Abstract
Background:
Psychiatrists frequently struggle with how to sequence treatment for depressed adolescents who do not respond to an adequate trial of a selective serotonin reuptake inhibitor (SSRI). This study leveraged recent statistical and computational advances to create Bayesian Hierarchal Models (BHMs) of response in the Treatment of SSRI-resistant Depression (TORDIA) study to inform treatment planning.
Methods:
BHMs of individual treatment trajectories were developed and estimated using Hamiltonian Monte Carlo No U-Turn Sampling. From the Monte Carlo pseudorandom sample, 95% credible intervals, means, posterior tail probabilities, etc. were determined. Then, for random effects models, posterior tail probabilities were used to create Bayesian two-tailed p-values to evaluate the null hypotheses: no difference in efficacy between SSRIs and venlafaxine. The robustness of the results was examined using fixed effects models of treatment comparisons.
Results:
In patients not receiving cognitive behavioral therapy (CBT) (n=168), SSRIs produced greater and faster improvement in depressive symptoms compared to venlafaxine (p=0.015). No differences in response or trajectory of response for symptoms of anxiety were detected between SSRIs and venlafaxine (p=0.168). For patients receiving CBT (n=162), SSRIs and venlafaxine produced similar improvements in symptoms of anxiety and depression.
Conclusions:
Findings from this novel computational approach suggest that a second trial of an SSRI is warranted for depressed adolescents who fail to respond to initial SSRI treatment.
Key words and abbreviations: depression, major depressive disorder, paroxetine, sertraline, fluoxetine, TORDIA, clinical trial
1 |. INTRODUCTION
What to do when a depressed adolescent fails to respond to an adequate trial of a selective serotonin reuptake inhibitor (SSRI) is a common challenge for child and adolescent psychiatrists. Unfortunately, more than a decade since the largest trial comparing antidepressant switching, the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study, the evidence base has little to offer clinicians, beyond adding cognitive behavioral therapy (CBT)—regardless of whether these youth are switched to another SSRI or to a serotonin norepinephrine reuptake inhibitor (SNRI)—improves outcomes (Brent et al., 2008a; Strawn and Croarkin, 2020; Strawn et al., 2020). In TORDIA, adolescents with major depressive disorder (MDD) aged 12–18 (N=334) who did not respond to an adequate dose of an SSRI, for at least 8 weeks, were randomized to one of 4 treatments over 12 weeks (March et al., 2004). Patients could switch to (1) a different SSRI (paroxetine, citalopram or fluoxetine); (2) a different SSRI + cognitive behavioral therapy (CBT); (3) venlafaxine; or (4) venlafaxine + CBT. CBT + medication switch (either to another SSRI or venlafaxine) was associated with a marked decrease in depressive symptoms compared to simply changing medication. No differences in final outcome were detected between switching to venlafaxine relative to another SSRI, but patients who were switched to a second SSRI had less suicidal ideation than those treated with venlafaxine (Vitiello et al., 2011) and fewer adverse events (Sakolsky et al., 2011). Additionally, when tolerability and efficacy of the SSRIs were considered together, adolescents treated with citalopram and fluoxetine fared better than those treated with paroxetine (Strawn, Mills, & Croarkin, 2019).
TORDIA was elegantly designed, its participants were meticulously characterized, and multiple symptom measures were obtained. Treatment effects were examined using a random effects model—the standard approach at the time. Importantly, this approach dealt significant blows to the original aim of the study (Table 1). First, the random effects model included multiple parameters. However, only four assessments of depressive symptoms were available to evaluate the random effects coefficient for each patient. This reduced the degrees of freedom and degraded the ability to detect differences among treatments. Second, standard random effects models require asymptotic assumptions (i.e., assuming that the study sample is large enough to approximate the true population distribution) whereas contemporary approaches (e.g., Bayesian Hierarchal Modeling [BHM]) do not. Third, standard models of treatment effects over time that flexibly estimate the trajectory of improvement in clinical trials result in decreased precision relative to a model that specifies the functional form of improvement (Strawn, Mills, Sauley, & Welge, 2018; Varigonda et al., 2015). Leveraging a specific functional form (e.g., logarithmic trend) allows for greater degrees of freedom and more precisely estimates treatment effects. Fourth, as was common at the time, the effect of unnecessary variables on models was not examined when TORDIA was analyzed. Contemporary computational approaches leverage statistical methods that assess whether extraneous variables were included in the model and whether the models have omitted variables that introduce bias. The inclusion of extraneous variables decreases the precision of the model and reduces the power to detect treatment differences.
TABLE 1:
Comparison of Standard Random Effects Mixed Model with Repeated Measures (MMRM) and Time Trajectory Bayesian Hierarchical Model (BHM)
| Standard Random Effects MMRM | Time Trajectory BHM |
|---|---|
| Requires large sample assumption | Valid for small samples |
| Static model which does not incorporate individual correlation of symptom measures over time | Dynamic model which incorporates correlation of symptom measures over time |
| Requires complex autocovariance structure | Valid with simple, independently distributed error structure |
| Less computationally demanding | More computationally demanding |
| Allows for heterogeneity in sample | Allows for and estimates the degree of heterogeneity in the sample |
Since TORDIA was conceived, implemented and analyzed, there have been significant advances in statistical approaches for clinical trials. Today, computationally intensive approaches are common including Markov Chain Monte Carlo and Hamiltonian Monte Carlo (MCMC and HMC) (Gelfand & Smith, 1990; Hoffman & Gelman, 2014) which (1) allow more precise estimation of treatment effects; (2) do not require as restrictive assumptions and (3) facilitate more clinically meaningful model specifications. Further, a Bayesian Hierarchical Model (BHM) integrates multi-level information (e.g, individual patient, treatment, comorbidity) which allows multiple effects to be estimated in parallel (Figure 1) (McGlothlin & Viele, 2018). BHMs separate the observed variability into parts attributable to “random differences and true differences” (McGlothlin & Viele, 2018). These methods allow better assessment of heterogeneity and provide more precise estimates of the treatment effect without relying on large sample assumptions. BHMs do not require assuming complete heterogeneity, as has been common in prior analyses of clinical trials, instead modeling the degree of heterogeneity through estimation of an individual effects distribution (Table 1).
FIGURE 1.
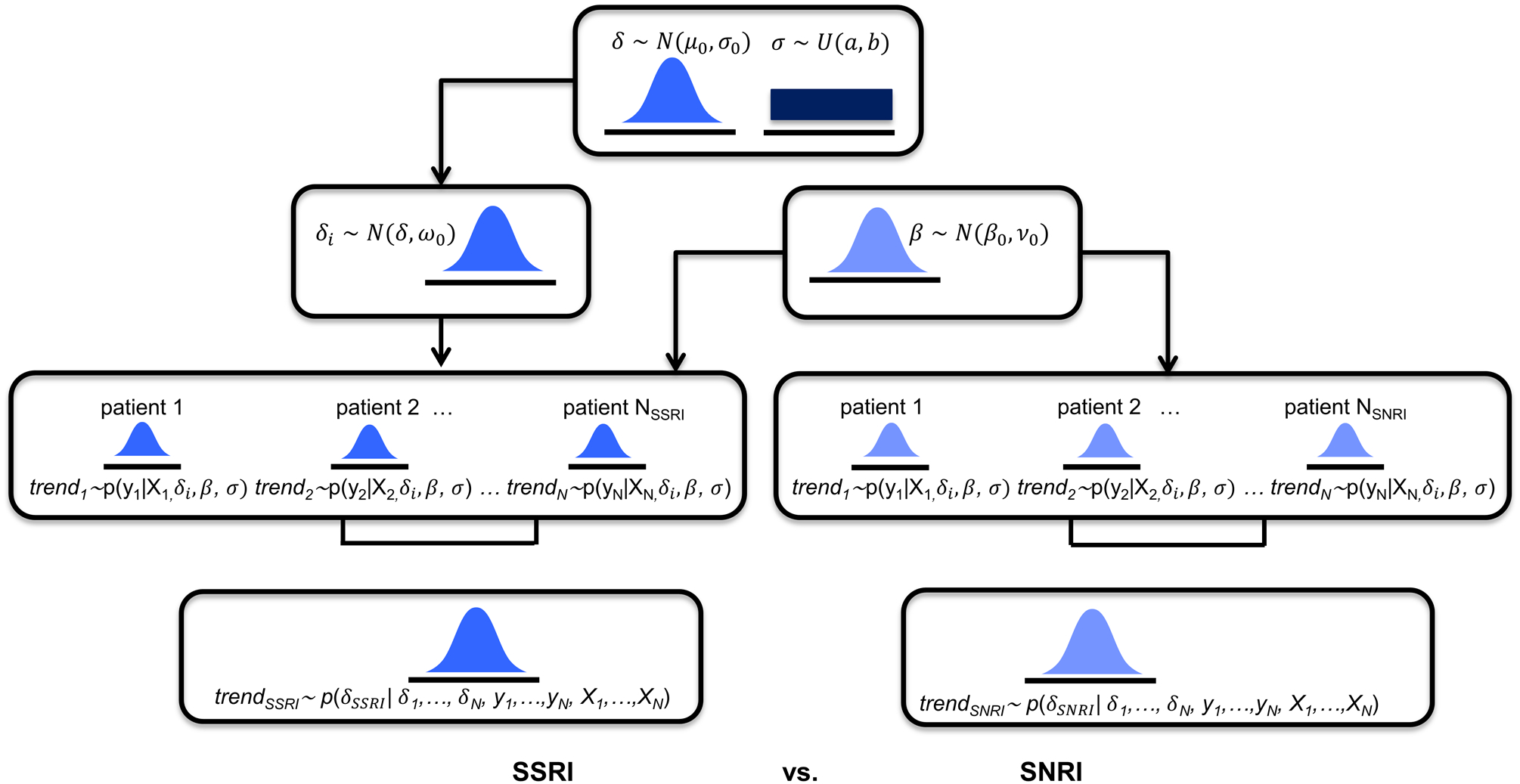
Bayesian Hierarchical Modeling of Children’s Depression Rating Scale-Revised (CDRS-R) and Screen for Child Anxiety and Related Disorders (SCARED) scores in patients who were switched to a selective serotonin reuptake inhibitor (SSRI) or to extended-release venlafaxine. N(μ, σ) represents the normal distribution with mean, μ and standard deviation, σ. U(a, b) represents the uniform distribution in the interval a to b. p(y | x) represents a normal likelihood distribution. p(δ | y, x) represents the numerically computed MCMC posterior distributions. SNRI, serotonin-norepinephrine reuptake inhibitor.
