Abstract
High intensity interval exercise (HIIE) improves aerobic fitness with decreased exercise time compared to moderate continuous exercise. A gap in knowledge exists regarding the effects of HIIE on cerebrovascular function such as cerebral blood velocity and autoregulation. The objective of this systematic review was to ascertain the effect of HIIE on cerebrovascular function in healthy individuals. We searched PubMed and the Cumulative Index to Nursing and Allied Health Literature databases with apriori key words. We followed the Preferred Reporting Items for Systematic Reviews. Twenty articles were screened and thirteen articles were excluded due to not meeting the apriori inclusion criteria. Seven articles were reviewed via the modified Sackett’s quality evaluation. Outcomes included middle cerebral artery blood velocity (MCAv) (n = 4), dynamic cerebral autoregulation (dCA) (n = 2), cerebral de/oxygenated hemoglobin (n = 2), cerebrovascular reactivity to carbon dioxide (CO2) (n = 2) and cerebrovascular conductance/resistance index (n = 1). Quality review was moderate with 3/7 to 5/7 quality criteria met. HIIE acutely lowered exercise MCAv compared to moderate intensity. HIIE decreased dCA phase following acute and chronic exercise compared to rest. HIIE acutely increased de/oxygenated hemoglobin compared to rest. HIIE acutely decreased cerebrovascular reactivity to higher CO2 compared to rest and moderate intensity. The acute and chronic effects of HIIE on cerebrovascular function vary depending on the outcomes measured. Therefore, future research is needed to confirm the effects of HIIE on cerebrovascular function in healthy individuals and better understand the effects in individuals with chronic conditions. In order to conduct rigorous systematic reviews in the future, we recommend assessing MCAv, dCA and CO2 reactivity during and post HIIE.
Introduction
High intensity interval exercise (HIIE) has emerged at the forefront of exercise regimens due to the shorter activity time needed to benefit [1–3]. HIIE confers similar or significant increased aerobic fitness compared to conventional moderate intensity continuous exercise [1, 4–7]. While aerobic fitness is a measure of increased cardiovascular health, the entire vascular system (including the cerebral vascular system) may be improved following increased aerobic fitness [8]. With aging, higher aerobic fitness is associated with a lower risk of stroke and dementia [9, 10]. A review and meta-analysis of HIIE in healthy adults has shown significant increases in aerobic fitness [1, 5, 6, 11]. Preliminary evidence has also shown HIIE may improve cognitive function [12]. However, the effects of HIIE on cerebrovascular function have not been systematically reviewed.
Cerebrovascular function is the ability of the cerebral blood vessels to deliver oxygen and nutrients for neuronal metabolism and maintain cerebral blood flow through dynamic autoregulation (dCA). dCA is the ability of the brain to sustain a constant cerebral blood flow despite large fluctuations in peripheral blood pressure [13, 14]. During resting conditions, cerebral blood flow responds to arterial blood pressure fluctuations, neuronal metabolism, cortical activation, arterial blood gases and cardiac output [15]. Cerebral blood flow can be measured at rest using magnetic resonance imaging or transcranial Doppler ultrasound (TCD). Middle cerebral artery blood velocity (MCAv) measured by TCD is the only technique to measure cerebral blood flow during exercise, with high temporal resolution [16]. MCAv is linearly related to cerebral blood flow with the caveat that the MCA diameter remains unchanged [17].
A normal cerebrovascular response to submaximal moderate continuous exercise results in increased MCAv [18–20], increased cerebral oxygenation [21, 22] and sustained dCA [23, 24]. MCAv has been shown to concomitantly increase as exercise intensity increases, up to moderate intensity [15, 18, 25–28]. MCAv is affected differently during high intensity exercise. During continuous high intensity exercise and hyperventilation, MCAv is decreased due to a reduction in arterial carbon dioxide (CO2) [29, 30] causing downstream arteriole constriction [15, 31]. Cerebrovascular reactivity is the ability of the small vessels in the brain to vasodilate and vasoconstrict in response to fluctuating CO2 levels [32, 33]. The cerebrovascular response to HIIE may differ from continuous high intensity exercise due to the repetitive short interval bouts that rapidly increase blood pressure which may cause cerebrovascular hyper-perfusion [34, 35]. If neuroprotective mechanisms of the brain, such as dCA, do not respond quickly to the repetitive and rapid increases in blood pressure, HIIE could elevate the risk for leakage within the blood brain barrier [34, 36]. For clinical populations with cerebrovascular impairment, such as stroke [37–39], the cerebrovascular response to HIIE may play an important role in guiding exercise prescription [36].
Previous scientific statements and narrative reviews have recounted the molecular, hemodynamic and structural processes (i.e. CO2, nitric oxide, systemic blood pressure, vessel compliance, glial cell integrity) associated with the cerebrovascular response that may occur during HIIE [36, 40]. However, these detailed narrative reviews [41, 42] did not report the statistical findings of previous studies showing cerebrovascular function during HIIE. To our knowledge, our current systematic review is the first to systematically search and report the results of the dynamic cerebrovascular response during HIIE. Reporting the cerebrovascular response during HIIE is important because it provides objective results to support the previously described narrative statements on hemodynamic processes during HIIE [41, 42]. The purpose of this systematic review was to address the gap in knowledge and report the various study results of HIIE on cerebrovascular function compared to moderate continuous exercise or rest conditions. We systematically examined the results of HIIE studies in healthy individuals based on the operationalization of cerebrovascular outcomes.
Methods
This review follows the guidelines for Preferred Reporting Items for Systematic Reviews [43]. Literature searches and reviews were performed using PubMed and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases. The University of Kansas Medical Center Online Library system was used to access these databases in February, March, and June 2020. In this systematic review, we included peer-reviewed manuscripts written in English from January 2010 to June 2020.
Key words used to search the databases included “high intensity interval training”, “HIIT”, “high intensity interval exercise”, “HIIE” AND “cerebral blood flow”, “cerebral blood velocity”, “dynamic autoregulation”. We believe these key words primarily reflect the high intensity interval intervention and cerebrovascular function outcome measures. The main outcomes of this systematic review were MCAv, an indirect measure of cerebral blood flow, and dCA, a measure of cerebrovascular homeostasis during peripheral blood pressure changes [30, 44–48]. MCAv supplies oxygen and nutrients to neurons while dCA maintains stable perfusion [49]. However, additional cerebrovascular measures were also included such as oxygenated hemoglobin [50–52], cerebrovascular reactivity [46, 53], cerebrovascular conductance index and cerebrovascular resistance index [45, 54]. Oxygenated hemoglobin is an important measure of aerobic metabolism within cerebral tissue using near-infrared spectrometry [55]. Cerebrovascular reactivity is a measure of cerebrovascular regulation [56] and shows the ability of the vessels to vasodilate or vasoconstrict to a stimulus [57]. Cerebrovascular conductance index is a measure of the conductance of peripheral blood pressure to cerebral blood velocity and is calculated as MCAv/mean arterial pressure (MAP) [45]. Cerebrovascular resistance index (MAP/MCAv) measures the resistance of cerebral perfusion pressure to cerebral blood velocity [45].
