Summary
A generalized absence of enteroendocrine cells characterizes 2 diarrheal/malabsorptive diseases, namely, enteroendocrine cell dysgenesis and autoimmune polyglandular syndrome 1. However, it is not routine for pathologists to examine mucosal biopsies for enteroendocrine cells in cases of chronic diarrheal illness. Our primary aim was to prospectively examine colonic mucosa for loss of enteroendocrine cells using chromogranin A immunohistochemistry for diagnostic purposes. Our secondary aim was to investigate enterochromaffin cells as a subset of enteroendocrine cells, using serotonin (5HT) immunohistochemistry; we hypothesized that other causes of diarrhea due to loss of enteroendocrine cell subsets are missed by evaluating enteroendocrine cells alone. Our approach was limited to patients with chronic unexplained diarrhea partly selected by referring physicians who considered the patients problematic. Seven problematic patients with reduced enteroendocrine or enterochromaffin cells were collected over a 9-month period and placed in group A. Three group A patients demonstrated reduced enteroendocrine cells relative to controls, and they were later diagnosed as having enteroendocrine cell dysgenesis (n = 1) and autoimmune polyglandular syndrome 1 (n = 2). Four group A patients had reduced enterochromaffin cells but normal enteroendocrine cells. These 4 patients had conditions such as congenital diarrhea, mild graft-versus-host disease, acquired childhood chronic diarrhea, and diarrhea post lung transplant. The reduced enterochromaffin cells in the graft-versus-host disease patient inspired a third aim, that is, to investigate whether a loss of enterochromaffin cells would be a generalized defect seen in patients with mild colonic graft-versus-host disease (group B). However, no loss of enterochromaffin cells was detected in group B. Two methods of enumerating endocrine cells were used and demonstrated 67% agreement.
Keywords: Colon, Intestine, Pathology, Enteroendocrine, Endocrine, Malabsorption, Diarrhea, Image analysis, Immunohistochemistry, Enteroendocrine dysgenesis, Autoimmune polyglandular syndrome, Quantitative
1. Introduction
Chronic diarrhea and malabsorption are manifestations of gastrointestinal disease. Chronic diarrhea can have osmotic or secretory mechanisms, and often both [1]. With gastrointestinal inflammation, both secretory and osmotic mechanisms conspire to cause diarrhea. The pathologist has a role in diagnosing causes of diarrhea and/or malabsorption (D/M), often examining for common inflammatory offenders such as infection, gluten-sensitive enteropathy, inflammatory bowel disease, and microscopic colitis. Structural and metabolic diseases such as microvillus inclusion disease or abetalipoproteinemia need to be considered as well. It is not routine to examine colonic mucosal biopsies for the presence of enteroendocrine (EE) cells other than in cases of EE cell hyperplasia or tumors [2-4]. Identifying a paucity of endocrine cells in D/M has been recommended by some [5-7].
The importance of EE cells in gut physiology cannot be overemphasized. Their role in gut pathophysiology is likely just as significant. In a state-of-the-art reference text, it is professed, “Most clinically significant diarrheas are complex; rather than being produced by a single mechanism, they are caused by several mechanisms that may include the effects of substances released by enteroendocrine cells, cytokines released by local and remote immunologically reactive cells, the activity of the enteric nervous system and peripherally released peptides and hormones…” [1]. Thus, the endocrine cells and their products are prime candidates for examination when searching for causes of D/M.
In 1993, leaders in the field of the gastrointestinal endocrine system offered a challenge that “no endocrine deficiency has been identified in the gastrointestinal tract” [4]. However, it is now known that EE cell abnormalities figure prominently in some rare causes of D/M; such is the case in autoimmune polyglandular syndrome 1 (APS1), enteroendocrine cell dysgenesis (ECD; clinically referred to as enteric anendocrinosis), and autoimmune enteropathy [5-10]. In those diseases, EE cells are markedly reduced or absent; in APS1 and ECD, the near absence of endocrine cells is the only abnormality in the intestines. Whereas APS1 and ECD are inherited, it is possible that an acquired state of EE cell loss exists, for instance, small intestinal graft EE cell reduction with secretory diarrhea was reported by Thomas Fishbein at the Xth International Small Bowel Transplant Symposium (Los Angeles, CA, 2007).
APS1 is an inherited autoimmune disease caused by a defective AIRE gene, the function of which is required by regulatory T cells to prevent autoimmunity [11-14]. Affected patients have mucocutaneous candidiasis, hypo-parathyroidism, and adrenocortical failure [12], as well as gastrointestinal disturbances such as malabsorption [5,8]. ECD is a relatively recently described defect in the development of EE cells [6,7]. It is a congenital generalized malabsorptive disease associated with a lack of EE cells in the small intestine and colon secondary to mutations in NEUROG3. NEUROG3 is required by the secretory lineage intestinal epithelial cells to commit to the endocrine phenotype [15].