With these considerations in mind, we employed BHMs (Gelman, Carlin, Stern, & Rubin, 2004; McGlothlin & Viele, 2018) to determine the difference in treatment effect for changing to an SSRI or venlafaxine in TORDIA. Second, we examined whether adding CBT modified these effects. Third, we evaluated the robustness of heterogeneity assumptions employed in the original analyses of TORDIA on detection of treatment response in adolescents with SSRI-resistant major depressive disorder. We hypothesized--based on accumulating data that SSRIs are more efficacious than SNRIs--that switching to an SSRI would produce greater improvement (Dobson, Bloch, & Strawn, 2019; Locher et al., 2017; Strawn, Mills, Sauley, & Welge, 2018) in depressive symptoms compared to switching to venlafaxine.
2 |. METHODS
2.1 |. Source of data
We accessed the complete, de-identified, original TORDIA data set from the NIMH data archive ( Brent et al., 2008). The analyses focused on the acute phase of the trial in which depressive symptoms, as reflected by Children’s Depression Rating Scale-Revised (Mayes, Bernstein, Haley, Kennard, & Emslie, 2010) score were determined at baseline, week 4, week 8 and week 12 and anxiety symptoms, as reflected by Screen for Child Anxiety and Related Disorders (Birmaher et al., 1999, 1997) score were obtained at baseline, week 6 and week 12.
2.2 |. Study details
TORDIA was a prospective, randomized controlled trial that involved 6 sites in the United States. Outpatients (N=334) aged 12 to 18 years (mean age 15.9±1.6 (SD) years, 70% female, 83% Caucasian) with a DSM-IV diagnosis of MDD, established by the Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL) (Kaufman et al., 1997) were included. Exclusion criteria included ≥ 2 two adequate trials of an SSRI or non-response to venlafaxine (≥4 weeks at a dosage of ≥150 mg) or CBT (≥7 sessions). Patients could also not be currently treated with antipsychotics, mood stabilizers, non-SSRI antidepressants, although they could be treated with stable doses of stimulants, hypnotics (trazodone, zolpidem, zaleplon), or anxiolytics (clonazepam, lorazepam).
Patients who failed at least 8 weeks of an SSRI trial (dose equivalent of at least 40 mg of fluoxetine or 20 mg daily, if tolerability concerns were present) were randomly assigned, using a 2×2 balanced factorial design, to a switch to a different SSRI (paroxetine, citalopram, or fluoxetine, 20–40 mg daily) or venlafaxine (150–225 mg) or to switch to a different SSRI plus CBT or to a switch to venlafaxine plus CBT. Depressive and anxiety symptoms were measured using the Children’s Depression Rating Scale-Revised (CDRS-R) and the Screen for Child Anxiety and Related Disorders (SCARED), respectively. Additional details about the trial (Emslie., 2008) and its results have been described elsewhere (Brent et al., 2009a; Emslie et al., 2010; Emslie, 2008; Kennard et al., 2009; Mansoor et al., 2013; Sakolsky et al., 2011; Strawn, Mills, & Croarkin, 2019).
Institutional review boards at each site approved and monitored the protocol in addition to external monitoring by a data safety and monitoring board. Patients and at least one parent per participant provided informed assent and consent. For this study, no additional ethics approval or informed consent was required; this secondary analysis used publicly available data.
2.3 |. Response Modeling
Individual trajectory of treatment response was modeled using an individual logarithmic trend “random effects” coefficients BHM. The logarithmic trend models, and specifically the individual trends BHM, has several advantages over the standard mixed model with repeated measures (MMRM) (Walker & Shostak, 2010). MMRM produces individual errors that are correlated over time (Gosho, Hirakawa, Noma, Maruo, & Sato, 2017). As such, a covariance structure that allows for serially correlated errors is needed in MMRM. However, dynamic variation in the data is best modeled explicitly rather than as a model error that is dealt with by specifying a general covariance matrix (Mizon, 1995). Incorporating an error term to allow for serially correlated errors requires a sandwich estimator (e.g., heteroskedasticity and autocorrelation consistent (HAC) covariance matrix estimation) (Gosho et al., 2017). Importantly, HAC estimators rely on large sample assumptions and perform poorly in simulations (Müller, 2014). The BHM logarithmic trend approach explicitly models a patient’s correlation in symptoms over time rather than attempting to adjust for estimation bias. Further, the logarithmic trend and BHM approaches do not rely on asymptotic theory—there is no large sample assumption (Gelman et al., 2004). In TORDIA, the assessment of depressive and anxiety symptoms over time was limited, albeit standard for a clinical trial of this magnitude: four measurements per patient for depressive symptoms and three measures per patient for anxiety symptoms. Additionally, there were cross sectional limitations, especially when subgroups were examined (e.g., patients who received an SSRI but no CBT, or those who received venlafaxine and no CBT). Thus, the validity of large sample assumptions is questionable.
Models were estimated using the Hamiltonian Monte Carlo No U-Turn Sampler in the package Turing.jl, in Julia (version 1.3) (Bezanson, Edelman, Karpinski, & Shah, 2014; Ge, Xu, & Ghahramani, 2018). Specifically, the BHM individual treatment trajectories model was specified as follows:
where μit = δitrendt + (Xitrendt)β, trendt = log(t), t = 1, 2, 3, 4. Improvement in depressive symptoms was defined as: yit = CDRSRit − CDRSRi1 = change in CDRSR from baseline. Similarly, improvement in anxiety symptoms (i.e., total SCARED score) was defined as: yit = SCAREDit − SCAREDi1 = change in SCARED from baseline.
Additionally, to evaluate the robustness of the results, a logarithmic trend with individual fixed effects and the sensitivity of the results to variations in the degree of unobserved heterogeneity across individuals was examined. The log trend individual “fixed effects” model was specified using a differences in differences approach, which cancels out the individual fixed effects from the model. The fixed effects log trend model used for estimation purposed is, yit = Xitβ + δtrendt + εit, εit = (uit − ui1) ~ N(0, σ2), where the δi s capture unobserved heterogeneity in individual patients, and the interaction terms Xit = (Xi × trendt) capture variations in trajectories across individuals due to observed individual characteristics, with Xi containing individual covariates (i.e., a treatment type indicator for SSRI vs. SNRI, age, sex, anxiety level, household income level).
Prior parameter values used for all BHM estimation were: μ0 = 0, σ0 = 10, a = 0.01, b = 30, β0 = 0, v0 = 10, ω0 = 5. Prior and posterior densities were compared to ensure the priors were relatively uninformative (i.e. reasonably flat over the domain of the posterior with non-negligible probability). Given concern that the results of the linear models may be difficult to interpret clinically (Bondar, Caye, Chekroud, & Kieling, 2020), we calculated effect sizes to reflect the change in CDRS-R and SCARED scores for each treatment (at week 12) by multiplying slope difference coefficients (β) by the natural log of treatment duration (trendweek 12), as previously described (Bondar et al., 2020).
For all analyses, Bayesian posterior p values ≤0.05 were considered statistically significant. Statistical analyses were performed using Julia (version 1.3).
