ABSTRACT
Background:
Acute bacterial skin and skin structure infections (ABSSSI) cause significant morbidity and mortality in hospitalized patients and outpatients as well. Newer fluoroquinolones such as delafloxacin might be a useful medication for treating infections of skin caused by gram-positive bacterial species that are resistant.
Aims and Objectives:
The aim of this study was to evaluate all the literature on delafloxacin in databases and make comparisons of its efficacy with antimicrobial drugs routinely used to treat skin infections.
Materials and Methods:
A detailed search on different databases was conducted using, Cochrane Central Register of Controlled Trials, PubMed, and Embase. Primary outcome was microbiological cure at the end of the follow-up period. Absence of the signs and symptoms at the termination of the follow-up period and clinical response to medications was regarded as the secondary outcome.
Results:
The pooled efficacy of delafloxacin was at 80% (95% confidence interval 1.01 [0.97, 1.06]; P = 0.51). No statistically significant difference was found between intravenous delafloxacin and comparator drugs.
Conclusion:
The effectiveness of delafloxacin was found to be non-inferior to tigecycline and linezolid. Efficacy and pooled cure rate of delafloxacin was also found to be superior to vancomycin.
KEYWORDS: Bacteria, delafloxacin, efficacy, infections, skin, systematic review
INTRODUCTION
According to the newly proposed guidelines, acute bacterial skin and skin structure infections (ABSSSI) can be defined as a bacterial skin lesion greater than 75 cm2. The definition includes three major types of skin infections; that is, cellulitis/erysipelas, wound infection, and major cutaneous abscess.[1] It also includes less severe infections of skin such as impetigo, animal bites, necrotizing fasciitis, ecthyma gangrenosum, and myonecrosis.[2] ABSSSI have been divided into either complicated or uncomplicated infections.[3] Complicated infections usually manifest themselves in the deeper layers of the skin and its structures, are more deep seated, and mostly require surgical intervention, intravenous (IV) treatment, or both.[1,2,3]
Uncomplicated infections are confined to the superficial layers and these may include minor abrasions, insect bites, cellulitis, and even impetigo. The etiology of ABSSSI can involve gram-positive pathogens such Staphylococcus aureus. But other gram-negative and gram-positive pathogens such as enterococci, beta-hemolytic streptococci, and Pseudomonas aeruginosa can also be a cause of infection.[4,5,6] Standard treatment guidelines as suggested by the Infectious Diseases Society of America and British infection association recommend oxacillin, clindamycin, and cefazolin for the management of ABSSSI by meticillin-susceptible S. aureus (MSSA). However, serious infections caused by meticillin-resistant Stapylococcus aureus (MRSA) should be treated with vancomycin, tigecycline, linezolid, daptomycin, ceftaroline, and telavancin [Table 1].[7,8,9,10]
Table 1.
Treatment guidelines for ABSSSI caused by Staphylococcus aureus (adapted from Russo et al.[1])
| MSSA infections | Oxacillin | 1–2g every 4h IV |
| Clindamycin | 1–2g every 4h IV | |
| Cefazolin | 600mg every 8h IV or 450mg qid | |
| MRSA infections | Vancomycin | 30mg/kg/day in two divided doses IV |
| Daptomycin | 4mg/kg every 24h IV | |
| Ceftaroline | 600mg BID IV | |
| Tigecycline | 100mg loading dose, followed by 50mg every 12 h | |
| Linezolid | 600mg every 12h IV or 600mg BID PO |
BID = bis in die, twice a day
PO = per orally
qid: quater in die, four times a day
For polymicrobial and mixed infections, the mainstay of treatment includes vancomycin plus piperacillin/tazobactam. Delafloxacin is a fluoroquinolone that has been authorized for the treatment of ABSSSI in June 2017 in the United States. The drug has a wide spectrum of activity as it is active against both gram-negative and gram-positive pathogens including MRSA.[4] Regulatory authorities have also shown interest in new drugs going through the earlier stages of clinical trials and have hence issued guidance for the assessment of these drugs. Gram-positive organisms have been estimated to cause more than 60% of skin infections. The most common bacterial specie responsible is S. aureus in 66% of the cases.[4,5] The goal of this systematic review is to filter all of the available proof in literature on delafloxacin and compare its efficacy and adverse events with a range of comparator drugs (vancomycin, tigecycline, and linezolid) currently being used for treating acute skin infections.
MATERIALS AND METHODS
An extensive literature search was performed and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement was used to report this systematic review.
