Abstract
Signalling through the B cell receptor (BCR) is central to the development and maintenance of B cells. In light of the numerous proliferative and survival pathways activated downstream of the BCR, it comes as no surprise that malignant B cells would co-opt this receptor to promote their own growth and survival. However, direct evidence for BCR signalling in human lymphoma has only come to light recently. Roles for antigen-dependent and antigen-independent, or tonic, BCR signalling have now been described for several different lymphoma subtypes. Furthermore, correlative data implicate antigen-dependent BCR signalling in many other forms of lymphoma. A host of therapeutic agents targeting effectors of the BCR signalling pathway are now in clinical trials and have shown initial success against multiple forms of lymphoma.
Lymphoma is a malignancy of mature lymphocytes that manifests in secondary lymphoid organs or extranodal tissues. In the United States, lymphoma is the sixth most common form of cancer, affecting 22.5 per 100,000 people (see the SEER Cancer Statistics Review 1975–2009). Lymphoma itself is an umbrella term applied to over 30 distinct clinical entities1 (TABLE 1. Most lymphomas originate from B lymphocytes, and their diversity can often be traced to the developmental stage of a normal precursor B cell, with the bulk being derived from antigen-experienced germinal centre or post-germinal centre B cells. Although the standard of care for each type of lymphoma varies, most regimens use combinations of cytotoxic drugs along with the anti-CD20 monoclonal antibody rituximab. For example, the most common type of lymphoma, diffuse large B cell lymphoma (DLBCL), is treated with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). Owing to the inherent heterogeneity of lymphoma, there are very different responses to treatments. Generally, indolent lymphomas such as follicular lymphoma and chronic lymphocytic leukaemia (CLL) have less robust responses to immunochemotherapies than aggressive lymphomas such as Burkitt’s lymphoma and DLBCL. However, many patients with aggressive lymphomas do not show a durable response to treatments, necessitating salvage therapies such as autologous stem cell transplant that often have poor patient outcomes. Moreover, many of the first-line immunochemotherapy regimens are too toxic to be tolerated by the elderly or by people in the developing world, where infectious diseases are more difficult to manage following treatment. Clearly, novel approaches to lymphoma treatment are needed.
Table 1 ∣.
Evidence supporting BCR signalling in lymphoma
| Malignancy | Frequency* | Clinical features |
Presumed cell of origin |
Pre- dominant IgH isotype |
SHM | Putative BCR signalling |
Putative antigens |
Evidence supporting BCR activity |
|---|---|---|---|---|---|---|---|---|
| Activated B cell-like diffuse large B cell lymphoma (ABC DLBCL) | 15% | Median age 66; nodal and extranodal; survival ~40% at 5 years | Post-germinal centre; plasmablast | IgM | Yes | Chronic Active‡ | Auto-antigens | CD79B and CD79A ITAM mutations; RNAi: CD79A, IgM, IgL, SYK, BTK and CARD11; BCR clusters; ibrutinib response |
| Germinal centre B cell-like diffuse large B cell lymphoma (GCB DLBCL) | 17% | Median age 61; nodal and extranodal; survival ~60% at 5 years | Germinal centre B cell | IgG | Yes | None or tonic | None | |
| Burkitt’s lymphoma | <1% | Median age 11 (children), 24 (adult); extranodal and nodal; survival >80% at 5 years | Germinal centre B cell | IgM | Yes | Tonic‡ | None | RNAi: CD79A and SYK |
| Follicular lymphoma | 25% | Median age 57; nodal and bone marrow; survival 73% at 10 years | Germinal centre B cell | IgM | Yes | Chronic active | IgH–mannose–lectin interactions§ | Nonrandom IgH V region mutation; phospho-flow analysis |
| Chronic lymphocytic leukaemia (CLL) | 8% | Median age 65; blood, bone marrow, spleen and nodal; survival 50% at 7 years | Pre-germinal centre mature B cell or memory B cell | IgM | M-CLL: yes; U-CLL: no | Chronic active | Auto-antigens and IgH V region framework 2 | IgH V segment usage and stereotyped receptors; ibrutinib response |
| Mantle cell lymphoma (MCL) | 7% | Median age 55; nodal, intestinal, blood and bone marrow; survival 50% at 3–5 years | Pre-germinal centre mature B cell | IgM | No | Chronic active | Unknown | IgH V segment usage and stereotyped receptors; ibrutinib response |
| Mucosa-associated lymphoid tissue lymphoma/marginal zone lymphoma (MALT/MZL) | 9% | Median age 60; extranodal and bone marrow; survival >80% at 5 years | Marginal zone B cell | IgM | Yes | Chronic active | Bacterial and viral infection | IgH V segment usage and stereotyped receptors |
| Primary mediastinal B cell lymphoma (PMBL) | 6% | Median age 33; mediastinal thoracic; survival >60% at 5 years | Thymic B cell | IgG¶ | Yes | None | None | - |
| Hodgkin’s lymphoma | 11% | Bimodal median age: 25 and 60 years; mediastinal and nodal; survival 85% at 15 years | Thymic B cell | IgG¶ | Yes | None | None | - |
Adapted from REF. 12. BCR, B cell receptor; BTK, Bruton tyrosine kinase; CARD11, caspase recruitment domain-containing protein 11; IgH, immunoglobulin heavy chain; ITAM, immunoreceptor tyrosine-based activation motif; M-CLL, mutated CLL; RNAi, RNA interference; SHM, somatic hypermutation; U-CLL, unmutated CLL; V, variable.
Approximate percentage of the particular lymphoma subtype among all patients with B cell lymphoma.
Indicates known mechanism.
Not an antigen, but can cause BCR crosslinking.
These malignancies express little or no surface BCR expression.
Recent advances spurred by functional and structural genomics have enriched our understanding of the pathogenesis underlying various lymphomas. Half a century after the notion that antigenic stimulation could induce lymphomagenesis was first articulated2, B cell receptor (BCR) signalling has now emerged as a central oncogenic pathway that promotes growth and survival in various lymphoma types. The cascade of effectors downstream of the BCR includes many kinases that are amenable to therapeutic intervention. Here we discuss how various lymphoma subtypes use qualitatively different modes of BCR signalling that engage different downstream pathways, necessitating a nuanced approach to the therapeutic inhibition of oncogenic BCR signalling. We first review the basics of BCR signalling in normal B cells and highlight how lymphomas adopt and pervert this signalling pathway. We then focus on BCR-pathway-targeting small molecules that are entering clinical trials, which have the potential to transform the standard of care for patients with lymphoid malignancies.
B cell receptor signalling in normal lymphocytes
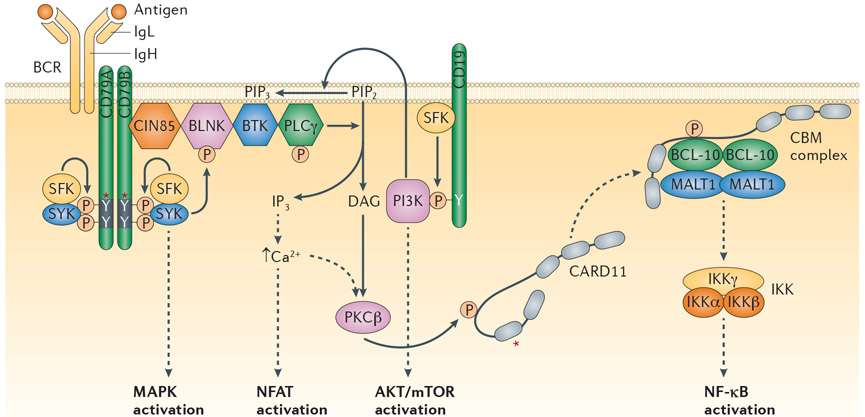
Every normal B cell, and consequently every lymphoma, has a unique BCR consisting of pairs of immunoglobulin heavy (IgH) and light (IgL) chains. Each IgH and IgL has a unique variable (V) region that allows the BCR to bind to diverse antigens in the extracellular environment. This antibody portion of the BCR is coupled non-covalently with an invariant, disulphide-linked heterodimer of the CD79A and CD79B (Igα and Igβ) subunits, which mediates plasma membrane expression, signal transduction and receptor internalization3. Both CD79A and CD79B have similar signalling modules, termed immunoreceptor tyrosine-based activation motifs (ITAMs), each containing two tyrosine residues4. Antigen-induced aggregation of the BCR leads to phosphorylation of ITAM tyrosines by the SRC-family kinases, including LYN, FYN and BLK5. The tyrosine kinase SYK is then recruited to the dually phosphorylated ITAMs through its tandem SRC homology 2 (SH2) domains, resulting in SYK phosphorylation and activation by SRC-family kinases and by autophosphorylation6. The SRC-family kinases and SYK nucleate a signalosome composed of various other kinases and adaptor proteins. SYK recruits a complex of Cbl-interacting protein of 85 kDa (CIN85; also known as SH3KBP1) and B-cell linker protein (BLNK), which in turn coordinates the phosphorylation and activation of Bruton tyrosine kinase (BTK) and phospholipase Cγ2 (PLCγ2)7. PLCγ2 then catalyses the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2; also known as PtdIns(4,5)P2) into diacylglycerol (DAG) and inositol trisphosphate (IP3), resulting in increased intracellular calcium levels. The combination of DAG and increased intracellular calcium activates protein kinase Cβ (PKCβ), which in turn phosphorylates many substrates, including caspase recruitment domain-containing protein 11 (CARD11), a key signalling adaptor that coordinates a signalling complex that activates the nuclear factor-κB (NF-κB) pathway8. The transmembrane protein CD19 is phosphorylated by the SRC family kinase LYN during BCR signalling, recruiting phosphoinositide 3-kinase (PI3K) to the BCR. PI3K phosphorylates PI(4,5)P2 to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3; also known as PtdIns(3,4,5)P3), which recruits effectors including BTK and AKT to the inner leaflet of the plasma membrane via interactions with the plextrin homology (PH) domains of these effectors9. The net result of proximal BCR signalling is the activation of the NF-κB, PI3K, mitogen-activated protein kinase (MAPK), nuclear factor of activated T cells (NFAT) and RAS pathways, which promote proliferation and survival of normal and malignant B cells10 (FIG. 1).
Figure 1∣. B cell receptor signalling basics.
