ABSTRACT
Background:
Dental ceramics are known for their natural appearance and their durable chemical and optical properties, and their uses mainly reflect their excellent aesthetic properties, biocompatibility and resistance to wear. In addition, dental ceramics are considered to be chemically inert restorative material with large differences in the chemical compositions and microstructures. Although ceramics exhibit excellent physical properties and biocompatibility, the oral environment presents a series of external factors that affect its physical and mechanical properties in its long term function.
Aims and Objectives:
The aim of this study is to compare and evaluate the surface roughness of 2 dental ceramics (Noritake® & IPS Emax ceram) before and after exposure to an aerated drink, a mouthwash and simulated vomit solution with distilled water.
Materials and Methods:
128 ceramic discs were made, 16 for each of the 4 groups of 2 ceramics to be tested. It was fabricated in the form of discs of diameter 10 mm × 2 mm thickness. The test specimens were calibrated by grinding, using a medium grit diamond bur. Both the above specimens were divided into 4 groups of 16 each. They were then subjected to test solutions. Each specimen in a group was immersed in the following solutions: Coca-Cola, chlorhexidine mouthwash, simulated vomit solution and distilled water.
Results:
The results analyzed statistically using one way ANOVA (analysis of variance), paired t test and independent t test.
Conclusion:
Within the limitations of this study, it was concluded that the surface roughness of the evaluated ceramics increased upon exposure to Coca-Cola, chlorhexidine mouthwash and simulated vomit solution. Simulated vomit solution caused the maximum change in surface roughness followed by coca cola and then chlorhexidine mouthwash. No changes were observed after immersion in distilled water. Noritake® showed slightly more resistance to change in surface profile when compared to IPS e.max.
KEYWORDS: Chlorhexidine, Coca-Cola, dental ceramics, gastric acid
INTRODUCTION
Dentistry has seen the advent of various types of ceramics.[1] Feldspathic porcelain has been used in metal ceramic prostheses for more than 35 years. Feldspars have the tendency to form leucite, a crystalline phase formed due to melting. Formation of leucite is important for metal bonding to the porcelain. During prolonged firing cycles, changes occur in the leucite content, which produce tensile stresses and cause crack propagation in the ceramic; therefore, a newer ceramic system, Noritake® Super Porcelain EX-3 (Guangdong, China), with very fine particle size was introduced that remains stable during repeated firing cycles because of its higher coefficient of thermal expansion. It can hence be used in long span bridges with minimal risk of cracking.
For improved esthetics, a ceramic system containing fluorapatite crystals has been introduced. The glass–ceramic is composed of dispersed fluorapatite crystals [Ca10(PO4)6F2], which is present in a feldspathic glassy matrix, and the microstructure is different when compared to all other presently available ceramics. Fluorapatite crystals are needle shaped, and vary in length between 2 and 5 μm and are roughly 300 nm in diameter. They are also present in natural bone and teeth. The minute crystals present the special optical properties, of translucence and opalescence. IPS e.max Ceram (Ivoclar Vivadent, Schaan, Liechtenstein) is one such material, which is widely used as a veneering ceramic.[2] Together with color and translucency, surface glazing is one of the characteristics that determines the appearance of ceramic restorations.[3]
Changing lifestyles and food habits have resulted in considerable variation in the diets of people, with beverages forming a part of their routine diet.[4] Large-scale consumption of carbonated aerated water and beverages is one side of this change and on the other side is consumption of fruit juices containing citric acid as a part of health drinks. In this process, the oral cavity is exposed to various dietary agents, which include substances that are acidic in nature and that etch the surface of the tooth and restoration, which results in dental erosion.[5] Erosion causes an increase in surface roughness, which in turn causes increased microbial plaque retention and enamel wear.[6]
Chlorhexidine, a diguanidohexane with improved antiseptic properties, is commonly used as a mouthwash for achieving good oral hygiene, comfort, treatment predictability, and longevity of the fixed and removable prosthesis. As the durability of dental ceramics is influenced by aqueous solutions, temperature, and the time it has been exposed to, the effect of chlorhexidine on ceramics has to be investigated.[5]
The aim of this study was to compare and evaluate the surface roughness of two dental ceramics (Noritake and IPS e.max) before and after exposure to an aerated drink, mouthwash, and simulated vomit solution with distilled water.