3 |. RESULTS
3.1 |. Assessment of heterogeneity
The estimated response coefficients for response in SSRI and venlafaxine-treated patients are shown in Figure 2A,C. Figures 2B and D show the substantial variability in individual response trajectories among patients based on the response coefficient estimates. Despite this variability, the mean trend coefficient estimates for improvement in depressive symptoms were similar in the models with individual trends (βtrend = −17.94, p<0.001; βSSRI = −2.33, p=0.015) and those that assume a homogeneous trend (βtrend = −18.07, p<0.001; βSSRI = −2.25, p=0.024). Similarly, for improvement in anxiety symptoms the individual trend models (βtrend = −3.69, p<0.001; βSSRI = −1.27, p=0.017) and models assuming a homogeneous trend were similar (βtrend = −3.68, p<0.001; βSSRI = −1.29, p=0.038). These models were estimated with and without covariates (e.g., age, sex, household income, anxiety indicator variables) and the results were robust across specifications.
FIGURE 2.
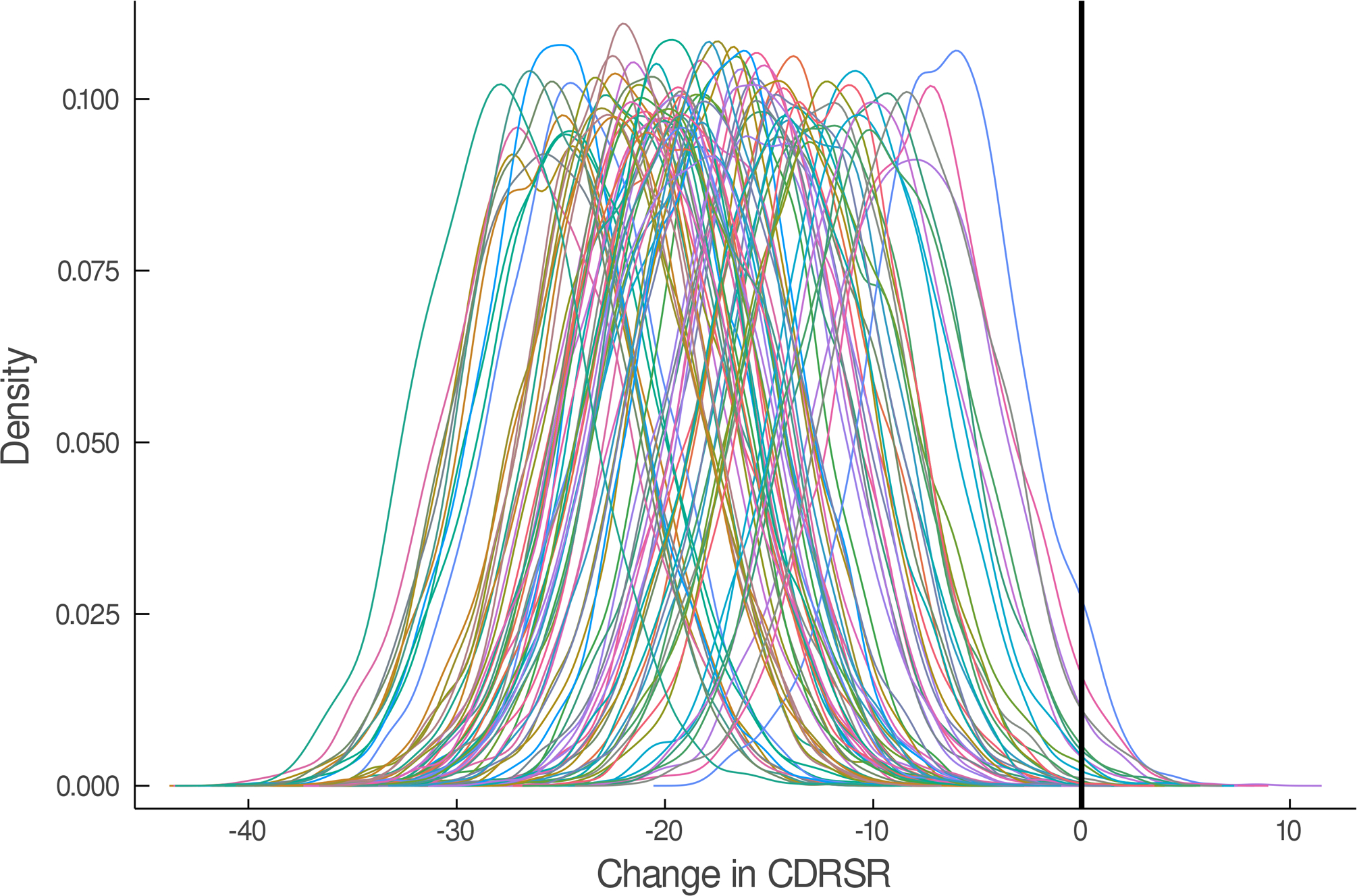
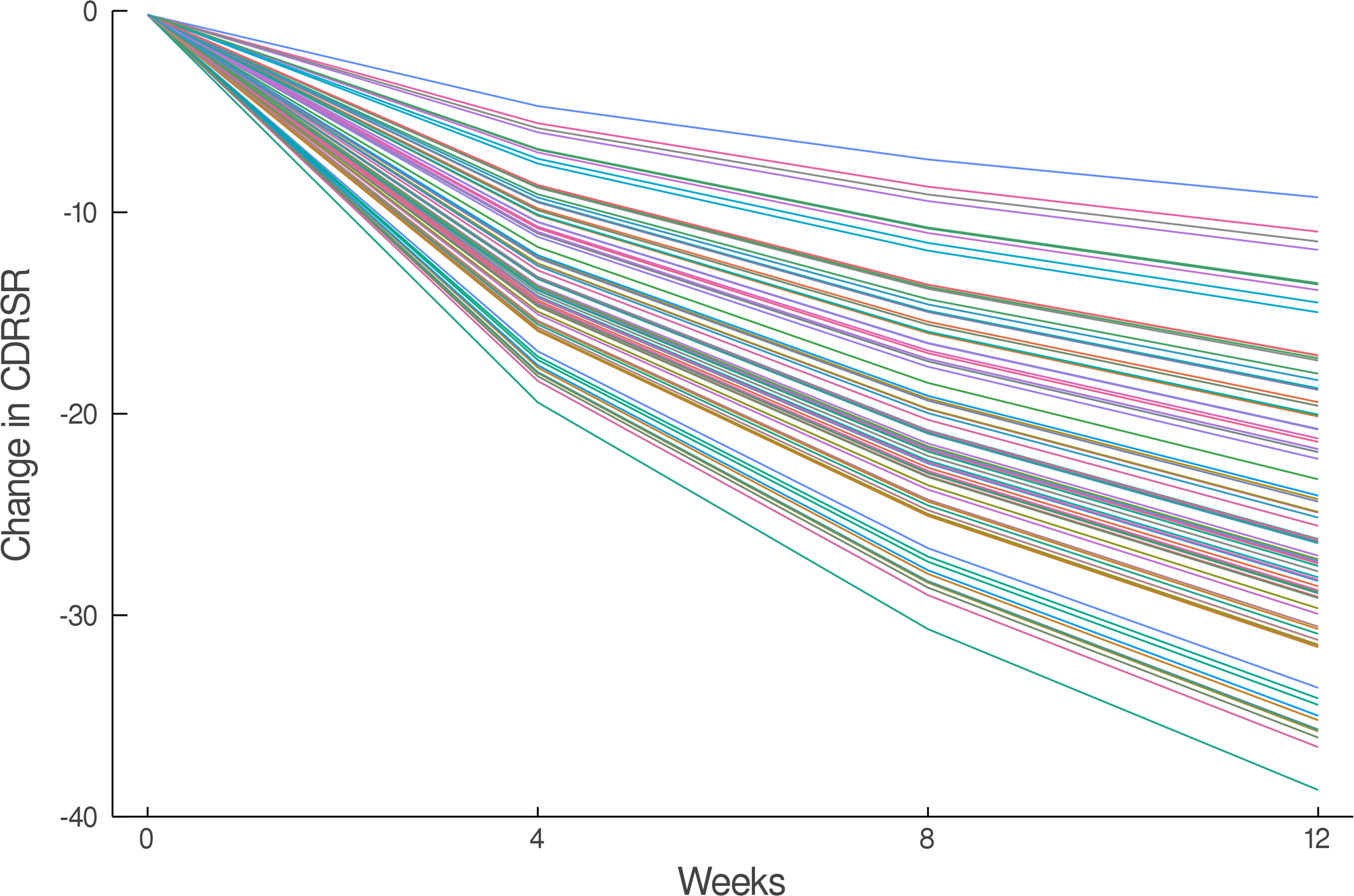
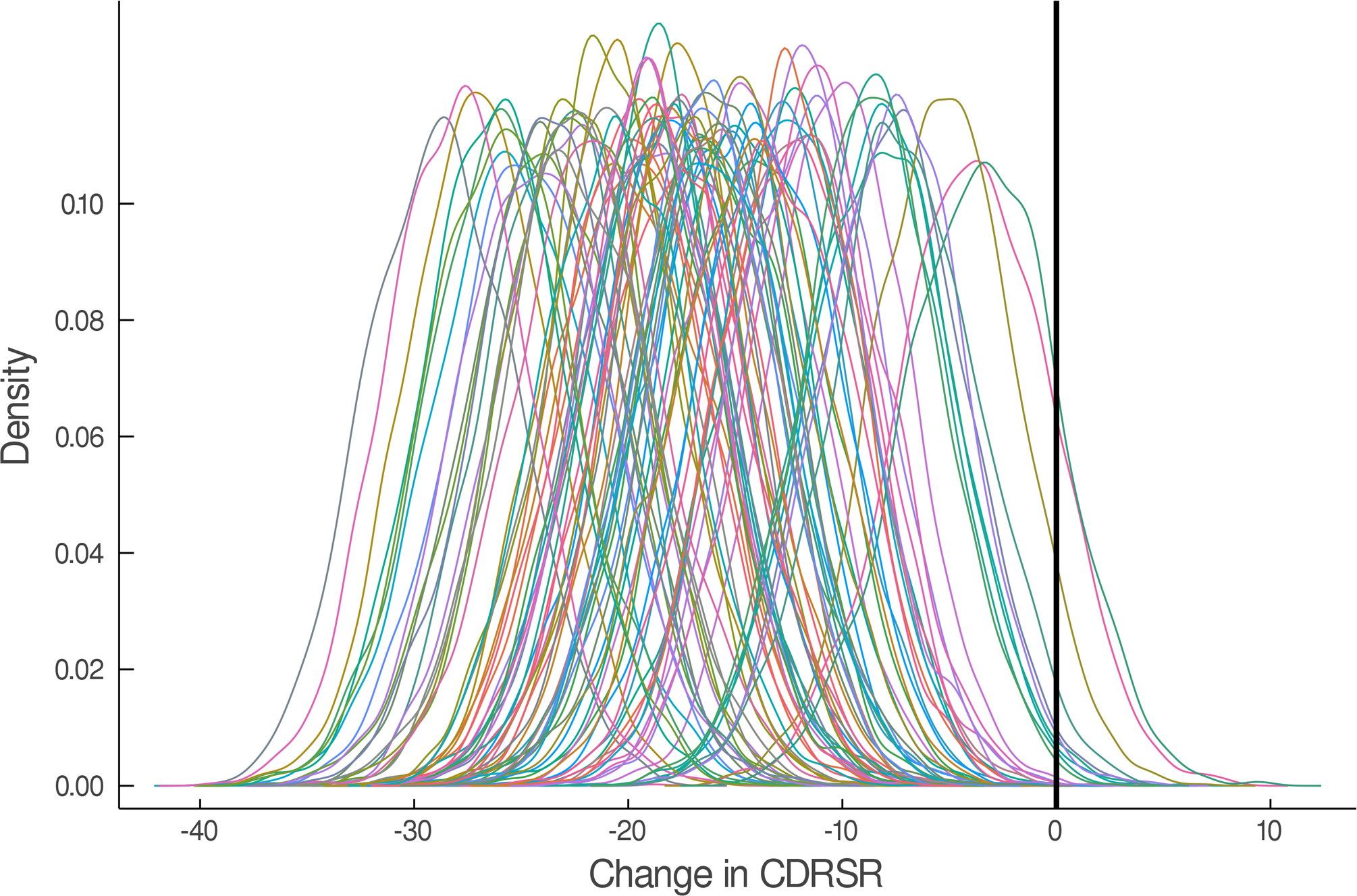
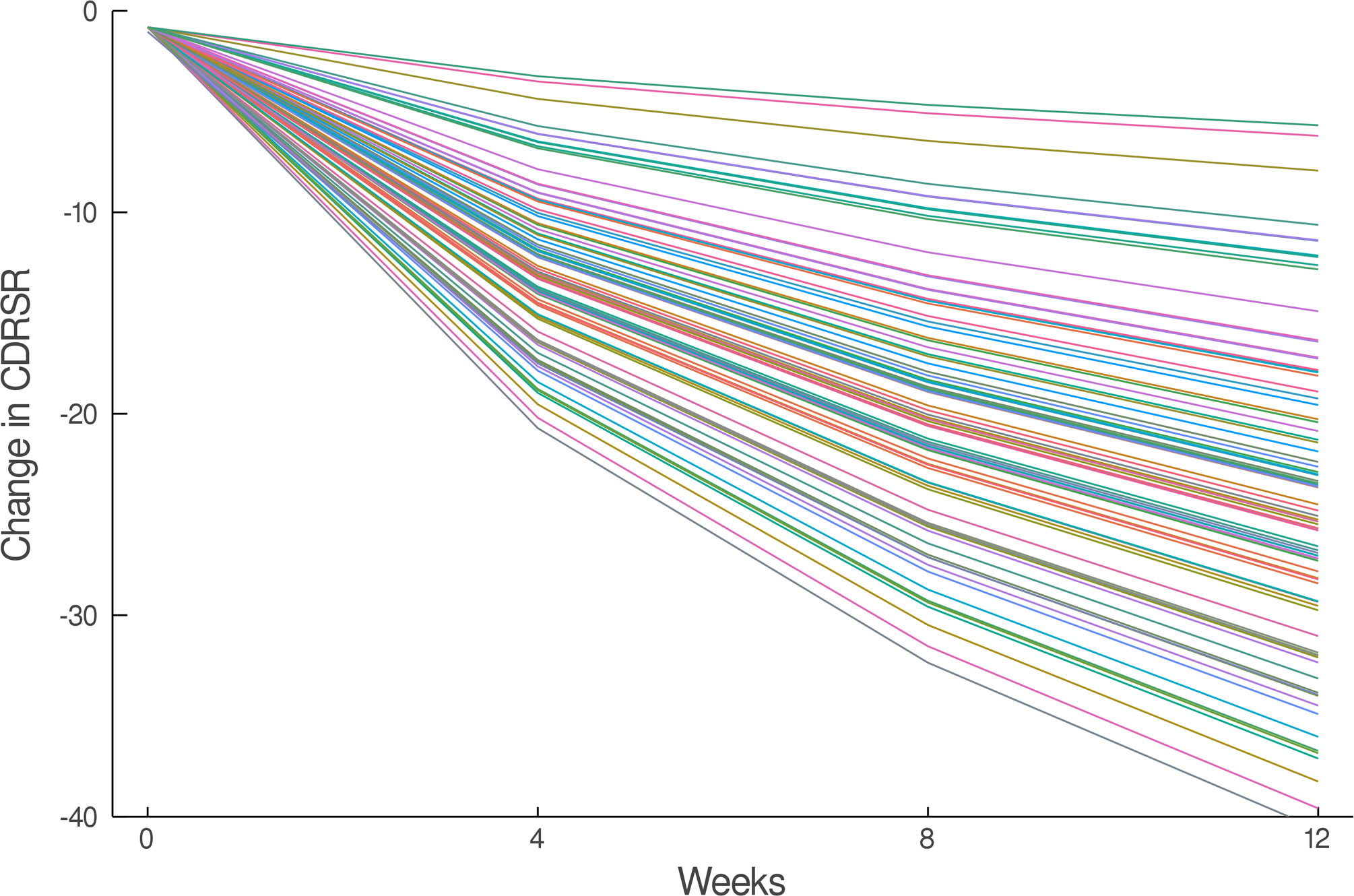
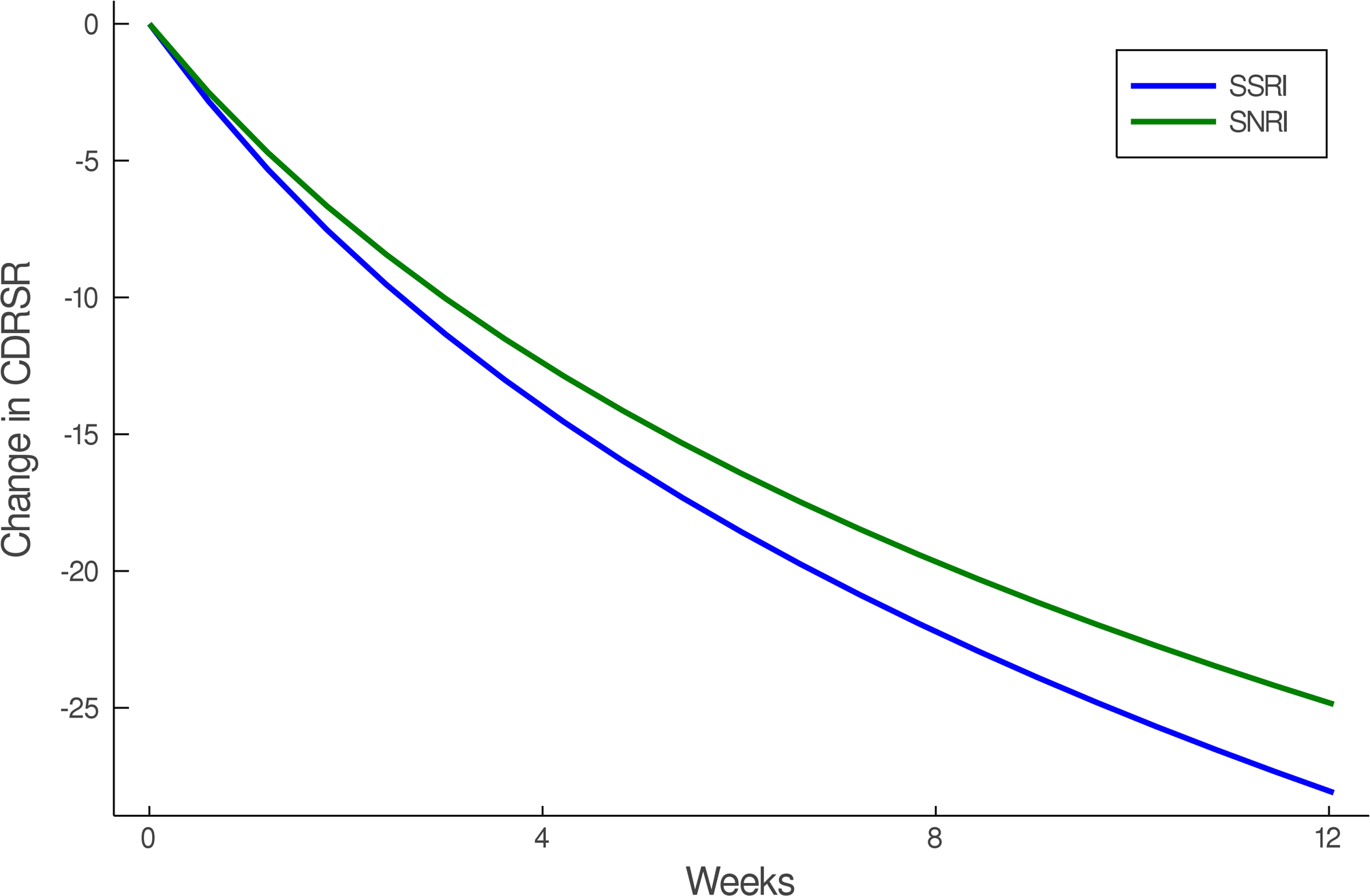
Improvement in Depressive Symptoms in Adolescents with SSRI-Resistant Depression. Heterogeneity trend parameters for individual response are shown for SSRI-treated patients (A) and individual responses are shown in (B). For, SNRI-treated youth, heterogeneity trend parameters for response are shown in (C) in addition to individual responses over time (D). Patients treated with a SSRI demonstrated faster and greater response than those treated with a SNRI (E).
