 (8.2) |
| Native American | 1,619 (0.2) |
| Other nonmissing | 67,615 (10.4) |
| Missing/refused/unknown | 2,121 (0.3) |
| Father’s race, N (%) | |
| White | 436,527 (70.4) |
| African American | 56,066 (9.0) |
| Asian/Pacific Islander | 46,977 (7.6) |
| Native American | 1,445 (0.2) |
| Other nonmissing | 69,348 (11.2) |
| Missing/refused/unknown | 9,576 (1.5) |
| Mother’s education, N (%) | |
| Less than high school | 70,886 (10.9) |
| High school/general education degree | 148,094 (22.8) |
| Some college | 144,549 (22.3) |
| Bachelor’s degree | 169,104 (26.0) |
| More | 116,799 (18.0) |
| Kotelchuck index, N (%) | |
| 0 | 10,018 (1.5) |
| 1 | 53,194 (8.2) |
| 2 | 44,822 (6.9) |
| 3 | 307,597 (47.2) |
| 4 | 236,536 (36.3) |
| Prenatal care paid by government, N (%) | 221,331 (34.0) |
| Season of conception, N (%) | |
| Fall | 172,438 (26.4) |
| Spring | 150,379 (23.1) |
| Summer | 167,412 (25.7) |
| Winter | 161,938 (24.8) |
| Maternal diseases and previous birth conditions | |
| Lung, N (%) | 24,683 (3.8) |
| Cardiac, N (%) | 3,793 (0.6) |
| Diabetes, N (%) | 4,221 (0.6) |
| Chronic hypertension, N (%) | 6,932 (1.1) |
| Incompetent cervix, N (%) | 3,053 (0.5) |
| Previous infant with birth defect, N (%) | 1,851 (0.3) |
| Previous infant over 4000 g, N (%) | 3,604 (0.6) |
| Previous infant of small for gestational age, N (%) | 6,120 (0.9) |
| Renal, N (%) | 2,505 (0.4) |
| rH sensitization, N (%) | 14,663 (2.2) |
| Sickle cell. N (%) | 654 (0.1) |
| Community contextual characteristics | |
| Population density, population per square mile, median (range) | 1,508.8 (102.1–12,415.7) |
| Median household income, USD/yr, median (range) | 69,068 (49,956–88,262) |
| Black or African American percent, median (range) | 6% (1%–25%) |
Preterm birth is defined as babies born alive before 37 weeks of pregnancy are completed (https://www.who.int/news-room/fact-sheets/detail/preterm-birth). Kotelchuck index, also called Adequacy of Prenatal Care Utilization (APNCU) Index, is a more accurate and comprehensive set of measures of prenatal care utilization compared with the widely used Kessner index, here 0 = no information, 1 = inadequate, 2 = intermediate, 3 = adequate, 4 = adequate plus; Clinical factors strongly associated with preterm birth: maternal age, smoking, maternal race, maternal education, preexisting lung, cardiac, renal and sickle cell disease, rH sensitization, chronic hypertension, incompetent cervix, fetus small or large for gestational age, and previous delivery with birth defects.
Figure 1.
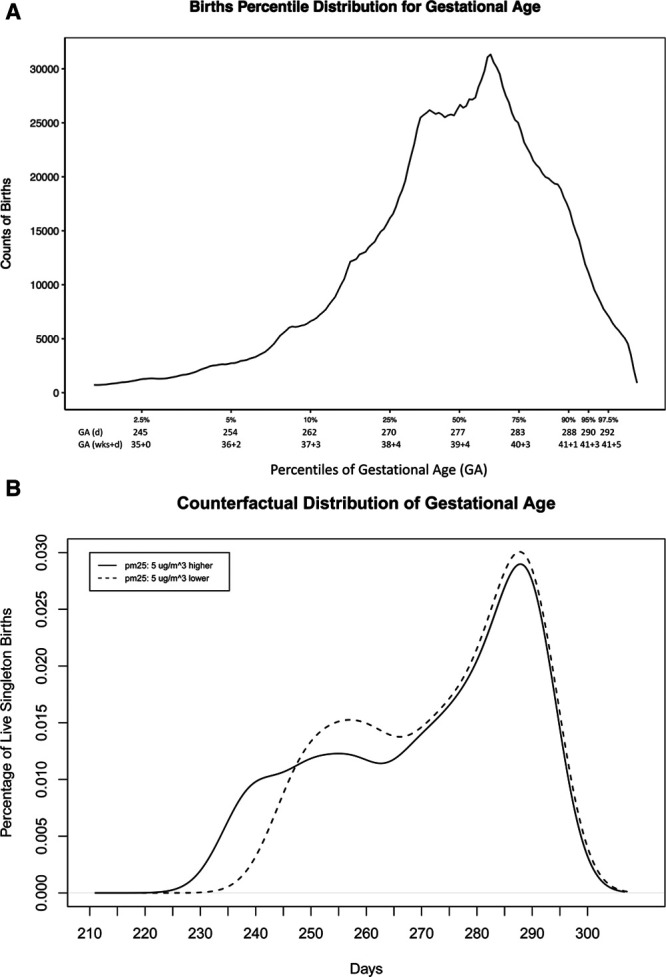
A, Births percentile distribution for GA. The counts of births distributed for percentiles of GA at delivery and its corresponding GA at delivery levels. B, Counterfactual distribution of GA. We used the effect estimates in the third trimester to do counterfactual predictions comparing the distribution of GA within Massachusetts newborns population under ambient PM2.5 levels 5 μg/m3 lower than current situation and 5 μg/m3 higher than current situation from 2001 to 2015.
