Introduction
Candida albicans is a polymorphic fungus that causes a wide spectrum of complex diseases ranging from superficial mucocutaneous disorders to life-threatening invasive and disseminated infections, particularly in immunocompromised individuals. Understanding this complex Candida–host interaction and the mechanisms that favour protective immunity over immune pathology may provide valuable insights for the rational design of new immunotherapies. Immunity to C. albicans during mucosal and disseminated infections involves the cooperative action of innate and adaptive immune effectors that ultimately determines patient outcome. In recent years, huge progress has been made in our understanding of Candida immunity, particularly with regard to inflammasome activation, characterised by the release of the cytokines interleukin (IL)-1β and IL-18.
IL-1β and IL-18 are potent pro-inflammatory cytokines that coordinate the activation of innate and adaptive immune cells. They are produced as inactive cytoplasmic precursors (pro-IL-1β and pro-IL-18) in response to danger- or pathogen-associated molecular patterns (DAMPs/PAMPs; priming step) and must be posttranslationally processed by multimeric complexes, termed ‘inflammasomes’, to generate the mature, biologically active cytokines [1]. Inflammasomes typically consist of a sensor protein, an adaptor (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD) (ASC)), and an effector caspase. Inflammasome assembly and activation of downstream caspases are triggered by several molecular and cellular signalling events (activation step) that include ion flux, reactive oxygen species (ROS), mitochondria dysfunction, or lysosomal destabilisation [1]. In addition to cytokine secretion, pro-inflammatory caspases localised on inflammasomes can also cleave and activate the pore-forming protein gasdermin D (GSDMD). Cleaved GSDMD oligomerises and forms pores in the plasma membrane, leading to an inflammatory form of programmed cell death called pyroptosis, which functions as a host defence mechanism in a wide range of microbial infections [2]. However, inflammasome activation may be a double-edged sword and thus requires tight regulation to be protective rather than immunopathogenic [3]. Accordingly, determining the role played by inflammasomes during infectious diseases will be essential to identify intervention strategies aimed at boosting or inhibiting inflammasome-mediated immune responses.
Inflammasome activation during C. albicans infection
Multiple inflammasomes can be activated as a result of the complex interplay between host receptors and C. albicans cell wall components and secreted molecules. Thus far, C. albicans has been shown to activate nucleotide-binding oligomerisation domain (NOD)-like receptor family pyrin domain-containing 3 (NLRP3), NOD-like receptor family CARD domain-containing protein 4 (NLRC4), and noncanonical/caspase-8 or caspase-11 inflammasomes in myeloid or epithelial cells (see PLOS Pathogens Pearls [4] for further details and references). Furthermore, accumulating evidence has demonstrated that Candida-induced inflammasome activation can lead to pyroptotic cell death (see PLOS Pathogens Pearls [5] for further details and references). Although the precise signalling sensed by cytosolic NLRs remains unclear, several molecular and cellular events leading to Candida-induced inflammasome activation are emerging. Fungal cell wall components can be recognised by cell surface C-type lectin receptors (CLRs) and Toll-like receptors (TLRs), which mainly provide a priming signal leading to the synthesis of pro-IL-1β and NLRP3 inflammasome components. For instance, engagement of the CLR dectin-1 and TLR2 by β-glucan and zymosan has been demonstrated to prime macrophages for subsequent IL-1β release by C. albicans, whilst the 2 stimuli were unable to elicit this response by themselves [6]. Similarly, Myd88 and B cell lymphoma 10 (BCL10), which mediate signals downstream of TLRs and CLRs, respectively, have been recently demonstrated to be necessary (induction of NLRP3 and IL-1β transcripts) but not sufficient to trigger inflammasome activation and pyroptosis in macrophages, suggesting that inflammasome activation and priming can be decoupled in response to C. albicans [7]. However, C. albicans activation of spleen tyrosine kinase (Syk)-coupled CLRs was found to induce both pro-IL-1β synthesis and NLRP3 inflammasome activation through ROS production and potassium efflux in murine dendritic cells [8]. Furthermore, β-glucan and both heat-killed and live Candida can induce the assembly and activation of a noncanonical CARD-9/BCL10/mucosa-associated lymphoid tissue lymphoma translocation (MALT)-1/ASC/caspase-8 inflammasome in human dendritic cells independently from dectin-1 internalisation or an intracellular NLR [9]. This alternative pathway may represent a rapid mechanism through which human dendritic cells release IL-1β to initiate and polarise appropriate adaptive immune responses. These findings also suggest that different signalling pathways may lead to inflammasome activation in distinct mononuclear phagocyte subsets during C. albicans infection.
