Abstract
As human life expectancy keeps increasing, ageing populations present a growing challenge for clinical practices. Human ageing is associated with molecular, structural, and functional changes in a variety of organ systems, including the kidney. During the ageing process, the kidney experiences progressive functional decline as well as macroscopic and microscopic histological alterations, which are accentuated by systemic comorbidities like hypertension and diabetes mellitus, or by preexisting or underlying kidney diseases. Although ageing per se does not cause kidney injury, physiologic changes associated with normal ageing processes are likely to impair the reparative capacity of the kidney and thus predispose older people to acute kidney disease, chronic kidney disease and other renal diseases. Mechanistically, cell senescence plays a key role in renal ageing, involving a number of cellular signaling mechanisms, many of which may be harnessed as interventional targets for slowing or even reversing kidney ageing. This review summarizes the clinical characteristics of renal ageing, highlights the latest progresses in deciphering the role of cell senescence in renal ageing, and envisages potential interventional strategies and novel therapeutic targets for preventing or improving renal ageing in the hope of maintaining long-term kidney health and function across the life course.
Keywords: Senescence, Nephrosclerosis, Kidney diseases, Kidney transplantation, Glomeruli, Renal tubules
1. Introduction
Ageing is defined as a progressive loss of functional reserve with a significant decline in adaptive homeostasis capacity to external or internal stress, resulting in a rise in the risk of disease and death (Grimley Evans, 2000). Due to improved living conditions, socioeconomic status and health care, human life expectancy has increased dramatically while mortality has significantly reduced in the past several decades. As a result, rapid ageing of the world population is a major global demographic trend. The U.S. Census Bureau and the National Center for Health Statistics predict that 80.8 million Americans will be 65 years old or older by 2040, representing approximately 21.6 % of the population. Among them, the number of people 85 years and older is projected to be 14.4 million in 2040, a 123 % increase from 6.5 million in 2017 (Administration for Community Living, 2019). In Europe, the population aged 65 and over will account for 28 % of Europeans by 2060 (Martin et al., 2014). Likewise, in China, the world’s most populous country, people aged 65 and above will increase to 400 million by 2050, accounting for 26.9 % of the population, and 150 million will be 80 years old or older (Fang et al., 2015). Human ageing is associated with molecular, structural, and functional changes in a variety of organ systems, including the kidney. During the ageing process, the kidney experiences progressive functional decline as well as macroscopic and microscopic histologic alterations. Age-related renal impairment has become an imminent challenge to clinical practice. To improve age-related renal impairment, it is essential to decipher the pathobiology of kidney ageing.
2. Basic changes in the ageing kidney
The kidneys are the key organs responsible for removing metabolic waste products and extra fluid from the body. The kidneys receive approximately 20–25 % of the cardiac output, filter about 200 L of blood every day and generate roughly 1.5 L of waste-containing urine. Thus, under physiologic conditions, the kidneys are highly metabolic organs, which withstand considerable oxidative stress and are susceptible to the ageing process. In actuality, the kidneys are among the organs with the most prominent changes during the normal ageing process (Long et al., 2005).
2.1. Macroscopic and microscopic structural changes
On the macroscopic scale, renal ageing manifests as roughness of the kidney surface as well as elevated formation and size of simple renal cysts (Hommos et al., 2017). Simple kidney cysts can form in one or both kidneys but do not result in enlargement of the kidneys. Their formation is seemingly associated with ageing because they are common among people greater than 40 years old. Simple cysts are usually harmless and have long been considered of little clinical significance. However studies have demonstrated that simple renal cysts correlated with hypertension (Chin et al., 2006), decreased renal size and functional changes(Al-Said et al., 2004; Al-Said and O’Neill, 2003), and may be an early sign of potential damage (Grantham, 2012). Kidney volume is an important indicator of renal impairment. Two early studies used ultrasound or computed tomography scan in hundreds of adult volunteers and patients with no kidney disease and demonstrated that kidney volume progressively declined with age (Emamian et al., 1993; Gourtsoyiannis et al., 1990). It was estimated that the parenchymal thickness of a kidney decreased 10 % per older decade of age regardless of gender. Recent studies with larger sample sizes replicated the above findings. Roseman et al.(Roseman et al., 2017) assessed kidney volume by magnetic resonance imaging in 1852 adults and found that the kidney volume declines by about 16 cm3 per decade after 60 years of age. In addition, Wang et al.(Wang et al., 2014) evaluated 1344 potential kidney donors by contrast-enhanced CT imaging and demonstrated that kidney volume declines at 22 cm3 per decade after the age of 50. Interestingly, Wang and colleagues also found that renal cortical volume progressively declines with age whereas medullary volume increased until 50 years of age, resulting in a net decline in total kidney volume after 50 years of age in normal individuals. Collectively, there is roughly a 20~25 % difference in kidney mass between the ages of 30 and 80 years of age (McLachlan and Wasserman, 1981).
These macrostructural alterations are associated with underlying histologic changes on the microscopic level including nephrosclerosis, glomerular basement membrane thickening (Nyengaard and Bendtsen, 1992), mesangial broadening, and increased accumulation of extracellular matrix in ageing kidneys (Silva, 2005b). Nephrosclerosis (Fig. 1) is the pathologic hallmark of ageing kidneys, characterized by nephron loss, hypertrophy of remaining nephrons, and arteriosclerosis, which is thought to be the initiating factor for ageing-related renal changes (Silva, 2005b; Takazakura et al., 1972; Zhou et al., 2008). Other features of nephrosclerosis include global glomerulosclerosis, tubular atrophy, and interstitial fibrosis, which are reminiscent of the changes observed in progressive chronic kidney disease (Sethi et al., 2017). After 30 years of age, approximately 6,000–6,500 nephrons will be lost every year due to nephrosclerosis, or more specifically glomerulosclerosis (Denic et al., 2016, 2017). The upper limit of glomerular sclerosis in normal renal ageing was estimated to exceed 10 % according to cadaver studies (Chan et al., 1990). However, the estimation of nephron loss in renal ageing may have been considerably underappreciated if it is solely based on the extent of glomerulosclerosis found in kidney biopsies (Keller et al., 2003). In support of this contention, a recent study of 1638 healthy kidney donors showed that the number of nephrons was reduced by 48 % in 70–75 year-olds as compared with 18–29 year-olds, whereas the number of globally sclerotic glomeruli increased only 15 % (Denic et al., 2017).
Fig. 1.
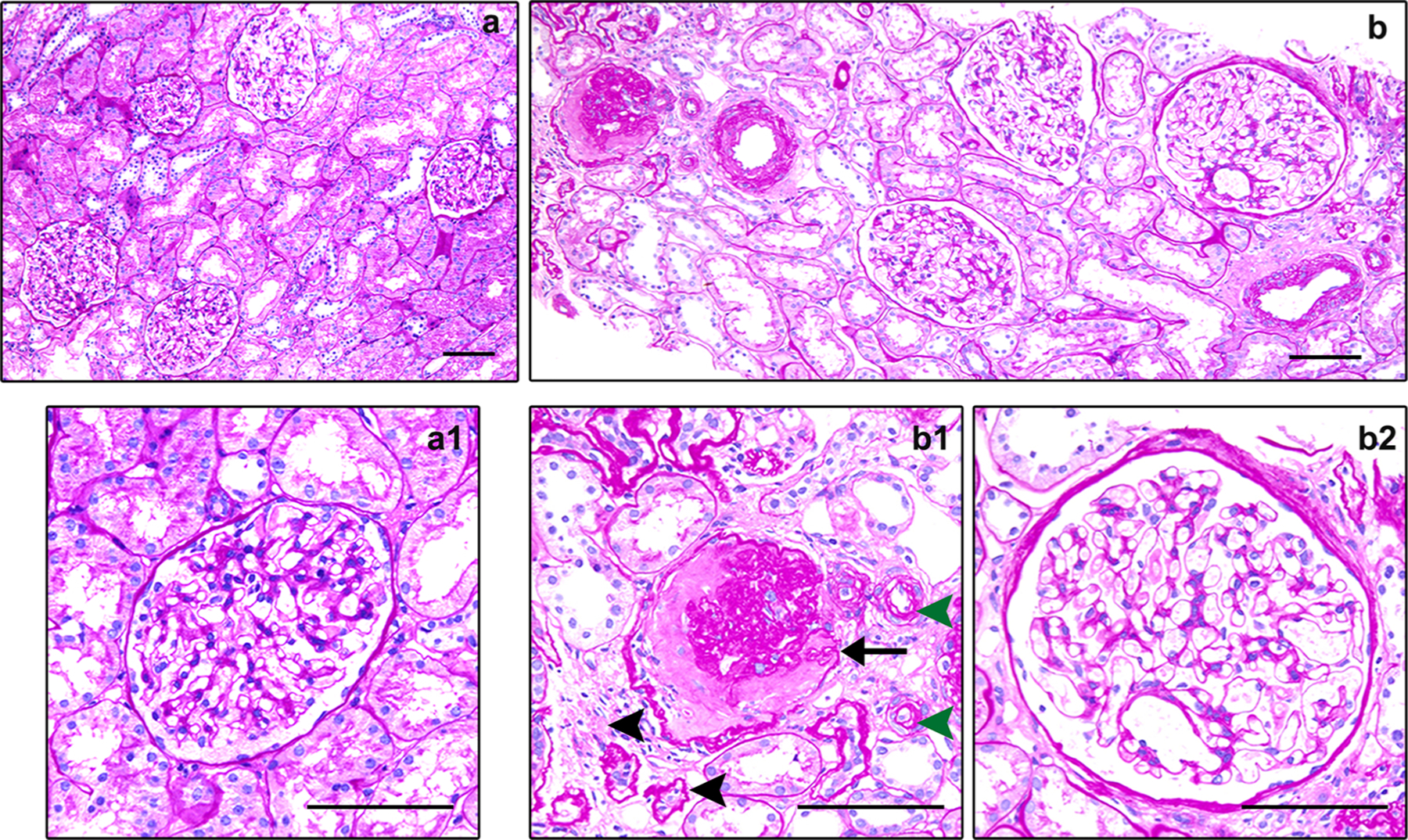
Histological characteristics of the ageing kidney. (a) Representative light micrographs showing normal histological characteristics in the healthy young kidney (periodic acid-Schiff staining, ×100). (a1) The enlarged view showing a normal glomerulus. Scale bar, 100 μm. (b) Representative light micrographs showing histological characteristics in the ageing kidney, including ischemic global glomerulosclerosis, focal tubular atrophy and interstitial fibrosis, and arteriolar hyalinosis (periodic acid-Schiff staining, ×100). (b1) Representative light micrograph highlighting nephrosclerosis in the ageing kidney. The arrow indicates global glomerulosclerosis, black arrowheads indicate focal tubular atrophy and interstitial fibrosis, and green arrowheads indicate arteriolar hyalinosis. (b2) The enlarged view showing a moderately hypertrophic glomerulus. Scale bar, 100 μm.