The identified abstracts from PubMed and CINAHL were screened using the following inclusion criteria: 1) experimental or quasi-experimental, 2) aerobic exercise identified as the primary means of performing HIIE, 3) cerebrovascular measures were primary or secondary outcomes and 4) human subjects across the lifespan with no current disease. After the removal of duplicates, two researchers screened titles/abstracts for inclusion criteria (A.W. and M.A.). The full texts were examined, and data extracted (A.W. and M.A.). If the authors were unable to come to an agreement, a third author moderated incongruity (A.F.).
A quality review was performed for each article using the modified version of Sackett’s 1981 criteria [58]. We critically analyzed each article’s study design, population, HIIE protocols, cerebrovascular outcomes and results. If an article did not report enough information to determine sufficient quality criteria a “No” rating was given. Articles were rated based on the level of evidence including level I for large randomized control trials, level II for small randomized trial, level III for nonrandomized design, Level IV for case series and Level V for case reports [59].
Results
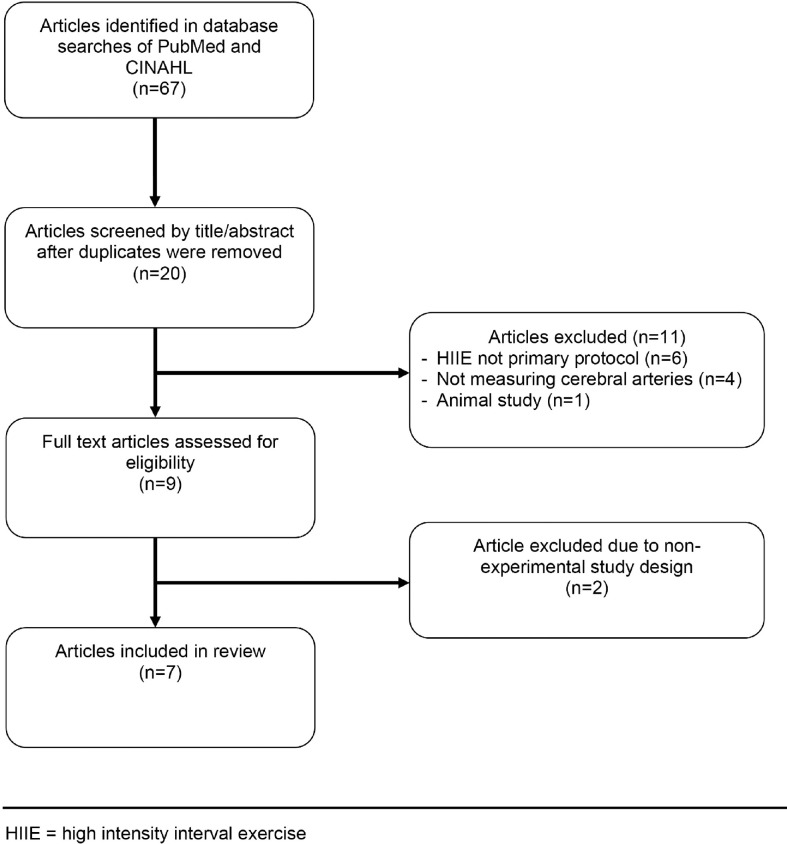
The search methods resulted in 67 articles. After removal of duplicates, 15 [45, 46, 50, 53, 60–69] articles were identified in PubMed and 5 [47, 51, 70–72] new articles in CINAHL. During the initial screening of titles/abstracts, 11 articles were excluded due to HIIE not being the primary experimental protocol performed (n = 6), studies not measuring cerebral arteries (n = 4) and an animal study (n = 1). Studies that combined other interventions with HIIE were excluded due to the confounding variables that could affect cerebrovascular outcomes (see S1 Table). After the full text assessment, two articles were excluded due to not meeting experimental or quasi experimental criteria (n = 2). See Fig 1 for flow diagram of article selection.
Fig 1. Flow diagram of article selection.
We included seven articles describing cerebrovascular outcomes following HIIE within this review [44–47, 50, 51, 53]. The full texts are described in Table 1. Of the articles reviewed, six were small, randomized trials and one nonrandomized cross-over trial. All the studies involved healthy individuals, although some studies only included men (n = 1) [45], women (n = 1) [46], or children (n = 1) [47]. Prior activity levels of participants ranged from inactive [50], recreationally active [51] and endurance trained [45].
Table 1. Summary of reviewed articles.
| Study | Design | Level of Evidence | Subjects | Intervention | Outcome measures | Results |
|---|---|---|---|---|---|---|
| Burma et al, March 2020 | Small Randomized Cross-Over Trial | II | 9 Young Adults (age 26 ± 5 years old) | 3 cycling conditions: • High intensity interval training (HIIT, 1-min interval at 85–90% predicted heart rate reserve with 1-min active recovery 15% power output for 10 intervals) • Moderate intensity continuous exercise (MICT, 50–60% predicted heart rate reserve for 45 min) • No-exercise control |
TCD measured: Average Exercise MCAv dCA via Transfer Function Analysis during forced MAP oscillations (repeated squat-stand maneuver) |
Significant increase in exercise MCAv during MICT compared to HIIT (p<0.05) and baseline (p<0.05). No change in exercise MCAv during HIIT compared to baseline (p>0.05). Significantly higher systolic gain/phase compared to diastolic/mean gain/phase at 0.05 and 0.10 Hz during control (p<0.05). Decreased systolic phase in 0.05 Hz immediately following HIIT (p>0.102) and MICT until hour 4 (p>0.079). Decreased systolic phase in 0.10 Hz immediately following HIIT until hour 2 (p>.11) and immediately following MICT until hour 4 (p>0.079). No change in gain or coherence in 0.05 Hz or 0.10 Hz during HIIT or MICT. |
| Burma et al, June 2020 | Secondary Analysis of the above Small Randomized Cross-Over Trial | II | Same as above | Same as above | TCD measured: Cerebrovascular reactivity to hypercapnia Cerebrovascular reactivity to hypocapnia |
Significantly decreased absolute and relative MCA reactivity to hypercapnia immediately following HIIT up to hour 2 (p<0.018) compared to MICT and control (p<0.022). Significantly decreased relative MCA reactivity to hypercapnia immediately following MICT up to hour 1 (p<0.024). No significant differences in MCA reactivity to hypocapnia between conditions (p>0.31). |
| Coetsee et al, 2017 | Small Randomized Controlled Trial | II | 67 Inactive Adults HIIT (age 64.5 ± 6.3 years old) MCT (age 61.6 ± 5.8 years old) CON (age 62.5 ± 5.6 years old) |
16-week intervention Treadmill 30 min, 3x/week 4 groups: • High intensity Interval training (HIIT, 4 min interval at 90–95% HRmax with 3 min active recovery 70% HRmax) • Moderate continuous training (MCT, 70–75% HRmax) • No-exercise control (CON). |
Near-Infrared Spectroscopy measured during Cognitive Stroop test: • Oxygenated Hemoglobin • Deoxygenated Hemoglobin • Total Hemoglobin Index |
No significant differences in oxygenated (effect size = .45, p = .3), deoxygenated (effect size = 0.67, p = .14), or total hemoglobin (effect size < .6, p>.18) after HIIT. Significant decrease in deoxygenated hemoglobin (effect size = 1.14, p = .01) and total hemoglobin index (effect size = 1.49, p < .01) after MCT. Significant increase in oxygenated hemoglobin in CON (effect size = .76, p = .03). |