Figure 2.
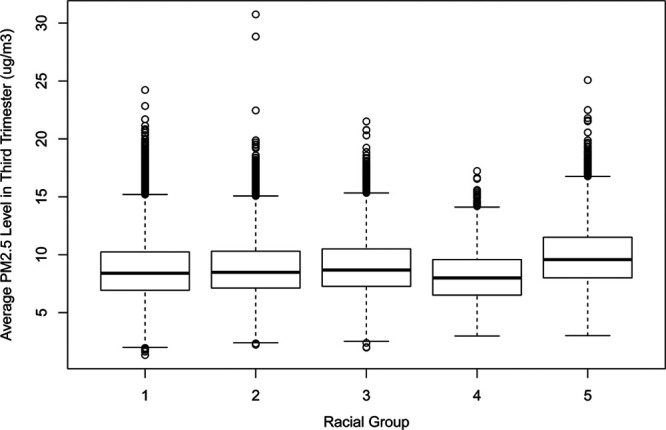
Boxplot distribution of average ambient PM2.5 levels during the third trimester at maternal residential address for various maternal racial groups. Racial groups represent 1: Caucasians, 2: African Americans, 3: Asian and Pacific Islanders, 4: Native Americans, and 5: other nonmissing race, respectively.
Our main analyses (Table 2, eFigure 1; http://links.lww.com/EE/A106) demonstrated a significant GA reduction per 5 μg/m3 increase in PM2.5 levels in the third trimester and preceding month before delivery. For neonates at the 2.5th, 10th, 25th, 50th, 75th, 90th and 97.5th percentiles of GA at delivery, we estimated a GA reduction of 4.2 days (95% CI = 3.4, 5.0), 1.9 days (95% CI = 1.6, 2.1), 1.2 days (95% CI = 1.0, 1.4), 0.82 days (95% CI = 0.72, 0.92), 0.74 days (95% CI = 0.54, 0.94), 0.00 days (95% CI = −0.10, 0.10), and 0.54 days (95% CI = 0.15, 0.93) for each 5 μg/m3 increase in average PM2.5 levels in the third trimester and a GA reduction of 7.5 days (95% CI = 6.7, 8.3), 3.7 days (95% CI = 3.4, 4.0), 2.2 days (95% CI = 2.1, 2.3), 1.2 days (95% CI = 1.1, 1.3), 0.74 days (95% CI = 0.64, 0.84), 0.65 days (95% CI = 0.36, 0.94), and 0.50 days (95% CI = 0.11, 0.89) for each 5 μg/m3 increase in average PM2.5 levels in the preceding month before delivery, adjusting for individual risk factors, maternal education, contextual variables, season of conception, and calendar year. Overall, we observed no significant effects of PM2.5 on the reduction of GA at delivery in association with first and second trimester exposure to PM2.5. Exceptions were that small GA decrease was identified for 10th percentile and GA increase for 2.5th percentile associated with increased second trimester exposure to PM2.5. Effect estimates for third trimester were consistently larger at lower percentiles, indicating stronger effect for preterm and early-term births. Minimally adjusted models (excluding adjustment for maternal diseases and previous birth conditions) showed similar results.
Table 2.