Shown are the B cell receptor (BCR), the co-receptor CD19 and various signalling intermediates that are engaged following binding of the BCR to antigen. Several downstream pathways are ultimately triggered, as indicated. The asterisk indicates protein regions affected by recurrent somatic alterations in human lymphomas. See main text for details. BLNK, B-cell linker protein; BTK, Bruton tyrosine kinase; CARD11, caspase recruitment domain-containing protein 11; CBM, CARD11–BCL-10–MALT1; CIN85, Cbl-interacting protein of 85 kDa; DAG, diacylglycerol; IKK, inhibitor of NF-κB kinase; IgH, immunoglobulin heavy chain; IgL, immunoglobulin light chain; IP3, inositol trisphosphate; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NFAT, nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PKCβ, protein kinase Cβ; PLCγ, phospholipase Cγ; SFK, SRC family kinase.
The assembly of the IgH and IgL components of the BCR begins with the V(D)J recombination-activating protein 1 (RAG1) and RAG2-mediated recombination of V, D (diversity) and J (joining) gene segments to create a complete V region. The assembled V regions include three hypervariable subregions, termed complementarity-determining regions (CDRs), which have diverse amino acid sequences and hence can recognize diverse antigens. The V regions of the IgH and IgL chains are fused to constant (C) regions. In the case of IgH, the C region belongs to one of several ‘classes’ — including IgM, IgD, IgG, IgA and IgE — that can be interchanged by a DNA rearrangement process known as class switch recombination. The IgH class influences the signalling output of the BCR (see below).
Non-self foreign antigens induce a productive immune response by engaging both helper T cells and mature B cells, thereby causing the formation of a new microenvironmental niche, known as the germinal centre, which consists of germinal centre B cells, T follicular helper (TFH) cells and follicular dendritic cells (reviewed in REF. 11). B cells within the germinal centre have a distinct developmental phenotype dictated by a set of characteristic transcription factors (reviewed in REF. 12). Whereas naive mature B cells have BCRs with IgM or IgD heavy chains, the germinal centre response triggers IgH class switching to IgG, IgA or IgE. In addition, germinal centre B cells diversify their IgH and IgL V regions by somatic hypermutation (SHM), a specialized mutational mechanism that is catalysed by activation-induced cytidine deaminase (AID). Antigen trapped on follicular dendritic cells is grabbed by the germinal centre B cells and presented to the TFH cells, producing an immunological synapse that activates the B cell. Germinal centre B cells that have mutated their BCRs to bind antigen with high affinity are the evolutionary winners; they are positively selected to differentiate terminally into antibody-secreting plasma cells.
In contrast to the ‘active’, antigen-dependent form of BCR signalling that initiates the germinal centre response, mature B cells utilize the BCR in a qualitatively distinct fashion known as ‘tonic’ BCR signalling, a process that is required for B cell survival. Evidence for this comes from experiments in which IgM or CD79A was conditionally inactivated in mature mouse B cells, resulting in a profound reduction of peripheral B cells numbers over several weeks13,14. Provision of a truncated CD79A construct lacking an ITAM was unable to rescue the CD79A-deficient B cells, indicating a requirement for signals emanating from the BCR to sustain B cell survival14. Such tonic signalling is likely to be antigen-independent as it affects all B cells, regardless of their IgH and IgL V regions, although formal proof of this hypothesis is lacking. Transgenic expression of a constitutively active form of the PI3K catalytic subunit was able to rescue mature B cells following conditional BCR ablation, but constitutively active forms of inhibitor of NF-κB kinase-β (IKKβ), MAPK/extracellular signal-regulated kinase (ERK) kinase 1 (MEK1; also known as MAPKK1) or Ras-related C3 botulinum toxin substrate 1 (RAC1) were ineffective15. Although these experiments implicated the PI3K pathways as a key component of tonic BCR signalling, the biochemical basis of PI3K activation is less clear. SYK may have a role in PI3K activation, as it does during strong antigenic stimulation16, but other evidence suggests that the small GTPase TC21 (also known as R-RAS2) may directly bridge CD79A to PI3K activation in tonic BCR signalling17. PI3K also associates directly with non-phosphorylated CD79A, suggesting yet another avenue for tonic BCR signalling through PI3K18. The biophysical basis of tonic BCR signalling is still debated. Early studies suggested that before antigen exposure, the BCR of mature B cells is pre-associated with SRC-family kinases as well as protein tyrosine phosphatases such as SHP1 (also known as PTPN6), which constantly extinguish most but not all signalling from the BCR19. Recent evidence suggests that the BCRs on a resting B cell do not exist as monomers but rather as auto-inhibited oligomers20, which might produce a tonic BCR signal.
Several mechanisms exist to prevent B cells from reacting with self antigens, which is a substantial threat because most developing B cells express a BCR that is autoreactive21. Exposure of immature B cells in the bone marrow to self antigens triggers an attempt to alter the specificity of the BCR, known as ‘receptor editing’, or results in cell death (reviewed in REF. 22). Autoreactive B cells that escape these ‘central tolerance’ mechanisms can be rendered functionally inactive by encounter with self antigens in the periphery, a process known as ‘anergy’ (reviewed in REF. 23). The hallmarks of anergic B cells are low expression of IgM on the cell surface and reduced responsiveness to further stimulation through the BCR. In humans, perhaps 30% of mature peripheral B cells are anergic24, and failures in maintaining the anergic phenotype are thought to contribute to the development of human autoimmune diseases, including systemic lupus erythematosis and type 1 diabetes. Continuous exposure to auto-antigens causes abnormally high activation of the SRC-family kinase LYN25. Sustained LYN activity attenuates BCR signalling by activating the lipid phosphatase SH2 domain-containing inositol 5′-phosphatase 1 (SHIP1; also known as INPP5D and PIP3 5-phosphatase 1), which can reduce PI3K activity26, causing the protein phosphatase SHP1 to be recruited to the inhibitory BCR co-receptors CD22 and CD72, culminating in CD79A and CD79B ITAM dephosphorylation.
BCR signalling in lymphoid malignancies
Most B cell lymphomas maintain BCR expression on the cell surface (reviewed in REF. 27). This is surprising as the enzymes that generate and refine the BCR — AID, RAG1 and RAG2 — also cause chromosomal translocations that disrupt the immunoglobulin loci, placing oncogenes under the control of the powerful immunoglobulin enhancers (reviewed in REF. 28). Despite these genetic assaults on the immunoglobulin loci, lymphomas retain one intact set of IgH and IgL alleles, allowing the lymphoma to form a BCR. These observations suggest that malignant B cells benefit from the proliferation and survival pathways that are triggered by the BCR.
Many B cell lymphomas utilize IgM constant regions to form their BCR despite the fact that most of these lymphomas are derived from germinal centre cells that typically switch their BCRs from IgM to IgG. This observation may be explained by the fact that IgM and IgG BCRs produce qualitatively distinct signalling outputs: IgM-BCR signalling promotes the survival and proliferation of B cells by activating many pathways, including NF-κB, whereas IgG-BCR signalling favours plasmacytic differentiation through the activation of ERK and MAPK pathways29-31.
The selective pressure to retain IgM-BCR expression is exemplified by follicular lymphoma (reviewed in REF. 32). Follicular lymphoma is characterized by a t(14;18) translocation that places BCL-2 expression under control of the IgH locus enhancers, destroying expression of immunoglobulin from the translocated allele in the process. In most B cells, one IgH allele is termed ‘productive’ because it encodes an in-frame V-D-J region, whereas the other is termed ‘non-productive’ because its V-D-J region is out of frame and cannot make a full-length IgH chain. In follicular lymphoma, the productive allele is never translocated to BCL-2 and remains as IgM, but the non-productive allele is translocated and undergoes class switch recombination to IgG33, demonstrating the selective pressure to maintain surface IgM expression in follicular lymphoma.
A second example is provided by DLBCL, an aggressive lymphoma diagnostic category that is comprised of two major molecular subtypes: germinal centre B cell-like (GCB) DLBCL and activated B cell-like (ABC) DLBCL34. GCB DLBCLs typically express an IgG-BCR35 and do not require BCR signalling for survival36. By contrast, ABC DLBCLs rely on BCR signalling (see below) and retain expression of an IgM-BCR36 as a consequence of genetic deletions within the IgH ‘switch’ regions (Sμ and Sγ), which are necessary for class switch recombination35. These deletions in the switch regions occur selectively on the productive IgH allele, blocking class switch recombination, whereas the non-productive allele lacks these mutations and can undergo class switch recombination37.
Chronic active BCR signalling
Genomic investigations of ABC DLBCL have provided clear genetic evidence that BCR signalling is central to the pathogenesis of human lymphoma36,38,39. Early studies noted that ABC DLBCL tumours rely on the anti-apoptotic NF-κB pathway for survival, unlike GCB DLBCLs, but the mechanisms responsible for this constitutive NF-κB pathway activation were unclear40. Evidence that BCR signalling might play a part in this constitutive NF-κB activity came from an RNA interference screen, which revealed that the CBM (CARD11–BCL-10–mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1)) complex is required for NF-κB activity and survival in ABC DLBCL38. In normal B cells, the CBM complex is an essential signalling intermediate between the BCR and IKK, the central regulatory kinase of the NF-κB pathway (reviewed in REF. 41). In ~10% of ABC DLBCL cases, activation of the CBM complex could be traced to mutant isoforms of CARD11 (REF. 39). These tumours acquire somatic mutations affecting the coiled-coil domain of CARD11, causing it to form spontaneous aggregates that recruit all downstream signalling components of the NF-κB pathway.
However, in most ABC tumours CARD11 is not mutated, but the tumour cells still require CARD11 for survival38. In these cases, RNA interference screening revealed that BTK is essential for NF-κB activation and cell survival36. In normal B cells, BTK is activated by BCR stimulation and is required for downstream NF-κB pathway engagement42,43, raising the possibility that its activity in ABC DLBCL was caused by BCR signalling. Indeed, these ABC DLBCLs were killed by knockdown of any BCR component (IgH, Igκ, CD79A or CD79B) or proximal BCR signalling effectors (SYK, BLNK, PLCγ2, PI3Kδ or PKCβ)36. Genetic or pharmacologic disruption of BCR signalling in ABC DLBCL cell lines abrogated multiple downstream effectors of BCR signalling, including NF-κB, AKT, ERK and NFAT signalling, underscoring the potent consequences of BCR signalling on cellular growth and survival pathways36,44. Total internal reflection fluorescence (TIRF) microscopy showed that the BCRs in ABC DLBCL are organized in clusters on the cell surface, which is reminiscent of BCR clusters that form on normal B cells when they are exposed to antigen36,45. Phosphotyrosine was localized underneath these BCR clusters, suggesting that they are the sites of constitutive BCR signalling.