| Drapeau et al, 2019 | Small Randomized Clinical Trial | II | 17 Endurance Trained Males HIIT85 (age 26 ± 6 years old) HIIT115 (age 28± 6 years old) |
6-week intervention.Cycled until exhaustion, 3x/week2 groups: • HIIT85 (1–7 min interval at 85% of maximal aerobic power, with active recovery of 50% of maximal aerobic power) • HIIT115 (30sec– 1min interval at 115% of maximal aerobic power, with active recovery of 50% of maximal aerobic power). |
TCD measured: Resting MCAv Resting CVCi Resting CVRi dCA via Transfer Function Analysis during forced MAP oscillations (repeated squat-stand maneuver) |
Significant decrease in phase at 0.10 Hz in HIIT85 and HIIT115 (p = .048) with no differences between intensity groups. No significant difference in power spectral density (p > .39), gain (p > .05), or coherence (p>.05) between time or intensity. No significant differences in MCAv (p = .4), CVCi (p = .87), or CVRi (p = .87). |
| Northey et al, 2019 | Small Randomized Controlled Trial | II | 17 Female Breast Cancer Survivors HIIT (age 60.3 ± 8.1 years old) MOD (age 67.8 ± 7.0 years old) CON (61.5 ±7.8 years old) |
12-week intervention Cycled 20–30 min 3x/week 3 groups: • High intensity interval training (HIIT, 30 sec intervals at ~90% maximal heart rate or ~105% peak power with 2 min active recovery) • Moderate intensity continuous exercise (MOD, 55–65% peak power) • No-exercise control (CON) |
TCD measured: Resting MCAv, Cerebrovascular Reactivity to CO2 |
No significant differences in resting MCAv (p = .24) or cerebrovascular reactivity (p = .54) after HIIT compared to MOD. No significant differences in resting MCAv (p = .86) or cerebrovascular reactivity (p = .72) after HIIT compared to CON. |
| Tallon et al, 2019 | Small Randomized Cross-over Trial | II | 8 Prepubertal Children (age 10 ± 1.9 years old) | 2 Cycling conditions: • High intensity interval exercise (HIIE, 1 min interval at 90%max watt with 1 min active recovery at 20%max watt for 6 intervals) • Moderate-intensity steady-state exercise (MISS, 15 min at 44%max watt) |
TCD measured: Exercise MCAv during each interval Immediate post-exercise MCAv 30-minutes post-exercise MCAv |
Significant decrease in exercise MCAv during the 6th interval of HIIE compared to baseline (10.7%, p = .004). Significant decrease in exercise MCAv during the 3rd and 4th intervals of HIIE compared to MISS (p = .001). Significant decrease in MCAv immediately post-exercise following HIIE and MISS (p < .001). No significant difference in MCAv at 30-minutes post-exercise following HIIE and MISS compared to baseline (p>.05). Significant increase in exercise MCAv during the 2nd minute of MISS compared to baseline (5.8%, p = .004). |
| Monroe et al, 2016 | Nonrandomized Cross-Over Trial | III | 15 Recreationally Active Adults (age 21.3 ± 2.4 years old) | 2 cycling conditions: • Sprint Interval Cycling (SIC, 30 second all-out sprint interval with 4 min active recovery for 4 intervals) • Constant Resistance Cycling (CRC, 18 min at 70rpm with resistance set by matching total work performed during SIC) |
Near-Infrared Spectroscopy measured: Oxygenated Hemoglobin (HbO2) Deoxygenated Hemoglobin (HHb) |
Significant increase in average HbO2(effect size = .536, p = .001), minimum HbO2 during recovery (effect size = .392, p < .001) and maximum HbO2 during recovery (effect size = .588, p = .001) in SIC compared to CRC. Significant increase in average HHb during SIC compared to CRC (effect size = .386, p = .003). |
MCAv = middle cerebral artery blood velocity, dCA = dynamic cerebral autoregulation, min = minute, HRmax = maximum heart rate, CVCi = cerebrovascular conductance index, CVRi = cerebrovascular resistance index, CO2 = carbon dioxide.
High intensity interval protocols
Methods of prescribing HIIE varied greatly and made comparisons between studies difficult. HIIE protocols included 6- to 16-week exercise interventions (n = 3) [45, 46, 50] or one single bout of exercise(n = 4) [44, 47, 51, 53]. By examining 6- to 16-weeks of HIIE, the long-term or chronic effects of this intervention were studied. By examining a single bout of HIIE, the immediate or acute effects of the exercise were reported. In addition to the duration variability, we found that the mode of HIIE also differed across the included studies. One study used a treadmill as the mode of exercise with 4-minute intervals of 90–95% maximal heart rate for 30 minutes [50]. The remaining six studies used cycling as the mode of exercise but differed in parameters ranging from 30 seconds [46, 51] to 7-minute intervals [45] at 85% to 115% of maximal watts [45, 47, 51] or ~ 85% to 90% maximal heart rate [44, 46, 50, 53]. A constant between all studies included an active (rather than passive) recovery interval between sprints. However, the intensity and duration of recovery intervals differed greatly.
Cerebrovascular outcome measures
The results of this review can be operationalized based on the outcome variables measured during HIIE such as MCAv (n = 4) [44–47], dCA (n = 2) [44, 45], cerebral de/oxygenated hemoglobin (n = 2) [50, 51], cerebrovascular reactivity to CO2 (n = 2) [46, 53] and cerebrovascular conductance/resistance index (n = 1) [45]. Table 2 describes whether HIIE increased, decreased or had no influence on the operationalized cerebrovascular measures. A meta-analysis was not performed due to low number of studies (≤ 2) reporting each operationalized cerebrovascular measure.