2.2. Clinical and functional changes
Age-related structural changes to the kidney are concomitant with a decline in kidney function, which is primarily assessed by total glomerular filtration rate (GFR). The exact rate of renal function decline associated with ageing has been extensively studied (Epstein, 1996; Silva, 2005a, b). Bolignano et al. examined three cohorts and nine cross-sectional studies on age-related decline in renal function carried out from 1950 to 2012 and showed that the annual mean reduction in GFR ranges from 0.4 to 2.6 mL/min /year (Bolignano et al., 2014). After 35 years of age, the GFR falls by about 5–10 % per decade, as reported by Glassock and Rule (Glassock and Rule, 2012, 2016). Approximately half of the people aged 70 and above had an estimated GFR (eGFR) <60 mL/min/1.73 m2, as shown by a study involving 610 older community residents (Schaeffner et al., 2012). Theoretically, age-related decline of GFR is attributable to nephron loss, which mimics the pathobiology of a remnant kidney and increases the risk for kidney diseases (Denic et al., 2017). However, the decline in GFR is disproportionate to and much less than the loss of nephrons. This may plausibly be explained by hypertrophy and functional compensation of remnant nephrons (Schmitt and Melk, 2017), but is also likely due to sampling bias/error of kidney biopsies. As a matter of fact, it has been highly controversial whether age-related decline of kidney function is actually associated with nephron loss (Glassock et al., 2015). Hayman et al. suggested that it is hard to measure the loss of nephrons merely from biopsy sections because obsolete glomeruli could disappear without leaving negligible traces (Hayman et al., 1939). In agreement, Denic A et al. found that glomeruli may undergo sclerosis and atrophy in the process of kidney ageing and disappear most likely via tissue resorption (Denic et al., 2017). Aside from glomerular filtration function, another critical function of the kidney is exerted by renal tubules and includes urine concentrating capacity, sodium reabsorption, and potassium excretion (Michelis, 1990; Mimran et al., 1992; Sands, 2012). Though it has been less studied, there is evidence suggesting that renal tubular function also progressively declines with ageing.
Age-related decline of renal function has many implications. Due to a gradually impaired renal functional reserve, kidney ageing undoubtedly leads to increased susceptibility to acute kidney injury (AKI) (James et al., 2010) and chronic kidney disease (CKD) (Nitta et al., 2013). Indeed, Americans aged 65 and above are at a heightened risk of end-stage renal disease, and drug-related nephrotoxicity (Nitta et al., 2013). This may affects the healthcare providers’ choice of medications when treating diseases, and is a major determinant of the outcome of both recipients and donors in kidney transplantation (Denic et al., 2016).
3. Cell senescence in renal ageing
Despite the progress made to increase our understanding of the pathophysiology of kidney ageing, the cellular and molecular mechanisms responsible for age-related processes in the kidney, such as nephron loss and extracellular matrix accumulation, remain largely elusive. A growing body of evidence suggests that renal cellular senescence may play a critical role in mediating kidney ageing and age-related diseases (Yang and Fogo, 2010). In addition, since human lifespan is a complex trait, the ageing processes of diverse organ systems including the kidney are also likely multifactorial and involve the intricate orchestration of genetic, environmental, and socioeconomic effects that regulate cellular senescence (Zhou et al., 2008).
3.1. Features of cell senescence
Cellular senescence is defined as irreversible cell cycle arrest in response to different types of cellular stresses, resulting in phenotypic changes of the cells featured by the inter-dependent triad characteristics of senescence, including arrested cell growth, resistance to apoptosis, and senescence-associated secretory phenotype (SASP), in addition to macromolecular damage and altered metabolism (Campisi and d’Adda di Fagagna, 2007; Gorgoulis et al., 2019). Cellular senescence is a central causative process of ageing and may lead to exhaustion of reparative potentials in the cell (O’Sullivan et al., 2017). Some unifying hallmarks have been commonly employed and allow the evaluation of cell senescence and age-related changes in diverse organ systems, including the kidney (Lopez-Otin et al., 2013; Sturmlechner et al., 2017). Among these, the acidic senescence-associated β-galactosidase (SA‑β‑gal) activity detectable at pH 6.0 reflects an increase in lysosomal mass and is the most reliable and commonly used biomarker of senescent cells (Dimri et al., 1995; Kurz et al., 2000). In addition, the expression levels of cyclin-dependent kinase (CDK) inhibitors, including p16INK4a, p21CIP1, p19ARF (in mouse), p14ARF (in human), p27KIP1 and p15INK4b, significantly increase, while proliferation markers like Ki67 is absent in senescent cells, which may be harnessed as parameters that reflect the magnitude of senescence (Krishnamurthy et al., 2004; Sharpless and Sherr, 2015; Wiley et al., 2017). In addition, senescent cells seem to be more resistant to apoptosis and have the potential to influence neighboring cells through secreted soluble factors, which are collectively known as the SASP (Hernandez-Segura et al., 2017). However, all these indicators are nonspecific and not unique for senescence. Hence, combined biomarkers are recommended to validate the presence of senescence (Matjusaitis et al., 2016). In various kidney diseases, SA-β-gal and p16INK4a appear to be detectable prior to the development of morphologic lesions, entailing that cell senescence is involved in the pathogenesis of renal diseases (Li and Wang, 2018).
3.2. Signaling pathways of cell senescence in renal ageing
A number of cellular signaling pathways have been implicated in cell senescence and renal ageing (Sturmlechner et al., 2017), mainly including the p53/p21 and p16/Rb pathways (Fig. 2). In the initial response to DNA damages triggered by various stresses, DNA damage response signaling cascades, such as ATM, ARF, or the p53 network, are activated and increase p21CIP1 expression and/or induce the expression of p16INK4a (el-Deiry et al., 1993; Harper et al., 1993; Rayess et al., 2012). In turn, activated p21CIP1 and p16INK4a suppress the phosphorylation of CDK complexes and retinoblastoma protein (Rb) (Serrano et al., 1993). Ultimately, cell proliferation is halted by Rb via inhibiting the activity of E2F, resulting in renal cell senescence and kidney ageing, which predispose the kidney to diverse injuries and impair the reparative capacity (Fig. 2) (Yang and Fogo, 2010). Klotho is an anti-ageing single-pass transmembrane protein related to longevity and expressed mainly in the kidney (Lee et al., 2007). Klotho regulates the p53/p21 signaling pathway and is a major modulator of cellular senescence (Sopjani et al., 2015). Additionally, Klotho provides control over the sensitivity of many other signaling pathways, such as FGF23, trans-forming growth factor-β (TGF-β), cAMP, PKC, Wnt, and insulin/IGF-1 signaling (Kurosu et al., 2005; Sopjani et al., 2015). Another important regulator of cellular senescence is SIRT1. Podocyte-specific knockdown of SIRT1 accelerated age-related glomerulosclerosis and podocyte loss in mice kidneys (Chuang et al., 2017). In contrast, a number of signaling molecules have been identified to exacerbate renal cell senescence and renal ageing. For instance, Wnt9a/β-catenin signaling seems to promote renal tubular senescence and renal fibrosis in diseased kidneys as evidenced by the upregulated expression of p16INK4a, p53, and p21, and increased SA‑β‑gal activity in renal tubules (Luo et al., 2018). More recently, converging evidence suggests that glycogen synthase kinase 3 (GSK3) plays a key role in cell senescence and ageing. GSK3 is a highly conserved, ubiquitously expressed serine/threonine protein kinase that was originally characterized to be a key transducer of the insulin signaling cascade and governs glycogenesis. Interest in GSK3 increased greatly with the realization that it also acts as a convergence point for multiple cell signaling pathways involved in inflammation, immunomodulation, embryogenesis, tissue injury, repair, and regeneration (Jope and Johnson, 2004). In C. elegans (McColl et al., 2008; Zarse et al., 2011) and drosophila (Castillo-Quan et al., 2016), inhibition of GSK3 by lithium has been demonstrated to drastically increase lifespan. In addition, large-scale population-based epidemiological studies revealed that lithium levels in drinking water significantly correlate with longevity (Zarse et al., 2011), suggesting that GSK3 is likely a pro-ageing factor. In the kidney, particularly in glomeruli, the β isoform of GSK3 seems to be predominantly expressed (Zhou et al., 2016). The role of GSK3β in renal cell senescence and kidney ageing has been barely studied and warrants further research in future studies.