Time window-specific gestational age change (d) per 5 μg/m3 increase in PM2.5 levels
| Percentile | 2.5th | 10th | 25th | 50th | 75th | 90th | 97.5th |
|---|---|---|---|---|---|---|---|
| Minimal adjustment | |||||||
| First trimester | 0.00 (−0.78, 0.78) | 0.00 (−0.29, 0.29) | 0.00 (−0.20, 0.20) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (−0.10, 0.10) | 0.00 (−0.39, 0.39) |
| Second trimester | 0.91 (0.13, 1.7) | 0.00 (−0.29, 0.29) | 0.00 (−0.29, 0.29) | 0.00 (−0.20, 0.20) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (−0.20, 0.20) |
| Third trimester | −2.8 (−3.5, −2.1) | −1.8 (−2.1, −1.5) | −1.2 (−1.3, −1.1) | −0.85 (−0.95, −0.75) | −0.80 (−0.90, −0.70) | −0.75 (−1.0, −0.46) | −0.67 (−1.1, −0.28) |
| Last month | −7.4 (−8.2, −6.7) | −3.7 (−4.0, −3.4) | −2.2 (−2.3, −2.1) | −1.2 (−1.3, −1.1) | −0.74 (−0.84, −0.64) | −0.65 (−0.94, −0.35) | −0.50 (−0.89, −0.11) |
| Full adjustment | |||||||
| First trimester | 0.00 (−0.39, 0.39) | 0.00 (−0.29, 0.29) | 0.00 (−0.20, 0.20) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0 (−0.10, 0.10) | 0.00 (−0.10, 0.10) |
| Second trimester | 0.78 (0.19, 1.4) | −0.65 (−1.0, −0.26) | 0.00 (−0.29, 0.29) | 0.00 (−0.20, 0.20) | 0.00 (0.00, 0.00) | 0.00 (−0.10, 0.10) | 0.00 (−0.20, 0.20) |
| Third trimester | −4.2 (−5.0, −3.4) | −1.9 (−2.1, −1.6) | −1.2 (−1.4, −1.0) | −0.82 (−0.92, −0.72) | −0.74 (−0.94, −0.54) | 0.00 (−0.10, 0.10) | −0.54 (−0.93, −0.15) |
| Last month | −7.5 (−8.3, −6.7) | −3.7 (−4.0, −3.4) | −2.2 (−2.3, −2.1) | −1.2 (−1.3, −1.1) | −0.74 (−0.84, −0.64) | −0.65 (−0.94, −0.36) | −0.50 (−0.89, −0.11) |
Point estimates (95% confidence interval) for each percentile are reported here. Each time window effect estimate is for neonates with information on that time window. Minimal adjustment included adjustment for individual covariates excluding maternal risk factors (maternal lung diseases, cardiac diseases, diabetes, chronic hypertension, incompetent cervix, with previous infant with birth defect, with previous infant over 4000 g, with previous infant of small for gestational age, renal diseases, rH sensitization, and sickle cell), contextual variables, season of conception, and calendar year described in Methods section. Full adjustment included adjustment for individual covariates, contextual variables, season of conception, and calendar year described in Methods section.
Adjusted stratified analyses were conducted to explore potential health disparities by fetal sex, maternal age at delivery, and maternal race. All of the effect estimates were reported per 5 μg/m3 increase in average ambient PM2.5 levels in the third trimester. Consistently larger decreases in GA at delivery were observed for male neonates compared with female neonates at lower percentiles (2.5th, 10th, and 25th). Consistently larger decreases in GA at delivery were also observed among neonates of younger mothers (younger than 35 years) than older mothers (35 years or older) for lower percentiles (2.5th, 10th, and 25th). An exception was for percentile 90th, neonates of older mothers and female neonates instead experienced a significant GA reduction compared with the null results for younger mothers and male neonates. However, the opposite results for the 90th percentile compared with the lower percentiles may not be true because the upper bounds for the estimates on female neonates and neonates of maternal older age were close to 0. For each 5 μg/m3 increase in average PM2.5 levels in the third trimester, neonates of African American mothers were expected to experience approximately 1 day to 1 week reduction of GA at delivery for the point estimates across percentile 2.5th to percentile 75th with higher reduction at the lower percentiles [i.e., −7.2 days (95% CI = −10, −4.2] for babies at 2.5th percentile). However, for neonates born to White mothers, Asian mothers, or mothers with other nonmissing race, the effect estimates were much smaller (approximately 1 to 4 days GA reduction for the point estimates at 2.5th, 10th, and 25th percentiles with larger effects for babies with earlier term at delivery). Due to the limited sample size (1,619, 0.2% of the total births), for neonates with maternal race of Native Americans, we found null results across the GA percentiles and the stratified analyses for them were underpowered. Maternal education level did not create much disparity for the effects of third trimester exposure to PM2.5 on GA at delivery. A 5 μg/m3 increase in average third trimester PM2.5 exposure is linked to point estimates of 1.7 to 10 GA reduction across the GA percentiles for subpopulation with high average ambient temperature (≥21°C) in the third trimester and also linked to 0 to 6.8 days reduction for subpopulation with low average ambient temperature (≤−0.59°C) in the third trimester. The neonatal GA at delivery seemed to be affected to a larger extent when exposed to increased levels of PM2.5 in extreme high and low tempered third trimester window than what we found for the main results without stratification (Table 2). More details were presented in Table 3.
Table 3.