MATERIALS AND METHODS
Two ceramic dentin materials Noritake and IPS e.max Ceram, of shades A3 and D4, respectively, were chosen for the fabrication of ceramic discs. The difference in shades allowed ease of identification between the two ceramics. A stainless-steel metal mold was used to facilitate fabrication of ceramic discs (10 mm diameter × 2 mm thick). The specimen former was a two-piece metal slab with four disc shapes of dimensions 12 mm × 4 mm and a base. The larger mold space (12 mm × 4 mm) compensated for the shrinkage of ceramic specimens during firing. Of 128 ceramic discs that were fabricated, 64 of each to be tested. The mold is fabricated by drilling a hole of diameter 12 mm onto a metallic plate of 4 mm thickness.
A plastic (agate) spatula was used to mix the porcelain powder on the Renfert® (Hilzingen, Germany) classic ceramic tray, with the mixing liquid as recommended by the manufacturers of each ceramic material. The creamy mix was then poured into the mold and condensed.
An absorbent paper on the specimen surface was used to remove any excess liquid. The mold was removed after condensation, leaving the non-sintered specimen on the base, which was then transferred to a firing tray. The firing was performed as per the manufacturer’s instructions in a ceramic furnace (Programat P310, Ivoclar Vivadent). This was then followed by glazing according to the manufacturer’s instructions.
Of the 128 ceramic discs that were made, 16 for each of the 4 groups of 2 ceramics need to be tested. The test specimens were calibrated by grinding using a medium grit diamond bur.
They were then subjected to test solutions. Each specimen in a group was immersed in the following solutions:
Group A: Coca-Cola (Atlanta, GA) for 60h[7]
Group B: Chlorhexidine mouthwash for 24h equivalent to 2 years of 2 min of daily use[8]
Group C: Simulated gastric acid-artificial vomit solution that had pH 3.8 for 24 h[9]
Group D: Distilled water
The simulated vomit solution was prepared using 1000 mL of artificial saliva (pH = 5.5) and 4.5 mL of simulated gastric fluid (pH = 1.2).[9] Surface roughness of the ceramic discs was analyzed using surface profiler (Mitutoyo surface profilometer SDA350, Kawasaki, Japan) before and after exposure to each solution.
The values were noted and the data were analyzed statistically with one-way ANOVA (analysis of variance), paired t test, and independent t test.
RESULTS
Observations were tabulated and analyzed statistically. Paired t test compared roughness before and after immersion into each solution. One-way ANOVA with Tukey’s post hoc test compared surface roughness between the groups and independent t test compared surface roughness between the two ceramics.
The surface roughness values of Noritake before and after immersion in Coca-Cola solution [Table 1] chlorhexidine mouth wash solution [Table 2] simulated vomit solution [Table 3], distilled water [Table 4] are enlisted. Surface roughness values of IPS Emax ceram on immersion in coca-cola [Table 5], chlorhexidine mouthwash [Table 6], simulated vomit solution [Table 7] and distilled water [Table 8] are also enlisted. Comparison of roughness values of Noritake and IPS Emax ceram between the different solutions before and after immersion is represented graphically in Graph 1 and Graph 2, respectively. Comparison of roughness between Noritake and IPS Emax ceram before and after immersion in each solution is given in Graph 3.
Table 1.
Surface roughness values of Noritake before and after immersion in Coca-Cola (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.81 | 0.83 |
| 2 | 0.62 | 0.67 |
| 3 | 0.54 | 0.60 |
| 4 | 0.48 | 0.51 |
| 5 | 0.82 | 0.87 |
| 6 | 0.77 | 0.79 |
| 7 | 0.91 | 0.96 |
| 8 | 0.99 | 1.01 |
| 9 | 0.66 | 0.69 |
| 10 | 0.83 | 0.89 |
| 11 | 0.53 | 0.57 |
| 12 | 0.89 | 1.01 |
| 13 | 0.91 | 0.97 |
| 14 | 0.76 | 0.81 |
| 15 | 0.61 | 0.69 |
| 16 | 0.68 | 0.75 |
Table 2.
Surface roughness values of Noritake before and after immersion in chlorhexidine mouthwash solution (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.81 | 0.83 |
| 2 | 0.62 | 0.67 |
| 3 | 0.54 | 0.60 |
| 4 | 0.48 | 0.51 |
| 5 | 0.82 | 0.87 |
| 6 | 0.77 | 0.79 |
| 7 | 0.91 | 0.96 |
| 8 | 0.99 | 1.01 |
| 9 | 0.66 | 0.69 |
| 10 | 0.83 | 0.89 |
| 11 | 0.53 | 0.57 |
| 12 | 0.89 | 1.01 |
| 13 | 0.91 | 0.97 |
| 14 | 0.76 | 0.81 |
| 15 | 0.61 | 0.69 |
| 16 | 0.68 | 0.75 |
Table 3.