Fig. 2.
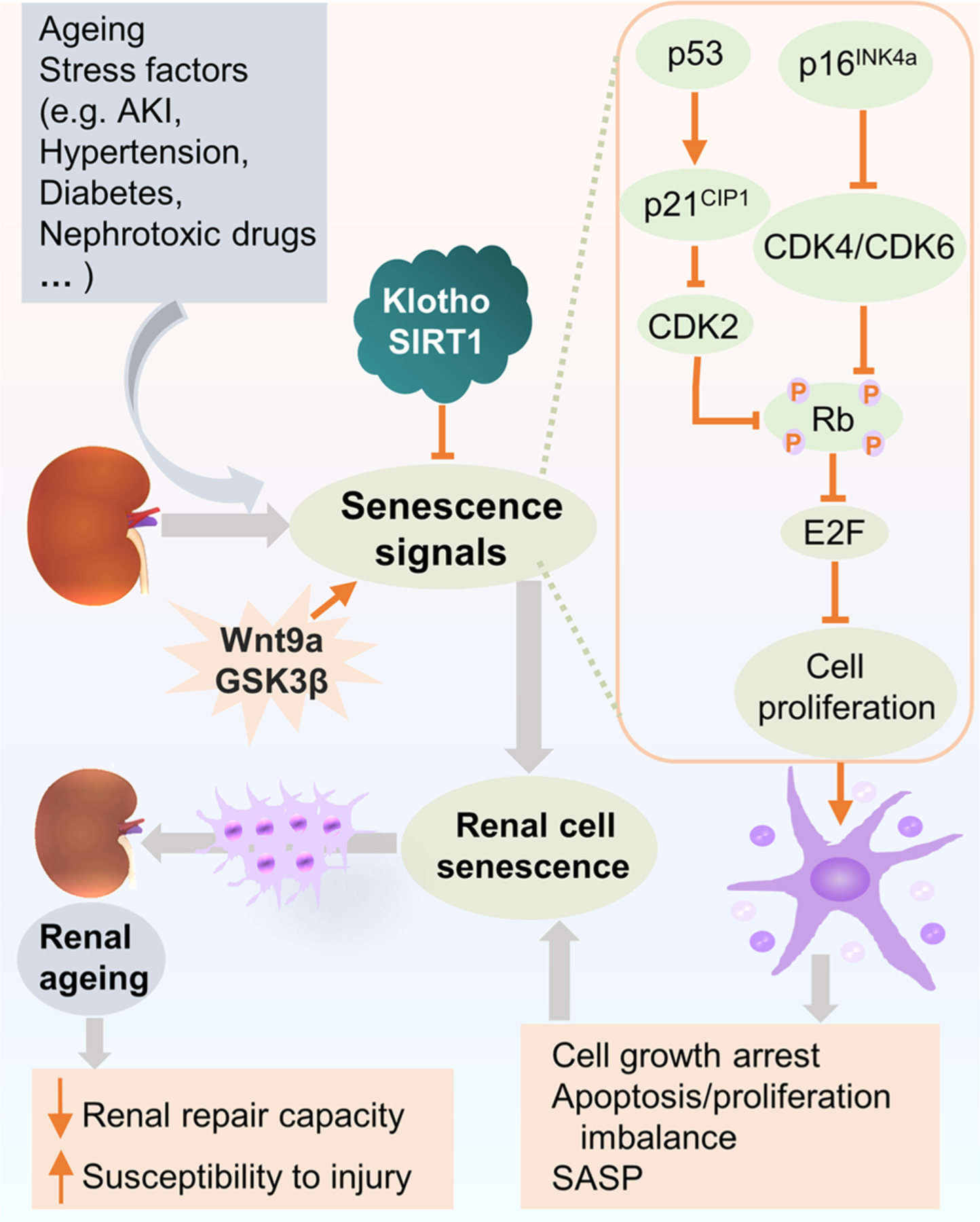
Cellular signaling pathways involved in cell senescence in renal ageing. In renal ageing, cell senescence signaling pathways are activated not only by stress factors or diseases, such as AKI, hypertension, diabetes, and cytotoxic drugs, etc, but also by ageing per se. The key signaling cascades implicated in renal cell senescence are p53/p21CIP1 and p16INK4a/Rb pathways, which in turn inhibit CDK complexes and Rb phosphorylation. Ultimately, these signals execute cell senescence via Rb suppression of the activity of E2F, characterized by cell proliferation arrest, apoptosis/proliferation imbalance and secretion of SASP factors. Hence, the repair capacity declines and the ageing kidney becomes more susceptible to injury. Klotho and SIRT1 have been shown as main modulators of cell senescence and inhibit cell senescence through regulating p53/p21CIP1 pathway. Whereas, Wnt9a accelerates renal fibrosis via promoting cell senescence signaling pathways, so does GSK3β. Abbreviations: AKI, acute kidney disease; CDK, cyclin-dependent kinase; GSK3β, glycogen synthase kinase 3β; Rb, retinoblastoma protein; SASP, senescence-associated secretory phenotype.
3.3. Different types of cell senescence
Cell senescence was first observed by Hayflick and Moorhead in human diploid cells (Hayflick and Moorhead, 1961), and later termed replicative senescence, characterized by low response in proliferation and ultimately cell-cycle arrest due to telomere attrition (Bodnar et al., 1998) (Fig. 3). In addition, cell senescence could also be triggered by stress scenarios such as oxidative stress (von Zglinicki, 2002), DNA damage (Sedelnikova et al., 2004), mitochondrial dysfunction (Wiley et al., 2016), epigenetic stress (Petrova et al., 2016), SASP elicited by primary senescent cells (Acosta et al., 2013), or diseases such as hypertension and diabetes (Westhoff et al., 2008), resulting in the activation of the p16/Rb pathway or ARF/p53 pathway and leading to stress-induced premature senescence (Fig. 3) (van Deursen, 2014). As a part of the homeostatic biological processes, acute senescence (Fig. 3) is a programmed event transiently activated in response to discrete stressors, and exerts beneficial effects on renal regenerative capacity after injury (Wen et al., 2015), limiting renal fibrosis (Wolstein et al., 2010), improving immune surveillance (Sturmlechner et al., 2017), and wound healing (Jun and Lau, 2010). In contrast, chronic senescence (Fig. 3) is induced through a prolonged period of cellular stress or slow macromolecular damage, and sustained accumulation of senescent cells during chronic senescence is detrimental to natural renal ageing and may cause age-related kidney diseases (Baker et al., 2016). Moreover, evidence suggests that chronic cellular senescence contributes to reduce renal function, drive age-associated glomerulosclerosis (Baker et al., 2016), impair regenerative capacity of kidneys (Sturmlechner et al., 2017), and promote renal allograft rejection (Schmitt et al., 2015). Collectively, cell senescent may play a role not only in renal ageing but also in the pathogenesis of kidney diseases.
Fig. 3.

Categorization of cell senescence. Two main types of cell senescence have been identified according to the different causes, i.e. replicative senescence, and stress-induced premature senescence (SIPS). Replicative senescence is caused by telomere attrition and characterized by low response in proliferation and ultimately cell-cycle arrest. SIPS is induced by various stressors, such as oxidative stress, DNA damage, mitochondrial dysfunction, epigenetic stress, and senescence-associated secretory phenotype (SASP) generated by primary senescent cells. There are also two classes of senescent cells in the process of senescence, i.e. acute and chronic senescent cells, which play different roles in kidney ageing. Acute senescence acts a beneficial role in renal regeneration after injury, renal fibrosis, immune surveillance, and wound healing, where the senescent cells transiently present and eventual eliminated by immune cells through immune surveillance process. In contrast, chronic senescence is elicited due to abnormal accumulation of senescent cells, inefficient clearance, or prolonged senescent signaling. It exerts deleterious effects in natural kidney ageing and age-related kidney diseases.
3.4. Senescence in renal parenchymal cells
As alluded to above, the kidney is constantly processing circulating blood and thereby is subjected to immense physiologic, metabolic, and hemodynamic stress. Renal parenchymal cells are challenged by both replicative senescence and stress-induced senescence (Sturmlechner et al., 2017).