Source of data
An extensive literature search was undertaken using three major databases in order to conclude the clinical efficacy of delafloxacin. Databases used were “PubMed” (1942 to 2017 December) “EMBASE” (1973 to 2017 December), and Cochrane Central Register of Controlled Trials (Central; 1972 to 21st December 2017). Other databases utilized for conducting an extensive literature search were clinical trial registers of the United States, China, India, and Europe.
Key words included in the search strategy were “delafloxacin,” “baxdela,” “ABSSSI,” “Skin infection,” “Abscess,” “Cellulitis,” “Efficacy,” “Randomized Controlled Trial,” and “Delafloxacin meglumine.”
Inclusion criteria
Inclusion criteria included patients older than 18 years with one of the mentioned symptoms and sign such as fever, purulent drainage, swelling, pain, erythema, and tenderness and patients diagnosed by culture and sensitivity with one of the following conditions: postsurgical wound infection, insect bite or post-traumatic injury, cellulitis, or abscess or oral abscess.
Exclusion criteria
Exclusion criteria included patients younger than 18 years; patients with hypersensitivity to fluoroquinolones, tetracycline, and its derivates; patients who were pregnant; patients who were lactating; patients with conditions such as prosthetic device infections, diabetic foot ulcer, osteomyelitis, septic arthritis, necrotizing fasciitis, and decreased perfusion.
Types of outcome
Primary outcome
Primary outcome was defined as microbiological cure proven by negative culture at the termination of follow-up period, response to antimicrobials clinically, and absence of the aforementioned symptoms at termination of follow-up period.
Secondary outcome
The secondary outcome, which was assessed in the studies, included adverse events related to the drug, such as diarrhea, nausea, vomiting, and hepatic insufficiency, and clinical response of the patients to drug at the end of treatment period.
Types of interventions
Studies where patients were administered delafloxacin following the mentioned route and dosage:
(1) 300mg IV twice a day (BID) for 5–14 days
(2) 450 mg IV BID for 5–14 days
Quality assessment of randomized and non- randomized observational studies
To evaluate the quality of included studies, a modified Downs and Black method checklist was used. The checklist contained 27 different items. The questions were adapted to assess the confounding, selection, internal validity, and reporting bias. The final question was used to measure the power of the study. It was adapted to from another research [Table 1].[11] Risk of bias was separately assessed using Cochrane risk of bias assessment tool. Risk of bias figure was generated using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK).[12]
Data collection and analysis
SBT and SSB (two authors) independently reviewed the studies for eligibility. Study selection was concluded on the content of titles and abstracts of research articles. Any other conflict or disagreement on inclusion criteria of selected studies was solved by mutual consensus.
Extraction of data and management
A form was customized to extract different parameters of the studies selected for inclusion in this review. Data extraction template was based on the forms already mentioned by the Cochrane Collaboration for Systematic Reviews. The data extracted from the study included year the study was published, study design, dosage and route of delafloxacin, dosage and route of comparator drug with length of treatment, and primary outcome measures such as cure rate, most common adverse events, length of treatment, follow-up duration, and method of diagnosis.
Data synthesis
To determine the major difference between delafloxacin and control drugs (vancomycin, aztreonam, and tigecycline), random effects model was used as risk ratio at 95% confidence intervals (CIs). Test for heterogeneity was checked using the chi-square test. To assess the level of uncertainty among studies, I2 test was used. Review Manager (RevMan) software (Copenhagen, Denmark), version 5.0 for windows, was used to perform all calculations. All of the studies where the probability value was less than 0.05 were considered as statistically significant.
RESULTS
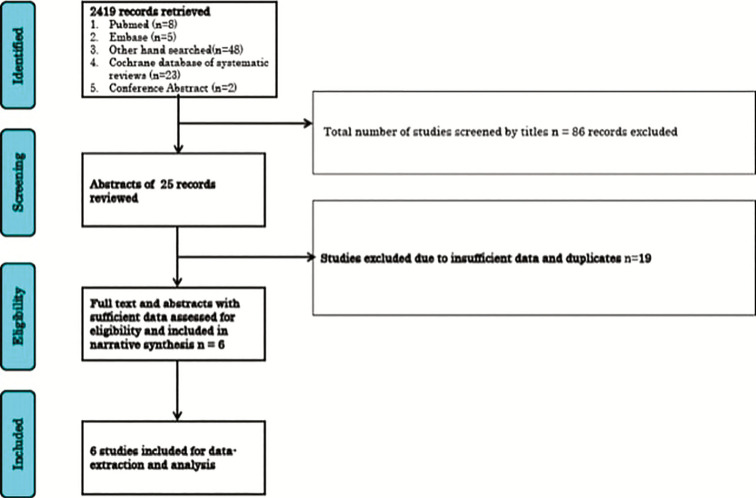
After screening for 86 abstracts, 25 relevant abstracts were added after applying the exclusion and inclusion criteria and consensus. Of the 25 relevant abstracts, only six randomized controlled trials were added in the final review and meta-analysis [Figure 1]. Nineteen studies were excluded from this review due to the following reasons:
Figure 1.