We chose to prospectively examine colonic mucosa in selected patients with chronic D/M for EE cells and enterochromaffin (EC) cells. The primary aim was to potentially add to the diagnostic yield in the pathological evaluation of chronic diarrhea. The secondary aim was to investigate if a loss of EC cells might characterize a subset of enteric endocrinopathies. The third aim was to examine the nearly normal mucosa of patients who have mild graftversus-host disease (GVHD) with acquired chronic diarrhea for possible EC deficiencies.
2. Materials and methods
This study was approved by the institutional review board at the David Geffen School of Medicine at UCLA.
2.1. Patients
There were 2 sets of patients with chronic D/M illnesses separated into group A and group B. A third set of patients without chronic D/M illnesses was employed as controls, referred to as group C. None of the patients had been part of any prior study by the investigators.
Group A consisted of 7 patients with unexplained chronic D/M who had undergone colonoscopy with biopsies (n = 6) or resection (n = 1). They were selected in an ad hoc fashion without a formal enrollment protocol over a 9-month period. Typically, these patients were particularly intractable, and some discussion between the referring physician and pathologist (G. C.) had prompted a more thorough review of the mucosal biopsies for potentially missed etiologies. No record of the total number of patients so discussed was maintained, only that the number of such discussions was few. Seven patients were found to have visibly rare EE and/or EC cells during the 9-month study. Mucosal biopsies were otherwise normal or nearly normal in all cases.
Group B consisted of 7 patients with GVHD with persistent diarrhea who had undergone colonoscopy with biopsies. They were randomly selected from the archive using the pathology laboratory information system (Power-Path, Impac Medical Systems, Sunnyvale, CA). Criteria for selection included chronic diarrhea, nearly normal mucosa with only minimal involvement by GVHD such as rare apoptotic bodies without crypt loss.
Group C (the control group) consisted of 7 patients with colonic biopsies with normal histology. Patients with a history of chronic diarrhea, severe colitis, endocrine disorder, or immunodeficiency were excluded.
2.2. Histology and Immunohistochemistry
Colonic mucosal biopsies (and 1 resection) were stained with hematoxylin-eosin (H&E) and by immunohistochemistry (IHC) with antibodies to chromogranin A (CGA) and 5HT, as previously described [7]. Briefly for IHC, sections were cut at 2 to 3 μm, deparaffinized, and endogenous peroxidase activity was quenched using 0.5% hydrogen peroxide. Heat-induced epitope retrieval was performed. Slides were placed on a DAKO Autostainer and then incubated sequentially in primary antibody for 30 minutes in rabbit antimouse immunoglobulin (DakoCytomation, Carpinteria, CA), followed by Envision+ (DakoCytomation). Diaminobenzidine (DAB) and hydrogen peroxide were used as the substrates for the peroxidase enzyme. Slides were counterstained with hematoxylin except those used for image analysis where no counterstain was used. Primary antibodies included antibodies against CGA and 5HT (DakoCytomation).
2.3. Manual count of endocrine cells
The populations of CGA and 5HT-positive endocrine cells in an area bounded by 50 crypts in well-oriented colonic mucosa were enumerated, in a fashion identical to our prior study [7]. If the section contained fewer than 50 crypts, then the number was normalized to 50 crypts based on a calculation of mean endocrine cells per crypt. This normalized adjustment was in specimens showing 45 crypts (n = 2). Two pathologists (S. O. and G. C.) separately counted the test and control cases. Any staining of an epithelial cell compatible with an endocrine cell was counted, even faint staining. We defined a count value 2 SDs below the mean of controls as paucity of EE or EC cells. One SD below the mean was considered borderline paucity.
2.4. Image analysis for EC cells
Image analysis for 5HT immunoreactivity was performed on all group A and group C cases. Slides were scanned using an Aperio ScanScope XT (Aperio Technologies, Inc, Vista, CA). Images were analyzed using the Metamorph software package (MDS, Incorporated, Mississauga, Ontario, Canada). Briefly, the layer of epithelial cells was specifically selected so as not to include lamina propria or submucosa using the pen tool. A customized image protocol referred to as a journal by the Metamorph software was applied. The detection thresholds were gated for the DAB chromogen by fixing the red, green, and blue detectors. Manual adjustments to eliminate background staining were applied as needed. We defined measurements of immunoreactivity 2 SDs below the mean of controls as paucity of EC cells. One SD below the mean was considered borderline paucity.
2.5. Mutational analysis
Mutational analysis for patients with suspected ECD was performed as described previously [6]. Mutational analysis for patients with suspected APS1 was performed commercially at Athena Diagnostics, Worcester, MA.
3. Results
3.1. Group A patient characteristics and brief case histories
There were 7 cases having nearly normal mucosa but with few EE or EC cells. They all had chronic unexplained D/M. In the cases that were eventually traced to APS1 or ECD, the clinical diagnosis was not known or was suspected but unconfirmed before the histopathologic examination. The histopathologic examination was a major contributing factor to the eventual diagnosis. The patient characteristics are summarized in Table 1.
Table 1.