Stratified results for gestational age change (d) per 5 μg/m3 increase in PM2.5 levels in the third trimester by neonatal sex, maternal age at delivery, maternal race, maternal education, and high versus low 10th temperature
| Percentile | 2.5th | 10th | 25th | 50th | 75th | 90th | 97.5th |
|---|---|---|---|---|---|---|---|
| Neonatal sex | |||||||
| Male | −5.0 (−6.1, −3.9) | −2.2 (−2.6, −1.8) | −1.3 (−1.5, −1.1) | 0.00 (−0.20, 0.20) | 0.00 (−0.10, 0.10) | 0.00 (0.00, 0.00) | 0.00 (−0.49, 0.49) |
| Female | −3.4 (−4.4, −2.4) | −1.7 (−2.1, −1.3) | −0.90 (−1.1, −0.70) | 0.00 (−0.10, 0.10) | 0.00 (−0.10, 0.10) | −0.63 (−0.92, −0.34) | 0.00 (−0.49, 0.49) |
| Maternal age at delivery | |||||||
| <35 yrs | −4.7 (−5.6, −3.8) | −2.0 (−2.3, −1.7) | −1.2 (−1.4, −1.0) | 0.00 (−0.10, 0.10) | 0.00 (−0.10, 0.10) | 0.00 (−0.29, 0.29) | 0.00 (−0.20, 0.20) |
| ≥35 yrs | −2.9 (−4.5, −1.4) | −1.5 (−2.1, −0.88) | 0.00 (−0.39, 0.39) | 0.00 (−0.20, 0.20) | 0.00 (−0.29, 0.29) | −0.61 (−1.0, −0.22) | 0.00 (0.00, 0.00) |
| Maternal race | |||||||
| African American | −7.2 (−10, −4.2) | −3.6 (−4.8, −2.4) | −1.6 (−2.2, −1.0) | −1.1 (−1.5, −0.74) | −0.86 (−1.3, −0.47) | 0.00 (−0.20,0.20) | 0.00 (0.00, 0.00) |
| White | −3.7 (−4.5, −2.9) | −1.7 (−2.0, −1.4) | −1.1 (−1.3, −0.94) | 0.00 (−0.10, 0.10) | 0.00 (−0.10, 0.10) | −0.60 (−0.89, −0.31) | 0.00 (−0.39, 0.39) |
| Asian/Pacific Islander | −3.4 (−6.4, −0.47) | −1.7 (−2.7, −0.70) | −0.94 (−1.4, −0.45) | 0.00 (−0.39, 0.39) | 0.00 (−0.39, 0.39) | 0.00 (−0.49, 0.49) | 0.00 (−0.69, 0.69) |
| Other nonmissing | −2.8 (−6.1, 0.58) | −1.8 (−2.8, −0.67) | −0.55 (−0.94, −0.16) | 0.00 (−0.29, 0.29) | 0.00 (−0.29, 0.29) | 0.00 (−0.20,0.20) | 0.68 (0.09,1.3) |
| Native American | 10 (−5.6, 27) | 0.88 (−3.8, 5.6) | −2.7 (−5.6, 0.24) | −1.4 (−3.5, 0.66) | −2.3 (−4.5, −0.16) | 0.64 (−1.5, 2.8) | 0.00 (−2.3, 2.3) |
| Maternal education | |||||||
| ≤High school | −3.8 (−4.7, −2.9) | −1.7 (−2.0, −1.4) | −1.2 (−1.4, −1.0) | 0.00 (−0.10, 0.10) | −0.73 (−0.83, −0.63) | 0.00 (−0.10, 0.10) | 0.00 (−0.39, 0.39) |
| >High school | −4.6 (−6.2, −3.0) | −2.2 (−2.8, −1.6) | −1.1 (−1.4, −0.81) | 0.00 (−0.20, 0.20) | 0.00 (−0.29, 0.29) | 0.00 (−0.39, 0.39) | 0.00 (−0.49, 0.49) |
| High versus low temperature | |||||||
| Highest 10th (≥21°C) | −10 (−13, −8.0) | −7.8 (−8.8, −6.8) | −5.4 (−5.9, −4.9) | −3.7 (−4.0, −3.4) | −3.0 (−3.3, −2.7) | −2.6 (−2.9, −2.3) | −1.7 (−2.0, −1.4) |
| Lowest 10th (≤−0.59°C) | −6.8 (−9.2, −4.3) | −5.9 (−6.0, −4.0) | −3.7 (−4.2, −3.2) | −2.4 (−2.8, −2.0) | −1.9 (−2.3, −1.5) | −1.4 (−1.8, −1.0) | 0.00 (−0.29, 0.29) |
Adjusted for individual covariates, contextual variables, season of conception, and calendar year described in Methods section. Point estimates (95% confidence interval) for each percentile are reported here. High versus low temperature stratified analyses were only conducted for 2004–2015 due to data availability for temperature.
Similar results and trends were found in low-exposure analyses (Table 4), with slightly reduced effect sizes for third trimester and the last month preceding delivery at identified percentiles. The assumptions for IPW modeling were met (eFigures 2 and 3; http://links.lww.com/EE/A106). Over the study period, among the Massachusetts birth registry cohort, we did see a downward trend of the annual mean PM2.5 levels going from 11.8 μg/m3 in 2001 to 6.3 μg/m3 in 2015, while the population average GA at delivery remained relatively stable (eTable 1; http://links.lww.com/EE/A106). Sensitivity analyses by adding paternal age and race or adding previous time window exposures showed no significant change in effect estimates for each percentile examined compared with main analyses in third trimester (eTable 2; http://links.lww.com/EE/A106). Additional adjustment for temperature yielded similar results compared with the main analyses for all trimesters and preceding month time window (eTable 3; http://links.lww.com/EE/A106).