After recognition of Candida PAMPs, host cell surface receptors mediate the internalisation of the pathogenic fungus. Phagocytosis of C. albicans and host lysosomal dysfunction were shown to be required for NLRP3 inflammasome activation [10]. Indeed, treatment of macrophages with cytochalasin D or CA-074-Me, which inhibit phagocytosis and cathepsin B, respectively, inhibited IL-1β production following C. albicans infection [10]. Furthermore, the yeast-to-hypha transition [10] and changes in the profile of PAMPs expressed in the hyphal cell wall, for example, the absence of a fully matured, branched outer mannan layer and the exposure of inner hyphal cell wall components (e.g., β-glucan and chitin), have been proposed to enhance recognition by pattern recognition receptors (PRRs) and IL-1β production by macrophages [11]. Recently, however, the importance of C. albicans morphogenesis per se in the induction of inflammasome activation and pyroptosis has been placed under scrutiny. Indeed, filamentation-deficient C. albicans mutants capable of triggering IL-1β secretion and pyroptosis [12,13] and C. albicans mutants that retain the ability to form hyphae but induce decreased IL-1β secretion and macrophage pyroptosis [13,14] have been identified. These findings have also highlighted that biochemical features of the fungal cell wall, rather than just the physical morphology of Candida, likely contribute to inflammasome activation and pyroptosis. For instance, srb9Δ/Δ mutant hyphae, which show reduced surface-exposed β-1,3-glucan, also exhibit reduced ability to cause IL-1β secretion and macrophage death post-phagocytosis [14]. Furthermore, fungal cell wall remodelling within the macrophage phagosome and exposure of glycosylated mannoproteins were required to trigger macrophage pyroptosis [7,12]. Additionally, increased biosynthesis of fungal plasma membrane ergosterol during the yeast-to-hypha transition within phagosomes and its association with the outer mannoprotein layer have been shown to trigger inflammasome activation and pyroptosis [15]. Fungal cell wall remodelling was also observed to be dispensable for the priming step but crucial for inflammasome activation and macrophage pyroptosis, in the absence of phagolysosomal rupture [7]. However, how the signal moves from the phagosome to the cytoplasm to activate the cytosolic NLRP3 remains unclear. Indeed, whether the fungal moieties interact with membrane-bound receptors and/or enter the cytosol to trigger inflammasome activation has not yet been determined.
Apart from cell wall components, secreted molecules from C. albicans can also act as triggers of inflammasome activation. For instance, clathrin-dependent internalisation of secreted aspartyl proteinases (Sap2p and Sap6p) by human mononuclear phagocytes induces an early cascade of events (potassium efflux, ROS production, and lysosomal damage) leading to canonical NLRP3/caspase-1 activation and IL-1β/IL-18 production [16]. Additionally, studies with murine macrophages reveal that Sap-induced type I interferon production activated a noncanonical/caspase-11 inflammasome, which enhanced caspase-1 activation and cytokine production [16]. Furthermore, secretion of the peptide toxin candidalysin can also induce NLRP3/ASC/caspase-1 assembly and IL-1β maturation in human and murine macrophages via a mechanism requiring potassium efflux [17]. Candidalysin is secreted by hyphae, and phagocytes can be exposed to hyphae either pre- or post-phagocytosis. However, experiments conducted in the presence of cytochalasin D have shown inhibition of candidalysin-induced inflammasome activation, suggesting that toxin internalisation is required [17]. Thus, although a rapidly growing body of literature has begun to unravel the regulation and molecular mechanisms responsible for inflammasome activation during Candida infection, much remains to be learned, and further investigations are required for a better understanding of the precise mechanistic details.