Group A, B, C patient characteristics
| No. | Age | Sex | History of consanguinity |
Symptomatology and clinical diagnosis(es) | Site of colonic biopsy |
|---|---|---|---|---|---|
| A1 | 3 mo | Female | Yes | Congenital malabsorptive diarrhea, pathological examination suggested ECD | Transverse |
| A2 | 6 mo | Male | Yes | Congenital malabsorptive diarrhea | 25 cm |
| A3 | 1 y | Male | No | Acquired childhood chronic diarrhea | 40 cm |
| A4 | 6 y | Female | No | GVHD, chronic diarrhea | Ascending |
| A5 | 63 y | Male | No | s/p lung transplant, chronic diarrhea | Transverse |
| A6 | 11 y | Male | Yes | Malabsorptive diarrhea, clinically suspected APS1, later confirmed | Not known |
| A7 | 15 y | Female | Yes | Gut motility disorder, growth hormone deficiency, hypocalcemia, malabsorption, APS1 suggested based on mucosal histopathology, later confirmed | Right |
| B1 | 44 y | Male | No | GVHD, acute myelogenous leukemia | Descending |
| B2 | 24 y | Male | No | GVHD, ALL | 25 cm |
| B3 | 42 y | Female | No | GVHD, ALL | Ascending |
| B4 | 54 y | Female | No | GVHD, ALL | 40 cm |
| B5 | 26 y | Male | No | GVHD, Hodgkin lymphoma | Rectum |
| B6 | 1y | Male | No | GVHD, juvenile myelomonocytic leukemia | Right |
| C1 | 75 y | Male | No | GI bleed | Transverse |
| C2 | 72 y | Female | No | Incontinence | Not known |
| C3 | 36 y | Female | No | Nausea, vomiting, abdominal pain | Left |
| C4 | 59 y | Female | No | Screening examination, remote history of constipation predominant IBS | Not known |
| C5 | 15 y | Female | No | Hematochezia | Not known |
| C6 | 21 y | Female | No | Abdominal pain | Not known |
| C7 | 33 y | Female | No | Constipation predominant IBS | Not known |
Abbreviations: ALL, acute lymphocytic leukemia; s/p, status post; GI, gastrointestinal; IBS, irritable bowel syndrome.
Patient A1 had congenital diarrhea and was found to have only rare EE cells in the small bowel and none in the colon, consistent with a dysgenesis of EE cells. The gastric endocrine cells were present in a normal distribution. Subsequent DNA sequencing revealed a homozygous mutation in NEUROG3, confirming a diagnosis of ECD. This is the first report of a female with ECD.
Patient A2, a 6-month-old male infant, also had congenital diarrhea and reportedly normal small bowel and colonic mucosal biopsies on 2 separate occasions. Electron microscopy was negative and specifically excluded microvillus inclusion disease; disaccharidase deficiencies were also excluded in metabolic assays. However, the number of EC cells was markedly diminished in the colon, small intestine, and stomach on both sets of biopsies. Serum 5HT was 34 ng/mL (reference range, 50-220).
A3 is a 14-month-old boy with acquired chronic diarrhea and TPN (total parenteral nutrition) dependence beginning at 8 months. Upper and lower endoscopies with biopsies were repeatedly normal. Electron microscopy specifically ruled out microvillus inclusion disease. There were reduced EC cells in the colonic biopsies only.
A4 is a 6-year-old girl with a history of acute myelogenous leukemia and status post bone marrow transplant, chronic diarrhea, and GVHD. Her immunosuppressive medications at the time of diarrheal illness included cyclosporine, tacrolimus, mycophenolate mofetil, and prednisone. The colonic biopsies showed only slightly increased apoptotic bodies on H&E. Colonic EE cells were within normal limits, but EC cells were reduced.
A5 is a 63-year-old man, status post lung transplant for emphysema who had chronic diarrhea beginning 6 months after transplantation. The diarrhea went unresolved for 1 year despite extensive workup. His immunosuppressive medications at the time of diarrheal illness included tacrolimus, mycophenolate mofetil, and prednisone. Colonoscopy revealed diverticular disease and an endoscopic impression of colitis. Multiple biopsies showed normal histology on H&E. EE cells were within normal limits, but EC cells were reduced.
A6 is an 11-year-old boy with failure to thrive, chronic diarrhea, elevated serum transaminases, and borderline diabetes. Other medical problems included alopecia, nail dystrophy, juvenile rheumatoid arthritis, and vitamin D deficiency. Upper endoscopy revealed candidiasis, which was suspicious for APS1 in this setting. Biopsies from the stomach, small intestine, and colon had nearly absent EE and EC cells, but no other abnormalities. Subsequent genetic testing revealed mutations in the AIRE gene.