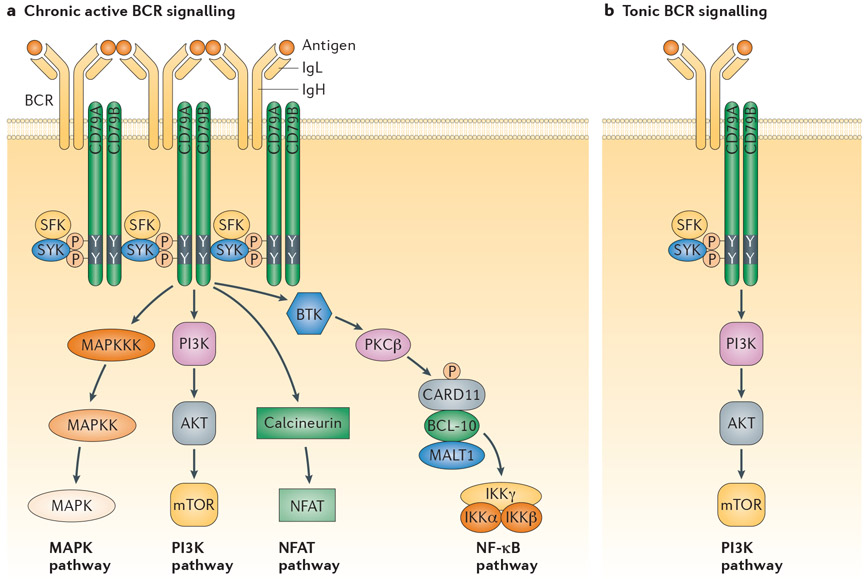
By several criteria, the BCR signalling in ABC DLBCL resembles active rather than tonic BCR signalling. First, the NF-κB dependence of ABC DLBCLs is not a feature of tonic BCR signalling, which instead relies on PI3K signalling15. Second, the CBM complex is required in ABC DLBCL but is not essential for tonic BCR signalling, as knockout of CBM components does not affect the survival of mouse follicular B cells (reviewed in REF. 46). Third, the BCR clusters in ABC DLBCL are present in antigen-stimulated B cells but not in naive B cells, even though they rely on tonic BCR signalling for survival. For these reasons, the term ‘chronic active BCR signalling’ was used to describe the constitutive BCR activity in ABC DLBCL36 (FIG. 2).
Figure 2 ∣. Two forms of pathological B cell receptor signalling in lymphoid malignancies.
Chronic active B cell receptor (BCR) signalling, which typifies activated B cell-like diffuse large B cell lymphoma (ABC DLBCL), engages multiple downstream pathways, including nuclear factor-κB (NF-κB). In normal B cells, antigen triggers this form of signalling, and antigen may also have a role in lymphoid malignancies. Tonic BCR signalling, which typifies Burkitt’s lymphoma, engages the phosphoinositide 3-kinase (PI3K) pathway only. Antigen most likely does not contribute to BCR signalling in this context. See main text for details. BTK, Bruton tyrosine kinase; CARD11, caspase recruitment domain-containing protein 11; IgH, immunoglobulin heavy chain; IgL, immunoglobulin light chain; IKK, inhibitor of NF-κB kinase; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; MAPKKK, MAPK kinase kinase; mTOR, mammalian target of rapamycin; NFAT, nuclear factor of activated T cells; PKCβ, protein kinase Cβ.
Mutations affecting the ITAM motifs of CD79A and CD79B in ABC DLBCL establish a genetic basis for chronic active BCR signalling. Over 20% of ABC DLBCL tumours have ITAM mutations, most of which change the first amino-terminal ITAM tyrosine of CD79B to another residue36. Mutations affecting other ITAM residues of CD79B are less common, and mutations in the CD79A ITAM are rarer still. By contrast, CD79 mutations are rare or absent in other lymphoma subtypes, highlighting the importance of BCR signalling in ABC DLBCL.
CD79A and CD79B mutations do not initiate BCR clustering or chronic active signalling in ABC DLBCL, but rather augment chronic active BCR signalling in two ways36. First, the mutations prevent BCR endocytosis, leading to increased expression of the BCR on the cell surface. Second, the CD79B mutants blunt the activity of LYN, a SRC-family tyrosine kinase that delivers negative feedback signals that attenuate BCR activity (see above). Indeed, LYN knockout mice develop a severe autoimmune disease stemming from aberrant BCR-dependent activation of B cells47.
The role of antigen in lymphomas
Many lymphomas may sustain BCR signalling by responding to foreign or self antigens in the tumour microenvironment. A foreign antigen has clearly been implicated in splenic marginal zone lymphoma (SMZL) arising in patients with hepatitis C virus (HCV) infection48. Treatment of these patients with type I interferon clears HCV and eradicates the lymphoma in over 75% of cases49. In one case, the BCR from HCV-associated lymphoma could directly bind the HCV-E2 envelope glycoprotein50, demonstrating a potential role for antigen-dependent BCR signalling in this case.
However, in most lymphomas, evidence favours BCR reactivity to a self antigen. In ABC DLBCL, the presence of BCR clusters on the cell surface raises the possibility that the BCR is engaged by a self antigen51. If this cancer indeed derives from autoreactive B cells, the presence of CD79A and/or CD79B mutations may aid and abet malignant transformation. As described above, normal B cells with a low avidity for a self antigen persist as anergic B cells, and high LYN kinase activity characterizes this state23. Conceivably, the CD79A and CD79B mutations in ABC DLBCL may quell LYN activity in such anergic cells, redirecting BCR signalling towards BTK activation and NF-κB engagement, thereby breaking the anergic state. In epidemiological studies, several systemic autoimmune diseases are risk factors for the development of DLBCL, supporting the hypothesis that ABC DLBCL is a malignancy that may utilize some of the same pathological mechanisms as the autoreactive B cells in these diseases52.
Strong circumstantial evidence for antigenic signalling in CLL comes from immunoglobulin V region analysis. Two main forms of CLL have been discerned: an indolent subtype with highly mutated V regions in the CLL BCR (M-CLL) and an aggressive subtype with few or no BCR mutations (U-CLL)53,54. ZAP70, a paralogue of SYK that can augment BCR signalling, is expressed in U-CLL but not M-CLL55,56. Of the ~65 functional human IgH V gene segments, only a handful are found in CLL (for example, variable heavy (VH)1-69, VH3-21, VH3-07 and VH4-34)57,58. In one-third of CLL cases, including both U-CLL and M-CLL, the malignant cells express ‘stereotyped’ BCRs in which the V regions from unrelated patients are nearly identical58. Stereotypical BCRs converge on these identical sequences despite the V region diversification that occurs during V(D)J recombination and during SHM.
The limited repertoire of V regions in CLL and the selection of stereotyped BCRs imply a role for antigen, and many candidates have been proposed. Both U-CLL and M-CLL seem to be derived from normal B cells with BCRs that react weakly with diverse self antigens59 and are often reactive with antigens that are exposed on apoptotic cells60,61. It is likely that CLL cells encounter these self antigens at high concentration when they reside in the lymph node microenvironment, as it is at this site, rather than in the peripheral blood, that BCR signalling to NF-κB is detectable in the malignant clone62. A recent study proposed a novel form of autoreactivity in CLL, in which the BCR reacts with an invariant epitope on its own V region located in the framework 2 region63. This reactivity caused all CLL-derived BCRs tested to spontaneously signal when introduced into BCR-deficient mouse B cells, a property not shared by BCRs derived from other lymphoid malignancies or from normal B cells. It is not yet clear whether the same CLL-derived BCRs that recognize their own framework 2 region can also recognize other auto-antigens, but these two reactivities are not mutually exclusive. It may be the case that the malignant B cell clone relies on BCR recognition of its own framework 2 regions to sustain itself initially, but that BCR recognition of more classical self antigens, which may help to break tolerance by integrating additional signalling in the malignant B cell, is required to develop CLL.
An unusual form of BCR signalling may occur in follicular lymphoma owing to the frequent introduction of N-linked glycosylation acceptor sites in the V region by SHM64 Consequently, these BCRs are modified by high-mannose oligosaccharides65, leading to the proposal that mannosylated BCRs can interact with mannose-binding lectins present on stromal cells in the tumour microenvironment, thereby crosslinking the BCR and initiating BCR signalling66. Consistent with this, analysis of somatic mutations in the IgH V regions of follicular lymphoma reveals a selection against deleterious mutations in the conserved framework region, suggesting a pressure to maintain a structurally intact BCR on the cell surface67. More recently, one-quarter of follicular lymphoma BCR clones were shown to have some autoreactivity despite ongoing SHM68. However, BCR signalling in follicular lymphoma appears to be more complicated, as malignant subclones exist that cannot not be activated through the BCR upon receptor crosslinking69. Phosphatase inhibition with hydrogen peroxide could reverse this deficit, suggesting an active suppression mechanism in the tumour subclone. Future work will be needed to determine whether this phenotype is a form of B cell anergy or whether this subclone represents a different stage of B cell differentiation, as it also has low CD20 expression69. Importantly, follicular lymphoma tumours with a high proportion of these BCR-inhibited cells were associated with a shorter overall survival69. It will be interesting to investigate whether this subpopulation of BCR-inhibited cells emerges as a mechanism of resistance in patients treated with BCR pathway inhibitors.