Inflammasomes are immunological weapons in the fight against C. albicans infection
In vivo studies have confirmed that inflammasomes are critical for mounting an effective anti-Candida response and in restricting fungal growth and dissemination. Using a murine model of oropharyngeal candidiasis (OPC) and Il1r-/-, Nlrp3-/-, Asc-/-, and caspase-1-/- mice, Hise and colleagues showed that inflammasome components were critical for controlling C. albicans burdens in tongue tissue, preventing dissemination to the kidneys and enhancing host survival [6]. In a follow-up study, NLRC4 inflammasome activation was also demonstrated to prevent OPC and early systemic dissemination of C. albicans infection by coordinating both innate and adaptive immune responses [18]. Neutrophils are essential for innate immunity and resistance to fungal pathogens, and IL-1β participates in driving neutrophil recruitment at the site of infection. Neutrophil influx into the Nlrc4-/- tongue was drastically reduced compared to either wild-type (wt) or Nlrp3-/- mice, and NLRC4 activation was crucial for the trafficking of neutrophils to the site of active Candida infection. Furthermore, antimicrobial peptides are important effector molecules of innate immunity that disrupt pathogen function and act as regulators of inflammation. Interestingly, the expression of antimicrobial peptides in the buccal mucosal tissue of Nlrc4-/-, Nlrp3-/-, and Asc-/- mice was strongly reduced compared to wt mice following C. albicans infection. Similarly, mucosal IL-17 responses to Candida were dependent on both NLRP3 and NLRC4 [18]. OPC was more severe in Nlrc4-/- mice compared with either wt or Nlrp3-/- mice, suggesting that NLRC4 plays a more prominent role than NLRP3 against oral infection. In addition, murine bone marrow chimaera experiments showed that OPC is controlled by NLRC4 functioning in the stromal and epithelial compartment and by NLRP3 functioning in hematopoietic-derived inflammatory cells, whilst protection against disseminated fungal infection was driven by NLRP3 functioning in both hematopoietic and stromal cell lineages [18].
Currently, the fungal components that activate inflammasomes at mucosal surfaces are unclear, but oral epithelial cells are known to secrete IL-1β in response to candidalysin [19]. Notably, IL-1β release may occur through epidermal growth factor receptor (EGFR) activation [20] and drives the proliferation of innate IL-17+TCRαβ+lymphocytes in tongue tissue [21], which are essential for the resolution of murine OPC [22]. These data strongly suggest a central role for candidalysin in inflammasome-mediated defences against OPC. Epithelial inflammasomes may also be activated through the EphA2 receptor, since oral tissues of EphA2−/− mice expressed lower levels of IL-1β, interferon gamma (IFN-γ), and IL-17A and harboured higher fungal burdens and greater fungal dissemination to the liver [23].
Inflammasome activation is also critical for protection against (intravenous) disseminated C. albicans infection. Nlrp3-/- mice rapidly succumb to C. albicans infection compared with wt mice [8] and are associated with significantly increased fungal burdens in the kidneys, liver, spleen, and lungs [8,10]. Likewise, Asc-/- and Caspase-1-/- mice were more susceptible to disseminated candidiasis, with bone marrow–derived dendritic cells releasing significantly reduced levels of IL-1β and IL-18 following C. albicans challenge [24]. In addition, impaired production of IL-17 and IFN-γ was observed in C. albicans–challenged splenocytes isolated from Il-1β-/- and Il-18-/- mice, respectively [24].
Together, these findings suggest that the activation of inflammasomes plays a prominent role in driving protective innate and adaptive antifungal immune responses during mucosal and invasive Candida infections (Fig 1A). However, the precise role of C. albicans–triggered pyroptosis has yet to be elucidated. Importantly, a recent study has shown that pyroptosis occurs in vivo in the kidneys of infected mice during the early stages of infection [15]. Furthermore, neutrophil recruitment in the kidneys of infected mice was dependent on C. albicans mutant strains capable of inducing inflammasome activation and pyroptosis [7]. Thus, although pyroptosis may represent an immune evasion strategy to overcome killing by macrophages, this inflammatory form of programmed cell death may also favour neutrophil recruitment to eliminate the pathogen.