A7 is a 15-year-old girl with a complex medical history. She had a remote diagnosis of a gastrointestinal motility disorder and a right hemicolectomy for possible pseudoobstruction at age 6. The pathological examination of the resection was unremarkable. She developed growth retardation (10th-25th percentile height, 3rd percentile weight), endocrine deficiencies, and malabsorption. Upper and lower endoscopy revealed esophageal candidiasis but no other abnormality. Biopsies showed a lack of endocrine cells in the small intestine and stomach. Colonic biopsies were not taken; however, the prior ileocolonic resection was reexamined, and only rare EE cells were present. APS1 was suggested and later confirmed by AIRE gene sequencing.
3.2. Group B patient characteristics
All patients had chronic diarrhea, bone marrow transplants, GVHD, and nearly normal colonic mucosal biopsies. They are summarized in Table 1.
3.3. Group C patient characteristics
The group C patients served as controls lacking D/M illness. They had normal colonic mucosa. Their characteristics are summarized in Table 1. Although most of the controls were adults, we reported no difference in child EE cell numbers when compared to adults [7].
3.4. Qualitative assessment of colonic endocrine cells
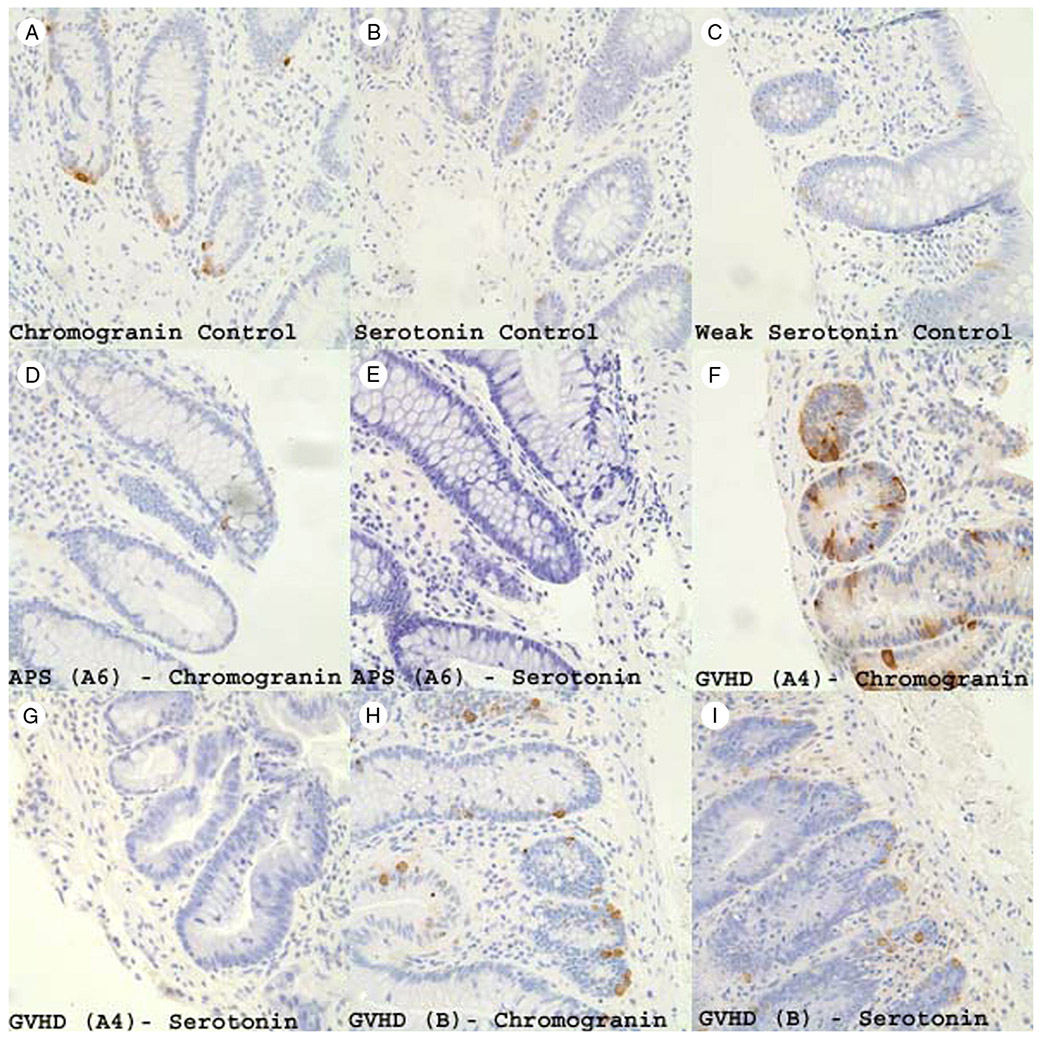
The EE cells of the group C control samples typically showed robust staining (Fig. 1A). The EC cell staining of controls was qualitatively of moderate intensity (most) to faint (occasional), and this is shown in Fig. 1B and C. In the cases of EE cell loss, the occasional EE cells detected often had diminished immunoreactive material in the cytoplasm (Fig. 1D). An example of complete loss of EC cells in patient A6 (APS1) is shown in Fig. 1E. A marked contrast in preserved EE cell staining (numerous, strong) was seen versus selectively reduced EC cell staining (nearly undetectable) in the group A patient with GVHD (Fig. 1F and G). Conversely, the group B GVHD patients had abundant strongly staining EE and EC cells (Fig. 1H and I).