3.4.1. Glomerular cell senescence
Glomerular podocytes are a critical structural constituent of the glomerular filtration barrier (GFB) and determine the glomerular permselectivity. They are terminally differentiated neuron-like cells with limited potential for cell division and regeneration (Asanuma and Mundel, 2003; Pabst and Sterzel, 1983). Injury and loss of podocytes directly cause damage of the GFB and result in proteinuria and kidney diseases (Hara et al., 2001; Vogelmann et al., 2003; Ziyadeh and Wolf, 2008) featured by progressive glomerulosclerosis (Kim et al., 2001; Wharram et al., 2005). Podocytes also play a crucial role in age-related glomerular changes such as global glomerulosclerosis during renal ageing (Floege et al., 1997). In support of this notion, an analysis of normal human kidney specimens demonstrated that the podocyte nuclear density was more than 300 per 106 μm3 at less than 20 years of age compared with less than 100 per 106 μm3 at 70~80 years of age, corresponding to a rate of decline in podocyte density to be approximately 0.9 % per year (Hodgin et al., 2015) possibly due to either reduced podocyte number per glomerulus or enlarged glomerular volume (Wiggins et al., 2005). As the major cause of age-related podocyte loss, podocyte senescence (Fig. 4) may manifest in the ageing kidney as hypertrophy, binucleate, detachment, cytoplasmic resorption droplets, and foot processes effacement, in parallel with increased expression of SASP (Ortmann et al., 2004; Verzola et al., 2008), ultimately leading to podocytes depletion and age-related global glomerulosclerosis (Wiggins, 2012). Recent evidence indicates that podocytes may be regenerated from other sources, such as parietal epithelial cells and bone marrow cells (Ronconi et al., 2009). Nevertheless, during renal ageing under normal conditions, podocyte proliferation and regeneration are likely negligible (Wanner et al., 2014). Aside from podocytes, glomeruli are composed of glomerular capillary endothelial cells and mesangial cells. With ageing, endothelial and mesangial cell numbers proportionally increases leading to mesangial matrix expansion along with the enlargement of glomeruli (Wiggins, 2012). However, the role of endothelial or mesangial cell senescence in kidney ageing has not been fully investigated.
Fig. 4.
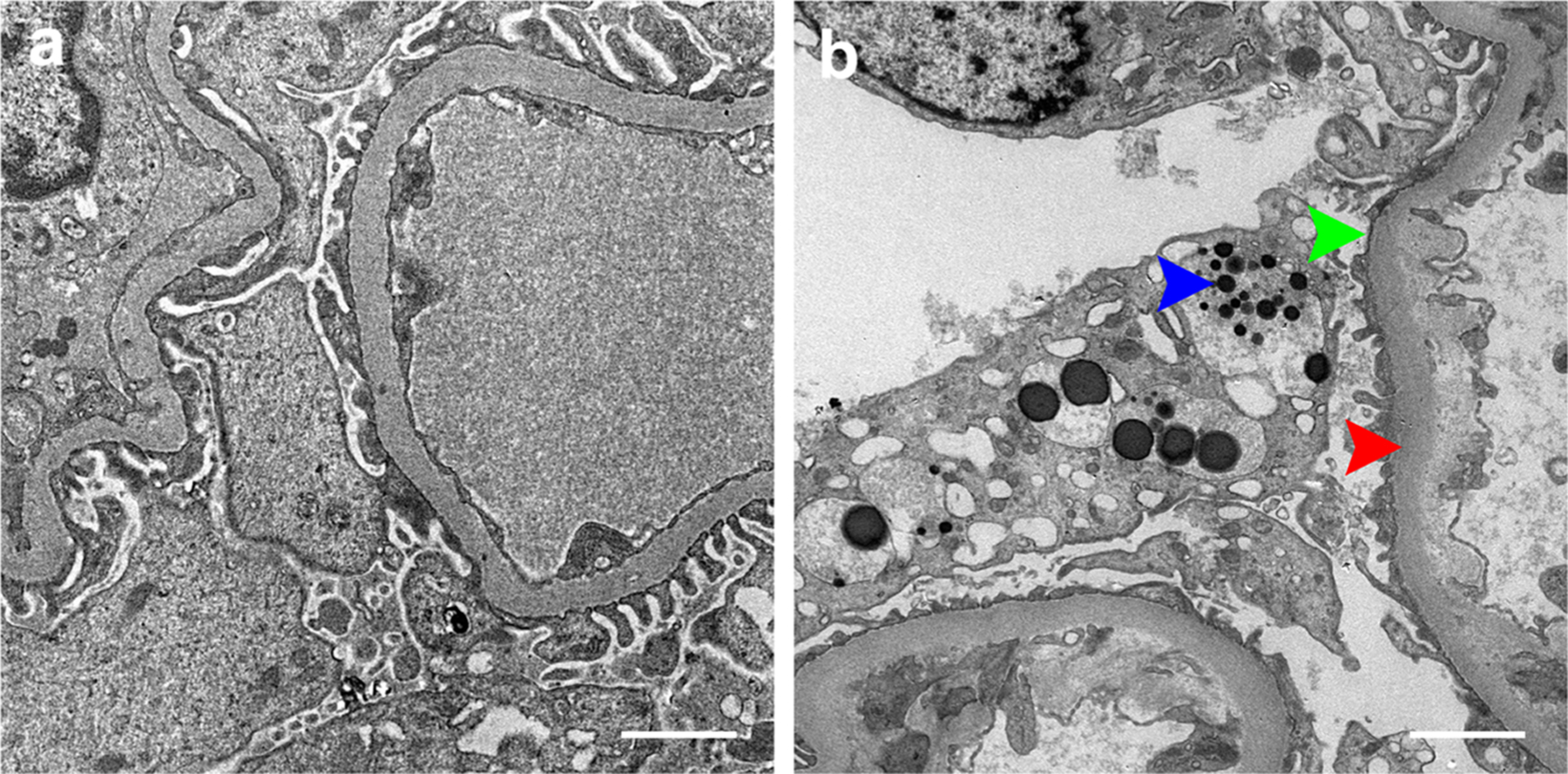
Changes of glomerular podocytes in kidney ageing. Transmission electron micrographs show (a) the typical morphology of normal glomerular podocytes in the healthy young kidney. Scale bar, 2 μm. (b) ultrastructural changes of glomerular podocytes in the ageing kidney, characterized by variable foot processes effacement (green arrowhead), podocyte detachment, cytoplasmatic absorption droplets (blue arrowhead), concomitant with thickening of glomerular basement membrane (red arrowhead). Scale bar, 2 μm.
3.4.2. Tubular cell senescence
Renal tubules account for over 90 % of renal mass and are mostly affected by kidney ageing. Indeed, hallmarks of cellular senescence, such as nuclear expression of p16INK4a, were found to be more pronounced in renal tubular cells than in other renal cell types during ageing (Melk et al., 2004). Experimental models demonstrated that a variety of injuries could induce cellular senescence and subsequent fibrosis in renal tubular epithelial cells (Jin et al., 2019). In the ageing kidney, the regenerative capability of renal tubular cells after acute insults significantly declines, concomitant with increased cell senescence and augmented SASP, which may impair renal tubular cell repopulation and promote fibrotic maladaptive renal repair, ultimately leading to an exacerbated AKI to CKD transition and accelerated kidney ageing (Li and Wang, 2018; Luo et al., 2018).
4. Renal ageing and kidney diseases
Although ageing per se does not cause kidney disease, ageing-related structural and functional changes in the kidney may predispose people to kidney diseases (Schmitt and Melk, 2017). As a matter of fact, renal ageing is an independent risk factor for various diseases of the kidney and other organ systems (Sturmlechner et al., 2017).
4.1. Acute kidney injury
Advanced age is a primary risk factor for AKI. Increased susceptibility to AKI, especially nephrotoxic AKI or ischemia reperfusion injury, as well as diminished potential of recovery after AKI in older people are related to the decline of nephron numbers and GFR in aged kidneys (O’Sullivan et al., 2017; Sturmlechner et al., 2017). A study of Medicare patients in the United States showed that the prevalence of AKI for different age groups increased progressively from 26.8 (66–69 years of age) to 37.4 (70–74 years of age), 55.4 (75–79 years of age), 77.1 (80–84 years of age), and 110.5 (≥85 years of age) per 1000 patient-years (Saran et al., 2018). Another study showed that dialysis-requiring AKI was more common in the elderly patients, indicating that AKI in the ageing population is likely more severe (Hsu et al., 2013).
4.2. Chronic kidney disease
CKD is diagnosed and staged according to levels of GFR and albuminuria based on the Kidney Disease Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines. About half of people older than 70 years of age have an eGFR less than 60 mL/min/1.73 m2 and thus meet the diagnostic criteria of CKD (Rule, 2018). Moreover, the incidence of CKD in the elderly is 3–13 times higher than that in younger individuals (Minutolo et al., 2015). A large study involving 47,204 Chinese adults showed that the rate of CKD for 18–39 year-old females was 7.4 %, increasing to 18.0 % and 24.2 % for 60–69 and >70 year-old females, respectively (Zhang et al., 2012). However, because the changes of the ageing kidney are similar to CKD pathology, the high incidence of CKD in the elderly population has been a topic of debate. Also, there is controversy over whether age-related decline in GFR represents kidney disease or merely functional decline of an older kidney (Minutolo et al., 2015). Regardless, the amount of elderly people with CKD will continuously increase along with the progressively ageing population (Tonelli and Riella, 2014).