Surface roughness values of Noritake before and after immersion in simulated vomit solution (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.47 | 1.31 |
| 2 | 0.86 | 1.91 |
| 3 | 0.52 | 1.06 |
| 4 | 0.72 | 1.66 |
| 5 | 0.61 | 1.12 |
| 6 | 0.41 | 0.98 |
| 7 | 0.59 | 1.22 |
| 8 | 1.09 | 1.97 |
| 9 | 0.87 | 1.76 |
| 10 | 0.96 | 1.72 |
| 11 | 0.62 | 1.33 |
| 12 | 0.73 | 1.58 |
| 13 | 0.57 | 1.23 |
| 14 | 0.90 | 1.83 |
| 15 | 0.69 | 1.79 |
| 16 | 0.89 | 1.87 |
Table 4.
Surface roughness values of Noritake before and after immersion in distilled water (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 1.39 | 1.39 |
| 2 | 0.65 | 0.65 |
| 3 | 0.66 | 0.66 |
| 4 | 0.85 | 0.87 |
| 5 | 0.72 | 0.73 |
| 6 | 0.55 | 0.55 |
| 7 | 0.77 | 0.77 |
| 8 | 0.99 | 1.01 |
| 9 | 0.59 | 0.59 |
| 10 | 1.21 | 1.21 |
| 11 | 0.84 | 0.84 |
| 12 | 0.62 | 0.62 |
| 13 | 0.89 | 0.89 |
| 14 | 0.59 | 0.59 |
| 15 | 0.71 | 0.72 |
| 16 | 0.91 | 0.91 |
Table 5.
Surface roughness values of IPS e.max Ceram before and after immersion in Coca-Cola (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.61 | 0.91 |
| 2 | 0.87 | 1.36 |
| 3 | 0.73 | 1.21 |
| 4 | 0.51 | 0.98 |
| 5 | 0.47 | 0.91 |
| 6 | 0.90 | 1.43 |
| 7 | 1.21 | 1.72 |
| 8 | 0.96 | 1.31 |
| 9 | 0.75 | 1.07 |
| 10 | 1.03 | 1.81 |
| 11 | 0.81 | 1.51 |
| 12 | 0.61 | 1.21 |
| 13 | 0.69 | 1.25 |
| 14 | 0.82 | 1.41 |
| 15 | 0.97 | 1.75 |
| 16 | 1.19 | 1.91 |
Table 6.
Surface roughness values of IPS e.max Ceram before and after immersion in chlorhexidine mouthwash solution (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.96 | 0.99 |
| 2 | 0.83 | 0.84 |
| 3 | 0.57 | 0.67 |
| 4 | 0.51 | 0.52 |
| 5 | 0.82 | 0.84 |
| 6 | 1.13 | 1.21 |
| 7 | 0.41 | 0.46 |
| 8 | 0.59 | 0.63 |
| 9 | 0.99 | 1.06 |
| 10 | 0.73 | 0.79 |
| 11 | 0.81 | 0.88 |
| 12 | 0.47 | 0.56 |
| 13 | 1.32 | 1.44 |
| 14 | 0.89 | 0.91 |
| 15 | 0.97 | 1.05 |
| 16 | 0.68 | 0.75 |
Table 7.
Surface roughness values of IPS e.max Ceram before and after immersion in simulated vomit solution (in µm)
| Sample no. | Roughness before immersion (µm) | Roughness after immersion (µm) |
|---|---|---|
| 1 | 0.72 | 1.39 |
| 2 | 0.65 | 1.29 |
| 3 | 0.81 | 1.69 |
| 4 | 0.52 | 1.11 |
| 5 | 0.49 | 0.98 |
| 6 | 0.91 | 1.78 |
| 7 | 1.27 | 1.97 |
| 8 | 1.08 | 1.71 |
| 9 | 0.68 | 1.07 |
| 10 | 0.77 | 1.82 |
| 11 | 0.87 | 1.63 |
| 12 | 0.99 | 1.98 |
| 13 | 0.59 | 1.07 |
| 14 | 0.61 | 1.28 |
| 15 | 0.74 | 1.41 |
| 16 | 0.69 | 0.99 |
Table 8.