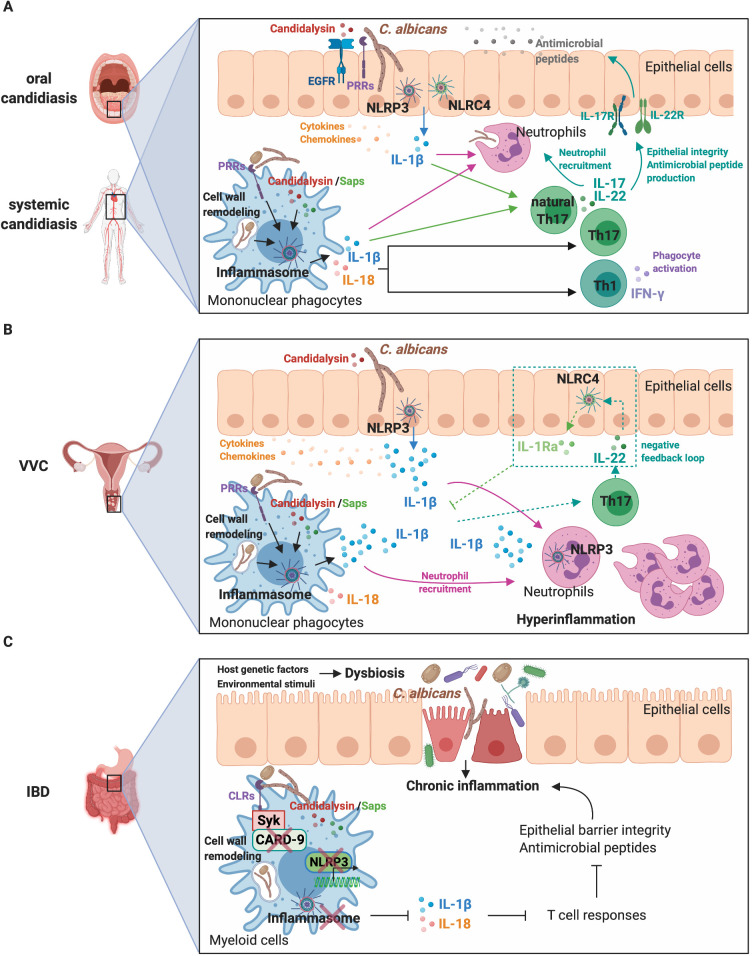
Fig 1. Interplay between C. albicans, inflammasomes, and the host immune system during infection.
(A) NLRP3 and NLRC4 inflammasome activation in epithelial and myeloid cells and the release of IL-1β and IL-18 during oral and systemic candidiasis are instrumental components of the inflammatory response, ultimately orchestrating both innate and adaptive immunity to eliminate the fungus. (B) During VVC, hyphae- and hypha-associated virulence factors induce a powerful NLRP3 inflammasome and inflammatory response that drives the recruitment of large numbers of neutrophils to the site of inflammation. Host genetic variations in inflammasome and other pathways likely influence VVC susceptibility and hyperreactivity to the fungus. An imbalance of NLRP3 inflammasome activation and its IL-22/NLRC4/IL-1Ra negative feedback pathway also contributes to hyperinflammation and disease pathogenesis. (C) A combination of environmental (e.g., diet, antibiotics, and antifungals) and host genetic factors can promote dysbiosis and loss of intestinal immune homeostasis. Impaired mucosal barrier function and dysregulated antimicrobial immune responses result in uncontrolled chronic inflammation. In the genetically susceptible host, impaired inflammasome-mediated anti-Candida responses contribute to intestinal inflammation and IBD pathogenesis. CARD-9, caspase recruitment domain-containing protein 9; CLRs, C-type lectin receptors; EGFR, epidermal growth factor receptor; IBD, inflammatory bowel disease; IFN-γ, interferon gamma; IL, interleukin; IL-1Ra, IL-1 receptor antagonist; NLRC4, NOD-like receptor family CARD domain-containing protein 4; NLRP3, NOD-like receptor family pyrin domain-containing 3; PRRs, pattern recognition receptors; Saps, secreted aspartyl proteinases; Syk, spleen tyrosine kinase; Th, T-helper; VVC, vulvovaginal candidiasis.