Fig. 1.
CGA staining (A) and 5HT staining (B) of normal colonic mucosa showing a normal distribution of endocrine and EC cells. Panel (C) shows several faintly staining EC cells in normal colonic mucosa highlighting the variation that may be encountered. CGA staining (D) and 5HT staining (E) of patient A6 with APS1 shows only rare endocrine cells and no EC cells. CGA staining (F) and 5HT staining (G) of patient A4 with GVHD showing a normal distribution of EE cells but a severe and almost complete loss of EC cells. CGA staining (H) and 5HT staining (I) of a patient with GVHD from group B highlighting a significant increase in EE cells mildly increased EC cells (all photographs are original magnification ×400).
3.5. Quantified colonic endocrine cells by the manual count method for groups A, B, and C
Table 2 shows the aggregate findings of EE and EC counts for the group C controls. The mean group C EE cells enumerated by the 2 pathologists were 72.3 and 60.9 in 50 crypts; this is similar to a prior report where 84 EE cells were detected in 50 crypts [7]. Similarly, for EC cells, the results were 58.3 and 49.0 EC cells. The number of EC cells appears valid because 50% or more of colonic EE cells are known to be EC cells [16]. The correlation coefficient for enumerating endocrine cells between the 2 pathologists was 0.96 and 0.98 for EE and EC cells, respectively.
Table 2.
Manually quantified EE and EC cells
| Pathologist 1, CGA-positive cells per 50 crypts |
Pathologist 2, CGA-positive cells per 50 crypts |
Paucity (2 SD below mean) |
Pathologist 1, 5HT-positive cells per 50 crypts |
Pathologist 2, 5HT-positive cells per 50 crypts |
Paucity (2 SD below mean) |
|
|---|---|---|---|---|---|---|
| Group C patients (controls, n = 7) |
Average = 72.3 | Average = 60.9 | Average = 58.3 | Average = 43.0 | ||
| SD = 25 | SD = 24 | SD = 24 | SD = 18 | |||
| Range = 49-120 | Range = 36-100 | Range = 36-99 | Range = 22-77 | |||
| Group A patients (chronic unexplained diarrhea) | ||||||
| A1 (ECD) | 0 | 0 | Yes | 0 | 0 | Yes |
| A2 (congenital diarrhea) | 62 | 54 | No | 7 | 6 | Yes |
| A3 (acquired childhood chronic diarrhea) | 69 | 63 | No | 10 | 12 | Yes/Borderline |
| A4 (GVHD) | 44 | 45 | No | 3 | 1 | Yes |
| A5 (lung transplant) | 85 | 78 | No | 19 | 18 | Borderline |
| A6 (APS1) | 10 | 11 | Yes | 0 | 0 | Yes |
| A7 (APS1) | 12 | 11 | Yes | 0 | 0 | Yes |
| Group B patients (GVHD and diarrhea) | ||||||
| B1 | 270 | 241 | No | 175 | 119 | No |
| B2 | 307 | 206 | No | 100 | 68 | No |
| B3 | 75 | 34 | No | 43 | 44 | No |
| B4 | 135 | 93 | No | 78 | 63 | No |
| B5 | 367 | 288 | No | 152 | 85 | No |
| B6 | 85 | 54 | No | 41 | 35 | No |
| All group B | Average = 206.5 | Average = 152.6 | Average = 98.1 | Average = 69.0 | ||
| SD = 113 | SD = 97 | SD = 51 | SD = 28 | |||
Patients A1, A6, and A7 showed EE cell numbers 2 SDs below the mean. None of the other patients in group A were so affected with respect to EE cells. Likewise, patients A1, A6, and A7 all had EC cell counts 2 SDs below the mean. In addition, all 4 remaining group A patients had reduced EC cell counts compared with controls. Of the 4, 3 had paucity of EC cells by one pathologist’s count (A2, A3, and A4). Of 4 remaining group A patients, 2 had paucity of EC cells by the other pathologist’s count (A2 and A4). Patients A2 (congenital diarrhea) and A4 (GVHD) clearly had paucity of EC cells by both enumerations. Only patient A5 (lung transplant) was determined to be in the borderline paucity category for EC cells by both pathologists.
The group B colonic GVHD biopsies showed only a mildly increased number of crypt apoptotic bodies and otherwise had nearly normal colonic mucosal histology. The group B patients had no reduction in EE or EC cells. However, they did show a statistically significant increase in EE cells over controls (P = .011 and .034 for pathologists S. O. and G. C., respectively). There was no statistically significant increase in EC cells (P = .091 and .068 for S. O. and G. C., respectively). The group B GVHD EC cell count was markedly different than the EC cell count in patient A4 (GVHD).