Surface roughness values of IPS e.max Ceram before and after immersion in distilled water (in µm)
| Sample No | Roughness before Immersion (µm) | Roughness after Immersion (µm) |
|---|---|---|
| 1 | 0.65 | 0.65 |
| 2 | 0.48 | 0.49 |
| 3 | 0.81 | 0.82 |
| 4 | 0.62 | 0.62 |
| 5 | 0.57 | 0.57 |
| 6 | 0.51 | 0.51 |
| 7 | 0.77 | 0.78 |
| 8 | 0.85 | 0.87 |
| 9 | 0.93 | 0.93 |
| 10 | 0.65 | 0.67 |
| 11 | 0.86 | 0.86 |
| 12 | 0.61 | 0.63 |
| 13 | 0.79 | 0.82 |
| 14 | 0.91 | 0.91 |
| 15 | 0.49 | 0.51 |
| 16 | 0.57 | 0.57 |
Graph 1.
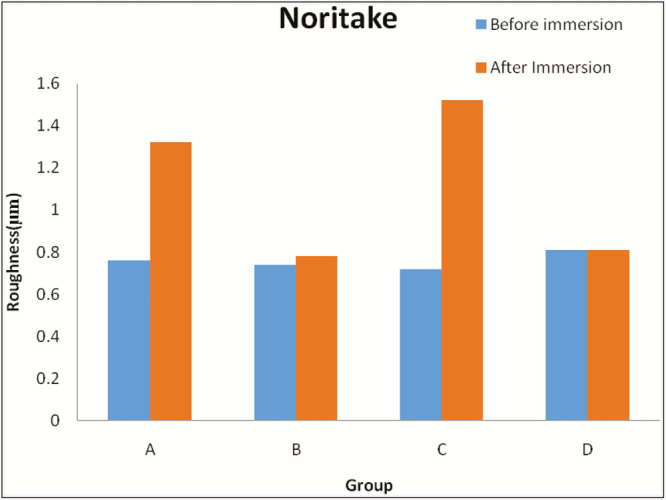
Comparison of roughness value of Noritake between the groups before and after immersion in each solution (in µm). Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
Graph 2.
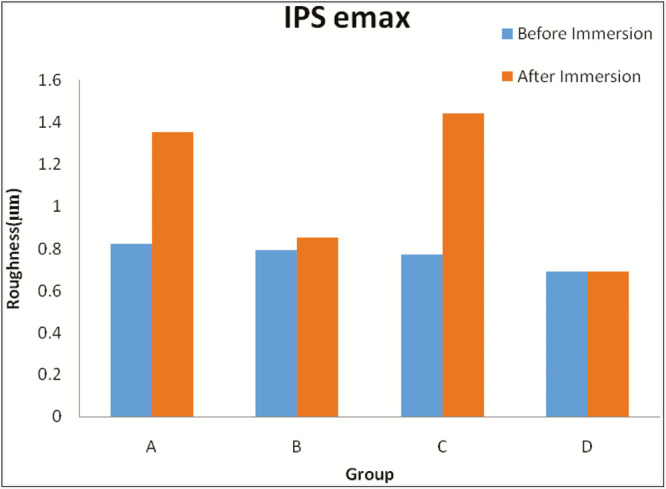
Comparison of roughness value of IPS e.max Ceram between the groups before and after immersion in each solution (in µm). Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
Graph 3.