Flow Chart showing study selection process
(1) randomized controlled trials assessing only the pharmacokinetics and pharmacodynamics of delafloxacin
(2) studies where the primary outcome of the clinical trials was to evaluate the adverse events and safety
(3) studies where access to the full text of the articles was not available
Of the six studies included in this review, five studies collated and compared the efficacy of delafloxacin against vancomycin and aztreonam. One trial also collated and compared the efficacy of delafloxacin against linezolid. The remaining study collated and compared the efficacy of delafloxacin against tigecycline.[6,7,8,9,10,13]
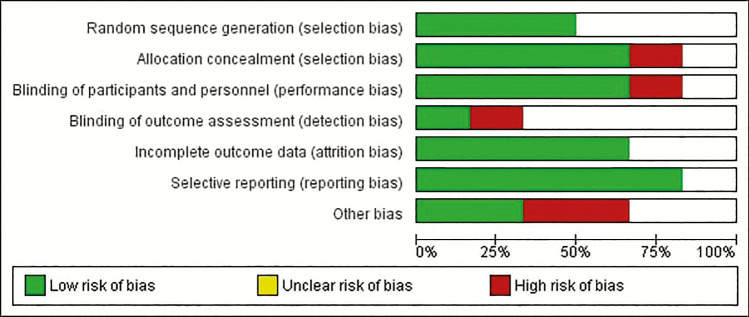
In trials estimating the efficacy of delafloxacin versus vancomycin + aztreonam, four trials compared the efficacy of 300mg IV delafloxacin versus 15mg/kg IV vancomycin with aztreonam,[6,7,8] whereas only one trial compared the efficacy of 450mg IV delafloxacin versus 15mg/kg IV vancomycin with aztreonam. Two trials also compared the efficacy of 300mg IV delafloxacin versus 100mg IV tigecycline and 300mg IV delafloxacin versus 600mg IV linezolid.[7,8,9] The risk of bias was estimated [Figure 2].
Figure 2.
Risk of Bias across all studies
Characteristics of the included studies
A sum of 2048 patients were added in this review. The average mean of age of the patients was 45 years. While 1012 patients were provided treatment with vancomycin and aztreonam, 957 patients underwent treatment with delafloxacin. More than 50% of the patients were administered vancomycin and aztreonam, whereas only 74 patients received treatment with tigecycline and 39 patients with linezolid [Table 2].[7,8,9,10,13,14]
Table 2.
Characteristics of included studies
| Author and year | Study design | Dosage and route of delafloxacin | Dosage and route of comparator drug | Outcome measure (primary) | Adverse events | Organism isolated | Follow-up duration | Method of diagnosis |
|---|---|---|---|---|---|---|---|---|
| Cammarata et al. 20156 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 97.76% treatment success | Nausea, diarrhea, and infusion extravasation most common | 98% S. aureus 2% gram negatives | 14 days average | Clinical and culture and susceptibility |
| Kemeny et al. 20157 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 72.5% treatment success | Nausea, diarrhea, and infusion extravasation most common | 100% S. aureus | 14 days average | Clinical and culture and susceptibility |
| Kemeny et al. 20157 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 70% treatment success | Nausea, diarrhea, and infusion extravasation most common | 100% S. aureus | 14 days average | Clinical and culture and susceptibility |
| Kingsley et al. 20168 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 70% treatment success | Nausea, diarrhea, and infusion extravasation most common | 90.9% S. aureus 9.1% gram negatives | 14 days average | Clinical and culture and susceptibility |
| Kingsley et al. 20168 | RCT | 300mg IV BID for 5–14 days | Linezolid 600mg IV BID 5–14 days | 70% treatment success | Nausea, diarrhea, and vomiting | 90.9% S. aureus 9.1% gram negatives | 14 days average | Clinical and culture and susceptibility |