3.6. Quantified colonic EC cells by image analysis of group A patients
Manually quantifying EC cells was more challenging than EE cells because of staining variation that occasionally appeared faint and near the limit of detection. Subjectively, it appeared that the group A patients had lighter 5HT staining than the controls. It was supposed that image analysis may be able to more accurately measure 5HT immunoreactivity by analog measurement in contrast to the manual count method where any stainable cell was weighted as equally positive no matter how faint. Scanned control and group A slides stained for 5HT by IHC were analyzed for the red-brown color of DAB. Slides from the colonic resection specimen for patient A7 could not be scanned because the automated slide scanner was unable to compensate for depth of field variations present in the much larger area of tissue; therefore, patient A7 was excluded from analysis. Depth of field variations did not affect the scanning of the relatively smaller mucosal biopsy specimens for cases A1 to A6. The total quantity of signal was low, less than 0.5 optical density units based on the arbitrary scale of the software. The area immunoreactive material corresponding to just endocrine cells was also small measuring 0.0031 mm2. The results of the analysis are shown in Table 3. Paucity of 5HT reactivity was seen in patients A1 (ECD), A4 (GVHD), and A6 (APS1). Borderline paucity was seen in patient A5 (lung transplant). In contrast to the manual count method, a loss of 5HT reactivity was not measured by image analysis in A2 (congenital diarrhea) and A3 (acquired childhood chronic diarrhea). This discrepancy is addressed in the discussion.
Table 3.
Image analysis of EC cells by 5HT immunoreactivity
| Patient | Average optical density (×10 000) |
Area of 5HT reactivity (mm2) |
Total area measured (mm2) |
Result | Paucity | Agreement with manual count method |
|---|---|---|---|---|---|---|
| Controls (n = 7) | 1412.4 | 0.02926 | 7.42 | 5.57, SD = 1.4 | – | – |
| A1 (ECD) | 3720.1 | 0.00006 | 0.375 | 0.64 | Yes | Yes |
| A2 congenital diarrhea | 1515.1 | 0.00222 | 0.779 | 4.31 | No | No |
| A3 (acquired childhood chronic diarrhea) | 1699.7 | 0.00191 | 0.574 | 5.68 | No | No |
| A4 (GVHD) | 2050.6 | 0.00004 | 0.445 | 0.19 | Yes | Yes |
| A5 (lung transplant) | 1471.2 | 0.00313 | 1.468 | 3.13 | Borderline | Yes |
| A6 (APS1) | 4662.9 | 0.00001 | 1.149 | 0.02 | Yes | Yes |
| A7 (APS1) | N/Aa | – | – | – | – | – |
N/A not applicable secondary to inability to scan slide from resection specimen.
3.7. Summary of results
Diagnosing a paucity of EE cells in cases of nearly normal colonic mucosa and chronic D/M was instrumental in identifying ECD and APS1 diseases in 3 patients. A paucity of EC cells was identified by the manual count method in other congenital and acquired chronic diarrheal conditions and may herald a subset of colonic endocrinopathies distinct from ECD and APS1. A paucity of EC cells was not found to be a generalized feature of mild colonic GVHD, but an increase of EE cells was found. The manual count method and image analysis method agreed in 4 of 6 cases tested for EC cell loss.
4. Discussion
There are 4 points that merit discussion. They are (1) the diagnostic utility of examining colonic mucosa for endocrine cells in cases of unexplained D/M, (2) the investigational promise of quantifying EC cells, (3) the technical aspects of quantification of endocrine cells, and (4) the pathophysiological implications EE cell deficiencies.
4.1. Diagnostics
For diagnostic purposes, EE cell assessment is important in cases of unexplained chronic D/M. Reduced EE cell numbers is strongly suggestive of at least 2 disorders, namely, APS1 and ECD [5-9]. We previously proposed a quantitative approach when examining known cases of ECD [7]. We took the opportunity to define categories of ‘paucity’ of endocrine cells (2 SD below controls) and ‘borderline’ paucity of endocrine cells (1 SD below controls). In patients A1, A6, and A7, the EE cell assessment was critical in defining the anatomic defect and referring the patients for genetic testing for the NEUROG3 and AIRE mutations in ECD and APS1, respectively. In each of these cases, the histopathologic assessment was the driving force in defining the nature of the malabsorptive syndrome and the impetus for genetic testing. Because an undiagnosed APS1 patient may present with D/M before other recognized symptoms, this approach to the mucosal biopsy would direct the patient to definitive testing and treatment. For patients with congenital diarrhea, this approach will identify ECD, which has clinical aspects distinct from microvillus inclusion disease or tufting enteropathy such as a risk of type 1 diabetes [6]. For these reasons, the diagnostic aspects our primary aim were satisfied.