NLRP3 inflammasome: A double-edged sword in Candida infection
Whilst inflammasome activation is generally considered beneficial for mounting protective anti-Candida responses, it may also be detrimental and drive immunopathology, as seems to be the case in vulvovaginal candidiasis (VVC). Symptomatic VVC infection is linked to immune hyperreactivity to the fungus and characterised by significant vaginal neutrophil infiltration, NLRP3 inflammasome activation, and increased cytokine production. Notably, polymorphisms and variable number tandem repeats in the NLRP3 gene are associated with VVC [25,26]. Likewise, overexpression of NLRP3, caspase-1, and elevated IL-1β secretion are observed in vaginal epithelial cells isolated from symptomatic VVC patients [27]. In murine studies, neutrophil numbers and IL-1β levels were reduced in vaginal lavage fluid of Nlrp3-/- mice challenged with C. albicans [28]. Interestingly, both filamentation [29] and candidalysin secretion [30] are critical in driving inflammation (including IL-1β), neutrophil recruitment, and pathology in VVC, suggesting a primary role for candidalysin in vulvovaginal immunopathogenesis (Fig 1B).
IL-22 also appears to play a role in VVC. A functional single nucleotide polymorphism (SNP) in the human IL-22 gene was found to be associated with a decreased risk for VVC and correlated with increased IL-22 expression [31]. Borghi and colleagues demonstrated that an IL-22/NLRC4/IL-1 receptor antagonist (IL-1Ra) axis controls NLRP3 activation during Candida infection [32]. In this model, IL-22 production via the aryl hydrocarbon receptor (AhR) activates epithelial NLRC4, which, in turn, restrains NLRP3 activity by inducing sustained production of IL-1Ra. Similarly, NLRC4, but not NLRP3 expression, was increased by IL-22 in response to Candida in human vulvovaginal A431 cells. Moreover, high levels of IL-1β and low levels of IL-1Ra and IL-22 were observed in vaginal fluids of patients with recurrent VVC, suggesting that defective production of IL-1Ra and IL-22 and the impairment of this axis may contribute to disease pathogenesis [32] (Fig 1B). These data highlight the importance of regulating inflammasome activation to fine tune inflammatory processes during C. albicans infection. However, whether the pro-inflammatory nature of C. albicans–induced pyroptosis [7] contributes to disease pathogenesis is still largely unknown and is thus an important direction for future research.
Candida, inflammasomes, and inflammatory bowel disease
Inflammatory bowel disease (IBD) is characterised by chronic inflammation of the gastrointestinal (GI) tract, which includes ulcerative colitis (UC) and Crohn’s disease (CD). Notably, alterations in the biodiversity and composition of fungal microbiota, with an expansion of C. albicans, are observed in IBD patients [33,34]. Although the cause and the exact mechanism of IBD pathogenesis remain to be elucidated, environmental stimuli (e.g., diet, antibiotics, and antifungals), imbalanced interactions with commensal gut microbes, including fungi, and aberrant immune responses appear to contribute to the development of the chronic intestinal inflammation in genetically susceptible individuals [35]. Interestingly, IBD is associated with SNPs in dectin-1, which is a critical CLR required for an effective antifungal immune response [36]. Consistently, dextran sulfate sodium (DSS)-induced colitis was more severe following Candida tropicalis supplementation in dectin-1-/- mice. In addition, fluconazole treatment reduced colitis symptoms, inflammatory cell infiltration, and production of IL-17 and IFN-γ by T cells, confirming that impaired antifungal immunity leads to increased disease severity [36]. Similarly, higher levels of neutrophil infiltration, colon lesions, and IL-1β expression were observed in DSS-treated mice following GI colonisation with C. albicans [37].