Table 4.
Time window-specific gestational age change (d) per 5 μg/m3 increase in PM2.5 levels (restricting to population in areas with annual exposures ≤12 μg/m3)
| Percentile | 2.5th | 10th | 25th | 50th | 75th | 90th | 97.5th |
|---|---|---|---|---|---|---|---|
| Third trimester | −2.4 (−3.1, −1.6) | −1.4 (−1.7, −1.1) | −0.70 (−0.80, −0.60) | 0.00 (−0.10, 0.10) | 0.00 (−0.10, 0.10) | −0.62 (−0.91, −0.33) | 0.00 (−0.39, 0.39) |
| Last month | −6.2 (−6.9, −5.5) | −3.4 (−3.7, −3.1) | −2.1 (−2.3, −1.9) | −1.3 (−1.4, −1.2) | −0.54 (−0.64, −0.44) | −0.48 (−0.58, −0.38) | 0.00 (−0.10, 0.10) |
Adjusted for individual covariates, contextual variables, season of conception, and calendar year described in Methods section. Point estimates (95% confidence interval) for each percentile are reported here for the third trimester and last month preceding delivery.
Discussion
In this cohort study with a follow-up of 15 years using Massachusetts Birth Registry from 2001 to 2015, we observed that third trimester average maternal exposure to PM2.5 levels and preceding month before delivery was associated with significant reduction of neonatal GA at delivery with an application of causal modeling and quantile regression. Effect estimates were consistently higher at lower percentiles, indicating stronger effects for preterm and early-term births, which is the population with the worst prognosis. Women younger than 35 years, African American, and male neonates were shown to be more vulnerable. Exposure to high and low extreme ambient temperature in the third trimester amplified the effects of air pollution on GA. Similar results were found in low-level analyses below the current EPA fine particulate matter long-term standard. Although we did not detect significant changes in the population average GA at delivery in Massachusetts newborns over the study period with temporally decreasing PM2.5 exposure, this does not mean regulating ambient air pollution brings no benefits. The counterfactual population GA distribution predictions as well as the estimates presented indicated that newborns with GA at delivery s at the lower ends of population distribution, who were very preterm, preterm, or early term babies, were at a higher risk of GA reduction resulted from increased PM2.5 levels in third trimester and can still benefit from air pollution regulation.
Evidence on the association between ambient air pollution on preterm birth or gestational length is mixed. Some studies supported an association,18,32–38 whereas more recent large studies showed no evidence of increased risk.39–42 Studies have been conducted in different countries, including the United States,43,44 China,33 and Europe.12 A study in Hong Kong (1997)13 showed that PM10 (per 5.7 μg/m3 higher) was associated with a shorter average GA at delivery by 2.1 day (95% CI = 1.7, 2.4). Another study in Barcelona, Spain (2002–2005), also reported a 1.3 days (95% CI = 0.6, 1.9) reduction in average GA in women with preterm premature rupture of the membranes associated with combined air pollutants, containing PM2.5 absorbance, NO, and NO2.12
A key feature of our study is that we employed causal modeling, attempting to provide casual and population-level marginal effect estimates to better facilitate risk assessment. The basic idea is that causal models analyze observational data to emulate a randomized experiment. Randomization makes exposure independent of all potential confounders, and causal methods attempt to replicate that scenario, rather than conditioning on them. Under specified assumptions,22 these methods yield causal estimates. Often, in contrast to the conventional linear or logistic regression, causal methods, including IPW modeling, provide marginal estimates, not conditional on the distribution of covariates and therefore more generalizable.