4.3. Diabetic nephropathy
The major changes in the kidney caused by diabetes or hypertension, such as glomerulosclerosis and arteriolosclerosis, are likely to be promoted by renal ageing (Martin and Sheaff, 2007). Renal tubular cells and glomerular cells positive for p16INK4a and SA‑β-gal have been found in patients with type 2 diabetic nephropathy (DN) (Verzola et al., 2008) and in a mouse model of streptozotocin-induced diabetes (Kitada et al., 2014). Furthermore, in mice with streptozotocin-elicited diabetes, genetic knockout (KO) of p21 prevented the development of proteinuria and glomerular hypertrophy, despite the increase in TGF-β1 expression levels, providing evidence that the cyclin kinase inhibitor p21 may be required for diabetic glomerular hypertrophy induced by TGF-β1 (Al-Douahji et al., 1999). These findings suggest that cell senescence is involved in the pathogenesis of DN and hyperglycemia accelerates senescence in DN.
4.4. Polycystic kidney disease
Renal cell senescence is a fundamental cause of age- or disease-related parenchymal glomerular or renal tubular cell dropout or loss, ensued by fibrotic maladaptive repair and decline in kidney function. However, cell senescence does not always play a bad role in all kidney diseases. For instance, the homeostatic cell senescence is repressed in autosomal dominant polycystic kidney disease (ADPKD), which is characterized by an uncontrolled renal tubular epithelial cell proliferation with fluid secretion. Indeed, in kidneys from ADPKD patients and in animal models of PKD, p21 expression levels were significantly reduced (Park et al., 2007). In contrast, in a murine model of PKD, the CDK inhibitor roscovitine was able to promote cell senescence, marked by increased p21 expression and enhanced SA-β-gal staining, resulting in a mitigated progression of PKD (Park et al., 2009).
4.5. Other glomerular disease
It is well known that the ageing kidney is susceptible for certain types of kidney diseases but protected against others. For instance, older people have significantly higher incidence rates of membranous nephropathy and crescentic glomerulonephritis (Silva, 2005a). The underlying mechanism is largely unknown, but there is evidence demonstrating the amplified expression of p16INK4a in kidney specimens procured from patients with glomerular diseases suggesting that cell senescence contributes to glomerular injury (Sis et al., 2007). In contrast, the prevalence rates decease sharply with age in minimal change disease and lupus nephritis (Silva, 2005a), which involve systemic immune dysregulation, implying that immunosenescence might protect against these disease. In addition, although the incidence of IgA nephropathy is independent of age (Tumlin et al., 2007), cell senescence in renal tubular cells in kidney biopsy specimens, marked by the expression of p21 and p16INK4a, increased significantly in older patients with IgA nephropathy, suggesting that cell senescence is associated with IgA nephropathy progression (Liu et al., 2012).
5. Ageing and kidney transplantation
Kidney transplant has been the standard choice for renal replacement therapy for patients with end-stage renal disease (ESRD). Age has become a subject of heated debate for recipient and donor selection in kidney transplantation, as well as for the choice of immunosuppressive regimens (Martin and Sheaff, 2007). A number of studies have revealed that the rate of survival decreased while the risk of infection and adverse drug reactions increased substantially in older kidney transplant recipients (Silva, 2005a). However, despite the lower survival rate, older ESRD patients do benefit from kidney transplant and demonstrate an extended lifespan and an improved quality of life as compared with their peers treated by hemodialysis (Macrae et al., 2005; Silva, 2005a; Wolfe et al., 1999). On the contrary, the risk of acute renal allograft rejection is likely lower, most likely associated with immunosenescence. In support of this, a study of 145,470 renal transplant recipients showed that patients aged 55–75 had the lowest risk of death-censored allograft failure (Molnar et al., 2012). In agreement with this, another study involving 108,188 recipients also demonstrated better graft survival in older recipients (Tullius et al., 2010). In contrast, the most common cause of allograft kidney failure in older recipients is comorbidity-related death of the patient (Zhou et al., 2008). To this end, a recent study of 3,597 adult kidney transplant recipients showed that older recipients (≥65 years) of older allograft kidneys experienced a higher risk of five-year mortality and were more likely to have acute rejection, delayed graft function, and lower kidney function, as compared with older recipients of young kidneys (Peters-Sengers et al., 2017). This is concerning because older ESRD patients are more likely to receive transplantation of older kidneys due to the increase in use of expanded criteria donor kidneys, including kidneys from older donors. As such, with the increasingly improved survival rate for aged ESRD patients on hemodialysis, the survival benefit of kidney transplant becomes less significant (Peters-Sengers et al., 2017). In order to improve the survival rate and minimize post-transplant mortality for older kidney transplant recipients, it is essential to carefully screen comorbidities and optimize the quality of donors.
In order to determine the mechanism underlying the poor performance of older allograft kidneys, Halloran and his colleagues examined the role of renal ageing (Silva, 2005a). It seems that kidneys from older donors have declined function, higher risk of graft failure and functional loss, reduced repair capacity after acute rejection, greater susceptibility to ischemia and drug toxicity, and higher incidence of immunogenicity (Li and Wang, 2018; Schmitt and Melk, 2017). A recent study of 133,824 living kidney donors showed that older age groups had higher risk of ESRD among nonblack donors (Massie et al., 2017). Another study including 889 living kidney donors showed that older age was associated with a lesser recovery of eGFR (Bellini et al., 2019). However, as compared with age-matched common people, older living donors did not demonstrate higher risk for renal failure or long-term mortality (Berger et al., 2011; Ibrahim et al., 2009). The controversies over the long-term consequences of kidney donation in older living donors warrants more large-scale studies. On the other hand, ageing allograft kidneys may have a better performance in young recipients. At least in experimental models, bone marrow-derived cells from young mice could alleviate renal ageing in aged mice (Yang et al., 2011). Consistent with this, in murine models of heterochronic parabiosis, youthful systemic milieu successfully alleviated renal injury in elderly mice after ischemia-reperfusion injury (Liu et al., 2018). These studies may provide a new avenue for making the best use of allograft kidneys from older donors and advancing our understanding of renal ageing.
Recently, some seminal findings suggest that cell senescence may explain some of the effects of ageing on graft outcome. In humans, increased cell senescence in kidneys from older donors was associated with impaired regenerative capacity of the allograft kidney in response to injury (Melk and Halloran, 2001) and thus increased the risk to ischemic injury and to develop post-transplant delayed graft function (Lim et al., 2013; Oberhuber et al., 2012). In consistency, in experimental animal models, inhibition of kidney cell senescence may improve the outcome of kidney transplantation. Indeed, p16INK4a KO mice developed less fibrosis, attenuated tubulointerstitial damage, more renal tubular epithelial cell proliferation, and improved graft survival when receiving kidneys transplant from p16INK4a KO mice (Braun et al., 2012).
6. Therapeutic strategies for renal ageing
Kidney function is a crucial predictor of longevity (Hediger, 2002). Hence, maintaining long-term homeostatic renal function is the primary goal of any interventions aimed at retarding or even reversing renal ageing (Schmitt and Melk, 2017). In recent years, great advances have been made to achieve this goal.
6.1. Calorie restriction
Almost 80 years ago, the beneficial effect of calorie restriction was first described. In rats subjected to life-long calorie restriction without malnutrition, median and maximal lifespan were considerably prolonged (McCay et al., 1989). Later, this finding was reproducibly corroborated by many other studies in a wide variety of species including nonhuman primates (Colman et al., 2009). The beneficial effect was extended from ageing to age-related diseases and tumorigenesis (Weindruch and Sohal, 1997). The age-related changes in the kidney can also be modulated by caloric restriction. In calorie-restricted animals, age-associated structural and functional changes in the kidney were mitigated and animals were resistant to diverse experimental kidney injuries (Calvo-Rubio et al., 2016). In humans, life-long caloric restriction is not feasible, but the beneficial effects of even short-term calorie restriction have been unequivocally demonstrated, including reduced blood pressure, body weight, blood cholesterol, blood glucose, and attenuated atherosclerosis, as well as a decrease in blood urea nitrogen, creatinine, and uric acid (Walford et al., 2002). Mechanistically, calorie restriction lessens cell senescence via suppressing the activation of the mTOR signaling pathway, a nutrient-sensing signaling network that controls cellular metabolism (Inoki et al., 2012). Other underlying mechanisms include the inhibition of insulin and IGF-1 signaling, suppression of oxidative stress, induction of antioxidants (De Cabo et al., 2004), and improved mitochondrial function and autophagy (Ning et al., 2013; Nisoli et al., 2005).