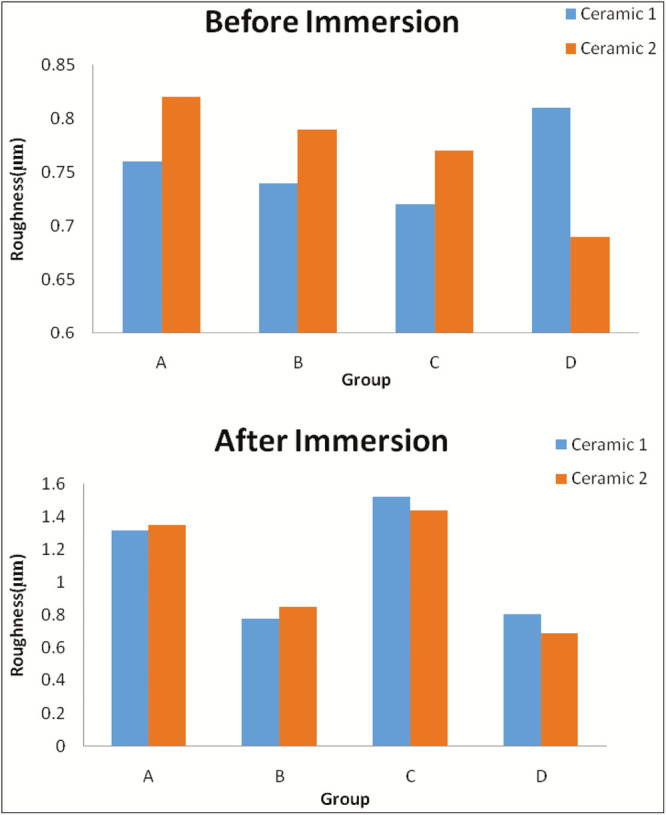
Comparison of roughness between Noritake and IPS e.max Ceram before and after immersion in each solution (in µm). Ceramic 1 = Noritake, Ceramic 2 = IPS e.max Ceram, Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
-
(1)
There is a statistically significant increase in surface roughness of Noritake after immersion in Coca-Cola, chlorhexidine mouthwash, and simulated vomit solution [Table 9].
-
(2)
There is no statistically significant change in surface roughness of Noritake after immersion in distilled water [Table 9]. Surface roughness values of IPS e.max Ceram before and after immersion in Coca-Cola [Table 6], chlorhexidine mouthwash solution [Table 7], simulated vomit solution [Table 8] and distilled water [Table 9] are enlisted.
-
(3)
There is a statistically significant increase in surface roughness of IPS e.max after immersion in Coca-Cola, chlorhexidine mouthwash, and simulated vomit solution [Table 10].
-
(4)
There is no statistically significant change in surface roughness of IPS e.max after immersion in distilled water [Table 10].
-
(5)
Between the two dental ceramics, IPS e.max Ceram showed more increase in surface roughness than Noritake, but the difference is not statistically significant [Table 11].
Table 9.
Comparison of roughness values of Noritake between the groups before and after immersion in each solution (in µm)
| Paired t test statistic | |||||
|---|---|---|---|---|---|
| Group | Before immersion | After immersion | t statistic | P value | |
| A | 0.76 ± 0.19 | 1.32 ± 0.35 | −9.957 | <0.001 | |
| B | 0.74 ± 0.15 | 0.78 ± 0.16 | −7.806 | <0.001 | |
| C | 0.72 ± 0.19 | 1.52 ± 0.34 | −17.535 | <0.001 | |
| D | 0.81 ± 0.23 | 0.81 ± 0.23 | −2.087 | 0.054 | |
| ANOVA | F | 0.626 | 27.456 | ||
| P value | 0.601 | <0.001 | |||
One-way ANOVA is used to compare between the groups. Paired t test is used to compare roughness before and after immersion. Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
Table 10.
Comparison of roughness values of IPS e.max Ceram between the groups before and after immersion in each solution (in µm)
| Group | Before immersion | After immersion | Paired t test statistic | ||
|---|---|---|---|---|---|
| t statistic | P value | ||||
| A | 0.82 ± 0.22 | 1.35 ± 0.32 | −14.235 | <0.001 | |
| B | 0.79 ± 0.25 | 0.85 ± 0.26 | −6.893 | <0.001 | |
| C | 0.77 ± 0.21 | 1.44 ± 0.35 | −13.126 | <0.001 | |
| D | 0.69 ± 0.15 | 0.69 ± 0.15 | −3.5 | 0.004 | |
| ANOVA | F | 1.02 | 27.26 | ||
| P value | 0.355 | <0.001 | |||
One-way ANOVA is used to compare between the groups. Paired t test is used to compare roughness before and after immersion. Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
Table 11.