IBD is also associated with SNPs in CARD-9 [38], and mice lacking CARD-9 or the kinase Syk show reduced inflammasome activation and IL-18 secretion during azoxymethane (AOM)–DSS-induced colitis-associated colon cancer [39]. These mice also exhibited reduced IFN-γ production by T cells and increased inflammation and epithelial hyperplasia. Notably, similar events occurred when wt mice were depleted of commensal fungi and disease severity significantly ameliorated by exogenous IL-18 supplementation. Furthermore, CARD-9 and Syk were required for caspase-1 activation and IL-18 release in bone marrow–derived myeloid cells in response to C. albicans. Thus, inflammasome-mediated IL-18 release through the activation of Syk/CARD-9 signalling by commensal gut fungi preserves epithelial barrier function, promotes CD8+ T cell responses, and ultimately, restrains colitis and colon tumorigenesis [39]. Interestingly, CD is also associated with SNPs in NLRP3 and concomitant reduction in IL-1β production [40]. Indeed, whilst the exact role of NLRP3 in IBD is not yet fully elucidated, increasing evidence has suggested that appropriate inflammasome activation has a protective effect in colitis and colitis-associated carcinogenesis [41]. For instance, Nlrp3-/-, Asc-/-, and caspase-1-/- mice were observed to be highly susceptible to DSS-induced colitis. Furthermore, these mice also exhibited commensal overgrowth and bacteremia, increased DSS-induced morbidity and lethality, and an exaggerated immune response, which may further worsen disease severity [42]. Similarly, a pathophysiological model linking impaired anti-Candida responses, including an impaired NLRP3 inflammasome activity, and the development of CD has been suggested. This model supports a scenario whereby the progression of IBD in genetically susceptible individuals results from a vicious cycle: inflammation and abnormalities in immune regulation promote proliferation and mucosal invasion by C. albicans, which, in turn, exacerbates the inflammatory process and tissue damage as a consequence of immune dysregulation [43].
Although animal models cannot fully reflect human diseases, they have greatly contributed to our understanding of the mechanism of IBD and the potential contribution of gut fungi and dysregulated immune responses to IBD pathogenesis. Interestingly, the anti-IL-17A monoclonal antibody secukinumab was ineffective in the treatment of CD in a randomised, double-blind, placebo-controlled proof-of-concept study and highlighted adverse effects in patients including a higher frequency of Candida infections compared to placebo [44]. This result further suggests that impaired anti-Candida immunity, including defective inflammasome-driven responses, may play a role in human IBD. Nonetheless, further investigation is required to confirm this model and the relationship between Candida, inflammasome dysregulation, and GI inflammation (Fig 1C).
Inflammasomes as therapeutic targets in Candida disease
Inflammasome activation plays a pivotal role in antifungal immune responses and is a tightly regulated process. Dysregulation of the inflammasome can lead to host damage and excessive inflammation, with inflammasome hyperactivation being a central player in several human autoinflammatory and autoimmune diseases. This has encouraged efforts to identify potent and specific ways to interfere with inflammasome activation or the activity of inflammasome-dependent cytokines [1]. Hence, targeting inflammasome pathways may provide novel and effective therapeutic approaches to neutralise potent inflammatory mediators that exacerbate the pathogenesis of C. albicans infection, as in the case of VVC. Notably, administration of recombinant IL-1Ra (anakinra) can reduce NLRP3-driven inflammation and protect against infection in mouse models of Candida vaginitis [32]. Furthermore, the NLRP3 inhibitor MCC950 and the adenosine triphosphate (ATP)-sensitive potassium channel inhibitor glyburide, which also inhibits inflammasome activation, significantly reduced IL-1β release by the human monocytic cell line THP-1 following Candida exposure or stimulation with candidalysin [45]. Likewise, intravaginal inoculation of glyburide in mice prior to C. albicans infection reduced neutrophil infiltration and IL-1β production in vaginal lavage fluid [28]. These findings demonstrate the potential role for inflammasome inhibitors or anti-IL-1β treatment in the control of hyperinflammation-driven C. albicans diseases. Future work will determine whether such a therapeutic approach can be translated to clinical care.