Table 2. Summary of the effects of HIIE on operationalized cerebrovascular measures.
| Resting MCAv | Exercise MCAv | Post-Exercise MCAv | dCA phase | dCA Gain | dCA Coherence | De/Oxygenated Hemoglobin | Cerebrovascular Conductance Resistance Index | Cerebrovascular Reactivity to CO2 | |
|---|---|---|---|---|---|---|---|---|---|
| Burma et al, March 2020 | ↓ To moderate |
↓ | # | # | |||||
| Burma et al, June 2020 | ↓ To moderate and control |
||||||||
| Coetsee et al, 2017 | # During cortical activation |
||||||||
| Drapeau et al, 2019 | # | ↓ | # | # | # | ||||
| Northey et al, 2019 | # | # | |||||||
| Tallon et al, 2019 | ↓ To moderate and rest |
↓ To rest |
|||||||
| Monroe et al, 2016 | ↑ During HIIE |
↓ = Decreased effect, ↑ = Increased effect, # = no effect
MCAv
Of the studies reporting MCAv outcomes, resting MCAv (n = 2) [45, 46], exercise MCAv (n = 2) [44, 47] and MCAv immediately post exercise (n = 1) [47] were used. No significant differences were found for resting MCAv after 6- or 12-weeks of HIIE when compared to moderate continuous exercise or control [45, 46]. During an acute bout of HIIE, exercise MCAv was significantly decreased compared to moderate continuous exercise [44, 47]. Conflicting results were found between two studies comparing exercise MCAv to rest. Burma et al. [44] reported no significant difference between average exercise MCAv and rest in adults. However, rather than reporting average exercise MCAv of the entire HIIE bout, Tallon et al. [47] reported exercise MCAv for each 1-minute sprint interval of HIIE. During the 6th sprint interval of HIIE, Tallon et al. [47] reported significantly decreased exercise MCAv compared to rest which remained immediately following exercise [47].
dCA
Transfer function analysis of dCA was reported in the very low and low frequency bands (n = 2) [44, 45]. Drapeau et al. [45] conducted a 6-week intervention of HIIE and reported a significant decrease in phase compared to rest with no significant change in coherence or gain. Burma et al. [44] conducted a single bout of HIIE and reported decreased MCAv systolic phase immediately following exercise that extended up to four hours later.
De/oxygenated hemoglobin
Oxygenated and deoxygenated hemoglobin were reported during a single bout of HIIE (n = 1) [51] and during a 16-week HIIE intervention (n = 1) [50]. Monroe et al. [51] conducted a single bout of HIIE and reported an increase in oxygenated and deoxygenated hemoglobin during HIIE compared to moderate continuous exercise. Coetsee et al. [50] conducted a 16-week intervention of HIIE and reported no significant lasting changes in oxygenated or deoxygenated hemoglobin during cortical activation.
Cerebrovascular reactivity
Cerebrovascular reactivity to CO2 were reported during a single bout of HIIE (n = 1) [53] and during a 12-week HIIE intervention (n = 1) [46]. After a single bout of HIIE, cerebrovascular reactivity to higher CO2, or hypercapnia, was significantly decreased by 37% and remained an hour later [53]. The reduced cerebrovascular reactivity to hypercapnia was also significantly different than moderate intensity and control. Cerebrovascular reactivity to lower CO2, or hypocapnia, was not significantly different following a single bout of HIIE [53]. Cerebrovascular reactivity to CO2 was also not significantly different following 12 weeks of HIIE [46].
Cerebrovascular conductance and resistance
Cerebrovascular conductance index and cerebrovascular resistance index were only reported in a single study [45]. A 6-week HIIE intervention reported no significant changes in cerebrovascular conductance index or cerebrovascular resistance index [45].
Quality review
The quality review of each study is presented in Table 3. Out of seven total quality criteria, 2 studies reported five quality criteria [44, 53], one study reported four quality criteria [45] and the remaining four studies reported three quality criteria [46, 47, 50, 51]. Therefore, the overall quality criteria results were moderately poor. All studies accounted for subjects and monitored the HIIE protocol parameters. No studies reported avoidance of contamination or co-intervention. No studies reported blinding of the outcome assessments. Only Burma et al. [44, 53] and Monroe et al. [51] reported their reliability via coefficient of reproducibility and intraclass coefficients of their measures. And only Burma et al. [44, 53] and Drapeau et al. [45] reported validity of their respective cerebrovascular outcomes.
Table 3. Summary of quality review.
| Avoided Contam-ination and Co-Intervention | Random Assignment to Conditions | Blinded Assessment | Monitored Intervention | Accounted for All Subjects | Reported Reliability of Measures Used | Reported Validity of Measures Used | Total Number of Criteria Met | |
|---|---|---|---|---|---|---|---|---|
| Burma et al, March 2020 | No | Yes | No | Yes | Yes | Yes | Yes | 5 |
| Burma et al, June 2020 | No | Yes | No | Yes | Yes | Yes | Yes | 5 |
| Coetsee et al, 2017 | No | Yes | No | Yes | Yes | No | No | 3 |
| Drapeau et al, 2019 | No | Yes | No | Yes | Yes | No | Yes | 4 |
| Northey et al, 2019 | No | Yes | No | Yes | Yes | No | No | 3 |
| Tallon et al, 2019 | No | Yes | No | Yes | Yes | No | No | 3 |
| Monroe et al, 2016 | No | No | No | Yes | Yes | Yes | No | 3 |
Discussion
This review met the objective of reporting the results of various HIIE studies and the effects on operationalized cerebrovascular function in healthy individuals. This review is the first to report the effects HIIE on cerebrovascular function compared to moderate continuous exercise and rest in healthy individuals. In general, we found that the acute and chronic effects of HIIE on cerebrovascular function vary largely depending on the methods and outcomes measured.
MCAv
In these studies, 6- to 12-week HIIE interventions had no effect on resting MCAv in healthy individuals. No significant change in resting MCAv may be due to the HIIE intervention duration being too short. Also a ceiling effect may be observed for young, healthy individuals and could explain no changes in resting MCAv [19]. During a single bout of HIIE, hyperventilation and downstream arteriole vasoconstriction may explain the acute decreases in exercise MCAv compared to moderate continuous exercise [15, 31, 73, 74]. Vasoconstriction may play a protective role during HIIE due to heightened peripheral blood pressure potentially causing hyper-perfusion [75] or damage to the blood brain barrier [36]. During a single bout of HIIE, there is contradictory evidence comparing exercise MCAv to rest. One study reported no change in average exercise MCAv compared to resting [44]. Another study reported decreased exercise MCAv after six sprint intervals of HIIE and remained decreased compared to rest immediately following HIIE [47]. The differences reported in exercise MCAv compared to rest could be due to age [19] (adults versus prepubertal children) or due to the analysis of MCAv during HIIE (average over entire exercise versus separate sprint intervals). Decreases in exercise MCAv compared to rest may only occur in the late intervals of HIIE, during hyperventilation [76]. Therefore, exercise MCAv should be reported for each interval of HIIE rather than an average of the entire exercise bout.
dCA
After a 6-week intervention and single bout of HIIE, dCA phase was decreased compared to rest. The chronic effects of HIIE on dCA phase may be due to elevated cardiorespiratory fitness in endurance trained individuals being associated with attenuated dCA [45, 48]. In healthy individuals, increased frequency within MCAv and MAP waveforms (that can occur with HIIE) may cause a reduction in phase due to dCA being a high-pass filter [77, 78]. Burma et al. [44] also suggests that systolic phase may reveal greater changes in dCA than both diastolic and mean phase. After a single bout of HIIE, reduction in systolic phase extended up to 4 hours and therefore the common approach of abstaining from exercise 12 hours before research studies [79–81] may be too conservative [44].