Comparison of roughness between Noritake and IPS e.max Ceram before and after immersion in each solution (in µm)
| Independent t test | |||||
|---|---|---|---|---|---|
| Group | Ceramic 1 | Ceramic 2 | t statistic (df = 30) | P value | |
| A | Before | 0.76 ± 0.19 | 0.82 ± 0.22 | −0.788 | 0.437 |
| After | 1.32 ± 0.35 | 1.35 ± 0.32 | −0.317 | 0.753 | |
| B | Before | 0.74 ± 0.15 | 0.79 ± 0.25 | −0.740 | 0.465 |
| After | 0.79 ± 0.16 | 0.85 ± 0.26 | −0.802 | 0.429 | |
| C | Before | 0.72 ± 0.19 | 0.77 ± 0.21 | −0.782 | 0.441 |
| After | 1.52 ± 0.34 | 1.45 ± 0.35 | 0.602 | 0.551 | |
| D | Before | 0.81 ± 0.23 | 0.69 ± 0.23 | 1.676 | 0.104 |
| After | 0.81 ± 0.23 | 0.69 ± 0.15 | 1.714 | 0.097 |
Ceramic 1 = Noritake, Ceramic 2 = IPS e.max, Group A = Coca-Cola, Group B = chlorhexidine mouthwash, Group C = simulated vomit solution, Group D = distilled water
DISCUSSION
Ceramics have become very popular because of their esthetics, strength, and known impervious nature. Dental porcelain when compared to other esthetic dental materials has a smooth and glossy surface finish, which is attained by glazing. Ideally ceramic restorations should retain their surface glaze even under function in the oral environment, where they are exposed to various food substances and acidic solutions. Etching of ceramic surfaces can occur on exposure to these acidic solutions, resulting in a rough surface, which is undesirable for maintaining esthetics.[10,11]
The two reasons behind selecting these bioceramic restorative materials were (1) they have been abundantly used in dentistry and there is (2) not enough scientific literature on the effects of Coca-Cola, chlorhexidine, and simulated vomit solutions on the surface roughness.[12,13] Increase in surface roughness causes increased plaque accumulation leading to increased gingival bleeding and increased color deterioration, which is one of the major reason (38%) for changing ceramic restorations.[14,15] Color changes can be caused due to the use of mouthwashes, colored acidic or alcoholic drinks, and also tea or coffee.[16,17]
There has been increased consumption of artificially sweetened soft drinks, sports drinks, high-energy beverages, and coffee products. Adolescents have shown an alarming increase in soft drink consumption.[18] It has also been proven that food and drinks can cause erosion on teeth.[19]
Variations in pH, solution chemistry, wear, and mechanical load make the oral cavity a complex environment. According to Kukiattrakoon et al.,[1] consequences of ceramic degradation include coarseness of the exposed surface, which promotes plaque accumulation; increase in wear of the antagonist teeth or restorative materials; and change to the color of dental ceramics, thereby compromising the aesthetic appearance of ceramic restorations. An aqueous environment increases crack propagation in ceramics when exposed.[1]
Surface roughness measurements are performed by contact and noncontact methods.[20] Noncontact methods use a light beam or a laser beam to obtain a surface profile. The disadvantage of this method is that shiny surfaces are sometimes difficult to measure due to the scattering effect of the reflected light, which can cause false values being documented.[20] The contact method works by moving the stylus across the surface in a vertical movement to follow the surface profile of the object. Therefore, a contact method with a profilometer was used in this study.
This study, therefore, compares and evaluates the surface roughness of two dental ceramics (IPS e.max Ceram and Noritake) before and after exposure to an aerated drink, mouthwash, and simulated vomit solution with distilled water (control).
The results of this study clearly showed that Coca-Cola, simulated vomit solution, and chlorhexidine mouthwash significantly increased surface roughness of the ceramics evaluated in all dimensions (P < 0.001).