Acknowledgments
We apologise to colleagues whose work was not cited in this article because of space limitations. The figure was created with BioRender.
Funding Statement
This work was supported by grants from the Wellcome Trust (214229_Z_18_Z), Biotechnology & Biological Sciences Research Council (BB/N014677/1), National Institutes of Health (R37-DE022550) and the NIH Research at Guys and St. Thomas’s NHS Foundation Trust and the King’s College London Biomedical Research Centre (IS-BRC-1215-20006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606. Epub 2018 Jul 22. 10.1038/nrd.2018.97 . [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17(3):151–164. Epub 2017 Feb 1. 10.1038/nri.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–159. Epub 2015 Nov 10. 10.1038/cmi.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares AH, Burgel PH, Bocca AL. Turning up the heat: inflammasome activation by fungal pathogens. PLoS Pathog. 2015;11(7):e1004948 Epub 2015 Jul 24. 10.1371/journal.ppat.1004948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krysan DJ, Sutterwala FS, Wellington M. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathog. 2014;10(6):e1004139 Epub 2014 Jun 27. 10.1371/journal.ppat.1004139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5(5):487–497. Epub 2009 May 21. 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Meara TR, Duah K, Guo CX, Maxson ME, Gaudet RG, Koselny K, et al. High-throughput screening identifies genes required for Candida albicans induction of macrophage pyroptosis. mBio. 2018;9(4). Epub 2018 Aug 23. 10.1128/mBio.01581-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. Epub 2009 Apr 3. 10.1038/nature07965 . [DOI] [PubMed] [Google Scholar]
- 9.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13(3):246–254. Epub 2012 Jan 24. 10.1038/ni.2222 . [DOI] [PubMed] [Google Scholar]
- 10.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183(6):3578–3581. Epub 2009 Aug 18. 10.4049/jimmunol.0901323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90(2):357–366. Epub 2011 May 3. 10.1189/jlb.1210702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6:6741 Epub 2015 Apr 1. 10.1038/ncomms7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellington M, Koselny K, Krysan DJ. Candida albicans morphogenesis is not required for macrophage interleukin 1β production. mBio. 2012;4(1):e00433–12.. Epub 2012 Dec 28. 10.1128/mBio.00433-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio. 2014;5(2):e00003–e00004. Epub 2014 Mar 29. 10.1128/mBio.00003-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koselny K, Mutlu N, Minard AY, Kumar A, Krysan DJ, Wellington M. A genome-wide screen of deletion mutants in the filamentous Saccharomyces cerevisiae background identifies ergosterol as a direct trigger of macrophage pyroptosis. mBio. 2018;9(4). Epub 2018 Aug 2. 10.1128/mBio.01204-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrielli E, Pericolini E, Luciano E, Sabbatini S, Roselletti E, Perito S, et al. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect Immun. 2015;83(5):1940–1948. Epub 2015 Feb 26. 10.1128/IAI.02895-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper L, Konig A, Koenig PA, Gresnigt MS, Westman J, Drummond RA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9(1):4260 Epub 2018 Oct 17. 10.1038/s41467-018-06607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7(12):e1002379 Epub 2011 Dec 17. 10.1371/journal.ppat.1002379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–68. Epub 2016 Mar 31. 10.1038/nature17625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho J, Yang X, Nikou SA, Kichik N, Donkin A, Ponde NO, et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10(1):2297 Epub 2019 May 28. 10.1038/s41467-019-09915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol. 2017;2(17). Epub 2017 Nov 5. 10.1126/sciimmunol.aam8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. Epub 2009 Feb 11. 10.1084/jem.20081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swidergall M, Solis NV, Lionakis MS, Filler SG. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat Microbiol. 2018;3(1):53–61. Epub 2017 Nov 15. 10.1038/s41564-017-0059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Veerdonk FL, Joosten LA, Shaw PJ, Smeekens SP, Malireddi RK, van der Meer JW, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41(8):2260–2268. Epub 2011 Jun 18. 10.1002/eji.201041226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaeger M, Carvalho A, Cunha C, Plantinga TS, van de Veerdonk F, Puccetti M, et al. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur J Clin Microbiol Infect Dis. 2016;35(5):797–801. Epub 2016 Mar 10. 10.1007/s10096-016-2600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200(3):303.e1–e6. Epub 2009 Mar 4. 10.1016/j.ajog.2008.10.039 . [DOI] [PubMed] [Google Scholar]