Although not included in this review due to the observational study design, contradictory evidence of sustained dCA during HIIE has been reported [63]. Differences in exercise parameters between HIIE may be the cause to contradictory findings due to exhaustive exercise showing decreased dCA [34, 82]. More studies are needed to confirm the acute and chronic decreases in dCA following HIIE.
De/oxygenated hemoglobin
After a 16-week HIIE intervention, oxygenated and deoxygenated hemoglobin during cortical activation did not change [50]. However, the 16-week HIIE intervention decreased reaction time during cortical activation and therefore may have increased efficiency of cortical oxygen use [50]. During a single bout of HIIE oxygenated and deoxygenated hemoglobin increased compared to moderate continuous exercise [51]. As suggested by Coetsee et al, increased oxygenated hemoglobin during neuronal activation may suggest engaging additional regions of the brain [50]; while decreased oxygenated hemoglobin may indicate reduced neuronal activity due to task-efficiency [50]. The acute and chronic effects of HIIE on oxygenated and deoxygenated hemoglobin still needs further investigation due to each only being reported in a single study.
Cerebrovascular reactivity
A 12-week HIIE intervention did not significantly change cerebrovascular reactivity which could be due to vascular desensitization from chronic exposure to CO2 during HIIE [36, 83]. Following a single bout of HIIE, cerebrovascular reactivity to hypercapnia was decreased showing the inability of the cerebrovascular system to maximally vasodilate. The maximal capacity for vasodilation after HIIE may be reduced following HIIE due to prolonged cerebrovascular vasoconstriction that occurs with hyperventilation during HIIE [36, 53]. Cerebrovascular reactivity to hypocapnia was not changed following a single bout of HIIE due to the ability of the vessels to vasoconstrict remaining intact [53]. The reduction in cerebrovascular reactivity to higher CO2 remains an hour after HIIE. Therefore, the authors conclude again that the common approach of abstaining from exercise 12 hours before research studies [54, 80, 84] may be too conservative [53].
Cerebrovascular conductance and resistance
Cerebrovascular conductance and resistance were not significantly changed following a 6-week HIIE intervention. While a 6-week HIIE intervention significantly improved peripheral arterial conductance and resistance [4], this change in the peripheral arteries may not be demonstrated in the cerebrovascular arterial conductance or resistance [41, 45]. However, due to the cerebrovascular conductance or resistance index being reported in only a single study, no conclusive effects of HIIE can be determined.
Future research
We recommend future research on the effects of HIIE on cerebrovascular function should include: 1) examining the cerebrovascular response during HIIE before and after an intervention of HIIE, 2) analyzing cerebrovascular outcomes during each separate interval of HIIE rather than an average of the entire bout, 3) simultaneously measuring MCAv, blood pressure, heart rate, and CO2 during HIIE, 4) measuring cerebrovascular outcomes during HIIE, immediately following HIIE and at a follow up 30 minutes to 4 hours post exercise.
Limitations
The authors acknowledge a risk of publication bias by only including peer-reviewed articles written in English and did not include grey literature. The cerebrovascular function measures included within this review vary greatly and have vast heterogeneity. The overall quality of studies is moderately poor due to the lack of avoiding contamination, not blinding the assessment, and scarce reporting of the reliability and validity of the outcomes measured. These studies report the effects of HIIE on cerebrovascular function in healthy young individuals which limits generalizability and cannot be translated to clinical populations with altered cerebrovascular function at baseline, such as stroke [37, 39, 85, 86].
While HIIE is not a new mode of exercise, studying cerebrovascular measures during HIIE is novel. There are potential limitations to using TCD during HIIE and MCAv may be underestimated [87]. Cerebral oxygenation may also be underestimated due to the two-channel near infrared spectrometer not measuring the motor, occipital, or parietal cortex [50, 51]. Authors could only identify seven small studies with the oldest article dating back to 2015. The primary outcome of MCAv (n = 4) and dCA (n = 2) were reported in few studies with low power. Therefore, a meta-analysis could not be performed due to insufficient mathematical combination.
Conclusion
This review has provided preliminary information studying the effects of HIIE on cerebrovascular function. Currently, there are a sparse number of research studies with moderately poor quality criteria that have reported the acute and chronic effects of HIIE on cerebrovascular function. An increased amount of studies and greater quality of research avoiding contamination, blinding the assessments, and reporting reliability and validity is needed. Randomized controlled trials with large sample sizes are needed to conduct a meta-analysis to combine and statistically analyze the summary results of HIIE on cerebrovascular function. Additionally, more studies are needed to determine the optimal interval parameters of HIIE to provide a consistent exercise dose between studies.
With increased interest in healthy brain aging and implementing interventions to maintain or improve brain health [88], studying the effects of HIIE interventions are critically needed [41, 42]. While this review only included healthy individuals, we provide an early reference to understanding “normal” physiological effects of HIIE on cerebrovascular function and the need to compare to clinical populations. Researchers should make further efforts to investigate and report the effects of HIIE on diverse measures of cerebrovascular function. To do so, it is imperative that researchers implement high quality criteria within the planning of future studies.