6.2. Pharmacologic therapy
Though calorie restriction seems beneficial, long-term chronic calorie restriction makes it impossible to implement and reap the health benefits of an active lifestyle with regular exercise, and causes severe health consequences for humans (Schmitt and Melk, 2017). As such, it is appealing to explore pharmacological interventions for ageing. Some studies have shown that blockade of the renin-angiotensin-aldosterone system (RAAS) could alleviate cardiovascular ageing, reduce renal senescence in hypertensive rats, and improve mitochondrial number and function (Benigni et al., 2009; de Cavanagh et al., 2003; Westhoff et al., 2008). Although angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have been used for hypertension or other indications widely, evidence of their effects on natural renal ageing in men is lacking at present. Nevertheless, there is ample data in support of the beneficial effect of ACEIs/ARBs on general ageing. For instance, ARBs have been shown to extend lifespan in both mice and hypertensive rats (Benigni et al., 2009; Linz et al., 2000). Likewise, in normotensive adult Wistar rats fed with standard or palatable hyper-lipidic diets, long term treatment with the ACEI enalapril prolonged lifespan (Santos et al., 2009). Therefore, it is tempting to speculate that ACEIs/ARBs may possess an anti-ageing effect on the kidney in men, though further studies are warranted. Pioglitazone, a proliferator-activated receptor-γ (PPARγ) agonist, has been shown to alleviate age-related renal changes, including proteinuria, sclerosis, cell senescence, and the decline of GFR in naturally aged rats (Yang et al., 2009). The underlying mechanisms involve mitochondrial protection by activation of PPARγ with associated increased klotho and reduced protein kinase C-β and p66Shc phosphorylation in the kidney. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, a new category of antidiabetic drugs, have been demonstrated to slow progression of kidney disease in type 2 diabetic patients significantly (Wanner et al., 2016) and alleviate cell senescence of renal tubular epithelial cells in animals with type 1 diabetes (Kitada et al., 2014). This class of drugs may be a possible therapeutic strategy for renal ageing, though no direct evidence in natural kidney ageing has been obtained.
Rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR), prevents cell senescence following activation of the mTOR pathway and delays premature ageing caused by phosphate (Iglesias-Bartolome et al., 2012; Kawai et al., 2016). Genetic or pharmacological inhibition of mTOR signaling has been reported to prolong lifespan of invertebrates (Lamming et al., 2013) and genetically heterogeneous mice (Harrison et al., 2009), and delay ageing and age-related pathologies in model organisms (Bjedov et al., 2010). Although the anti-ageing efficacy of rapamycin is conspicuous, it often has pleiotropic effects beyond ageing as an FDA-approved immunosuppressant. The side effects of long-term rapamycin treatment include viral or fungal infections due to its immunosuppressive activities in renal transplant recipients (Mahé et al., 2005), dermatological adverse events (McCormack et al., 2011), and metabolic changes such as hyperlipidemia, decreased insulin sensitivity and increased incidence of new-onset diabetes (Gyurus et al., 2011; McCormack et al., 2011). Thus, rapamycin is unlikely an optimal choice for the prevention of renal ageing in healthy individuals. AMPK is a major inhibitory signaling transducer upstream of mTOR. The AMPK activator metformin has been shown to prevent the induction of p16INK4a and p21CIP1 in cellular models of senescence (Noren Hooten et al., 2016). In vivo, metformin was shown to protect the kidney from diabetes-induced hypertrophy and also prevent cisplatin or gentamicin-induced renal injury in mice, suggesting a feasible beneficial effect in mitigating renal ageing (Li et al., 2016; Morales et al., 2010). Furthermore, metformin has minimal side effects that are likely reversible. Hence, it is promising that metformin may be used safely in healthy individuals to prevent ageing and possibly age-related renal changes (Barzilai et al., 2016). Recently, there is evidence suggesting that activation of the nuclear factor-erythroid 2-related factor 2 (Nrf2), the master regulator of anti-oxidative responses, is able to extend lifespan in mice (Strong et al., 2016) and reverse premature ageing in cells (Kubben et al., 2016). In this context, bardoxolone, a potent Nrf2 activator, has garnered much attention because of the exciting efficacy in patients with type 2 diabetes and CKD observed early in the Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON) Trial (Pergola et al., 2011). Unfortunately, this trial was later discontinued due to an unexpected significantly higher rate of heart failure events in patients randomized to bardoxolone treatment (de Zeeuw et al., 2013). Thus, further investigations are required to examine the efficacy and safety of bardoxolone or other Nrf2 activators in ageing and age-related renal changes due to their pleiotropic effects beyond ageing. In addition, lithium, an inhibitor of GSK3, has been revealed to drastically increase lifespan in C. elegans and drosophila (Castillo-Quan et al., 2016), and its levels in drinking water were associated with longevity in humans (McColl et al., 2008; Zarse et al., 2011). Despite the concern about nephrotoxicity of lithium at psychiatric doses, microdose lithium has been found to attenuate proteinuria, podocyte injury, and glomerulosclerosis in diverse experimental glomerular diseases (Xu et al., 2014; Zhou et al., 2016). However, the effect of microdose lithium on kidney ageing is unknown and merits further studies. Furthermore, therapeutic targeting of the anti-ageing gene klotho is under investigation via repurposing some existing approved drugs with klotho agonizing activities, including PPARγ agonists like thiazolidinediones (Youm et al., 2010).
Apart from the above, a seminal study by Baker et al. recently demonstrated that ablation of naturally occurring p16INK4a-positive cells in INK-ATTAC transgenic mice could attenuate age-related pathological changes in the kidney (Baker et al., 2016), suggesting that clearance of senescent cells is possibly a new therapeutic strategy for ageing and age-related diseases as mechanism-based targeted therapy (Childs et al., 2015). Senolytic drugs specifically targeting senescent cell apoptosis but not non-senescent cells have been invented, including navitoclax, dasatinib, and quercetin. A recent open label Phase 1 pilot clinical trial of dasatinib in combination with quercetin in patients with diabetic kidney disease demonstrated a considerable reduction in senescent cell burden in adipose tissue and skin biopsy specimens as quick as 11 days after completing 3 days’ treatment (Hickson et al., 2019). However, so far little is known about the effect of those drugs on actual renal ageing. Nevertheless, these findings suggest that senolytic drugs will likely be a promising therapeutic strategy for renal ageing (Zhu et al., 2015). Considering the essential role of cellular senescence in physiological homeostasis, senescent cell-targeted therapy may have side effect concerns. Indeed, there is evidence suggesting that ablation of senescent cells could delay the cutaneous wound healing process (Demaria et al., 2014). Collectively, although a number of anti-ageing strategies seem inspiring for the prevention of renal ageing and age-related kidney diseases in experimental models, more clinical research is required to verify their efficacy and safety in men.
7. Conclusion
Renal ageing is a complex process, and has attracted much attention as the global population increasingly ages. Although the characteristic alterations of ageing kidneys have been well known, the distinction between natural ageing and age-related kidney diseases merits further elucidation. The exact cellular and molecular signaling mechanisms of renal ageing are still uncertain. Cell senescence plays a key role in renal ageing and serves as a hallmark of renal ageing. A number of therapeutic interventions have been tested in the hope of slowing kidney ageing and some have shown promising results. Novel senolytic therapy may open a new door to maintain human kidney health during the ageing process.
Acknowledgments
Funding
R.G. was supported in part by the Foundation for Health and the U.S. National Institutes of Health grant DK092485. Y.F. was supported in part by the International Society of Nephrology Sister Renal Center Program for fellowship training.