4.2. Investigations
For investigational purposes, this was an attempt to take advantage of readily available colonic mucosal biopsies and assess them for one of the multitude endocrine cell types in other cases of D/M. Reduced EC cell number is potentially of diagnostic utility. EC cells represent a large fraction of EE cells in the colon and therefore seem an appropriate choice. EC cells secrete 5HT, and it is known from APS1 that tryptophan decarboxylase (an enzyme responsible for 5HT synthesis) is an autoantigen [17] and antibodies to it are associated with intestinal dysfunction. Using stringent criteria of EC cell values 2 SDs below the mean of control patients, 3 additional cases of endocrinopathy were characterized by the manual count method. One patient (A2) was diagnosed with congenital diarrhea, another with acquired childhood chronic diarrhea (A3), and a third with GVHD (A4). The lung transplant patient (A5) fell into the borderline category for EC cell loss, and the value of this finding requires further investigation. This approach also serves as the first step in establishing a mode of study regarding specific endocrine cell loss for a defined symptom. At the very least, it may serve as a way of classifying a disease if not providing a pathophysiological rationale for the dysfunction. We feel this satisfied the initial aspects of our secondary aim.
4.3. Technical considerations
Two techniques were used in this study, a manual count of EE and EC cells and image analysis for EC cells. The manual count method was moderately tedious but fairly reproducible among pathologists 1 and 2, also in prior work [7]. It suffers from an inability to discriminate heavily staining and lightly staining endocrine cells, all detectable cells are counted equally. This may result in lost information as to the state of the endocrine cell. It also does not lend itself to a high throughput mode of operation like one might expect for hundreds of clinical samples. The image analysis platform with IHC was meant to discriminate heavily and lightly staining EC cells and potentially lend itself to a high throughput mode of operation. It suffered from marked difficulties at the low end of signal, such is the situation of EE cell numbers relative to surrounding cells (<1%) [18]. There was greater difficulty in the cases of reduced or faint EC cells. Small areas of background staining, readily ignored by the trained eye, were still captured and then needed to be deselected. Other difficulties that could not be overcome included a very weak but diffuse discoloration of the slides relative to the rare focal signal produced by the endocrine cells. Even if unseen at the microscope, a ‘fog’ of faint color overlap with DAB chromogen could readily overwhelm the signal. Selecting only the epithelial cell layer from the scanned images became necessary for reducing similar background interference from the lamina propria. Eliminating the counterstain corrected for some of the fog and allowed for a degree of measurement; however, in cases A2 and A3, there was no way to further discriminate satisfactorily between the DAB visibly associated with the endocrine cells and diffuse faint background, and these cases measured as high as controls despite visual inspection being credibly different. The values were reported as measured without any further refinement of technique. The challenges of image analysis were reported by Ronnblom et al [19] who studied EE cells in rectal mucosa of patients with myotonic dystrophy found significant differences in CGA-positive cells but could not find differences when they examined for individual endocrine products including 5HT. Our 2 techniques agreed in 4 of the 6 cases subjected to the manual count and image analysis method. To some degree, the difficulties of using a composite color pigment like DAB in a low-intensity system on a bright background might be inferior to a primary color light emitting system such as immunofluorescence on a dark background [20,21].
4.4. Pathophysiology
The pathophysiology of a paucity of EE cells remains poorly understood. EE cells sense luminal contents and receive input from the enteric nervous system and the immune system [22]. It is understandable that the extreme paucity of nearly all EE cells seen in APS1 and ECD result in D/M, as a primary defect. It is likely that patient A2 with an undefined congenital D/M and loss of EC cells suffers a similar primary defect. However, the remaining group A cases with paucity of EC cells are all acquired conditions with no established genetic disease. It is unclear if the loss EC cells causes the diarrhea or if the loss of EC cells is secondary to diarrheal injury, or if this represents adaptation to altered colonic function. It is also not known if the EC cells are actually absent or if the 5HT staining is reduced to below detectable levels. Gut endocrine cells are known to adapt in a variety of gastrointestinal diseases: colonic EE and EC cells increase in ulcerative colitis [23], EC cells increase in the small bowel in celiac disease [24], somatostatin cells were noted to decrease in IBD colons [25], and G cells increase in cases of reduced gastric acid [26]. The notion that a paucity of EE cells and EE cell subsets contributes to disease seems likely because endocrine deficiencies in other organs abound as causes of disease [27].
Our third aim lead only to negative results. We did not find paucity of EC cells to be a generalized feature in patients with diarrhea and colonic GVHD. That was only seen in patient A4 but none of the group B patients. This implies that patient A4 had a different injury or adaptive pattern. Paradoxically, the number of EE cells was significantly higher in group B than in controls. To our knowledge, this is the first report of such a finding.
4.5. Conclusions
In conclusion, an evaluation of EE cells should be part of the examination of colonic mucosa in cases of unexplained D/M to assist in diagnosing APS1 and ECD. A paucity of EC cells suggests a similar defect, but further work in this area is necessary. We do not feel that our IHC image analysis method of measuring EC cells was superior to the manual count method.