Supporting information
(DOC)
(DOC)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund. JPP was supported in part by the National Institute on Aging of the National Institutes of Health [grant number P30AG035982]. AW was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number T32HD057850]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Batacan RB Jr., Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. Epub 2016/11/01. 10.1136/bjsports-2015-095841 . [DOI] [PubMed] [Google Scholar]
- 2.Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D, et al. Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training On Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018;48(9):2127–42. Epub 2018/06/28. 10.1007/s40279-018-0944-y . [DOI] [PubMed] [Google Scholar]
- 3.Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–92. Epub 2015/03/17. 10.1007/s40279-015-0321-z . [DOI] [PubMed] [Google Scholar]
- 4.Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R236–42. Epub 2008/04/25. 10.1152/ajpregu.00069.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M, et al. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med Sci Sports Exerc. 1996;28(10):1327–30. Epub 1996/10/01. 10.1097/00005768-199610000-00018 . [DOI] [PubMed] [Google Scholar]
- 6.Cunningham DA, McCrimmon D, Vlach LF. Cardiovascular response to interval and continuous training in women. Eur J Appl Physiol Occup Physiol. 1979;41(3):187–97. Epub 1979/07/02. 10.1007/BF00430011 . [DOI] [PubMed] [Google Scholar]
- 7.Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96(1):97–105. Epub 2005/11/12. 10.1007/s00421-005-0068-6 . [DOI] [PubMed] [Google Scholar]
- 8.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–10. Epub 2008/07/19. 10.1113/jphysiol.2008.158279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prestgaard E, Mariampillai J, Engeseth K, Erikssen J, Bodegard J, Liestol K, et al. Change in Cardiorespiratory Fitness and Risk of Stroke and Death. Stroke. 2018:STROKEAHA118021798. Epub 2018/12/26. 10.1161/STROKEAHA.118.021798 . [DOI] [PubMed] [Google Scholar]
- 10.Tari AR, Nauman J, Zisko N, Skjellegrind HK, Bosnes I, Bergh S, et al. Temporal changes in cardiorespiratory fitness and risk of dementia incidence and mortality: a population-based prospective cohort study. Lancet Public Health. 2019;4(11):e565–e74. Epub 2019/11/05. 10.1016/S2468-2667(19)30183-5 . [DOI] [PubMed] [Google Scholar]
- 11.Bayati M, Farzad B, Gharakhanlou R, Agha-Alinejad H. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble 'all-out' sprint interval training. J Sports Sci Med. 2011;10(3):571–6. Epub 2011/01/01. [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh SS, Chueh TY, Huang CJ, Kao SC, Hillman CH, Chang YK, et al. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J Sports Sci. 2020:1–13. Epub 2020/08/12. 10.1080/02640414.2020.1803630 . [DOI] [PubMed] [Google Scholar]
- 13.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. Epub 1989/01/01. 10.1161/01.str.20.1.45 . [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Zhang M, Xin Q, Luo S, Zhou W, Cui R, et al. Assessment of cerebral oxygenation oscillations in subjects with hypertension. Microvasc Res. 2013;88:32–41. Epub 2013/04/16. 10.1016/j.mvr.2013.04.003 . [DOI] [PubMed] [Google Scholar]
- 15.Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol. 2017;102(11):1356–71. Epub 2017/08/09. 10.1113/EP086249 . [DOI] [PubMed] [Google Scholar]
- 16.Brassard P, Ainslie PN, Secher NH. Cerebral oxygenation in health and disease. Front Physiol. 2014;5:458 Epub 2014/12/17. 10.3389/fphys.2014.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31(7):1672–8. Epub 2000/07/08. 10.1161/01.str.31.7.1672 . [DOI] [PubMed] [Google Scholar]
- 18.Witte E, Liu Y, Ward JL, Kempf KS, Whitaker A, Vidoni ED, et al. Exercise intensity and middle cerebral artery dynamics in humans. Respir Physiol Neurobiol. 2019;262:32–9. Epub 2019/02/03. 10.1016/j.resp.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward JL, Craig JC, Liu Y, Vidoni ED, Maletsky R, Poole DC, et al. Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am J Physiol Heart Circ Physiol. 2018;315(3):H492–H501. Epub 2018/05/19. 10.1152/ajpheart.00129.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brugniaux JV, Marley CJ, Hodson DA, New KJ, Bailey DM. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J Cereb Blood Flow Metab. 2014;34(12):1873–6. Epub 2014/10/02. 10.1038/jcbfm.2014.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev. 2012;40(3):153–8. Epub 2012/04/17. 10.1097/JES.0b013e3182553430 . [DOI] [PubMed] [Google Scholar]
- 22.Fisher JP, Hartwich D, Seifert T, Olesen ND, McNulty CL, Nielsen HB, et al. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol. 2013;591(7):1859–70. Epub 2012/12/12. 10.1113/jphysiol.2012.244905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brys M, Brown CM, Marthol H, Franta R, Hilz MJ. Dynamic cerebral autoregulation remains stable during physical challenge in healthy persons. Am J Physiol Heart Circ Physiol. 2003;285(3):H1048–54. Epub 2003/08/14. 10.1152/ajpheart.00062.2003 . [DOI] [PubMed] [Google Scholar]
- 24.Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans O, Secher NH, et al. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288(4):H1526–31. Epub 2004/12/14. 10.1152/ajpheart.00979.2004 . [DOI] [PubMed] [Google Scholar]
- 25.Steventon JJ, Hansen AB, Whittaker JR, Wildfong KW, Nowak-Fluck D, Tymko MM, et al. Cerebrovascular Function in the Large Arteries Is Maintained Following Moderate Intensity Exercise. Front Physiol. 2018;9:1657 Epub 2018/12/07. 10.3389/fphys.2018.01657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herholz K, Buskies W, Rist M, Pawlik G, Hollmann W, Heiss WD. Regional cerebral blood flow in man at rest and during exercise. J Neurol. 1987;234(1):9–13. Epub 1987/01/01. 10.1007/BF00314001 . [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol (1985). 1992;72(3):1123–32. Epub 1992/03/01. 10.1152/jappl.1992.72.3.1123 . [DOI] [PubMed] [Google Scholar]
- 28.Thomas SN, Schroeder T, Secher NH, Mitchell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol (1985). 1989;67(2):744–8. Epub 1989/08/01. 10.1152/jappl.1989.67.2.744 . [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom G, Wahlgren NG. Physical exercise increases middle cerebral artery blood flow velocity. Neurosurg Rev. 1993;16(2):151–6. Epub 1993/01/01. 10.1007/BF00258249 . [DOI] [PubMed] [Google Scholar]
- 30.Moraine JJ, Lamotte M, Berre J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol. 1993;67(1):35–8. Epub 1993/01/01. 10.1007/BF00377701 . [DOI] [PubMed] [Google Scholar]
- 31.Ogoh S, Ainslie PN. Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev. 2009;37(3):123–9. Epub 2009/06/25. 10.1097/JES.0b013e3181aa64d7 . [DOI] [PubMed] [Google Scholar]