Tonic BCR signalling in lymphoma
As tonic BCR signalling to the PI3K pathway is required to maintain the viability of all peripheral B cells in the mouse13-15, it would seem plausible that lymphoid malignancies would take advantage of this mechanism. The clearest example is Burkitt’s lymphoma, an aggressive malignancy derived from germinal centre B cells70. MYC translocations to the heavy chain locus are a hallmark of Burkitt’s lymphoma. However, conditional activation of MYC in mouse germinal centre B cells is insufficient to cause Burkitt’s lymphoma, suggesting that cooperating oncogenic mechanisms are required71. Despite their derivation from the germinal centre, where most B cells undergo class switch recombination, Burkitt’s lymphoma tumour cells almost invariably express IgM-BCRs72, suggesting selection against class switch recombination in Burkitt’s lymphoma and a possible role for signalling from the IgM-BCR. Consistent with this hypothesis, most Burkitt’s lymphoma cell lines die upon knockdown of the BCR component CD79A and the associated kinase SYK70. However, the survival of BCR-dependent Burkitt’s lymphoma lines does not depend on CARD11 or BTK, demonstrating that the BCR signal in Burkitt’s lymphoma is qualitatively distinct from chronic active BCR signalling in ABC DLBCL (FIG. 2). BCR signalling in Burkitt’s lymphoma activates the PI3K pathway, as detected by phosphorylation of AKT and p70 S6 kinase70. Moreover, pharmacologic inhibition of PI3K or treatment with the mammalian target of rapamycin complex 1 (mTORC1) inhibitor rapamycin kills Burkitt’s lymphoma cell lines70. Thus, tonic BCR signalling to PI3K may provide the necessary survival signals to all Burkitt’s lymphoma cells to allow them to tolerate ectopic MYC expression, which is lethal to many primary cells in the absence of growth factors73. Consistent with this hypothesis, conditional activation of PI3K along with MYC in mouse germinal centre B cells causes lymphomas that phenocopy human Burkitt’s lymphoma71.
A genetic basis for tonic BCR signalling in Burkitt’s lymphoma was revealed by genomic resequencing of human Burkitt’s lymphoma tumours, which uncovered many recurrent somatic mutations that distinguish Burkitt’s lymphoma from other lymphoma subtypes70. In particular, ~70% of Burkitt’s lymphoma cases harbour mutations affecting the transcription factor TCF3 (also known as E2A) or its negative regulator inhibitor of DNA binding 3 (ID3). Most of these mutations foster TCF3 transcriptional activity by preventing ID3 from inhibiting TCF3. TCF3 was originally identified and cloned as a factor required for expression of all immunoglobulin genes and TCF3 has been shown to increase expression of the BCR in Burkitt’s lymphoma70. Moreover, TCF3 represses expression of the phosphatase SHP1, a potent negative regulator of BCR signalling. Hence, TCF3 augments BCR signalling in two distinct ways and, accordingly, knockdown of TCF3 decreased PI3K activity in Burkitt’s lymphoma cell lines and was lethal.
A role for tonic BCR signalling has been postulated in GCB DLBCL based on the sensitivity of certain cell lines of this lymphoma subtype to R406, a small molecule inhibitor of SYK74. However, R406 targets several other kinases besides SYK75,76 and thus is not a perfect tool for probing tonic BCR signalling. Genetic knockdown of BCR components (IgM, Igκ, CD79A or CD79B) did not kill various GCB DLBCL cell lines36, suggesting that if SYK is indeed essential in GCB DLBCL, other receptors may have a role in its activation (reviewed in REF. 77).
BCR pathway inhibitors
As described above, many lymphoma subtypes subvert BCR signalling to their malignant purpose, suggesting that pharmacological inhibition of this pathway holds promise in these cancers. However, correct deployment of these inhibitors will require a careful understanding of the type of BCR signalling that is used in each lymphoma subtype (TABLE 2). For example, chronic active BCR signalling in ABC DLBCL engages the SRC-family kinases SYK and BTK to activate downstream NF-κB and PI3K pro-survival pathways. Although less certain, the same pathways are likely to be triggered in other lymphomas in which a foreign or self antigen stimulates the BCR. By contrast, lymphomas that rely upon tonic BCR signalling, such as Burkitt’s lymphoma, depend upon SRC-family kinases and SYK to activate the PI3K pathway, but BTK and the NF-κB pathway are dispensable.
Table 2 ∣.
BCR pathway inhibitors in the clinic
| Target | Inhibitor | Manufacturers | Clinical trials |
|---|---|---|---|
| Chronic active BCR signalling | |||
| BTK | Ibrutinib (PCI-32765) | Pharmacyclics/Janssen Research & Development | Phase III |
| Dasatinib | Bristol-Myers Squibb | Approved | |
| AVL-292 | Celgene | Phase I | |
| PKCβ | Sotrastaurin (AEB071) | Novartis | Phase I |
| PI3Kδ | GS-1101 (CAL-101) | Calistoga Pharmaceuticals/Gilead Sciences | Phase II |
| Chronic active and tonic BCR signalling | |||
| SYK | Fostamatinib (R788) | Rigel Pharmaceuticals/AstraZeneca | Phase II |
| PRT062607 | Portola Pharmaceuticals/Biogen Idec | Phase I | |
| Pan-PI3K | BKM120 | Novartis | Phase I* |
| GDC-0941 | Genentech | Phase Ib* | |
| XL147 | Exelixis | Phase I* | |
| ZSTK474 | Zenyaku Kogyo Co. | Phase I* | |
| SRC family | Saracatinib | AstraZeneca | Phase II |
| KX01 | Kinex Pharmaceuticals | Phase II | |
| Dasatinib | Bristol-Myers Squibb | Approved | |
| TORC1 | Rapamycin (sirolimus) | Wyeth/Pfizer | Approved |
| Everolimus | Novartis | Phase III | |
| Temsirolimus | Wyeth/Pfizer | Approved | |
BCR, B cell receptor; BTK, Bruton tyrosine kinase; PI3K, phosphoinositide 3-kinase; PKCβ, protein kinase Cβ; TORC1, target of rapamycin complex 1.
Clinical trials in solid tumours only.
As with any targeted agent in cancer, a crucial goal is to identify molecular predictors of therapeutic response so that the right drug can be chosen for each patient. In this regard, an important conceptual distinction exists between the development of kinase inhibitors in epithelial cancers and BCR signalling inhibitors in lymphoma. Whereas the addiction of epithelial tumours to a signalling pathway stems from oncogenic gain-of-function mutations in the pathway, such as in the epidermal growth factor receptor or B-RAF, the addiction of lymphomas to BCR signalling may not be directly related to oncogenic mutations but rather a consequence of BCR engagement by an antigen. Indeed, although CLL appears to be responsive to BCR pathway inhibitors (see below), genomic resequencing has failed to identify genetic aberrations that activate the BCR pathway78-80. Likewise, although CD79A and CD79B mutations are likely to identify ABC DLBCL tumours with chronic active BCR signalling, some ABC DLBCL cell lines with chronic active BCR signalling have wild-type CD79A and CD79B36.
SYK inhibitors
SYK is a non-receptor tyrosine kinase that is recruited and activated by phosphorylated ITAMs following BCR engagement (reviewed in REF. 81). As such, SYK is crucial to antigen-dependent BCR signalling, which includes chronic active BCR signalling in ABC DLBCL. Genetically, SYK is required for tonic BCR signalling in Burkitt’s lymphoma, but the role of SYK kinase activity in this setting has not been addressed. SYK has additional diverse roles in several haematopoietic lineages that can be traced to its involvement in integrin and selectin signalling and in certain innate immune receptors that sense fungal pathogens81. Thus, SYK inhibitors may have on-target side effects outside the B cell lineage that may or may not limit their clinical utility.
Rigel Pharmaceuticals and AstraZeneca developed fostamatinib (R788), the first SYK inhibitor to enter clinical trials for lymphoma and certain autoimmune and inflammatory disorders. Fostamatinib is an orally available pro-drug version of the active R406 compound, which is an ATP-competitive inhibitor. In vitro kinase assays demonstrated that R406 is only somewhat selective for SYK, and can inhibit Fms-like tyrosine kinase 3 (FLT3), KIT, lymphocyte cell-specific protein-tyrosine kinase (LCK), Janus kinase 1 (JAK1), JAK3, RET and the adenosine A3 receptor with similar potencies75,76. Constitutive phosphorylation of SYK on Y352 was observed in CLL cells, which is consistent with BCR signalling. R406 treatment induced apoptosis of CLL cells82 and also antagonized the beneficial influence of stromal cells on CLL viability83. Likewise, R406 is toxic to ABC DLBCL cell lines with chronic active BCR signalling36. In vitro studies determined that R406 could induce apoptosis in certain GCB DLBCL cell lines74, although as noted above, GCB DLBCL lines do not appear to rely upon BCR signalling.
In preclinical studies of mouse lymphoma models with antigen-dependent BCR signalling, fostamatinib prolonged survival84. Likewise, fostamatinib inhibited lymphoma growth in the Eμ-TCL1 model, which recapitulates certain aspects of CLL, including the use of stereotyped BCRs and BCRs that recognize antigens from dying cells85.
Fostamatinib has completed Phase I and Phase II clinical trials examining its toxicity and efficacy in the context of autoimmune diseases and haematological malignancies. A Phase I/II clinical trial of fostamatinib in relapsed or refractory non-Hodgkin’s lymphoma of various subtypes indicated significant clinical activity86. Common toxicities included diarrhoea, fatigue, leucopoenia, neutropoenia and anaemia. The study reported objective response rates of 55% in CLL, 24% in DLBCL, 11% in mantle cell lymphoma (MCL) and 10% in follicular lymphoma. It was noted in this study that all patients with CLL experienced an increase in circulating lymphocytes following initial treatment, a phenomenon that has also been noted in patients with CLL who were treated with other BCR pathway inhibitors87. The mutational status of the patients with CLL was not discussed in this study, but it would be of interest to know whether fostamatinib was selectively toxic to either U-CLL or M-CLL. The DLBCL tumours were not classified into ABC and GCB subtypes, so it is not clear whether fostamatinib was inhibiting chronic active BCR signalling in ABC DLBCL or exerting some other antitumour effect.
A second SYK inhibitor from Portola Pharmaceuticals, termed PRT062607, is being developed collaboratively with Biogen IDEC and is currently in Phase I trials. Initial trials will target autoimmune and inflammatory diseases based on preclinical activity in a mouse collagen antibody-induced arthritis model88. PRT062607 also has in vitro anti-proliferative activity against DLBCL cell lines and can inhibit BCR signalling89.
BTK inhibitors
BTK is a member of the TEC family of non-receptor kinases and contains an N-terminal PH domain followed by a proline-rich domain, an SH3 domain, an SH2 domain and a carboxy-terminal kinase domain. Inactivating mutations in BTK are the cause of X-linked agammaglobulinaemia (XLA)90, in which B cell development and antibody production is severely attenuated, rendering these patients susceptible to infection. Similar mutations in the mouse Btk gene cause X-linked immunodeficiency (XID)91, which is characterized by a partial block in B cell development, and the same phenotype occurs with a full Btk knockout92,93. Outside the B cell lineage, BTK deficiency has minimal consequences, suggesting that BTK inhibitors should have an excellent safety profile.