References
- [1].Schiller LR, Sellin JH. Diarrhea In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease pathophysiology diagnosis management. 8th ed. Philadelphia (Pa): Saunders Elsevier; 2006. p. 159–67. [Google Scholar]
- [2].Bernstein CN, Riddell RH. Colonoscopy plus biopsy in the inflammatory bowel diseases. Gastrointest Endosc Clin N Am 2000; 10:755–74. [PubMed] [Google Scholar]
- [3].Freeman HJ. Small intestinal mucosal biopsy for investigation of diarrhea and malabsorption in adults. Gastrointest Endosc Clin N Am 2000;10:739–53. [PubMed] [Google Scholar]
- [4].Solcia E, Fiocca R, Rindi G, Villani L, Cornaggia M, Capella C. The pathology of the gastrointestinal endocrine system. Endocrinol Metab Clin North Am 1993;22:795–821. [PubMed] [Google Scholar]
- [5].Hogenauer C, Meyer RL, Netto GJ, et al. Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N Engl J Med 2001;344:270–4. [DOI] [PubMed] [Google Scholar]
- [6].Wang J, Cortina G, Wu SV, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med 2006;355:270–80. [DOI] [PubMed] [Google Scholar]
- [7].Cortina G, Smart CN, Farmer DG, et al. Enteroendocrine cell dysgenesis and malabsorption, a histopathologic and immunohisto-chemical characterization. Hum Pathol 2007;38:570–80. [DOI] [PubMed] [Google Scholar]
- [8].Oliva MM, Yardley JH. Marked paucity of gastrointestinal endocrine cells (GIECs) in a child with polyglandular autoimmune (PGA) syndrome, and steatorrhea. Gastroenterology 1995;108:A743 (abstract). [Google Scholar]
- [9].Oliva-Hemker M, Berkenblit GV, Anhalt GJ, Yardley JH. Pernicious anemia and widespread absence of gastrointestinal endocrine cells in a patient with autoimmune polyglandular syndrome type I and malabsorption. J Clin Endocrinol Metab 2006;91:2833–8. [DOI] [PubMed] [Google Scholar]
- [10].Al Khalidi H, Kandel G, Streutker CJ. Enteropathy with loss of enteroendocrine and paneth cells in a patient with immune dysregulation: a case of adult autoimmune enteropathy. Hum Pathol 2006;37: 373–6. [DOI] [PubMed] [Google Scholar]
- [11].Peterson P, Peltonen L. Autoimmune polyendocrinopathy syndrome type 1 (APS1) and AIRE gene: new views on molecular basis of autoimmunity. J Autoimmun 2005;25(Suppl):49–55. [DOI] [PubMed] [Google Scholar]
- [12].Perheentupa J Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 2006;91:2843–50. [DOI] [PubMed] [Google Scholar]
- [13].Cheng MH, Shum AK, Anderson MS. What’s new in the AIRE? Trends Immunol 2007;28:321–7. [DOI] [PubMed] [Google Scholar]
- [14].An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997;17:399–403. [DOI] [PubMed] [Google Scholar]
- [15].Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 2002;21:6338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 1983;85:193–210. [PubMed] [Google Scholar]
- [17].Ekwall O, Hedstrand H, Grimelius L, et al. Identification of tryptophan hydroxylase as an intestinal autoantigen. Lancet 1998;352:279–83. [DOI] [PubMed] [Google Scholar]
- [18].Dahl J, Greenson JK. Colon. In: Mills SE, editor. Histology for pathologists. 3rd ed. Philadelphia (Pa): Lippincott Williams & Wilkins; 2007. p. 634. [Google Scholar]
- [19].Ronnblom A, Danielsson A, el-Salhy M. Intestinal endocrine cells in myotonic dystrophy: an immunocytochemical and computed image analytical study. J Intern Med 1999;245:91–7. [PubMed] [Google Scholar]
- [20].Walker RA. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment I. Histopathology 2006;49:406–10. [DOI] [PubMed] [Google Scholar]
- [21].Feldman MD. Beyond morphology: whole slide imaging, computer-aided detection, and other techniques. Arch Pathol Lab Med 2008;132: 758–63. [DOI] [PubMed] [Google Scholar]
- [22].Liddle RA. Gastrointestinal hormones and neurotransmitters In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease pathophysiology/diagnosis/management. 8th ed. Philadelphia (Pa): Saunders Elsevier; 2006. p. 3–25. [Google Scholar]
- [23].El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med 1997;242:413–9. [DOI] [PubMed] [Google Scholar]
- [24].Wheeler EE, Challacombe DN. Quantification of enterochromaffin cells with serotonin immunoreactivity in the duodenal mucosa in coeliac disease. Arch Dis Child 1984;59:523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Watanabe T, Kubota Y, Sawada T, Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum 1992;35:488–94. [DOI] [PubMed] [Google Scholar]
- [26].Rindi G, Solcia E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol Clin North Am 2007;36:851–65. [DOI] [PubMed] [Google Scholar]
- [27].Moran GW, Leslie FC, Levison SE, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Ther Adv Gastroenterol 2008;1:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]