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J, 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration for Community Living, 2019. 2018 profile of older Americans U.S. Department of Health and Human Services. [Google Scholar]
- Al-Douahji M, Brugarolas J, Brown PA, Stehman-Breen CO, Alpers CE, Shankland SJ, 1999. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int 56, 1691–1699. [DOI] [PubMed] [Google Scholar]
- Al-Said J, O’Neill WC, 2003. Reduced kidney size in patients with simple renal cysts. Kidney Int 64, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O’Neill WC, 2004. Reduced renal function in patients with simple renal cysts. Kidney Int 65, 2303–2308. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Mundel P, 2003. The role of podocytes in glomerular pathobiology. Clin. Exp. Nephrol 7, 255–259. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM, 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a tool to target aging. Cell Metab 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini MI, Charalampidis S, Stratigos I, Dor F, Papalois V, 2019. The Effect of Donors’ Demographic Characteristics in Renal Function Post-Living Kidney Donation. Analysis of a UK Single Centre Cohort. J. Clin. Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G, 2009. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest 119, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JC, Muzaale AD, James N, Hoque M, Wang JM, Montgomery RA, Massie AB, Hall EC, Segev DL, 2011. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin. J. Am. Soc. Nephrol 6, 2887–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L, 2010. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE, 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C, 2014. The aging kidney revisited: a systematic review. Ageing Res. Rev 14, 65–80. [DOI] [PubMed] [Google Scholar]
- Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A, 2012. Cellular senescence limits regenerative capacity and allograft survival. J. Am. Soc. Nephrol 23, 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Rubio M, Buron MI, Lopez-Lluch G, Navas P, de Cabo R, Ramsey JJ, Villalba JM, Gonzalez-Reyes JA, 2016. Dietary fat composition influences glomerular and proximal convoluted tubule cell structure and autophagic processes in kidneys from calorie-restricted mice. Aging Cell 15, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F, 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Castillo-Quan JI, Li L, Kinghorn KJ, Ivanov DK, Tain LS, Slack C, Kerr F, Nespital T, Thornton J, Hardy J, Bjedov I, Partridge L, 2016. Lithium promotes longevity through GSK3/NRF2-Dependent hormesis. Cell Rep 15, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KW, Leung CY, Chan CW, 1990. Age-related glomerular sclerosis: baseline values in Hong Kong. Pathology 22, 177–180. [DOI] [PubMed] [Google Scholar]
- Childs BG, Durik M, Baker DJ, van Deursen JM, 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin HJ, Ro H, Lee HJ, Na KY, Chae DW, 2006. The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney Int 70, 1468–1473. [DOI] [PubMed] [Google Scholar]
- Chuang PY, Cai W, Li X, Fang L, Xu J, Yacoub R, He JC, Lee K, 2017. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Renal Physiol 313, F621–f628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R, 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P, 2004. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp. Gerontol 39, 297–304. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG, 2003. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 17, 1096–1098. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, 2013. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med 369, 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic A, Glassock RJ, Rule AD, 2016. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis 23, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD, 2017. The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol 28, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. , 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S. A 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B, 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Emamian SA, Nielsen MB, Pedersen JF, Ytte L, 1993. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am. J. Roentgenol 160, 83–86. [DOI] [PubMed] [Google Scholar]
- Epstein M, 1996. Aging and the kidney. J. Am. Soc. Nephrol 7, 1106–1122. [DOI] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, Zhu X, Preedy V, Lu H, Bohr VA, Chan WY, Liu Y, Ng TB, 2015. A research agenda for aging in China in the 21st century. Ageing Res. Rev 24, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Hudkins KL, Seifert RA, Francki A, Bowen-Pope DF, Alpers CE, 1997. Localization of PDGF alpha-receptor in the developing and mature human kidney. Kidney Int 51, 1140–1150. [DOI] [PubMed] [Google Scholar]
- Glassock RJ, Rule AD, 2012. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 82, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassock RJ, Rule AD, 2016. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron 134, 25–29. [DOI] [PubMed] [Google Scholar]
- Glassock R, Delanaye P, El Nahas M, 2015. An age-calibrated classification of chronic kidney disease. Jama 314, 559–560. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M, 2019. Cellular senescence: defining a path forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- Gourtsoyiannis N, Prassopoulos P, Cavouras D, Pantelidis N, 1990. The thickness of the renal parenchyma decreases with age: a CT study of 360 patients. AJR Am. J. Roentgenol 155, 541–544. [DOI] [PubMed] [Google Scholar]
- Grantham JJ, 2012. Solitary renal cysts: worth a second look? Am. J. Kidney Dis. 59, 593–594. [DOI] [PubMed] [Google Scholar]
- Grimley Evans J, 2000. 21st century: review: ageing and medicine. J. Intern. Med 247, 159–167. [DOI] [PubMed] [Google Scholar]
- Gyurus E, Kaposztas Z, Kahan BD, 2011. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: a long-term analysis of various treatment regimens. Transplant. Proc 43, 1583–1592. [DOI] [PubMed] [Google Scholar]
- Hara M, Yanagihara T, Kihara I, 2001. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89, 342–347. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ, 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA, 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS, 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Hayman JM Jr, Martin JW Jr, Miller M, 1939. Renal function and the number of glomeruli in the human kidney. Arch. Intern. Med. 64, 69–83. [Google Scholar]
- Hediger MA, 2002. Kidney function: gateway to a long life? Nature 417 (393), 395. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M, 2017. Unmasking transcriptional heterogeneity in senescent cells. Current biology: CB 27, 2652–2660 e2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL, 2019. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC, 2015. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol 26, 3162–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommos MS, Glassock RJ, Rule AD, 2017. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol 28, 2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY, 2013. Temporal changes in incidence of dialysis-requiring AKI. J. Am. Soc. Nephrol 24, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ, 2009. Long-term consequences of kidney donation. N. Engl. J. Med 360, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS, 2012. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Kim J, Guan KL, 2012. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol 52, 381–400. [DOI] [PubMed] [Google Scholar]
- James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M, 2010. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet (London, England) 376, 2096–2103. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhang Y, Ding Q, Wang SS, Rastogi P, Dai DF, Lu D, Purvis M, Cao C, Wang A, Liu D, Ren C, Elhadi S, Hu MC, Chai Y, Zepeda-Orozco D, Campisi J, Attanasio M, 2019. Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Johnson GV, 2004. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95–102. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF, 2010. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol 12, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Kinoshita S, Ozono K, Michigami T, 2016. Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an alphaKlotho-Deficient model. J. Am. Soc. Nephrol 27, 2810–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G, Zimmer G, Mall G, Ritz E, Amann K, 2003. Nephron number in patients with primary hypertension. N. Engl. J. Med 348, 101–108. [DOI] [PubMed] [Google Scholar]
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R, 2001. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60, 957–968. [DOI] [PubMed] [Google Scholar]
- Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A, 2014. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications 28, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE, 2004. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest 114, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T, 2016. Repression of the antioxidant NRF2 pathway in premature aging. Cell 165, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M, 2005. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD, 2000. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell. Sci 113 (Pt 20), 3613–3622. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA, 2013. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Invest 123, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS, 2007. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol 292, F617–627. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Z, 2018. Aging kidney and aging-related disease. Adv. Exp. Med. Biol 1086, 169–187. [DOI] [PubMed] [Google Scholar]
- Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, He W, Yang J, Dai C, 2016. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci. Rep. 6, 23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WH, Clayton P, Wong G, Campbell SB, Cohney S, Russ GR, Chadban SJ, McDonald SP, 2013. Outcomes of kidney transplantation from older living donors. Transplantation 95, 106–113. [DOI] [PubMed] [Google Scholar]
- Linz W, Heitsch H, Schölkens BA, Wiemer G, 2000. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension 35, 908–913. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR, Zhan J, Zhang JH, Xiao HS, 2012. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Translational research: the journal of laboratory and clinical medicine 159, 454–463. [DOI] [PubMed] [Google Scholar]
- Liu D, Lun L, Huang Q, Ning Y, Zhang Y, Wang L, Yin Z, Zhang Y, Xia L, Yin Z, Fu B, Cai G, Sun X, Chen X, 2018. Youthful systemic milieu alleviates renal ischemia-reperfusion injury in elderly mice. Kidney Int. 94, 268–279. [DOI] [PubMed] [Google Scholar]
- Long DA, Mu W, Price KL, Johnson RJ, 2005. Blood vessels and the aging kidney. Nephron Exp. Nephrol. 101, e95–99. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Zhou S, Zhou Z, Liu Y, Yang L, Liu J, Zhang Y, Li H, Liu Y, Hou FF, Zhou L, 2018. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol 29, 1238–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae J, Friedman AL, Friedman EA, Eggers P, 2005. Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States. Int. Urol. Nephrol 37, 641–648. [DOI] [PubMed] [Google Scholar]
- Mahé E, Morelon E, Lechaton S, Sang KH, Mansouri R, Ducasse MF, Mamzer-Bruneel MF, de Prost Y, Kreis H, Bodemer C, 2005. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation 79, 476–482. [DOI] [PubMed] [Google Scholar]
- Martin JE, Sheaff MT, 2007. Renal ageing. J. Pathol 211, 198–205. [DOI] [PubMed] [Google Scholar]
- Martin N, Beach D, Gil J, 2014. Ageing as developmental decay: insights from p16 (INK4a.). Trends Mol. Med 20, 667–674. [DOI] [PubMed] [Google Scholar]