BTK is essential for antigen-stimulated BCR signalling. BTK is recruited to the BCR signalosome through two mechanisms. First, the BTK PH domain recognizes the lipid PIP3, which is produced by PI3K following BCR crosslinking, causing BTK to interact with the inner leaflet of the plasma membrane. Second, SYK phosphorylation of the adaptor BLNK causes BTK recruitment via the SH2 domain of BTK (reviewed in REF. 94). Once in the BCR signalosome, SRC-family kinases phosphorylate BTK on Y551 in its activation loop95,96, which in turn stimulates autophosphorylation of Y223 for full activation of BTK97. Active BTK phosphorylates and activates PLCγ2 (REFS 98,99), activating many downstream pathways, including NF-κB (reviewed in REF. 97). Thus, therapeutic targeting of BTK is rational in ABC DLBCL and other lymphoid malignancies that use an antigen-dependent chronic active form of BCR signalling. BTK inhibitors are likely to have little effect on tonic signalling, which relies on PI3K signalling, not NF-κB signalling. Indeed, the tonic BCR signalling in Burkitt’s lymphoma cell lines is not inhibited by genetic or pharmacologic inactivation of BTK (R.M.Y. and L.M.S., unpublished results).
Several kinase inhibitors capable of blocking BTK have recently entered clinical trials in lymphoid malignancies. Dasatinib (BMS-354825; Spycell) is a multi-kinase inhibitor used to target the BCR-ABL fusion protein produced by the t(9;22) translocation in chronic myelogenous leukaemia. Dasatinib also efficiently targets SRC-family kinases and BTK100. Treatment of ABC DLBCL lines with dasatinib blocked chronic active BCR signalling and induced cell death36. Although promising, trials of dasatinib in DLBCL have not been reported. A Phase II trial of dasatinib in CLL yielded a 20% objective response rate101.
A more selective and potent BTK inhibitor, ibrutinib (PCI-32765), that covalently modifies the enzyme has recently been developed by Pharmacyclics102. Ibrutinib is an irreversible BTK inhibitor because it forms a covalent bond with cysteine 481 near the active site of BTK. Only nine other kinases in the human genome have a similarly placed cysteine residue, lending ibrutinib a high level of specificity102. Celgene is developing another BTK inhibitor, AVL-292, that covalently attaches to the same cysteine in BTK as ibrutinib. In vitro studies of ibrutinib determined an IC50 of 0.5 nM for BTK102,103. In cell-based assays, ibrutinib was toxic for ABC DLBCL cell lines at low nanomolar concentrations36. Moreover, ibrutinib showed preclinical activity in canine lymphomas103. Preclinical studies in mouse models of lymphoma determined that ibrutinib treatment only affected mouse tumours that use chronic active BCR signalling and not tumours dependent on tonic BCR signals104.
In CLL, ibrutinib decreased DNA synthesis and viability of ex vivo cultured primary cells, whether or not supporting stromal cells or cytokines were present105,106. In a mouse model using Eμ-TCL1 transgenic CLL-like cells, ibrutinib prevented disease progression106. In early phase studies of patients with CLL, ibrutinib decreased plasma expression of the chemokines CLL3 and CLL4 (REF. 106), which is thought to be the consequence of BCR signalling in CLL cells within the lymph node microenvironment62. Ibrutinib inhibition of BTK may also block the ability of chemokines within the lymph node microenvironment to induce homing through C-X-C chemokine receptor type 4 (CXCR4) and CXCR5 and adhesion of CLL via integrins87, potentially explaining why ibrutinib treatment of patients with CLL induces a transient increase of CLL cells in the circulation107.
Both ibrutinib and AVL-292 are in clinical trials in lymphoid malignancies, but published data only exist for ibrutinib. An initial Phase I study used escalating doses of ibrutinib in a cohort of 47 patients with various forms of relapsed/refractory non-Hodgkin’s lymphoma108. No cumulative haematological toxicities were noted, and ibrutinib was generally well tolerated with some side effects that included neutropoenia and hypersensitivity reactions. An objective response (complete response (CR) or partial response (PR)) was observed in 20 of the 47 patients, including patients with CLL (9/15), MCL (3/4), follicular lymphoma (4/15), DLBCL (3/8) and marginal zone lymphoma (1/3).
Given the strong preclinical evidence that ibrutinib inhibits chronic active BCR signalling in ABC DLBCL, the Phase I trial was extended at the 560 mg per day dose to include ten patients with relapsed/refractory ABC DLBCL109. In this cohort, two patients achieved CR and one achieved PR, and the character of the responses was exceptional. One patient has remained in CR for over 2 years, taking ibrutinib daily as monotherapy without discernable side effects. Another patient with primary refractory disease that had not responded to any chemotherapy regimen had virtually complete resolution of massive abdominal disease.
Based on these results, a Phase II trial is being conducted at 560 mg per day to evaluate the efficacy of ibrutinib in patients with relapsed refractory DLBCL (n = 70), with the ABC and GCB subtypes identified by gene expression profiling110. Among patients with ABC DLBCL, an objective response rate of 40% was observed, including both complete and partial responses, suggesting that a substantial fraction of ABC DLBCL tumours depend upon BCR signalling. By contrast, only one partial response was observed among patients with GCB DLBCL (5%), which is consistent with the view that this subtype of DLBCL has minimal dependence on BCR signalling36. Sequence analysis was performed for genes in the BCR pathway that are frequently mutated in ABC DLBCL. Responses were more frequent among ABC DLBCLs with a CD79B ITAM mutation (60%), as expected. Importantly, responses were also frequent in patients whose tumours were wild type for CD79B (37%), demonstrating that chronic active BCR signalling in ABC DLBCL may not require oncogenic mutations. CARD11 mutant ABC DLBCL tumours did not respond to ibrutinib, consistent with CARD11 sitting downstream of BTK in the BCR signalling cascade. Together, these results argue for the use of the ABC versus GCB DLBCL distinction as a biomarker of ibrutinib response, with additional predictive value provided by mutational analysis.
As discussed above, abundant circumstantial evidence exists for BCR signalling in the pathogenesis of CLL, and clinical trials with ibrutinib provide strong support for this hypothesis. Phase II trials have administered ibrutinib with a median follow-up of 17.3 months with minimal and acceptable toxicity107,111. An objective response rate of 67% was seen in relapsed/refractory disease107,111. In elderly, treatment-naive patients for whom standard treatment is too toxic, a response rate of 71% was observed, with 61% PR and 10% CR111. Ibrutinib was effective in both the aggressive U-CLL subtype and the indolent M-CLL subtype. Importantly, ibrutinib responses occur in patients with known molecular risk factors, including inactivation of p53 (that is, del(17p)) and ataxia telangiectasia mutated (ATM; that is, del(11q))107.
Although less evidence exists for BCR signalling in MCL, recent support for this possibility came from V region sequencing, which revealed a restricted repertoire with stereotyped BCRs in some cases112. A Phase II trial of ibrutinib in relapsed/refractory patients with MCL at 560 mg per day yielded an objective response rate of 65.1% (n = 63) in a bortezomib-naive cohort and 67.4% (n = 46) in a cohort of patients previously treated with bortezomib113. This striking response rate suggests that further investigation is warranted into the nature of BCR signalling in MCL.
PI3K, AKT and mTOR inhibitors
The PI3K pathway is emerging as a promising therapeutic target in B cell lymphomas that utilize pathogenic chronic active or tonic BCR signalling. PI3K activity provides a direct conduit from diverse stimuli, including cell surface receptors and metabolic changes within the cell, to downstream pathways affecting cellular growth, size, survival and angiogenesis, among others (reviewed in REFS 9,114). It comes as no surprise then that PI3K and its downstream effectors and regulators, such as phosphatase and tensin homologue (PTEN), are frequently deregulated in human cancer114, and hence the development of PI3K pathway inhibitors has been an active area of research for many years.
PI3K initiates downstream signalling by recruiting signalling effectors to the plasma membrane (reviewed in REF. 115). PI3K is a lipid kinase that catalyses the phosphorylation of the 3-hydroxy position of phosphatidylinositols and phosphoinositides in the inner leaflet of the plasma membrane, most significantly phosphorylating PI(4,5)P2 to form PIP3. Conversely, PI3K activity is antagonized by PTEN, which is a PIP3 3-phosphatase, and by SHIP1, which is a PIP3 5-phosphatase. 3-phosphorylated phosphoinositides recruit cytosolic proteins containing PH, FYVE (FAB1, YOTB, VAC1 and EEA1) or PX (phox homology) domains to the plasma membrane, where they affect signalling. The PH domain of the kinase AKT mediates AKT recruitment to the plasma membrane in response to BCR signalling, resulting in AKT phosphorylation and activation. AKT in turn interacts with a multitude of signalling effectors that affect growth, proliferation and survival114. Prominent among these is mTORC1, a kinase that increases protein and lipid synthesis and energy production (reviewed in REF. 116). Besides the AKT–mTOR pathway, the generation of PIP3 by PI3K causes BTK to translocate to the plasma membrane, facilitating its activation during BCR signalling.
In vitro studies of lymphoma cell lines and primary cells have revealed PI3K pathway dependence in several subtypes. ABC DLBCL cell lines with chronic active BCR signalling engage PI3K, which augments anti-apoptotic NF-κB signalling and may also provide additional survival signals36,117. The tonic BCR signalling that characterizes some Burkitt’s lymphoma cell lines activates the PI3K pathway and downstream AKT and mTORC1 signalling70,71. In MCL, primary biopsy samples from highly proliferative (blastoid) cases showed AKT phosphorylation in all cases, and PI3K inhibition was toxic to MCL cell lines118. Although the molecular mechanisms that activate PI3K in MCL have not been fully elucidated, BCR signalling is plausibly involved given the efficacy of the BTK inhibitor ibrutinib in many cases (see above).