- 32.Maeda H, Matsumoto M, Handa N, Hougaku H, Ogawa S, Itoh T, et al. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke. 1993;24(5):670–5. Epub 1993/05/01. 10.1161/01.str.24.5.670 . [DOI] [PubMed] [Google Scholar]
- 33.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590(14):3261–75. Epub 2012/04/13. 10.1113/jphysiol.2012.228551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE, et al. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp Physiol. 2011;96(11):1196–207. Epub 2011/08/16. 10.1113/expphysiol.2011.060178 . [DOI] [PubMed] [Google Scholar]
- 35.Phillips AA, Matin N, Jia M, Squair JW, Monga A, Zheng MMZ, et al. Transient Hypertension after Spinal Cord Injury Leads to Cerebrovascular Endothelial Dysfunction and Fibrosis. J Neurotrauma. 2018;35(3):573–81. Epub 2017/11/17. 10.1089/neu.2017.5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas SJ, Cotter JD, Brassard P, Bailey DM. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab. 2015;35(6):902–11. Epub 2015/04/03. 10.1038/jcbfm.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41(11):2697–704. Epub 2010/10/12. 10.1161/STROKEAHA.110.594168 . [DOI] [PubMed] [Google Scholar]
- 38.Castro P, Azevedo E, Sorond F. Cerebral Autoregulation in Stroke. Curr Atheroscler Rep. 2018;20(8):37 Epub 2018/05/23. 10.1007/s11883-018-0739-5 . [DOI] [PubMed] [Google Scholar]
- 39.Kempf KS, Whitaker AA, Lui Y, Witte E, Perdomo SJ, Ward JL, et al. The Effect of Stroke on Middle Cerebral Artery Blood Flow Velocity Dynamics During Exercise. J Neurol Phys Ther. 2019;43(4):212–9. Epub 2019/08/27. 10.1097/NPT.0000000000000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert MF, Levine BD, et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective-An Update: A Scientific Statement From the American Heart Association. Circulation. 2020;141(13):e705–e36. Epub 2020/02/27. 10.1161/CIR.0000000000000749 . [DOI] [PubMed] [Google Scholar]
- 41.Lucas SJE, Cotter JD, Brassard P, Bailey DM. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cerebr Blood F Met. 2015;35(6):902–11. 10.1038/jcbfm.2015.49 WOS:000355575600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calverley TA, Ogoh S, Marley CJ, Steggall M, Marchi N, Brassard P, et al. HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol. 2020;598(13):2513–30. Epub 2020/04/30. 10.1113/JP275021 . [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535 Epub 2009/07/23. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burma JS, Copeland P, Macaulay A, Khatra O, Wright AD, Smirl JD. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep. 2020;8(5):e14367 Epub 2020/03/13. 10.14814/phy2.14367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drapeau A, Labrecque L, Imhoff S, Paquette M, Le Blanc O, Malenfant S, et al. Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol Rep. 2019;7(15):e14185 Epub 2019/08/03. 10.14814/phy2.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Northey JM, Pumpa KL, Quinlan C, Ikin A, Toohey K, Smee DJ, et al. Cognition in breast cancer survivors: A pilot study of interval and continuous exercise. J Sci Med Sport. 2019;22(5):580–5. Epub 2018/12/18. 10.1016/j.jsams.2018.11.026 . [DOI] [PubMed] [Google Scholar]
- 47.Tallon CM, Simair RG, Koziol AV, Ainslie PN, McManus AM. Intracranial Vascular Responses to High-Intensity Interval Exercise and Moderate-Intensity Steady-State Exercise in Children. Pediatr Exerc Sci. 2019;31(3):290–5. Epub 2019/03/06. 10.1123/pes.2018-0234 . [DOI] [PubMed] [Google Scholar]
- 48.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, et al. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep. 2017;5(21). Epub 2017/11/11. 10.14814/phy2.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics. 2016;3(3):031411 Epub 2016/07/13. 10.1117/1.NPh.3.3.031411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coetsee C, Terblanche E. Cerebral oxygenation during cortical activation: the differential influence of three exercise training modalities. A randomized controlled trial. Eur J Appl Physiol. 2017;117(8):1617–27. Epub 2017/06/02. 10.1007/s00421-017-3651-8 . [DOI] [PubMed] [Google Scholar]
- 51.Monroe DC, Gist NH, Freese EC, O'Connor PJ, McCully KK, Dishman RK. Effects of Sprint Interval Cycling on Fatigue, Energy, and Cerebral Oxygenation. Med Sci Sports Exerc. 2016;48(4):615–24. Epub 2015/11/13. 10.1249/MSS.0000000000000809 . [DOI] [PubMed] [Google Scholar]
- 52.Auger H, Bherer L, Boucher E, Hoge R, Lesage F, Dehaes M. Quantification of extra-cerebral and cerebral hemoglobin concentrations during physical exercise using time-domain near infrared spectroscopy. Biomed Opt Express. 2016;7(10):3826–42. Epub 2016/11/22. 10.1364/BOE.7.003826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burma JS, Macaulay A, Copeland P, Khatra O, Bouliane KJ, Smirl JD. Comparison of cerebrovascular reactivity recovery following high-intensity interval training and moderate-intensity continuous training. Physiol Rep. 2020;8(11):e14467 Epub 2020/06/09. 10.14814/phy2.14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol (1985). 2007;102(3):870–7. Epub 2006/11/18. 10.1152/japplphysiol.00906.2006 . [DOI] [PubMed] [Google Scholar]
- 55.Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26(4):279–87. Epub 2012/04/03. 10.1007/s10877-012-9348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffin J, Sobczyk O, McKetton L, Crawley A, Poublanc J, Venkatraghavan L, et al. Cerebrovascular Resistance: The Basis of Cerebrovascular Reactivity. Front Neurosci. 2018;12:409 Epub 2018/07/06. 10.3389/fnins.2018.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, et al. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol. 2013;591(23):5809–21. Epub 2013/10/02. 10.1113/jphysiol.2013.259150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sackett DL. How to read clinical journals. Can Med Assoc J. 1982;126(12):1373 Epub 1982/06/15. [PMC free article] [PubMed] [Google Scholar]
- 59.Sackett DL. Rules of evidence and clinical recommendations for the management of patients. Can J Cardiol. 1993;9(6):487–9. Epub 1993/07/01. . [PubMed] [Google Scholar]
- 60.Dupuy O, Tremblay J. Impact of Carbohydrate Ingestion on Cognitive Flexibility and Cerebral Oxygenation during High-Intensity Intermittent Exercise: A Comparison between Maple Products and Usual Carbohydrate Solutions. Nutrients. 2019;11(9). Epub 2019/08/31. 10.3390/nu11092019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willis SJ, Borrani F, Millet GP. Leg- vs arm-cycling repeated sprints with blood flow restriction and systemic hypoxia. Eur J Appl Physiol. 2019;119(8):1819–28. Epub 2019/06/13. 10.1007/s00421-019-04171-0 . [DOI] [PubMed] [Google Scholar]