3.2 |. Improvement in SSRI vs. SNRI (no CBT)
In the individual trajectory in improvement model (i.e., BHM), adolescents with SSRI-resistant MDD who were randomized to an SSRI experienced greater improvement in CDRS compared to those who received venlafaxine (difference in week 12 change in CDRS −3.23; p=0.015, Figure 2). These SSRI-treated patients also experienced faster improvement compared to those who received venlafaxine (p=0.015). Given the predicted average improvement in week 12 CDRS score for adolescents who received venlafaxine (-24.87) and this predicted improvement in CDRS for SSRI-treated adolescents (-28.10), the percent difference in improvement, at week 12, between SSRI and venlafaxine-treated patients is 13%. For comparison, a Minimal Clinically Important Difference (MCID) for pharmacologic treatments of depression in adolescents was 9±2.24. The MCID for comparisons of psychopharmacologic interventions with similar burden and cost in adolescents was determined from a survey of academic, board-certified child and adolescent psychiatrists at the associate professor level or higher (N=5) who received NIH-funding for treatment interventions in adolescent mood/anxiety disorders.
In the individual trajectory of anxiety improvement (as reflected by total SCARED score), no differences in response or response trajectory were detected between patients who randomized to SSRI compared to venlafaxine (trajectory difference = -1.27, p=0.168, Figure 3). Given the predicted average improvement in week 12 SCARED score for adolescents who received venlafaxine (-5.12) and this predicted improvement in SCARED for SSRI-treated adolescents (-6.88), the percent difference in improvement, at week 12, between SSRI and venlafaxine-treated patients is 63%. However, comparing this 63% difference relative to an MCID is difficult not only because of the lack of statistical significance but also because the improvement for both medications is small and distorts the magnitude of this difference.
FIGURE 3.
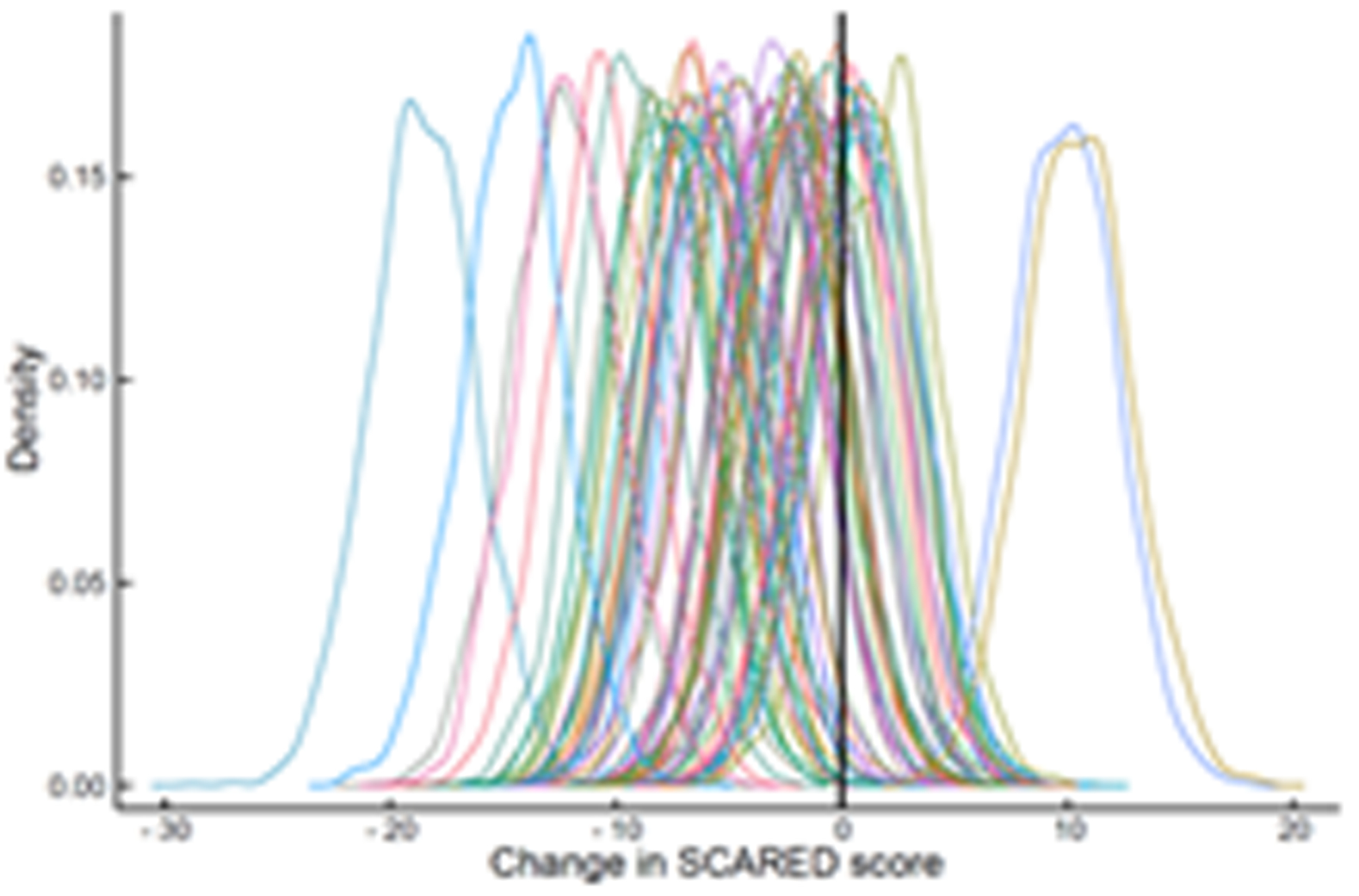
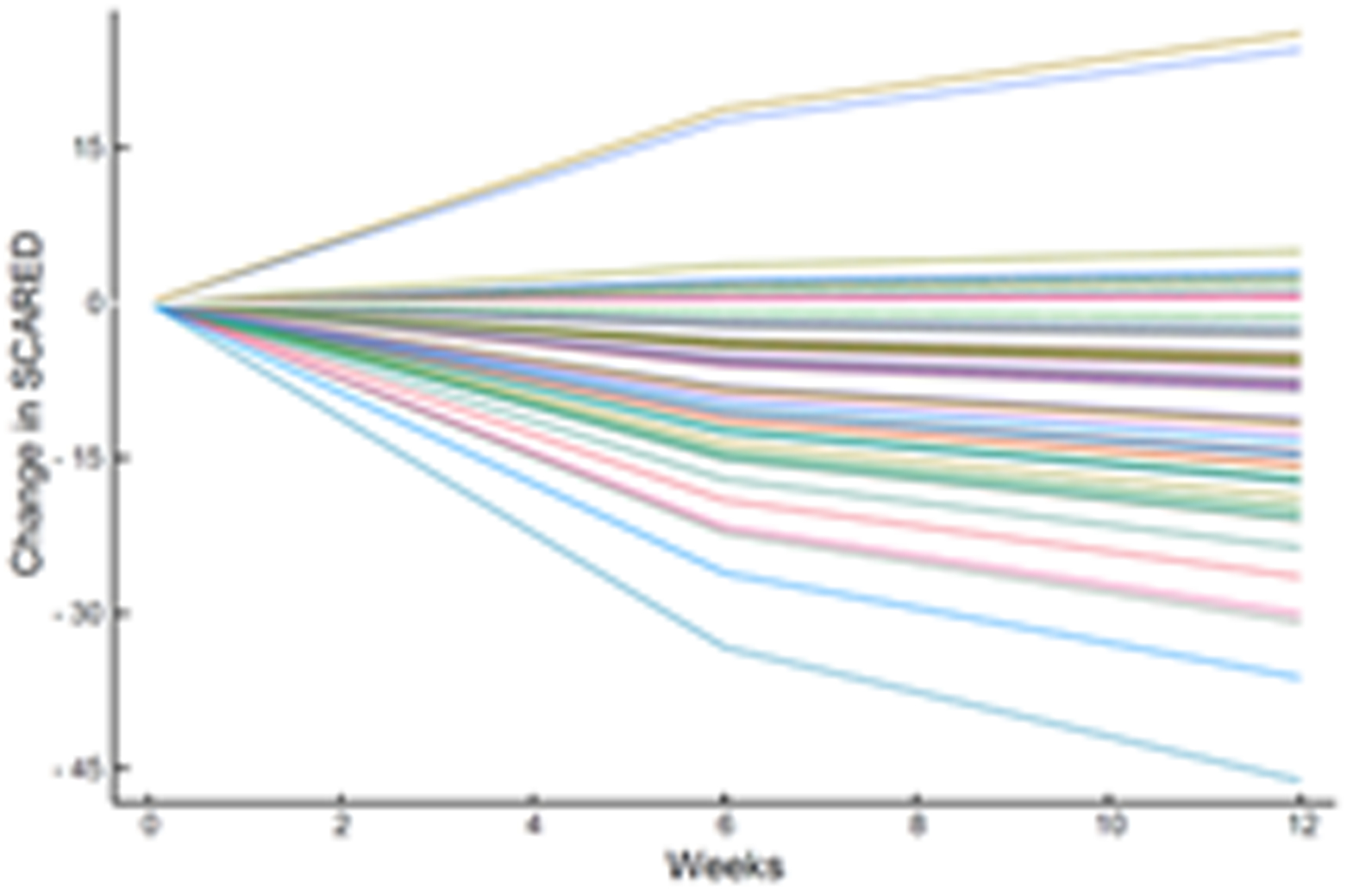
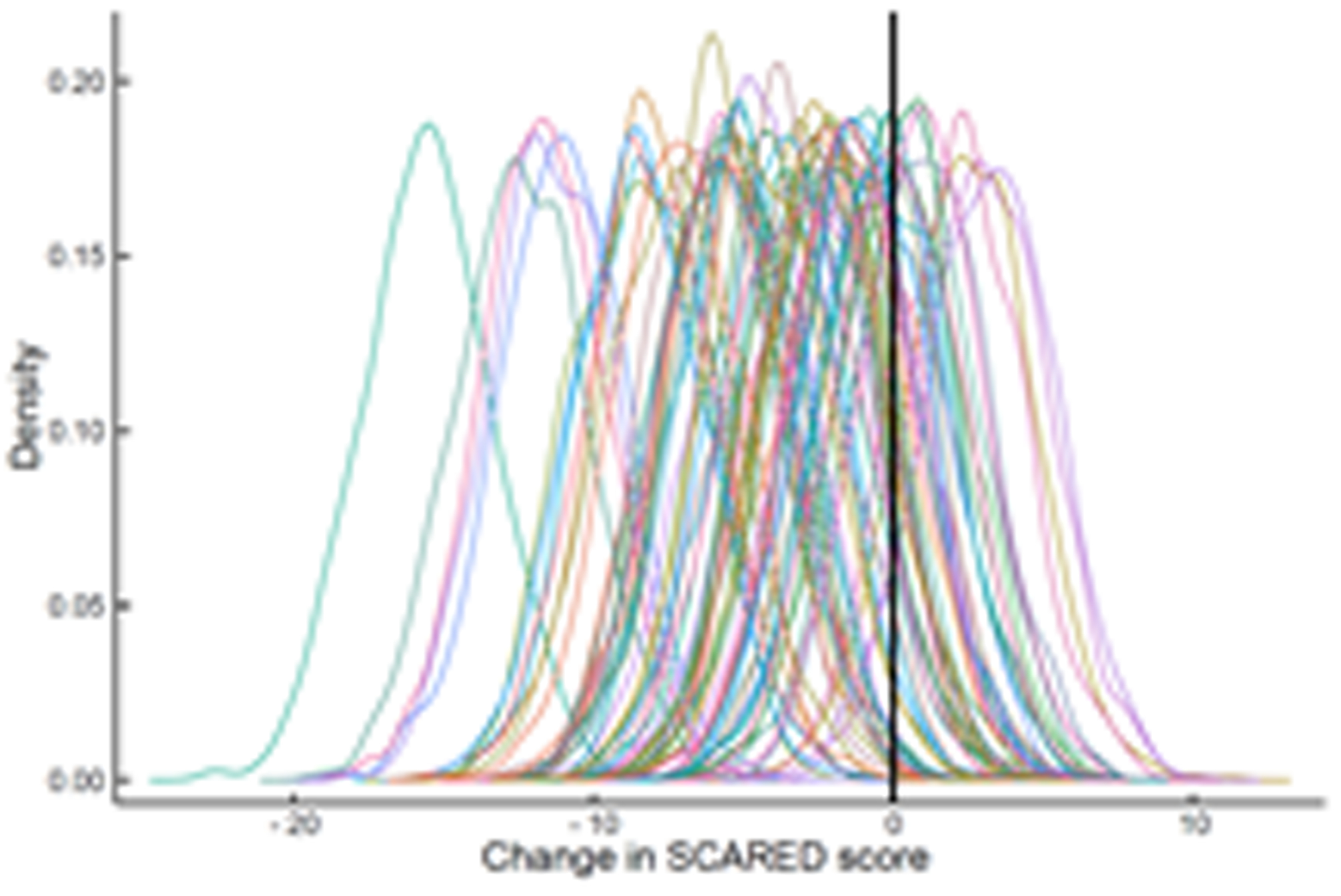
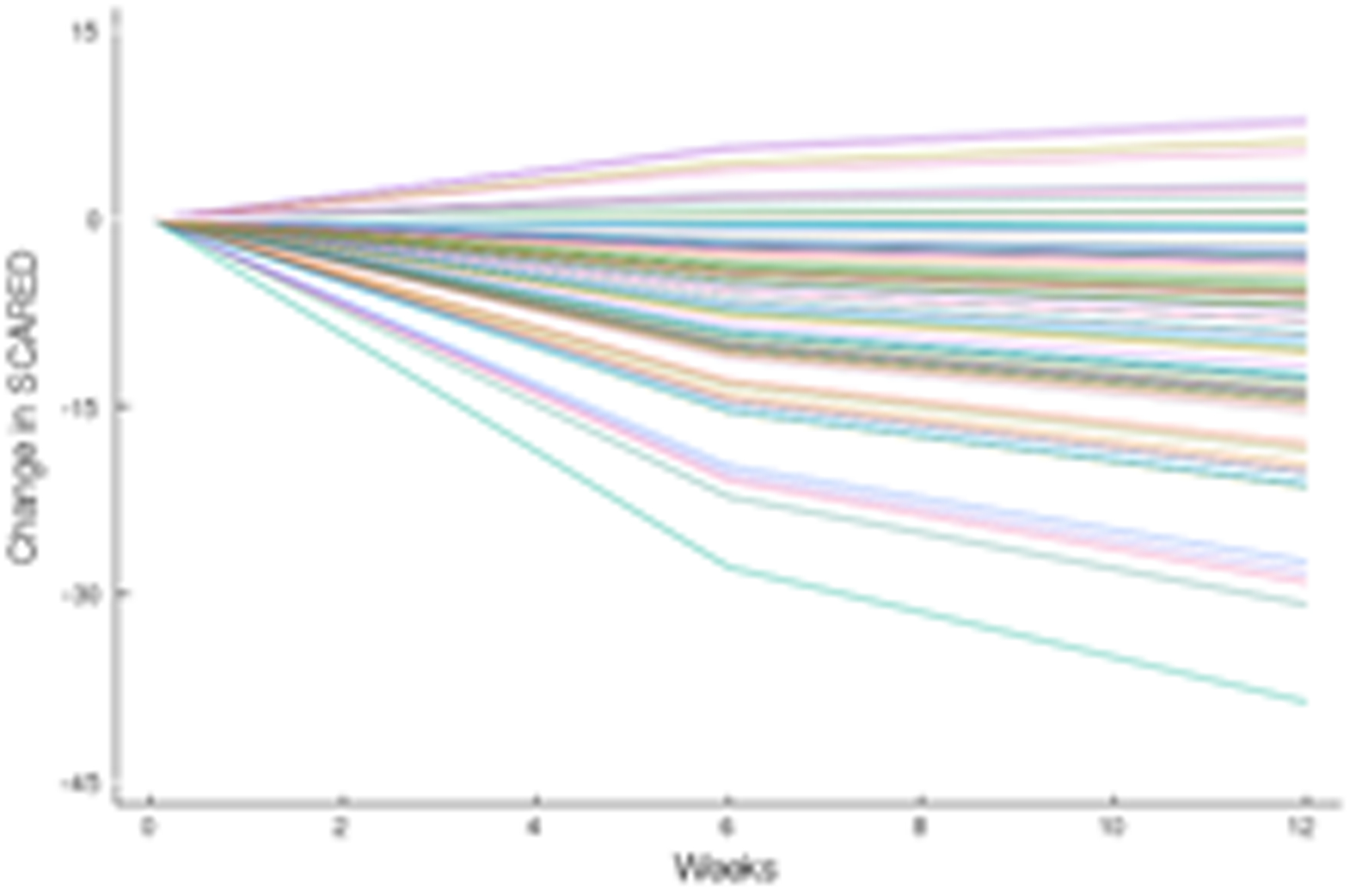
Improvement in Anxiety Symptoms in Adolescents with SSRI-Resistant Depression. Heterogeneity trend parameters for individual improvement in Screen for Child Anxiety and Related Disorders (SCARED) scores are shown for SSRI-treated patients (A) and individual responses are shown in (B). For, SNRI-treated youth, heterogeneity trend parameters for response are shown in (C) and individual responses over time are shown in (D).