- 27.Roselletti E, Perito S, Gabrielli E, Mencacci A, Pericolini E, Sabbatini S, et al. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci Rep. 2017;7(1):17877 Epub 2017 Dec 21. 10.1038/s41598-017-17649-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruno VM, Shetty AC, Yano J, Fidel PL Jr, Noverr MC, Peters BM. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. mBio. 2015;6(2). Epub 2015 Apr 23. 10.1128/mBio.00182-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL Jr, Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun. 2014;82(2):532–543. Epub 2014 Jan 31. 10.1128/IAI.01417-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson JP, Willems HME, Moyes DL, Shoaie S, Barker KS, Tan SL, et al. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun. 2018;86(2). Epub 2017 Nov 8. 10.1128/IAI.00645-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luca A, Carvalho A, Cunha C, Iannitti RG, Pitzurra L, Giovannini G, et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 2013;9(7):e1003486 Epub 2013 Jul 16. 10.1371/journal.ppat.1003486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghi M, De Luca A, Puccetti M, Jaeger M, Mencacci A, Oikonomou V, et al. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe. 2015;18(2):198–209. Epub 2015 Aug 14. 10.1016/j.chom.2015.07.004 . [DOI] [PubMed] [Google Scholar]
- 33.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. Epub 2016 Feb 5. 10.1136/gutjnl-2015-310746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatiades GA, Ioannou P, Petrikkos G, Tsioutis C. Fungal infections in patients with inflammatory bowel disease: a systematic review. Mycoses. 2018;61(6):366–376. Epub 2018 Feb 18. 10.1111/myc.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard ML, Lamas B, Liguori G, Hoffmann TW, Sokol H. Gut fungal microbiota: the Yin and Yang of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(3):656–665. Epub 2014 Dec 30. 10.1097/MIB.0000000000000261 . [DOI] [PubMed] [Google Scholar]
- 36.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–1317. Epub 2012 Jun 8. 10.1126/science.1221789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197(7):972–980. Epub 2008 Apr 19. 10.1086/528990 . [DOI] [PubMed] [Google Scholar]
- 38.Zhernakova A, Festen EM, Franke L, Trynka G, van Diemen CC, Monsuur AJ, et al. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82(5):1202–1210. Epub 2008 Apr 29. 10.1016/j.ajhg.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik A, Sharma D, Malireddi RKS, Guy CS, Chang TC, Olsen SR, et al. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity. 2018;49(3):515–530.e5. Epub 2018 Sep 21. 10.1016/j.immuni.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet. 2009;41(1):71–76. Epub 2008 Dec 23. 10.1038/ng.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32(4):171–179. Epub 2011 Mar 11. 10.1016/j.it.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. Epub 2010 Mar 23. 10.1016/j.immuni.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehaume LM, Jouault T, Chamaillard M. Lessons from the inflammasome: a molecular sentry linking Candida and Crohn’s disease. Trends Immunol. 2010;31(5):171–175. Epub 2010 Feb 13. 10.1016/j.it.2010.01.007 . [DOI] [PubMed] [Google Scholar]
- 44.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. Epub 2012 May 19. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowes DJ, Hevener KE, Peters BM. Second-generation antidiabetic sulfonylureas inhibit Candida albicans and Candidalysin-mediated activation of the NLRP3 inflammasome. Antimicrob Agents Chemother. 2020;64(2). Epub 2019 Nov 13. 10.1128/AAC.01777-19 [DOI] [PMC free article] [PubMed] [Google Scholar]