Three classes of PI3K exist, but only class I PI3Ks can phosphorylate PI(4,5)P2 to generate PIP3, which is perhaps the most important phosphoinositide species produced downstream of BCR signalling115. The several class I PI3Ks are composed of a regulatory (p85) subunit and one of four catalytic (p110) subunits to yield PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ. These different PI3K isoforms have both unique and redundant functions in B cells. PI3Kδ has a non-redundant role downstream of active BCR signalling119, whereas PI3Kα and PI3Kδ are both required for B cell development120, and a constitutively active form of PI3Kα can substitute for tonic BCR signalling in mice15. As discussed above, the mechanisms that engage PI3K during tonic BCR signalling have not been elucidated, and may either involve SYK16 or TC21 (REF. 17). Following antigen stimulation, BCR-dependent phosphorylation of CD19 and B cell adaptor for PI3K (BCAP; also known as PIK3AP1) recruits PI3Kδ via its SH2 domains, resulting in PI3Kδ activation. In addition to the BCR pathway, PI3Ks — including PI3Kδ — are effectors downstream of other cell surface receptors, including CD40, tumour necrosis factor receptors (TNFRs) and B cell activating factor (BAFF) receptors.
A variety of PI3K pathway inhibitors are available in the clinic, or are now in clinical trials. GS-1101 (CAL-101) from Calistoga Pharmaceuticals/Gilead Sciences is a potent and specific PI3Kδ inhibitor, achieving an IC50 of 2.5 nM in vitro versus 500–900 nM for PI3Kβ and PI3Kα, respectively121,122. GS-1101 induced apoptosis in ex vivo CLL cultures from both U-CLL and M-CLL tumours at concentrations approaching 10 μM, but this concentration of drug did not affect normal immune cell survival ex vivo123. Moreover, GS-1101 was preferentially toxic to CLL samples with high levels of PI3Kδ. Another study used ex vivo co-cultures of CLL cells and stromal cells to mimic the tumour microenvironment more closely124. In this setting, GS-1101 inhibited CLL cells blocking both BCR-dependent signalling and chemokine receptor signalling induced by C-X-C chemokine ligand 12 (CXCL12; also known as SDF1) and CXCL13 in the malignant cells. Interestingly, inhibition of stromal-dependent chemokine effects was achieved at a lower drug concentration than inhibition of BCR signalling124. GS-1101 also abolishes AKT phosphorylation (S573) in cell line models of DLBCL and in primary MCL cells ex vivo122.
An early Phase I dose escalation study in relapsed/refractory CLL reported that GS-1101 was well tolerated, decreased levels of phospho-AKT in patient tumour cells and reduced lymphadenopathy in all patients (32/32)125. Overall, the objective response rate in patients with CLL treated with GS-1101 was 26% at 11 months125. The increased levels of CXCL13 in sera from patients with CLL were reduced after one dose of GS-1101, correlating with an increase in peripheral lymphocytes and CLL cells. Thus, GS-1101 may abrogate chemokine signalling that keeps normal and malignant lymphocytes within the lymph node microenvironment. As the lymph node environment is crucial for BCR signalling and survival in CLL, the egress of CLL cells from their protective niche may contribute to the efficacy of GS-1101 (REF. 62).
Another Phase I clinical trial examined the effect of escalating doses of GS-1101 in indolent and aggressive lymphomas126. Only partial responses were seen, with rates of 62% (15/24) for indolent non-Hodgkin’s lymphoma, 62% (10/16) for MCL and 0% (0/9) for DLBCL. However, the DLBCL cases were not subclassified by their molecular subtype, so it is unclear whether PI3K-dependent ABC DLBCLs were included in this study.
In addition to isoform-specific PI3K inhibitors, several pan-PI3K inhibitors are in clinical trials for solid cancers. One of these, NVP-BKM120 from Novartis, was toxic to most Burkitt’s lymphoma cell lines that rely on PI3K activity for their survival70.
Downstream of PI3K, AKT is an attractive target, and many inhibitors are in development127. mTORC1 has been targeted by rapamycin and its analogues, yielding response rates of 20–38% in MCL128,129 and 30% in relapsed/refractory DLBCL130. Given that all Burkitt’s lymphoma cell lines were killed by rapamycin treatment in vitro, rapamycin analogues should be evaluated in this lymphoma as well. Of course, it should be noted that mTORC1 receives inputs from many signalling and metabolic pathways, so the activation of this kinase may not necessarily be a result of BCR signalling.
Other BCR pathway inhibitors
The initial biochemical step in BCR signalling is phosphorylation of ITAM tyrosines on CD79A and CD79B by SRC-family kinases. Moreover, SRC kinases are crucial to B cell development and have a presumed role in tonic BCR signalling5. As such, inhibiting these kinases would be a rational method for inhibiting BCR-dependent signalling in lymphoma. Owing to the functional redundancy of the SRC-family kinases, it is difficult to assess the contribution of individual family members to BCR signalling in lymphoma by genetic methods. Pharmacological approaches to inhibiting SRC kinases are complicated by off-target effects of many inhibitors. As described above, dasatinib is a multikinase inhibitor that can inhibit SRC kinase activity in the 50–200 nM range in ABC DLBCL lines, but as it is also able to inhibit BTK, it is unclear whether its toxicity for these cells is due to SRC kinase inhibition36. A new generation of more specific SRC kinase inhibitors, including SU6656 (Sugen, Inc.), saracatinib (Biovision), KX-01 (Kinex Pharmaceuticals), CGP76030 (Novartis) and bosutinib (Pfizer), is currently entering clinical trials in solid tumours. These new inhibitors may prove effective in lymphomas that rely on either chronic active or tonic BCR signalling, and they may be useful compounds for dissecting the biochemical details of BCR signalling in these malignancies.
PKCβ functions downstream of BTK and upstream of IKK and is therefore another potential therapeutic target in lymphomas with BCR signalling. PKCβ is a classical isoform of PKC that is activated by the products of PLCγ2 activity, DAG and increased intracellular calcium10. Activated PKCβ phosphorylates CARD11 to promote NF-κB activation8. Knockdown of PKCβ is toxic to ABC DLBCL cell lines that rely on chronic active signalling36, as is treatment with either pan-PKC inhibitors or PKCβ-specific inhibitors44. PKCβ may also have a role in CLL, as Eμ-TCL1 mice failed to develop a CLL-like disease when crossed with PKCβ-deficient mice131. The PKC inhibitor enzastaurin is in clinical development for lymphoma132,133, but enzastaurin did not selectively kill DLBCL cells that are dependent on BCR signalling44. However, a PKCβ-specific inhibitor from Novartis, sotrastaurin (AEB071), was selectively toxic to ABC DLBCL cell lines that rely on chronic active BCR signalling44, prompting Phase I trial evaluation in ABC DLBCL.
The CBM complex is required to recruit and activate IKKβ during antigen receptor signalling in normal and malignant lymphocytes. Recently, a catalytic function for MALT1 has emerged in which its caspase-like domain functions as a protease to cleave various protein substrates after arginine residues134-136. One substrate of MALT1 is A20, a negative regulator of NF-κB signalling, and another is RELB, an NF-κB subunit that is specific for the non-canonical NF-κB signalling cascade downstream of NF-κB-inducing kinase (NIK; also known as MAP3K14) and IKKα. As such, MALT1 catalytic activity preferentially activates the classical NF-κB pathway that is the consequence of IKKβ activity. As this limb of the NF-κB pathway is required for ABC DLBCL survival40,137, MALT1 inhibition is selectively toxic for these lymphoma cells138. These considerations support the identification and development of MALT1 inhibitors. As there are no other proteins encoded in the human genome with domains that resemble the MALT1 paracaspase domain, MALT1 inhibitors could have a good safety profile.
Finally, direct inhibition of IKKβ, which is activated by BCR signalling and is required for survival in many lymphoma subtypes, could be considered. Given the essential role of IKKβ and NF-κB in normal immune cell function and in a host of other stress responses, the development of drugs targeting this kinase has proceeded slowly (reviewed in REF. 139). Although long-term administration of IKKβ inhibitors in chronic autoimmune and inflammatory diseases might be problematic, their short-term use in cancer might be manageable. Given the ability of NF-κB to block the cytotoxic activity of conventional chemotherapeutic agents140, IKKβ inhibitors might be useful in combination with these drugs.
Combination therapies
Other oncogenic pathways in lymphoma might in some cases potentiate BCR signalling but could alternatively provide redundant proliferation and/or survival signals that might provide mechanisms of acquired resistance to BCR-directed therapies. A recent instructive example of these principles is oncogenic myeloid differentiation primary response protein 88 (MYD88) signalling in ABC DLBCL141. MYD88, a key adaptor protein in Toll receptor and interleukin-1 receptor signalling, is altered by gain-of-function somatic mutations in 39% of ABC DLBCL tumours. One highly recurrent mutation, L265P, accounts for 29% of cases. Notably, this same mutant has been identified at varying frequencies in CLL78-80, primary cutaneous DLBCL142, primary central nervous system lymphoma143, marginal zone lymphomas141,144,145 and Waldenstrom’s macroglobulinaemia146. In a similar way to BCR signalling, MYD88 signalling in ABC DLBCL activates NF-κB, but MYD88 uses a different pathway to IKKβ involving the kinases interleukin-1 receptor-associated kinase 1 (IRAK1) and IRAK4. From this perspective, oncogenic MYD88 mutations might make ABC DLBCL cells resistant to BCR pathway inhibitors by activating NF-κB in a parallel, redundant fashion. However, it is known that BCR and MYD88 signalling pathways can cooperate in normal and autoimmune B cells147,148. Of particular interest therefore was the significant co-occurrence in ABC DLBCL tumours of MYD88 L265P and CD79B mutations141. This genetic evidence suggests that the MYD88 pathway might in some way aid and abet BCR signalling. Consistent with this hypothesis, ABC DLBCL cell lines with MYD88 L265P are not resistant to inhibition of the BCR pathway141.
Ongoing experience with targeted agents in cancer teaches that single drugs are rarely effective in achieving long-term remission owing to existing or acquired compensatory pathways. From this perspective, cures in cancer will only come from the rational combination of targeted agents, informed by a deep knowledge of signalling pathways and their interconnectedness. For example, simultaneous inhibition of the BCR and MYD88 pathways by RNA interference was more toxic than inhibition of either pathway alone, probably due to the fact that both pathways activate pro-survival NF-κB signalling. Hence, a rational therapeutic cocktail might combine a BCR pathway inhibitor with an IRAK4 inhibitor, given the essential role of IRAK4 activity in MYD88 signalling141.