3.3 |. Improvement with SSRI vs. SNRI in patients receiving CBT
In the model of individual improvement trajectory (i.e., BHM), patients receiving CBT (n=162) who were randomized to an SSRI had similar improvements in CDRS (difference = -2.00, p=0.077) and SCARED (difference = -0.624, p=0.491) compared to those receiving venlafaxine.
3.4 |. Sensitivity analyses: robustness of improvement models.
For improvement in depressive symptoms, using a homogeneous (fixed effects) model, the trend was -18.07 (p<0.001) and the SSRI × trend interaction was -2.251 (p=0.024). For the heterogeneity model (random effects), the trend was -17.94 (p<0.001) and the SSRI × trend interaction was -2.33 (p=0.015). Similarly, robust findings were present for improvement in anxiety symptoms between models. For the homogeneous (fixed effects) model, the trend for improvement in SCARED score was -3.676 (p<0.001) and the SSRI × trend interaction was -1.291 (p=0.038). For the heterogeneity model (random effects), the trend in SCARED score improvement was -3.688 (p<0.001) and the SSRI × trend interaction was -1.269 (p=0.017).
4 |. DISCUSSION
In this re-examination of the first and only study to examine ‘next line’ interventions in youth with SSRI-resistant depression, SSRIs produced faster, and greater improvement compared to the SNRI, venlafaxine. Patients who were treated with either paroxetine, fluoxetine or sertraline began to improve more quickly compared to those who received venlafaxine. While endpoint differences are clinically important, faster improvement is also very important for clinicians, particularly as those patients with treatment resistant depression who improve earlier have triple the likelihood of achieving remission over longer-term follow-up (Emslie et al., 2010).
In TORDIA, venlafaxine was not as well tolerated as SSRIs. It was associated with more treatment-emergent suicidality (Brent et al., 2009) and hemodynamic side effects (Sakolsky. et al. 2011) compared to SSRIs, and in network meta-analyses of SSRIs and SNRIs in youth with MDD has greater adverse event related discontinuation relative to other antidepressants (Cipriani et al., 2016). Further, until now, its efficacy in adolescents with SSRI-resistant MDD was thought to be ‘equivalent’ to SSRIs despite secondary analyses that jointly consider tolerability and efficacy (Strawn, Mills, et al., 2019). However, our results suggest that venlafaxine is not only less tolerable than SSRIs but is less effective in adolescents with treatment resistant MDD. For clinicians, the take home message is clear: a second trial of an SSRI likely represents the best second medication option when a patient fails to respond to an initial SSRI.
How much an adolescent with SSRI-resistant MDD improves when switched to a second SSRI or venlafaxine-treated varies considerably whether we are evaluating depressive (Figure 2) or anxiety (Figure 3) symptoms, which underscores the substantial heterogeneity of response trajectories among patients. Yet, when clinical trial data are presented, clinicians are accustomed to trajectory plots showing ‘mean improvement’ with a measure of precision (e.g., error bars representing 95% confidence intervals). The spaghetti plots shown in Figures 2 and 3 remind us that there is substantial heterogeneity in response even in a well-controlled, masterfully implemented federally sponsored clinical trial. Understanding this heterogeneity of treatment response is critically important to advance the goal of precision medicine in pediatric mental health. Large trials that allow the parsing of heterogeneity are needed to develop personalized treatments—to know which patients will respond to what treatment.
An open question focuses on the optimal treatment of adolescents with treatment-resistant depression and predominant symptoms of anxiety. Notably, SSRIs are arguably the most well studied agents for the treatment of anxiety disorders in adolescents with the exception that duloxetine is FDA approved for the treatment of pediatric generalized anxiety disorder (Strawn et al., 2015). The present findings suggest that while additional study is warranted, clinicians may consider SNRI treatment earlier in the treatment course of youth with treatment resistant MDD and predominant anxiety who have failed to respond to an initial SSRI trial. However, this finding contrasts with recent meta-analyses and clinical trials of pediatric patients with primary anxiety disorders that suggest that SSRIs may be more beneficial compared to SNRIs (Dobson et al., 2019; Locher et al., 2017; Strawn et al., 2018). Whether this discrepancy is related to the sequence of treatment or to the differences in pathophysiology of mixed anxiety and depression in adolescents (Wehry et al., 2014) compared to anxiety without co-occurring depression remains to be determined.
The Bayesian hierarchical approach coupled with Markov Chain Monte Carlo and Hamiltonian Monte Carlo (MCMC and HMC) (Gelfand & Smith, 1990; Hoffman & Gelman, 2014) used herein allows more precise estimation of treatment effects; does not require as restrictive assumptions; and facilitates more clinically meaningful modeling of outcomes. This approach integrates multi-level information (e.g, individual patient, treatment, comorbidity) which allows multiple effects to be estimated in parallel (McGlothlin & Viele, 2018). These computational approaches are critical to examining existing data sets to develop precision medicine approaches for the treatment of psychiatric disorders (Gardner & Kleinman, 2019; Gordon, Frost Bellgowan, Lawhorn, & Scheinert, 2018). Secondary analyses of large clinical trials such as those presented herein leverage existing resources and do this more cost-effectively and rapidly compared to designing, initiating and implementing new trials.
4.1 |. Limitations
While this is one of very few applications of BHMs in psychiatry and leverages computational advances to shed new light on treatment selection in treatment resistant pediatric depression, there are several important limitations that warrant additional discussion. First, this study did not examine individual SSRIs within the SSRI group. Second, there may have been differences in exposure, with some SSRIs, related to pharmacokinetic genes (Strawn, Poweleit, & Ramsey, 2019) and other factors, that contributed to variability in response (Sakolsky et al. 2011). Third, these findings are based on an assumption of a deterministic logarithmic trend model to gain degrees of freedom which imposes a more restrictive functional form for the trajectory of response but leads to greater precision in evaluating response. Fourth, we were limited in our ability to examine moderators of treatment response and, despite the impressive sample size of TORDIA, had a limited number of observations over time which precluded a ‘deeper’ analysis examining differential trajectories of improvement within putative subgroups.
4.2 |. Conclusions
Experts have concluded that modern computational approaches are critical in both refining the phenotypic characterization of psychiatric disorders and leveraging existing data sets to develop precision medicine approaches for the treatment of psychiatric disorders (Gardner & Kleinman, 2019; Gordon, Frost Bellgowan, Lawhorn, & Scheinert, 2018). Despite considerable prior work, resource investment, and progress over the past decades, there are substantial knowledge gaps in the treatment of child and adolescent mood and anxiety disorders particularly in patients with treatment resistant forms of these disorders. Novel secondary analyses of prior clinical trials present an opportunity to capitalize on existing resources while minimizing the societal ethical burdens inherent with exposing youth to prospective clinical trial participation. BHMs are an important tool in these endeavors. The current findings provide clinicians with much needed guidance in sequencing SSRIs and SNRIs in youth with treatment resistant MDD (Croarkin and Strawn, 2020).