The stratified analyses of this study uncovered reproductive health disparities with respect to prenatal exposure to ambient air pollution. Preterm (<37 weeks) or early-term (37–39 weeks) neonates had a higher risk of having a larger GA at delivery reduction when they were exposed to elevated levels of particulate air pollution. Male fetuses were shown to be more vulnerable than females. The underlying mechanism could be sex response differences in inflammation and/or infection process and a more aggressive inflammatory response to the male trophoblast.45–47 Younger women may have less developed Lactobacillus modulation system and thus are more susceptible to uterine infection induced by air pollution.48 It is possible that the community air pollution levels may be higher in general for African Americans than for Caucasians or other racial groups, or particle components may be quite different or even more toxic for African American communities due to social risk factors, such as emission source and regulated greenness levels.49,50 The results of our analysis on the distribution of average PM2.5 levels during third trimester across racial groups showed similar results, making the second explanation more valid. In addition, these differences could also be driven by the population genetic/physiological sensitivity, maternal body mass index (BMI), and other unmeasured factors such as psychological stress, unhealthy diet, and access to healthcare. Our stratified analyses examining maternal education did not show substantial differences between maternal education groups. This may be due to the broad categorization. Ambient temperature seemed to be a modifier for the effects of PM2.5 on GA at delivery in the third trimester. This is physiologically plausible based on evidence showing that extreme temperature (heat or cold stress) predisposed placenta at a higher risk of inflammation triggered by air pollutants.51,52
Most studies highlighted the importance of particulate air pollution exposure in the late pregnancy period.33,53–55 In this study, we also identified the third trimester to be the sensitive window for particulate air pollution to act on gestation duration compared with the other two trimesters, indicating that PM2.5 can trigger delivery through uterine infection. Effect estimates were even larger for the exposure window of preceding month before delivery. The inhalation of particles may lead to elevated oxidative stress within the maternal body, which leads to inflammation in the lungs and placenta, and there is evidence showing that the preinflammation or inflammation response is involved with the initiation of labor.56
This study has certain strengths. By estimating the change of percentiles of GA at delivery, we could identify different effect estimates for infants at different GA level and see how specific groups of infants were more impacted than others (i.e., preterm/early-term infants compared with full-term infants). Using quantile regression to achieve this is more efficient than simply applying stratified analyses for subgroups because it does not require normality assumption for the outcome of interest and utilizes the total population instead of truncated subgroups of people for stratified analyses. In addition, our analyses included all singleton live newborns of Massachusetts from 2001 to 2015 that met the inclusion criteria, allowing better generalizability and power. Community-level contextual variables used in our study are more representative of the community-level exposure in air pollution epidemiology studies than family socio-economic status. Causal inference methods were applied to obtain marginal population-level causal estimates. We also investigated various potential modifiers of the effect of air pollution on GA at delivery, including ambient temperature, which has rarely been done previously. The PM2.5 predictions were matched to the home addresses at delivery at the geospatial resolution of 1 × 1 km, which is the finest resolution we can get from the US national PM2.5 prediction modeling.
Our study also has a few limitations. Certain outcome measurement error is likely because we employed the LMP method due to a substantial number of missing values on ultrasound-based GA calculation which may be prone to selection bias, availability of gestational age only in weeks, and advanced GA of first visit.57 Exposure misclassification is possible because we used maternal address at delivery. The residential address could be misreported and thus leaded to not being reflective of the actual exposure. However, we expect that the misreporting portion is low and the chance for pregnant women to move is also relatively low. Besides, the exposure metrics we applied did not incorporate pregnant women’s time-space activity or indoor exposure levels. Residual confounding from more paternal factors, maternal BMI, physical activity, housing conditions, stress, and greenness exposure that we do not have well-documented information for the study population and study period cannot be ruled out. Excluding C-sections could lead to potential bias if they are on the causal pathway. However, the heterogeneity of the clinical indications of C-sections which we do not have data for could make this path relevant only to a very small number of women. Another limitation is that we did not have access to a direct indicator for preeclampsia or not. However, by excluding C-section births, most of the preeclampsia cases would be removed from the study population.
We did not further adjust for temperature, other meteorological variables, or greenness in our main analyses. Part of the reason is that we only have access to temperature data in limited years of the study period for the study population at 1 km2 resolution. Thinking from a modeling perspective, the effect of these variables would be modeled at average trimester window as well, which approximates the length of a season, indicating that controlling for season of conception could serve as an adjustment proxy for trimester averaged meteorological factors or greenness that change with season. Moreover, the effect estimates remained robust with or without ambient temperature adjustment in the sensitivity analyses. The current literature evidence for other meteorological variables and greenness is unsupportive of a link with GA.58
Minimal adjustment results did not support the hypothesis that maternal risk factors are strong mediators. If they are important mediators, adjusting for them would block the indirect pathway from (+) PM2.5 exposure to > (+) maternal risk factors to > (−) gestational age. We would only be able to obtain part of the total effect (omitting the relevant indirect pathway described here) and the estimates would be smaller in a fully adjusted model. One merit of controlling for maternal risk factors is that this could actually help block the backdoor pathway via. unmeasured/unadjusted confounders U (including unknown exposures from other sources, etc) if U is believed to be associated with GA mainly via. influencing the maternal risk factors (see eFigure 4; http://links.lww.com/EE/A106). We acknowledge that the list of maternal risk factors is not an exhaustive variable list. However, this is the best we could do regarding the data itself. Although we did not find temperature substantially confounded the PM2.5 effects based on the current IPW quantile regression modeling, future large prospective cohorts studies examining the role of temperature, greenness, or other meteorological factors on preterm birth risk or gestation length are still needed.20
Conclusions
This cohort study on particulate air pollution and newborns’ GA at delivery adds quantitative knowledge to the current understanding of the effect of air pollution on birth. Prenatal exposure to increased PM2.5 levels at late pregnancy reduced GA at delivery among Massachusetts newborns based on casual modeling. Larger decreases were observed among preterm and early-term births, male neonates, and neonates of younger mothers and African American mothers. Exposure to extremely high or low temperature amplifies the effect of air pollution on GA. The current US environmental long-term regulation standard for PM2.5 is not protective against these effects.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
This study was supported by the National Institutes of Health (NIH) grant (R01 ES024332-01A1, ES-000002). This publication was made possible also by US Environmental Protection Agency (EPA) grant (RD-835872-01). Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The funding sources support open access publishing.