- 62.Klein T, Bailey TG, Abeln V, Schneider S, Askew CD. Cerebral Blood Flow during Interval and Continuous Exercise in Young and Old Men. Med Sci Sports Exerc. 2019;51(7):1523–31. Epub 2019/02/16. 10.1249/MSS.0000000000001924 . [DOI] [PubMed] [Google Scholar]
- 63.Tsukamoto H, Hashimoto T, Olesen ND, Petersen LG, Sorensen H, Nielsen HB, et al. Dynamic Cerebral Autoregulation Is Maintained during High-Intensity Interval Exercise. Med Sci Sports Exerc. 2019;51(2):372–8. Epub 2018/09/27. 10.1249/MSS.0000000000001792 . [DOI] [PubMed] [Google Scholar]
- 64.Hanssen H, Minghetti A, Magon S, Rossmeissl A, Rasenack M, Papadopoulou A, et al. Effects of different endurance exercise modalities on migraine days and cerebrovascular health in episodic migraineurs: A randomized controlled trial. Scand J Med Sci Sports. 2018;28(3):1103–12. Epub 2017/11/22. 10.1111/sms.13023 . [DOI] [PubMed] [Google Scholar]
- 65.Kirkham AA, Shave RE, Bland KA, Bovard JM, Eves ND, Gelmon KA, et al. Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: A proof-of-concept RCT. Int J Cardiol. 2017;245:263–70. Epub 2017/07/25. 10.1016/j.ijcard.2017.07.037 . [DOI] [PubMed] [Google Scholar]
- 66.Gjellesvik TI, Becker F, Tjonna AE, Indredavik B, Nilsen H, Brurok B, et al. Effects of High-Intensity Interval Training after Stroke (The HIIT-Stroke study)—A Multicenter Randomized Controlled Trial. Arch Phys Med Rehabil. 2020. Epub 2020/03/08. 10.1016/j.apmr.2020.02.006 . [DOI] [PubMed] [Google Scholar]
- 67.Scott JM, Tucker WJ, Martin D, Crowell JB, Goetchius E, Ozgur O, et al. Association of Exercise and Swimming Goggles With Modulation of Cerebro-ocular Hemodynamics and Pressures in a Model of Spaceflight-Associated Neuro-ocular Syndrome. JAMA Ophthalmol. 2019;137(6):652–9. Epub 2019/04/19. 10.1001/jamaophthalmol.2019.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saoi M, Percival M, Nemr C, Li A, Gibala M, Britz-McKibbin P. Characterization of the Human Skeletal Muscle Metabolome for Elucidating the Mechanisms of Bicarbonate Ingestion on Strenuous Interval Exercise. Anal Chem. 2019;91(7):4709–18. Epub 2019/03/06. 10.1021/acs.analchem.9b00149 . [DOI] [PubMed] [Google Scholar]
- 69.Labrecque L, Drapeau A, Rahimaly K, Imhoff S, Billaut F, Brassard P. Comparable blood velocity changes in middle and posterior cerebral arteries during and following acute high-intensity exercise in young fit women. Physiol Rep. 2020;8(9):e14430 Epub 2020/04/29. 10.14814/phy2.14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peyravi A, Yazdanpanahi N, Nayeri H, Hosseini SA. The effect of endurance training with crocin consumption on the levels of MFN2 and DRP1 gene expression and glucose and insulin indices in the muscle tissue of diabetic rats. J Food Biochem. 2020;44(2):e13125 Epub 2019/12/19. 10.1111/jfbc.13125 . [DOI] [PubMed] [Google Scholar]
- 71.Komiyama T, Tanoue Y, Sudo M, Costello JT, Uehara Y, Higaki Y, et al. Cognitive Impairment during High-Intensity Exercise: Influence of Cerebral Blood Flow. Med Sci Sports Exerc. 2020;52(3):561–8. Epub 2019/10/15. 10.1249/MSS.0000000000002183 . [DOI] [PubMed] [Google Scholar]
- 72.Hansen RK, Nielsen PS, Schelske MW, Secher NH, Volianitis S. CO2 supplementation dissociates cerebral oxygenation and middle cerebral artery blood velocity during maximal cycling. Scand J Med Sci Sports. 2020;30(3):399–407. Epub 2019/10/28. 10.1111/sms.13582 . [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, et al. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557(Pt 1):331–42. Epub 2004/03/09. 10.1113/jphysiol.2004.060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linkis P, Jorgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol (1985). 1995;78(1):12–6. Epub 1995/01/01. 10.1152/jappl.1995.78.1.12 . [DOI] [PubMed] [Google Scholar]
- 75.Brassard P, Kim YS, van Lieshout J, Secher NH, Rosenmeier JB. Endotoxemia reduces cerebral perfusion but enhances dynamic cerebrovascular autoregulation at reduced arterial carbon dioxide tension. Crit Care Med. 2012;40(6):1873–8. Epub 2012/05/23. 10.1097/CCM.0b013e3182474ca7 . [DOI] [PubMed] [Google Scholar]
- 76.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985). 2009;107(5):1370–80. Epub 2009/09/05. 10.1152/japplphysiol.00573.2009 . [DOI] [PubMed] [Google Scholar]
- 77.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28(6):1071–85. Epub 2008/03/20. 10.1038/jcbfm.2008.13 . [DOI] [PubMed] [Google Scholar]
- 78.Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol. 2004;559(Pt 3):965–73. Epub 2004/07/16. 10.1113/jphysiol.2004.066969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ainslie PN, Barach A, Murrell C, Hamlin M, Hellemans J, Ogoh S. Alterations in cerebral autoregulation and cerebral blood flow velocity during acute hypoxia: rest and exercise. Am J Physiol Heart Circ Physiol. 2007;292(2):H976–83. Epub 2006/10/03. 10.1152/ajpheart.00639.2006 . [DOI] [PubMed] [Google Scholar]
- 80.Ainslie PN, Hamlin M, Hellemans J, Rasmussen P, Ogoh S. Cerebral hypoperfusion during hypoxic exercise following two different hypoxic exposures: independence from changes in dynamic autoregulation and reactivity. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1613–22. Epub 2008/09/05. 10.1152/ajpregu.90420.2008 . [DOI] [PubMed] [Google Scholar]
- 81.Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie PN. Relationship between blood pressure and cerebral blood flow during supine cycling: influence of aging. J Appl Physiol (1985). 2016;120(5):552–63. Epub 2015/11/21. 10.1152/japplphysiol.00667.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288(3):H1461–7. Epub 2004/10/23. 10.1152/ajpheart.00948.2004 . [DOI] [PubMed] [Google Scholar]
- 83.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging. 2013;38(5):1177–83. Epub 2013/03/26. 10.1002/jmri.24090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011;589(Pt 12):3039–48. Epub 2011/04/28. 10.1113/jphysiol.2011.206052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexandrov AV, Bladin CF, Norris JW. Intracranial blood flow velocities in acute ischemic stroke. Stroke. 1994;25(7):1378–83. Epub 1994/07/01. 10.1161/01.str.25.7.1378 . [DOI] [PubMed] [Google Scholar]
- 86.Aoi MC, Hu K, Lo MT, Selim M, Olufsen MS, Novak V. Impaired cerebral autoregulation is associated with brain atrophy and worse functional status in chronic ischemic stroke. PLoS One. 2012;7(10):e46794 Epub 2012/10/17. 10.1371/journal.pone.0046794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985). 2014;117(10):1090–6. Epub 2014/07/12. 10.1152/japplphysiol.00285.2014 . [DOI] [PubMed] [Google Scholar]
- 88.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–e303. Epub 2017/09/09. 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.