An emerging and powerful concept in cancer therapy is synthetic lethality, which describes the situation in which oncogenic mutations render cancer cells exquisitely sensitive to drugs that have little if any effect on normal cells that lack these mutations. In some instances, synthetic lethality occurs when the oncogene creates a gain-of-function signalling capability that can be exploited therapeutically. In others, a cancer mutation may remove one of two redundant pathways, making the cancer cell particularly vulnerable to drugs that attack the remaining pathway.
A surprising example of synthetic lethality was recently uncovered in ABC DLBCL with the MYD88 L265P mutation, leading to the demonstration that these cancer cells can be efficiently killed by combining BCR pathway inhibitors with lenalidomide, a cancer drug of relatively obscure function149. Lenalidomide has been approved for use in multiple myeloma and myelodysplatic syndrome and has recently been reported to produce complete and partial remissions in relapsed/refractory ABC DLBCL150. Unexpectedly, treatment of ABC DLBCL cell lines with lenalidomide induced secretion of interferon-β and the activation of the type I interferon response. The cytotoxic effect of lenalidomide on ABC DLBCL cells was traced to this interferon-β production, and MYD88 L265P was required for this dysregulated interferon response. In other words, lenalidomide was synthetically lethal for ABC DLBCL cells owing to their oncogenic MYD88 L265P mutations. The transcription factor interferon regulatory factor 4 (IRF4) is key to this dysregulated interferon response, as it directly represses the transcription of IRF7, which encodes a key positive regulator of interferon-β expression. By an as yet unclear mechanism, lenalidomide causes IRF4 mRNA and protein levels to drop, thereby relieving the IRF4 blockade of interferon-β expression. IRF4 is regulated in a separate fashion by BCR signalling, as it is a direct target of the NF-κB transcription factors137,151. Consequently, simultaneous treatment of ABC DLBCL cell lines with ibrutinib, to inhibit BCR signalling, and lenalidomide caused the virtual disappearance of IRF4, a dramatic induction of interferon-β secretion and synergistic killing in vitro and in vivo in xenograft models149. These results support clinical trials testing ibrutinib plus lenalidomide in ABC DLBCL, anticipating that tumours with MYD88 L265P and chronic active BCR signalling will be the most sensitive to this combination.
Conclusions
The definition of pathological BCR signalling in lymphoma has led to the rapid development of BCR pathway inhibitors for this indication. Thus far, these therapeutic agents have had remarkable success in treating patients with cancers that were in many cases resistant to conventional chemotherapeutic agents. Moreover, the side effect profile of BCR-targeted therapies appears to be easily manageable, although it remains to be seen what long-term toxicities might emerge if patients with lymphoma need to be maintained on BCR pathway inhibitors continuously.
Two paradigms of BCR signalling in lymphoma have emerged — chronic active BCR signalling and tonic BCR signalling — and each signalling pathway offers different opportunities for therapeutic intervention. An important goal for the future will be to learn how to identify lymphomas that depend on BCR signalling and to classify them according to the type of BCR signalling that they use. It is unlikely that the analysis of oncogenic mutations will be sufficient. For example, ABC DLBCL tumours that have CD79B mutations are responsive to the BTK inhibitor ibrutinib, but so are some ABC DLBCL tumours with no mutations in the BCR110. Likewise, ibrutinib has high activity in CLL, but no mutations that clearly activate BCR signalling have been identified in genomic resequencing studies. Surrogate markers of BCR signalling will be quite important, therefore, which might take the form of a gene expression signature of BCR signalling62, an analysis of phosphoproteins that are produced by BCR signalling69, or perhaps analysis of secreted cytokines107. Genetic analysis will still be important, however, as some oncogenic mutations make lymphomas resistant to BCR pathway inhibitors. For example, ~10% of patients with ABC DLBCL have oncogenic mutations in CARD11 that obviate the need for chronic BCR signalling, rendering the lymphoma cells resistant to ibrutinib39. Phase II trials with deep and sophisticated correlative science will be needed to optimize the promise of BCR pathway inhibitors in lymphoma.
Acknowledgements
This research was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Campo E et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117, 5019–5032 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dameshek W & Schwartz RS Leukemia and auto-immunization- some possible relationships. Blood 14, 1151–1158 (1959). [PubMed] [Google Scholar]
- 3.Clark MR, Tanaka A, Powers SE & Veselits M Receptors, subcellular compartments and the regulation of peripheral B cell responses: the illuminating state of anergy. Mol. Immunol 48, 1281–1286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reth M Antigen receptor tail clue. Nature 338, 383–384 (1989). [PubMed] [Google Scholar]
- 5.Saijo K et al. Essential role of Src-family protein tyrosine kinases in NF-κB activation during B cell development. Nature Immunol. 4, 274–279 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Rowley RB, Burkhardt AL, Chao HG, Matsueda GR & Bolen JB Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem 270, 11590–11594 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Oellerich T et al. The B-cell antigen receptor signals through a preformed transducer module of SLP65 and CIN85. EMBO J. 30, 3620–3634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinohara H et al. PKCβ regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med 202, 1423–1431 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deane JA & Fruman DA Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol 22, 563–598 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Dal Porto JM et al. B cell antigen receptor signaling 101. Mol. Immunol 41, 599–613 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Victora GD & Nussenzweig MC Germinal centers. Annu. Rev. Immunol 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Shaffer AL, Young RM & Staudt LM Pathogenesis of human B cell lymphomas. Annu. Rev. Immunol 30, 565–610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam KP, Kuhn R & Rajewsky K In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90, 1073–1083 (1997).This study established the notion of tonic BCR signalling in B cells.
- 14.Kraus M, Alimzhanov MB, Rajewsky N & Rajewsky K Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell 117, 787–800 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan L et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139, 573–586 (2009).This article identified the role of PI3K in tonic BCR signalling.
- 16.Monroe JG ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nature Rev. Immunol 6, 283–294 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Delgado P et al. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nature Immunol. 10, 880–888 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Cambier JC & Johnson SA Differential binding activity of ARH1/TAM motifs. Immunol. Lett 44, 77–80 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Wienands J, Larbolette O & Reth M Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc. Natl Acad. Sci. USA 93, 7865–7870 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J & Reth M Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 467, 465–469 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Wardemann H et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Yurasov S & Nussenzweig MC Regulation of autoreactive antibodies. Curr. Opin. Rheumatol 19, 421–426 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Yarkoni Y, Getahun A & Cambier JC Molecular underpinning of B-cell anergy. Immunol. Rev 237, 249–263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quach TD et al. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J. Immunol 186, 4640–4648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill SK et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity 35, 746–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne CD, Del Nagro CJ, Cato MH, Dengler HS & Rickert RC Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity 31, 749–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuppers R Mechanisms of B-cell lymphoma pathogenesis. Nature Rev. Cancer 5, 251–262 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Nussenzweig A & Nussenzweig MC Origin of chromosomal translocations in lymphoid cancer. Cell 141, 27–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin SW & Goodnow CC Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nature Immunol. 3, 182–188 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Horikawa K et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med 204, 759–769 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan I et al. Multiple layers of B cell memory with different effector functions. Nature Immunol. 10, 1292–1299 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Staudt LM A closer look at follicular lymphoma. N. Engl. J. Med 356, 741–742 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Vaandrager JW et al. DNA fiber fluorescence in situ hybridization analysis of immunoglobulin class switching in B-cell neoplasia: aberrant CH gene rearrangements in follicle center-cell lymphoma. Blood 92, 2871–2878 (1998). [PubMed] [Google Scholar]
- 34.Alizadeh AA et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Lenz G et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J. Exp. Med 204, 633–643 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis RE et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92 (2010).This is the first study to show direct evidence for BCR signalling in human lymphoma, including the discovery of CD79 mutations and sensitivity of ABC DLBCL to the BTK inhibitor ibrutinib.
- 37.Ruminy P et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia 25, 681–688 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Ngo VN et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Lenz G et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Davis RE, Brown KD, Siebenlist U & Staudt LM Constitutive nuclear factor κB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med 194, 1861–1874 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawlings DJ, Sommer K & Moreno-Garcia ME The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nature Rev. Immunol 6, 799–812 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Bajpai UD, Zhang K, Teutsch M, Sen R & Wortis HH Bruton’s tyrosine kinase links the B cell receptor to nuclear factor κB activation. J. Exp. Med 191, 1735–1744 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petro JB, Rahman SM, Ballard DW & Khan WN Bruton’s tyrosine kinase is required for activation of IκB kinase and nuclear factor κB in response to B cell receptor engagement. J. Exp. Med 191, 1745–1754 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naylor TL et al. Protein kinase C inhibitor Sotrastaurin selectively inhibits the growth of CD79-mutant diffuse large B-cell lymphomas. Cancer Res. 71, 2643–2653 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Tolar P, Sohn HW, Liu W & Pierce SK The molecular assembly and organization of signaling active B-cell receptor oligomers. Immunol. Rev 232, 34–41 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Thome M CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nature Rev. Immunol 4, 348–359 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Chan VW, Meng F, Soriano P, DeFranco AL & Lowell CA Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity 7, 69–81 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Marcucci F & Mele A Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 117, 1792–1798 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Gisbert JP, Garcia-Buey L, Pajares JM & Moreno-Otero R Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment. Pharmacol. Ther 21, 653–662 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Quinn ER et al. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood 98, 3745–3749 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Rui L, Schmitz R, Ceribelli M & Staudt LM Malignant pirates of the immune system. Nature Immunol. 12, 933–940 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Smedby KE et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J. Natl Cancer Inst 98, 51–60 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Damle RN et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 94, 1840–1847 (1999). [PubMed] [Google Scholar]
- 54.Hamblin TJ, Davis Z, Gardiner A, Oscier DG & Stevenson FK Unmutated Ig VH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 94, 1848–1854 (1999). [PubMed] [Google Scholar]
- 55.Rosenwald A et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J. Exp. Med 194, 1639–1648 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 100, 4609–4614 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Fais F et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Invest 102, 1515–1525 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agathangelidis A et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood 119, 4467–4475 (2012).The authors examined the IgH V region sequences from 7,424 CLL patients and found that one-third of them possessed stereotyped receptors.