The surface roughness increased when both Noritake and IPS e.max Ceram were immersed in simulated vomit solutions. This is according to the findings of Kukiattrakoon et al.[21] who evaluated the effect of acidic agents on surface roughness of dental ceramics. Surface roughness was evaluated by a profilometer at the intervals of 24, 96, and 168h. Results showed all surface roughness parameters were significantly increased after 168h of immersion in acids.[21]
The surface roughness increased when both Noritake and IPS e.max Ceram were immersed in Coca-Cola. The results are in accordance with the results of Al-Hiyasat et al.[22] who investigated the effect of a carbonated beverage (Coca-Cola) on the wear of human enamel and three dental ceramics: a conventional porcelain (Vitadur Alpha, Bad Säckingen, Germany), a hydrothermal low-fusing ceramic (Duceram-LFC-Ballantyne Corporate Pl, Charlotte, NC, USA), and a machinable ceramic (Vita Mark II, Bad Säckingen, Germany). Significant differences in the wear of tooth and ceramics after exposure to Coca-Cola were observed.[22]
The surface roughness also increased when both Noritake and IPS e.max Ceram were immersed in chlorhexidine mouthwash. These findings are in accordance with Soygun et al.[23] who compared three bioceramic materials (IPS Empress CAD [Ivoclar], IPS e.max CAD [Ivoclar], and Lava Ultimate CAD [3M ESPE] Maplewood, NJ, USA) with three commercial mouthrinses (Listerine [St. Louis, MO, USA], Tantum Verde [London, UK], and Klorhex-Dexcel® Pharma Technologies Ltd [Jerusalem, Israel]). Change in surface roughness values was measured by profilometer device (Mitutoyo Surftest SJ-301; Mitutoyo Europe, Neuss, Germany). The change of surface roughness was inspected by scanning electron microscopy (SEM) and atomic force microscopy. The study concluded that mouthrinse with higher alcohol content had more deteriorating effect on the surface morphology of the bioceramic materials.[23]
The simulated vomit solutions cause maximum increase in surface roughness followed by Coca-Cola and then chlorhexidine mouthwash solution. Distilled water had no change in the surface roughness of the ceramics. There was no significant difference in the surface roughness between IPS e.max and Noritake.
The few limitations of this study were the following:
-
(1)
A profilometer along with a simultaneous SEM analysis would have enabled a clearer picture of the surface roughness of the materials.
-
(2)
It has been claimed that when using this contact method, the stylus tip may damage or alter the surfaces tested.[24]
-
(3)
The effect of many more solutions on the ceramics would have to be tested to simulate the oral cavity.
-
(4)
The study included only two ceramic materials available in the market, that is, Noritake and IPS e.max Ceram. Many more ceramic options are available and routinely used in clinical practice; the effect of various solutions on the other ceramics should also be assessed.
SUMMARY
The purpose of this study was to compare the effects of an aerated drink, mouthwash, and simulated gastric acid on the surface roughness of two dental ceramics.
In this study, two dental ceramics—Noritake and IPS e.max Ceram—were compared for surface roughness before and after immersion in an aerated drink, chlorhexidine mouthwash, and simulated vomit solution with distilled water as control. Sample sizes were calibrated to a diameter of 10 mm × 2 mm. The surface roughness of the samples was measured using a Mitutoyo SDA 350 surface profilometer before and after exposure to the solutions, and the results were statistically analyzed. Results showed significant differences in the surface roughness of samples before and after exposure to the solutions, and out of the solutions tested, simulated vomit solution was the most destructive, followed by the aerated drink. Between the two dental ceramics, IPS e.max Ceram showed higher increase in surface roughness than Noritake, although it was not statistically significant.
CONCLUSION
Within the limitations of this study it was concluded that:
-
(1)
the surface roughness of both the evaluated ceramics (Noritake and IPS e.max Ceram) increased upon exposure to Coca-Cola, chlorhexidine mouthwash, and simulated vomit solution;
-
(2)
simulated vomit solution caused maximum change in surface roughness followed by Coca-Cola and chlorhexidine mouthwash; and
-
(3)
Noritake showed slightly more resistance to change in surface profile when compared to IPS e.max, although it was not statistically significant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. Chemical durability and microhardness of dental ceramics immersed in acidic agents. Acta Odontol Scand. 2010;68:1–10. doi: 10.3109/00016350903251321. [DOI] [PubMed] [Google Scholar]