ACKNOWLEDGEMENTS
The authors wish to acknowledge the seminal work of the original (Brent et al. 2008) study team in the conception, design, execution, and original data analyses of the TORDIA study. The TORIDA study was funded by NIH under the cooperative grant agreement U01MH061835. Dr. Croarkin was supported under R01MH113700. Dr. Strawn was supported by R01HD098757 and the Yung Family Foundation. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTERESTS
Mr. Suresh has no potential financial conflicts of interest. Dr. Mills receives research support from the Yung Family Foundation. Dr. Croarkin has received research support from the National Institute of Mental Health, Neuronetics, NeoSync, and Myriad Genetics. Dr. Croarkin has served as a paid consultant for Myriad Neuroscience and Proctor and Gamble Company. Dr. Strawn has received research support from Edgemont, Shire, Forest Research Institute, Otsuka, the Yung Family Foundation and the National Institutes of Health (NICHD, NIMH and NIEHS). He receives royalties from Springer Publishing for two texts and has received material support from Myriad genetics and honoraria from CMEology and Neuroscience Educational Institute. He provides consultation to the U.S. Food and Drug Administration as a Special Government Employee.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the NIMH Data Archive at https://nda.nih.gov/edit_collection.html?id=2150, reference number 2150 (Treatment of SSRI-Resistant Depression in Adolescents [TORDIA]).
REFERENCES
- Bezanson J, Edelman A, Karpinski S, & Shah VB (2014). Julia: A Fresh Approach to Numerical Computing, 59(1), 65–98. [Google Scholar]
- Birmaher B, Brent D. a, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38(10), 1230–1236. 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 545–553. 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- Bondar J, Caye A, Chekroud AM, & Kieling C (2020). Symptom clusters in adolescent depression and differential response to treatment: a secondary analysis of the Treatment for Adolescents with Depression Study randomised trial. The Lancet Psychiatry, 7(4), 337–343. 10.1016/S2215-0366(20)30060-2 [DOI] [PubMed] [Google Scholar]
- Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, … Keller MB (2009a). Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. American Journal of Psychiatry, 166(4), 418–426. 10.1176/appi.ajp.2008.08070976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, … Keller MB (2009b). Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. American Journal of Psychiatry, 166(4), 418–426. 10.1176/appi.ajp.2008.08070976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, … Zelazny J (2008a). Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA, 299(8), 901–913. 10.1001/jama.299.8.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, … Zelazny J (2008b). Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA, 299(8), 901–913. 10.1001/jama.299.8.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, … Al. E (2016). Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. The Lancet, 1263–1271. 10.1016/S0140-6736(16)30385-3 [DOI] [PubMed] [Google Scholar]
- S. DJ, P. JM, E. GJ, C. GN, W. KD, V. B, … P. G (2011). Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Journal of Clinical Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ET, Bloch MH, & Strawn JR (2019). Efficacy and tolerability of pharmacotherapy in pediatric anxiety disorders: a network meta-analysis. Journal of Clinical Psychiatry, 80(1), 17r12064. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, … Brent D (2010). Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. American Journal of Psychiatry, 167(7), 782–791. 10.1176/appi.ajp.2010.09040552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- E. G (2008). The treatment of treatment-resistant adolescents with depression. European Neuropsychopharmacology, 18(S4), S182 Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L70060744 [Google Scholar]
- Gardner C, & Kleinman A (2019). Medicine and the mind — The consequences of psychiatry’s identity crisis. New England Journal of Medicine. 10.1056/NEJMp1910603 [DOI] [PubMed] [Google Scholar]
- Ge H, Xu K, & Ghahramani Z (2018). Turing: A Language for Flexible Probabilistic Inference. Proceedings of the Twenty-First International Conference on Artificial Intelligence and Statistics, 84, 1682–1690. [Google Scholar]
- Gelfand AE, & Smith AFM (1990). Sampling-based approaches to calculating marginal densities. Journal of the American Statistical Association, 85(410), 398–409. 10.1080/01621459.1990.10476213 [DOI] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, & Rubin DB (2004). Bayesian Data Analysis. Chapman Texts in Statistical Science Series. 10.1007/s13398-014-0173-7.2 [DOI] [Google Scholar]
- Gordon JA, Frost Bellgowan JA, Lawhorn C, & Scheinert RB (2018). Challenges and opportunities in psychiatric neuroscience. Cold Spring Harbor Symposia on Quantitative Biology. 10.1101/sqb.2018.83.037523 [DOI] [PubMed] [Google Scholar]
- Gosho M, Hirakawa A, Noma H, Maruo K, & Sato Y (2017). Comparison of bias-corrected covariance estimators for MMRM analysis in longitudinal data with dropouts. Statistical Methods in Medical Research. 10.1177/0962280215597938 [DOI] [PubMed] [Google Scholar]
- Guy W (1976). CGI Clinical Global Impressions In ECDEU Assessment Manual (pp. 217–222). [Google Scholar]
- Hoffman MD, & Gelman A (2014). The no-U-turn sampler: Adaptively setting path lengths in Hamiltonian Monte Carlo. Journal of Machine Learning Research, 15, 1593–1623. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kennard BD, Clarke GN, Weersing VR, Asarnow JR, Shamseddeen W, Porta G, … Brent DA (2009). Effective Components of TORDIA Cognitive-Behavioral Therapy for Adolescent Depression: Preliminary Findings. Journal of Consulting and Clinical Psychology. 10.1037/a0017411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, … Kossowsky J (2017). Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo in Common Psychiatric Disorders A Meta-analysis in Children and Adolescents. Jama Psychiatry, 74(10), 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor B, Rengasamy M, Hilton R, Porta G, He J, Spirito A, … Brent D (2013). The Bidirectional Relationship Between Body Mass Index and Treatment Outcome in Adolescents with Treatment-Resistant Depression. Journal of Child and Adolescent Psychopharmacology, 23(7), 458–467. 10.1089/cap.2012.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, … Treatment for Adolescents With Depression Study (TADS) Team. (2004). Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA: The Journal of the American Medical Association, 292(7), 807–820. 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, & Emslie GJ (2010). Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. Journal of Child and Adolescent Psychopharmacology, 20(6), 513–516. 10.1089/cap.2010.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin AE, & Viele K (2018). Bayesian Hierarchical Models. JAMA - Journal of the American Medical Association. 10.1001/jama.2018.17977 [DOI] [PubMed] [Google Scholar]
- Mizon GE (1995). A simple message for autocorrelation correctors: Don’t. Journal of Econometrics. 10.1016/0304-4076(94)01671-L [DOI] [Google Scholar]
- Müller UK (2014). HAC Corrections for Strongly Autocorrelated Time Series. Journal of Business and Economic Statistics. 10.1080/07350015.2014.931238 [DOI] [Google Scholar]
- Sakolsky DJ, Perel JM, Emslie GJ, Clarke GN, Wagner KD, Vitiello B, … Brent DA (2011). Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Journal of Clinical Psychopharmacology. 10.1097/JCP.0b013e318204b117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Sauley BA, & Welge JA (2018). The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 57(4). 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, & Croarkin PE (2019). Switching Selective Serotonin Reuptake Inhibitors in Adolescents with Selective Serotonin Reuptake Inhibitor-Resistant Major Depressive Disorder: Balancing Tolerability and Efficacy. Journal of Child and Adolescent Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Poweleit EA, & Ramsey LB (2019). CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. Journal of Child and Adolescent Psychopharmacology. 10.1089/cap.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, & Findling RL (2015). A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(4), 283–293. 10.1016/j.jaac.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, & Croarkin PE (2019). Switching Selective Serotonin Reuptake Inhibitors in Adolescents with Selective Serotonin Reuptake Inhibitor-Resistant Major Depressive Disorder: Balancing Tolerability and Efficacy. Journal of Child & Adolescent Psychopharmacology. 10.1089/cap.2018.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR and Croarkin PE Commentary: Treatment Failure and Success: A Commentary on Defining and Treating Pediatric Treatment-Resistant Depression-Reflections on Dwyer et al. (2020). J Child Psychol Psychiatry, 61(3), 333–335. https://doi/full/10.1111/jcpp.13207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Aaronson ST, Elmaadawi AZ, Schrodt GR, Holbert RC, Verdoliva S, Heart K, Demitrack MA, Croarkin PE Treatment-Resistant Depression in Adolescents: Clinical Features and Measurement of Treatment Resistance (2020). J Child Adolesc Psychopharmacol, 30(4), 261–266. 10.1089/cap.2020.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, & Bloch MH (2015). Systematic Review and Meta-Analysis: Early Treatment Responses of Selective Serotonin Reuptake Inhibitors in Pediatric Major Depressive Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(7), 557–564. 10.1016/j.jaac.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Vitiello B, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller MB, … Brent DA (2011). Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: A follow-up study of the TORDIA sample. Journal of Clinical Psychiatry, 72(3), 388–396. 10.4088/JCP.09m05885blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G, & Shostak J (2010). Common Statistical Methods for Clinical Research with SAS Examples (3rd ed.). SAS Institute. [Google Scholar]
- Wehry AM, McNamara RK, Adler CM, Eliassen JC, Croarkin P, Cerullo MA, … Strawn JR (2014). Neurostructural impact of co-occurring anxiety in pediatric patients with major depressive disorder: A voxel-based morphometry study. Journal of Affective Disorders, 171, 54–59. 10.1016/j.jad.2014.09.004 [DOI] [PubMed] [Google Scholar]