Acknowledgments
Author contributions are as follows: X. Qiu, J. D. Schwartz, C. Messerlian, K. C. Fong, L. Shi, S. Papatheodorou involved in study concept and design. X. Qiu involved in drafting of the manuscript and statistical analysis. She had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data are under the regulation of Massachusetts Registry of Vital Records and Statistics. Access to the data should get approval from the Registry first. Request for analytical code can be sent to xqiu@g.harvard.edu. J. D. Schwartz obtained funding and involved in study supervision. A. Kosheleva involved in administrative, technical, or material support. All authors involved in acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content.
Supplementary Material
Footnotes
Published online 14 September 2020
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013; 309:2445–2446 [DOI] [PubMed] [Google Scholar]
- 2.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993; 306:817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016; 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawlor DA, Davey Smith G, Clark H, Leon DA. The associations of birthweight, gestational age and childhood BMI with type 2 diabetes: findings from the Aberdeen Children of the 1950s cohort. Diabetologia. 2006; 49:2614–2617 [DOI] [PubMed] [Google Scholar]
- 5.Figlio DN, Guryan J, Karbownik K, Roth J. Long-term cognitive and health outcomes of school-aged children who were born late-term vs full-term. JAMA Pediatr. 2016; 170:758–764 [DOI] [PubMed] [Google Scholar]
- 6.Boyle EM, Poulsen G, Field DJ, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. 2012; 344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002; 288:728–737 [DOI] [PubMed] [Google Scholar]
- 8.Berry MJ, Foster T, Rowe K, Robertson O, Robson B, Pierse N. Gestational age, health, and educational outcomes in adolescents. Pediatrics. 2018; 142 [DOI] [PubMed] [Google Scholar]
- 9.Yuan L, Zhang Y, Gao Y, Tian Y. Maternal fine particulate matter (PM2.5) exposure and adverse birth outcomes: an updated systematic review based on cohort studies. Environ Sci Pollut Res Int. 2019; 26:13963–13983 [DOI] [PubMed] [Google Scholar]
- 10.Shah PS, Balkhair T; Knowledge Synthesis Group on Determinants of Preterm/LBW births. Air pollution and birth outcomes: a systematic review. Environ Int. 2011; 37:498–516 [DOI] [PubMed] [Google Scholar]
- 11.Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadvand P, Basagaña X, Figueras F, et al. Air pollution and preterm premature rupture of membranes: a spatiotemporal analysis. Am J Epidemiol. 2014; 179:200–207 [DOI] [PubMed] [Google Scholar]
- 13.Huang JV, Leung GM, Schooling CM. The association of air pollution with birthweight and gestational age: evidence from Hong Kong’s ‘Children of 1997’ birth cohort. J Public Health (Oxf). 2017; 39:476–484 [DOI] [PubMed] [Google Scholar]
- 14.Sengupta S, Carrion V, Shelton J, et al. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr. 2013; 167:1053–1059 [DOI] [PubMed] [Google Scholar]
- 15.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006; 114:1636–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakshmanan A, Chiu YH, Coull BA, et al. Associations between prenatal traffic-related air pollution exposure and birth weight: modification by sex and maternal pre-pregnancy body mass index. Environ Res. 2015; 137:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavigne É, Burnett RT, Stieb DM, et al. Fine particulate air pollution and adverse birth outcomes: effect modification by regional nonvolatile oxidative potential. Environ Health Perspect. 2018; 126:077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavigne E, Yasseen AS, 3rd, Stieb DM, et al. Ambient air pollution and adverse birth outcomes: differences by maternal comorbidities. Environ Res. 2016; 148:457–466 [DOI] [PubMed] [Google Scholar]
- 19.Westergaard N, Gehring U, Slama R, Pedersen M. Ambient air pollution and low birth weight - are some women more vulnerable than others? Environ Int. 2017; 104:146–154 [DOI] [PubMed] [Google Scholar]
- 20.Kloog I. Air pollution, ambient temperature, green space and preterm birth. Curr Opin Pediatr. 2019; 31:237–243 [DOI] [PubMed] [Google Scholar]
- 21.Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly american men: the normative aging study. Environ Health Perspect. 2016; 124:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernan MA, Robins JM. Causal Inference. 2010, Boca Raton, FL: CRC [Google Scholar]
- 23.Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int. 2019; 130:104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eunice Kennedy Shriver National Institue of Child Health and Human Development. About Pregnancy. Available at: https://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo#f1
- 25.Kloog I, Nordio F, Coull BA, Schwartz J. Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the Northeastern USA. Remote Sens Environ. 2014; 150:132–139 [Google Scholar]
- 26.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health. 1994; 84:1414–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008; 168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai K, van Dyke DA. Causal inference with general treatment regimes: generalizing the propensity score. J Am Stat Assoc. 2004; 99:854–866 [Google Scholar]
- 29.Mittendorf R, Williams MA, Berkey CS, Lieberman E, Monson RR. Predictors of human gestational length. Am J Obstet Gynecol. 1993; 168:480–484 [DOI] [PubMed] [Google Scholar]