- 2.McGuckin RS, Babin JF, Meyer BJ. Alterations in human enamel surface morphology following vital bleaching. J Prosthet Dent. 1992;68:754–60. doi: 10.1016/0022-3913(92)90197-i. [DOI] [PubMed] [Google Scholar]
- 3.Shannon H, Spencer P, Gross K, Tira D. Characterization of enamel exposed to 10% carbamide peroxide bleaching agents. Quintessence Int. 1993;24:39–44. [PubMed] [Google Scholar]
- 4.Meurman JH, Härkönen M, Näveri H, Koskinen J, Torkko H, Rytömaa I, et al. Experimental sports drinks with minimal dental erosion effect. Scand J Dent Res. 1990;98:120–8. doi: 10.1111/j.1600-0722.1990.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 5.Copps DP, Lacy AM, Curtis T, Carman JE. Effects of topical fluorides on five low-fusing dental porcelains. J Prosthet Dent. 1984;52:340–3. doi: 10.1016/0022-3913(84)90440-2. [DOI] [PubMed] [Google Scholar]
- 6.Demirhanoglu ST, Sahin E. Effects of topical fluorides and citric acid on overglazed and autoglazed porcelain surfaces. Int J Prosthodont. 1992;5:434–40. [PubMed] [Google Scholar]
- 7.Kamala KR, Annapurni H. Evaluation of surface roughness of glazed and polished ceramic surface on exposure to fluoride gel, bleaching agent and aerated drink: an in vitro study. J Ind Prosthodon Soc. 2006;6:128–32. [Google Scholar]
- 8.Jafari K, Hekmatfar S, Badakhsh S. The effect of mouth washes on surface hardness of dental ceramics. J Dental Biomater. 2014;1:23–6. [Google Scholar]
- 9.Matsou E, Vouroutzis N, Kontonasaki E, Paraskevopoulos KM, Koidis P. Investigation of the influence of gastric acid on the surface roughness of ceramic materials of metal-ceramic restorations. An in vitro study. Int J Prosthodont. 2011;24:26–9. [PubMed] [Google Scholar]
- 10.Wunderlich RC, Yaman P. In vitro effect of topical fluoride on dental porcelain. J Prosthet Dent. 1986;55:385–8. doi: 10.1016/0022-3913(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 11.Gawriołek M, Sikorska E, Ferreira LF, Costa AI, Khmelinskii I, Krawczyk A, et al. Color and luminescence stability of selected dental materials in vitro. J Prosthodont. 2012;21:112–22. doi: 10.1111/j.1532-849X.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 12.Douglas RD. Color stability of new-generation indirect resins for prosthodontic application. J Prosthet Dent. 2000;83:166–70. doi: 10.1016/s0022-3913(00)80008-6. [DOI] [PubMed] [Google Scholar]
- 13.Samra AP, Pereira SK, Delgado LC, Borges CP. Color stability evaluation of aesthetic restorative materials. Braz Oral Res. 2008;22:205–10. doi: 10.1590/s1806-83242008000300003. [DOI] [PubMed] [Google Scholar]
- 14.Patel SB, Gordan VV, Barrett AA, Shen C. The effect of surface finishing and storage solutions on the color stability of resin-based composites. J Am Dent Assoc. 2004;135:587–94; quiz 654. doi: 10.14219/jada.archive.2004.0246. [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Urano M, Ebisu S. Effect of surface roughness of porcelain on adhesion of bacteria and their synthesizing glucans. J Prosthet Dent. 2000;83:664–7. [PubMed] [Google Scholar]
- 16.Ertaş E, Güler AU, Yücel AC, Köprülü H, Güler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–6. [PubMed] [Google Scholar]
- 17.Guler AU, Yilmaz F, Kulunk T, Guler E, Kurt S. Effects of different drinks on stainability of resin composite provisional restorative materials. J Prosthet Dent. 2005;94:118–24. doi: 10.1016/j.prosdent.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Kitchens M, Owens BM. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J Clin Pediatr Dent. 2007;31:153–9. doi: 10.17796/jcpd.31.3.1157l653t8206100. [DOI] [PubMed] [Google Scholar]
- 19.Van Eygen I, Vannet BV, Wehrbein H. Influence of a soft drink with low ph on enamel surfaces: an in vitro study. Am J Orthod Dentofacial Orthop. 2005;128:372–7. doi: 10.1016/j.ajodo.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead SA, Shearer AC, Watts DC, Wilson NH. Comparison of methods for measuring surface roughness of ceramic. J Oral Rehabil. 1995;22:421–7. doi: 10.1111/j.1365-2842.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 21.Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. Effect of acidic agents on surface roughness of dental ceramics. Dent Res J (Isfahan) 2011;8:6–15. [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Hiyasat AS, Saunders WP, Sharkey SW, Smith GM. The effect of a carbonated beverage on the wear of human enamel and dental ceramics. J Prosthodont. 1998;7:2–12. doi: 10.1111/j.1532-849x.1998.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 23.Soygun K, Varol O, Ozer A, Bolayir G. Investigations on the effects of mouthrinses on the colour stability and surface roughness of different dental bioceramics. J Adv Prosthodont. 2017;9:200–7. doi: 10.4047/jap.2017.9.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stout KJ. Surface roughness: measurement, interpretation and significance of data. Mater Eng. 1981;2:260–5. [Google Scholar]