- 59.Herve M et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J. Clin. Invest 115, 1636–1643 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catera R et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol. Med 14, 665–674 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu CC et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood 115, 3907–3915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herishanu Y et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-κB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 117, 563–574 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minden MD et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 489, 309–312 (2012).This study found that BCRs from CLL patients can recognize an internal epitope on their own BCR, which leads to chronic activation.
- 64.Zhu D, Ottensmeier CH, Du MQ, McCarthy H & Stevenson FK Incidence of potential glycosylation sites in immunoglobulin variable regions distinguishes between subsets of Burkitt’s lymphoma and mucosa-associated lymphoid tissue lymphoma. Br. J. Haematol 120, 217–222 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Radcliffe CM et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem 282, 7405–7415 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Coelho V et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc. Natl Acad. Sci. USA 107, 18587–18592 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuckerman NS et al. Ig gene diversification and selection in follicular lymphoma, diffuse large B cell lymphoma and primary central nervous system lymphoma revealed by lineage tree and mutation analyses. Int. Immunol 22, 875–887 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Sachen KL et al. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood 120, 4182–4190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irish JM et al. B-cell signaling networks reveal a negative prognostic human lymphoma cell subset that emerges during tumor progression. Proc. Natl Acad. Sci. USA 107, 12747–12754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitz R et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).This article demonstrates a role for tonic BCR signalling and PI3K activity in Burkitt’s lymphoma.
- 71.Sander S et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell 22, 167–179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein U, Klein G, Ehlin-Henriksson B, Rajewsky K & Kuppers R Burkitt’s lymphoma is a malignancy of mature B cells expressing somatically mutated V region genes. Mol. Med 1, 495–505 (1995). [PMC free article] [PubMed] [Google Scholar]
- 73.Evan GI et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992). [DOI] [PubMed] [Google Scholar]
- 74.Chen L et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111, 2230–2237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braselmann S et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther 319, 998–1008 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Clemens GR et al. Developmental toxicity associated with receptor tyrosine kinase Ret inhibition in reproductive toxicity testing. Birth Defects Res. A Clin. Mol. Teratol 85, 130–136 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Mocsai A et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nature Immunol. 7, 1326–1333 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quesada V et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nature Genet. 44, 47–52 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Wang L et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med 365, 2497–2506 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puente XS et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475, 101–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mocsai A, Ruland J & Tybulewicz VL The SYK tyrosine kinase: a crucial player in diverse biological functions. Nature Rev. Immunol 10, 387–402 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gobessi S et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 23, 686–697 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Quiroga MP et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood 114, 1029–1037 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young RM et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood 113, 2508–2516 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suljagic M et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eμ-TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 116, 4894–4905 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Friedberg JW et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non Hodgkin’s lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Rooij MF et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 119, 2590–2594 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Coffey G et al. Specific inhibition of spleen tyrosine kinase suppresses leukocyte immune function and inflammation in animal models of rheumatoid arthritis. J. Pharmacol. Exp. Ther 340, 350–359 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Cheng S et al. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood 118, 6342–6352 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Bruton OC Aγglobulinemia. Pediatrics 9, 722–728 (1952). [PubMed] [Google Scholar]
- 91.Rawlings DJ et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 261, 358–361 (1993). [DOI] [PubMed] [Google Scholar]
- 92.Kerner JD et al. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity 3, 301–312 (1995). [DOI] [PubMed] [Google Scholar]
- 93.Khan WN et al. Defective B cell development and function in Btk-deficient mice. Immunity 3, 283–299 (1995). [DOI] [PubMed] [Google Scholar]
- 94.Kurosaki T & Hikida M Tyrosine kinases and their substrates in B lymphocytes. Immunol. Rev 228, 132–148 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Rawlings DJ et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 271, 822–825 (1996). [DOI] [PubMed] [Google Scholar]
- 96.Afar DE et al. Regulation of Btk by Src family tyrosine kinases. Mol. Cell. Biol 16, 3465–3471 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohamed AJ et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev 228, 58–73 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Watanabe D et al. Four tyrosine residues in phospholipase C-γ2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J. Biol. Chem 276, 38595–38601 (2001). [DOI] [PubMed] [Google Scholar]
- 99.Ozdener F, Dangelmaier C, Ashby B, Kunapuli SP & Daniel JL Activation of phospholipase Cγ2 by tyrosine phosphorylation. Mol. Pharmacol 62, 672–679 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Hantschel O et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc. Natl Acad. Sci. USA 104, 13283–13288 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amrein PC et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin. Cancer Res 17, 2977–2986 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan Z et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2, 58–61 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Honigberg LA et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl Acad. Sci. USA 107, 13075–13080 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young R, Smith A, Honigberg L & Refaeli Y Abstract #1984: Btk is a novel therapeutic target to treat large B-cell lymphomas. AACR Meeting Abstracts, April 2009 Abstr.1984 (2009). [Google Scholar]
- 105.Herman SE et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 117, 6287–6296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ponader S et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 119, 1182–1189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Brien S et al. The Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 induces durable responses in relapsed or refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): follow-up of a Phase Ib/II study. Blood (ASH Annual Meeting Abstracts) 118, 983 (2011). [Google Scholar]
- 108.Advani RH et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol 31, 88–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staudt LM et al. The Bruton’s tyrosine kinase (Btk) inhibitor PCI-32765 modulates chronic active BCR signaling and induces tumor regression in relapsed/refractory ABC DLBCL. Blood (ASH Annual Meeting Abstracts) 118, 2716 (2011). [Google Scholar]
- 110.Wilson WH et al. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lymphoma (DLBCL): interim results of a multicenter, open-label, Phase 2 study. Blood (ASH Annual Meeting Abstracts) 120, 686 (2012). [Google Scholar]
- 111.Byrd JC et al. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) promotes high response rate, durable remissions, and is tolerable in treatment naive (TN) and relapsed or refractory (RR) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) patients including patients with high-risk (HR) disease: new and updated results of 116 patients in a Phase Ib/II Study. Blood (ASH Annual Meeting Abstracts) 120, 189 (2012). [Google Scholar]
- 112.Hadzidimitriou A et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood 118, 3088–3095 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Wang M et al. Interim results of an international, multicenter, Phase 2 study of Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), in relapsed or refractory mantle cell lymphoma (MCL): durable efficacy and tolerability with longer follow-up. Blood (ASH Annual Meeting Abstracts) 120, 904 (2012). [Google Scholar]
- 114.Yuan TL & Cantley LC PI3K pathway alterations in cancer: variations on a theme. Oncogene 27, 5497–5510 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.So L & Fruman DA PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem. J 442, 465–481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laplante M & Sabatini DM mTOR signaling in growth control and disease. Cell 149, 274–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kloo B et al. Critical role of PI3K signaling for NF-κB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc. Natl Acad. Sci. USA 108, 272–277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rudelius M et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood 108, 1668–1676 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okkenhaug K, Ali K & Vanhaesebroeck B Antigen receptor signalling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 28, 80–87 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramadani F et al. The PI3K isoforms p110α and p110δ are essential for pre-B cell receptor signaling and B cell development. Sci. Signal 3, ra60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ikeda H et al. PI3K/p110δ is a novel therapeutic target in multiple myeloma. Blood 116, 1460–1468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lannutti BJ et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117, 591–594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herman SE et al. Phosphatidylinositol 3-kinasδ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 116, 2078–2088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoellenriegel J et al. The phosphoinositide 3ʹ-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 118, 3603–3612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coutre SE et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110d, in patients with previously treated chronic lymphocytic leukemia. J. Clin. Oncol. Abstr 29, 6631 (2011). [Google Scholar]
- 126.Kahl B et al. Clinical safety and activity in a Phase 1 study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase p110δ, in patients with relapsed or refractory non-Hodgkin lymphoma. Blood (ASH Annual Meeting Abstracts) 116, 1777 (2010). [Google Scholar]
- 127.Mattmann ME, Stoops SL & Lindsley CW Inhibition of Akt with small molecules and biologics: historical perspective and current status of the patent landscape. Expert Opin. Ther. Pat 21, 1309–1338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Witzig TE et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J. Clin. Oncol 23, 5347–5356 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Renner C et al. A multicenter phase II trial (SAKK 36/06) of single-agent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica 97, 1085–1091 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Witzig TE et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 25, 341–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holler C et al. PKCβ is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCβ as a therapeutic target in chronic lymphocytic leukemia. Blood 113, 2791–2794 (2009). [DOI] [PubMed] [Google Scholar]
- 132.Morschhauser F et al. A phase II study of enzastaurin, a protein kinase C β inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann. Oncol 19, 247–253 (2008). [DOI] [PubMed] [Google Scholar]
- 133.Robertson MJ et al. Phase II study of enzastaurin, a protein kinase C β inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol 25, 1741–1746 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Coornaert B et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-κB inhibitor A20. Nature Immunol. 9, 263–271 (2008). [DOI] [PubMed] [Google Scholar]
- 135.Rebeaud F et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nature Immunol. 9, 272–281 (2008). [DOI] [PubMed] [Google Scholar]
- 136.Hailfinger S et al. Malt1-dependent RelB cleavage promotes canonical NF-κB activation in lymphocytes and lymphoma cell lines. Proc. Natl Acad. Sci. USA 108, 14596–14601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lam LT et al. Small molecule inhibitors of IκB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin. Cancer Res 11, 28–40 (2005). [PubMed] [Google Scholar]
- 138.Hailfinger S et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 106, 19946–19951 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baud V & Karin M Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev. Drug Discov 8, 33–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baldwin AS Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Invest 107, 241–246 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ngo VN et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pham-Ledard A et al. MYD88 somatic mutation is a genetic feature of primary cutaneous diffuse large B-cell lymphoma, leg type. J. Invest. Dermatol 132, 2118–2120 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Montesinos-Rongen M et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 122, 791–792 (2011). [DOI] [PubMed] [Google Scholar]
- 144.Yan Y et al. BCR and TLR signalling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 97, 595–598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li ZM et al. MYD88 somatic mutations in MALT lymphomas. Br. J. Haematol 158, 662–664 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Treon SP et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N. Engl. J. Med 367, 826–833 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Leadbetter EA et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002). [DOI] [PubMed] [Google Scholar]
- 148.Hou B et al. Selective utilization of toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34, 375–384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 21, 723–737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hernandez-Ilizaliturri FJ et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 117, 5058–5066 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Saito M et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell 12, 280–292 (2007). [DOI] [PubMed] [Google Scholar]