- 30.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009; 20:3–5 [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. Assessing covariate balance when using the generalized propensity score with quantitative or continuous exposures. Stat Methods Med Res. 2019; 28:1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YY, Li Q, Guo Y, et al. Association of long-term exposure to airborne particulate matter of 1 μm or less with preterm birth in China. JAMA Pediatr. 2018; 172:e174872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Benmarhnia T, Zhang H, et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ Int. 2018; 121pt 1317–324 [DOI] [PubMed] [Google Scholar]
- 34.Qian Z, Liang S, Yang S, et al. Ambient air pollution and preterm birth: a prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health. 2016; 219:195–203 [DOI] [PubMed] [Google Scholar]
- 35.Laurent O, Hu J, Li L, et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001-2008. Environ Health Perspect. 2016; 124:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao H, Chang HH, Holmes HA, et al. Air pollution and preterm birth in the U.S. State of Georgia (2002-2006): associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect. 2016; 124:875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Sun J, Liu Y, et al. Different exposure levels of fine particulate matter and preterm birth: a meta-analysis based on cohort studies. Environ Sci Pollut Res Int. 2017; 24:17976–17984 [DOI] [PubMed] [Google Scholar]
- 38.Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res. 2018; 167:144–159 [DOI] [PubMed] [Google Scholar]
- 39.Stieb DM, Chen L, Beckerman BS, et al. Associations of pregnancy outcomes and PM2.5 in a National Canadian Study. Environ Health Perspect. 2016; 124:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingsley SL, Eliot MN, Glazer K, et al. Maternal ambient air pollution, preterm birth and markers of fetal growth in Rhode Island: results of a hospital-based linkage study. J Epidemiol Community Health. 2017; 71:1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson S, Bobb JF, Ito K, et al. Ambient fine particulate matter, nitrogen dioxide, and preterm birth in New York City. Environ Health Perspect. 2016; 124:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giorgis-Allemand L, Pedersen M, Bernard C, et al. The influence of meteorological factors and atmospheric pollutants on the risk of preterm birth. Am J Epidemiol. 2017; 185:247–258 [DOI] [PubMed] [Google Scholar]
- 43.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM2 5 exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012; 11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009; 117:1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta. 2013; 34:95–99 [DOI] [PubMed] [Google Scholar]
- 46.Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC. The Alabama Preterm Birth Study: intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am J Obstet Gynecol. 2006; 195:1533–1537 [DOI] [PubMed] [Google Scholar]
- 47.Perni SC, Vardhana S, Kalish R, Chasen S, Witkin SS. Clara cell protein 16 concentration in mid-trimester amniotic fluid: association with fetal gender, fetal G>A +38 CC16 gene polymorphism and pregnancy outcome. J Reprod Immunol. 2005; 68:85–90 [DOI] [PubMed] [Google Scholar]
- 48.Fichorova RN, Onderdonk AB, Yamamoto H, et al. ; Extremely Low Gestation Age Newborns (ELGAN) Study Investigators. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. mBio. 2011; 2:e00280–e00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Browning M, Rigolon A. Do income, race and ethnicity, and sprawl influence the greenspace-human health link in city-level analyses? Findings from 496 cities in the United States. Int J Environ Res Public Health. 2018; 15:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities: modification by temperature and city characteristics. Epidemiology. 2016; 27:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Liu X, Dong M, et al. Associations of maternal ambient temperature exposures during pregnancy with the placental weight, volume and PFR: a birth cohort study in Guangzhou, China. Environ Int. 2020; 139:105682. [DOI] [PubMed] [Google Scholar]
- 52.Lian S, Guo J, Wang L, et al. Impact of prenatal cold stress on placental physiology, inflammatory response, and apoptosis in rats. Oncotarget. 2017; 8:115304–115314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu R, Pearson D, Ebisu K, Malig B. Association between PM2.5 and PM2.5 constituents and preterm delivery in California, 2000-2006. Paediatr Perinat Epidemiol. 2017; 31:424–434 [DOI] [PubMed] [Google Scholar]
- 54.Huynh M, Woodruff TJ, Parker JD, Schoendorf KC. Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006; 20:454–461 [DOI] [PubMed] [Google Scholar]
- 55.Nobles CJ, Grantz KL, Liu D, et al. Ambient air pollution and fetal growth restriction: physician diagnosis of fetal growth restriction versus population-based small-for-gestational age. Sci Total Environ. 2019; 650pt 22641–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003; 111:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008; 22:587–596 [DOI] [PubMed] [Google Scholar]
- 58.Banay RF, Bezold CP, James P, Hart JE, Laden F. Residential greenness: current perspectives on its impact on maternal health and pregnancy outcomes. Int J Womens Health. 2017; 9:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