- Massie AB, Muzaale AD, Luo X, Chow EKH, Locke JE, Nguyen AQ, Henderson ML, Snyder JJ, Segev DL, 2017. Quantifying postdonation risk of ESRD in living kidney donors. J. Am. Soc. Nephrol 28, 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matjusaitis M, Chin G, Sarnoski EA, Stolzing A, 2016. Biomarkers to identify and isolate senescent cells. Ageing Res. Rev 29, 1–12. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA, 1989. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition (Burbank, Los Angeles County, Calif.) 5, 155–171 discussion 172. [PubMed] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ, 2008. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem 283, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Young LR, Kinder BW, Downey GP, Sullivan EJ, Colby TV, McKay RT, Cohen MM, Korbee L, Taveira-DaSilva AM, Lee HS, Krischer JP, Trapnell BC, 2011. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med 364, 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan M, Wasserman P, 1981. Changes in sizes and distensibility of the aging kidney. Br. J. Radiol 54, 488–491. [DOI] [PubMed] [Google Scholar]
- Melk A, Halloran PF, 2001. Cell senescence and its implications for nephrology. J. Am. Soc. Nephrol 12, 385–393. [DOI] [PubMed] [Google Scholar]
- Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF, 2004. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65, 510–520. [DOI] [PubMed] [Google Scholar]
- Michelis MF, 1990. Hyperkalemia in the elderly. Am. J. Kidney Dis 16, 296–299. [DOI] [PubMed] [Google Scholar]
- Mimran A, Ribstein J, Jover B, 1992. Aging and sodium homeostasis. Kidney international Supplement 37, S107–113. [PubMed]
- Minutolo R, Borrelli S, De Nicola L, 2015. CKD in the Elderly: Kidney Senescence or Blood Pressure-Related Nephropathy? Am. J. Kidney Dis 66, 184–186. [DOI] [PubMed] [Google Scholar]
- Molnar MZ, Streja E, Kovesdy CP, Shah A, Huang E, Bunnapradist S, Krishnan M, Kopple JD, Kalantar-Zadeh K, 2012. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am. J. Kidney Dis 59, 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arevalo M, Leverve X, Lopez-Novoa JM, El-Mir MY, 2010. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int 77, 861–869. [DOI] [PubMed] [Google Scholar]
- Ning YC, Cai GY, Zhuo L, Gao JJ, Dong D, Cui S, Feng Z, Shi SZ, Bai XY, Sun XF, Chen XM, 2013. Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage. Mech. Ageing Dev 134, 570–579. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO, 2005. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317. [DOI] [PubMed] [Google Scholar]
- Nitta K, Okada K, Yanai M, Takahashi S, 2013. Aging and chronic kidney disease. Kidney Blood Press. Res 38, 109–120. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Martin-Montalvo A, Dluzen DF, Zhang Y, Bernier M, Zonderman AB, Becker KG, Gorospe M, de Cabo R, Evans MK, 2016. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 15, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyengaard JR, Bendtsen TF, 1992. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec 232, 194–201. [DOI] [PubMed] [Google Scholar]
- O’Sullivan ED, Hughes J, Ferenbach DA, 2017. Renal aging: causes and consequences. J. Am. Soc. Nephrol 28, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber R, Ge X, Tullius SG, 2012. Donor age-specific injury and immune responses. Am. J. Transplant 12, 38–42. [DOI] [PubMed] [Google Scholar]
- Ortmann J, Amann K, Brandes RP, Kretzler M, Münter K, Parekh N, Traupe T, Lange M, Lattmann T, Barton M, 2004. Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension 44, 974–981. [DOI] [PubMed] [Google Scholar]
- Pabst R, Sterzel RB, 1983. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 24, 626–631. [DOI] [PubMed] [Google Scholar]
- Park JY, Schutzer WE, Lindsley JN, Bagby SP, Oyama TT, Anderson S, Weiss RH, 2007. p21 is decreased in polycystic kidney disease and leads to increased epithelial cell cycle progression: roscovitine augments p21 levels. BMC Nephrol 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Park SH, Weiss RH, 2009. Disparate effects of roscovitine on renal tubular epithelial cell apoptosis and senescence: implications for autosomal dominant polycystic kidney disease. Am. J. Nephrol 29, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG, 2011. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med 365, 327–336. [DOI] [PubMed] [Google Scholar]
- Peters-Sengers H, Berger SP, Heemskerk MB, Al Arashi D, Homan van der Heide JJ, Hemke AC, Ten Berge IJ, Idu MM, Betjes MG, van Zuilen AD, Hilbrands LB, de Vries AP, Nurmohamed AS, Christiaans MH, Ernest van Heurn LW, de Fijter JW, Bemelman FJ, 2017. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J. Am. Soc. Nephrol 28, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova NV, Velichko AK, Razin SV, Kantidze OL, 2016. Small molecule compounds that induce cellular senescence. Aging Cell 15, 999–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayess H, Wang MB, Srivatsan ES, 2012. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 130, 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P, 2009. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol 20, 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman DA, Hwang SJ, Oyama-Manabe N, Chuang ML, O’Donnell CJ, Manning WJ, Fox CS, 2017. Clinical associations of total kidney volume: the Framingham Heart Study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 32, 1344–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule A.Dea., 2018. The Aging Kidney(UpToDate, 2018)
- Sands JM, 2012. Urine concentrating and diluting ability during aging. J. Gerontol. A Biol. Sci. Med. Sci 67, 1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos EL, de Picoli Souza K, da Silva ED, Batista EC, Martins PJ, D’Almeida V, Pesquero JB, 2009. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem. Pharmacol 78, 951–958. [DOI] [PubMed] [Google Scholar]
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V, 2018. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis 71, A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, Kuhlmann MK, Schuchardt M, Tolle M, Ziebig R, van der Giet M, Martus P, 2012. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med 157, 471–481. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Melk A, 2017. Molecular mechanisms of renal aging. Kidney Int 92, 569–579. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Susnik N, Melk A, 2015. Molecular aspects of renal senescence. Curr. Opin. Organ Transplant 20, 412–416. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC, 2004. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol 6, 168–170. [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D, 1993. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707. [DOI] [PubMed] [Google Scholar]
- Sethi S, D’Agati VD, Nast CC, Fogo AB, De Vriese AS, Markowitz GS, Glassock RJ, Fervenza FC, Seshan SV, Rule A, Racusen LC, Radhakrishnan J, Winearls CG, Appel GB, Bajema IM, Chang A, Colvin RB, Cook HT, Hariharan S, Herrera Hernandez LP, Kambham N, Mengel M, Nath KA, Rennke HG, Ronco P, Rovin BH, Haas M, 2017. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91, 787–789. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ, 2015. Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408. [DOI] [PubMed] [Google Scholar]
- Silva FG, 2005a. The aging kidney: a review–part II. Int. Urol. Nephrol 37, 419–432. [DOI] [PubMed] [Google Scholar]
- Silva FG, 2005b. The aging kidney: a review – part I. Int. Urol. Nephrol 37, 185–205. [DOI] [PubMed] [Google Scholar]
- Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF, 2007. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71, 218–226. [DOI] [PubMed] [Google Scholar]
- Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M, 2015. Intracellular signaling of the aging suppressor protein Klotho. Curr. Mol. Med 15, 27–37. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE, 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM, 2017. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol 13, 77–89. [DOI] [PubMed] [Google Scholar]
- Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, Takeuchi J, 1972. Intrarenal vascular changes with age and disease. Kidney Int 2, 224–230. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Riella MC, 2014. World Kidney Day 2014: CKD and the aging population. Am. J. Kidney Dis 63, 349–353. [DOI] [PubMed] [Google Scholar]
- Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E, 2010. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann. Surg 252, 662–674. [DOI] [PubMed] [Google Scholar]
- Tumlin JA, Madaio MP, Hennigar R, 2007. Idiopathic IgA nephropathy: pathogenesis, histopathology, and therapeutic options. Clin. J. Am. Soc. Nephrol 2, 1054–1061. [DOI] [PubMed] [Google Scholar]
- van Deursen JM, 2014. The role of senescent cells in ageing. Nature 509, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, Villaggio B, Gianiorio F, Tosetti F, Weiss U, Traverso P, Mji M, Deferrari G, Garibotto G, 2008. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol 295, F1563–1573. [DOI] [PubMed] [Google Scholar]
- Vogelmann SU, Nelson WJ, Myers BD, Lemley KV, 2003. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. Renal Physiol 285, F40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci 27, 339–344. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T, 2002. Calorie restriction in biosphere 2Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-Year period. J. Gerontol. A Biol. Sci. Med. Sci 57, B211–B224. [DOI] [PubMed] [Google Scholar]
- Wang X, Vrtiska TJ, Avula RT, Walters LR, Chakkera HA, Kremers WK, Lerman LO, Rule AD, 2014. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int 85, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB, 2014. Unraveling the role of podocyte turnover in glomerular aging and injury. J. Am. Soc. Nephrol 25, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, 2016. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med 375, 323–334. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS, 1997. Seminars in medicine of the beth israel deaconess medical center. Caloric intake and aging. N. Engl. J. Med 337, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Zeng M, Shu Y, Guo D, Sun Y, Guo Z, Wang Y, Liu Z, Zhou H, Zhang W, 2015. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age Dordr. (Dordr) 37, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A, 2008. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52, 123–129. [DOI] [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC, 2005. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol 16, 2941–2952. [DOI] [PubMed] [Google Scholar]
- Wiggins JE, 2012. Aging in the glomerulus. J. Gerontol. A Biol. Sci. Med. Sci 67, 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC, 2005. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J. Am. Soc. Nephrol 16, 2953–2966. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J, 2016. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S, Campisi J, 2017. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK, 1999. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med 341, 1725–1730. [DOI] [PubMed] [Google Scholar]
- Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD, 2010. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol 299, F1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Ge Y, Liu Z, Gong R, 2014. Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: implications for the protective effect of low-dose lithium in podocytopathy. Am. J. Pathol 184, 2742–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Fogo AB, 2010. Cell senescence in the aging kidney. J. Am. Soc. Nephrol 21, 1436–1439. [DOI] [PubMed] [Google Scholar]
- Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB, 2009. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J. Am. Soc. Nephrol 20, 2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Rossini M, Ma LJ, Zuo Y, Ma J, Fogo AB, 2011. Cells derived from young bone marrow alleviate renal aging. J. Am. Soc. Nephrol 22, 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Yang H, Amin R, Smith SR, Leff T, Dixit VD, 2010. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell 9, 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M, 2011. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr 50, 387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H, 2012. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet (London, England) 379, 815–822. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG, 2008. The aging kidney. Kidney Int 74, 710–720. [DOI] [PubMed] [Google Scholar]
- Zhou S, Wang P, Qiao Y, Ge Y, Wang Y, Quan S, Yao R, Zhuang S, Wang LJ, Du Y, Liu Z, Gong R, 2016. Genetic and pharmacologic targeting of glycogen synthase kinase 3β reinforces the Nrf2 antioxidant defense against podocytopathy. J. Am. Soc. Nephrol 27, 2289–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN, Wolf G, 2008. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr. Diabetes Rev 4, 39–45. [DOI] [PubMed] [Google Scholar]