| O’ Riordan et al. 20159 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 94% treatment success | Nausea, diarrhea, and hepatotoxicity | 64% S. aureus 26% gram negatives and other GPC | 28–35 days | Clinical and culture and susceptibility |
| O’ Riordan et al. 20159 | RCT | 450mg IV every BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 94% treatment success | Nausea, diarrhea, and hepatotoxicity | 64% S. aureus 26% gram negatives and other GPC | 28–35 days | Clinical and culture and susceptibility |
| Giordano et al. 201710 | RCT | 300mg IV BID for 5–14 days | Tigecycline 100mg IV 1, followed by 50mg IV every | 81.5% treatment success | Not mentioned | S. aureus most common | 14 days average | Clinical and culture and susceptibility |
| Pullman et al. 201713 | RCT | 300mg IV BID for 5–14 days | VAN 15mg/kg IV with aztreonam BID for 5–14 days | 81.5% treatment success | Nausea and diarrhea | 100% S. aureus | 14 days average | Clinical and culture and susceptibility |
ABSSSI = acute bacterial skin and skin structure infections, BID = twice daily, GPC = gram positive cocci, IV = intravenous, RCT = randomized controlled trials, S. aureus = Staphylococcus aureus, VAN = vancomycin
Primary analysis for cure rate
Overall cure rate
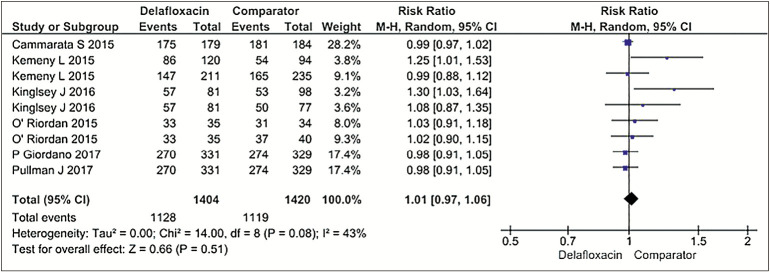
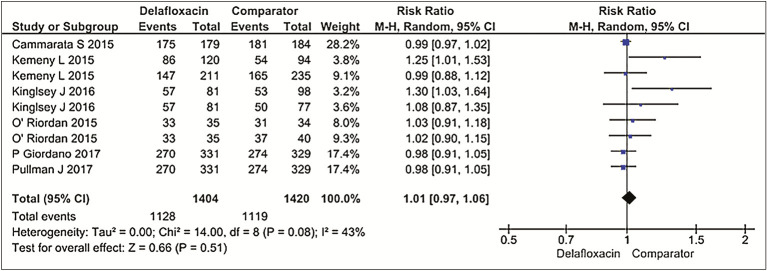
The pooled cure rate was 80% (1062/1334 patients) in delafloxacin group as opposed to 78.3% (1051/1341 patients) in comparator group (tigecycline, vancomycin + aztreonam, and linezolid), using dichotomous outcomes and random effects model at 95% CI 1.01 (0.97, 1.06), P = 0.51. Hence, no statistical difference was ascertained between the efficacy of delafloxacin and comparator drug. Heterogeneity among studies was at a moderate level: I2 = 43%, P = 0.08 [Figure 3].[6,7,8,9,10,13]
Figure 3.
Forest Plots for Efficacy (Delafloxacin versus Comparator group)
Delafloxacin versus vancomycin + aztreonam
Cure rate (five studies) was 80% (1062/1334 patients) in 300mg delafloxacin group compared to 78% (1051/1341 patients) in the 15mg/kg vancomycin and aztreonam group,[6,7,8,9,10,13] using dichotomous outcomes and random effects model at 95% CI 1.01 (0.96, 1.06), P = 0.64. Hence, the difference between the efficacy of delafloxacin and vancomycin + aztreonam was statistically nonsignificant. Heterogeneity among studies was at a moderate level; I2 = 48%, P = 0.07.
Delafloxacin versus tigecycline
Cure rate (one study) was 94% (66/70 patients) in 300 and 450mg delafloxacin group in contrast to 92% (68/74 patients) in 100mg tigecycline group, using dichotomous outcomes and random effects model at 95% CI 1.03 (0.94, 1.12), P = 0.57. Hence, the statistical difference between the efficacy of delafloxacin and tigecycline was not significant.[10]
Delafloxacin versus linezolid
Cure rate (one study) was 88% (30/34 patients) in 300mg delafloxacin group compared to 82% (32/39) in the linezolid group, using dichotomous outcomes and random effects model at 95% CI 1.08 (0.89, 1.80). Heterogeneity test was not applicable as there was only one trial comparing delafloxacin with linezolid.
Analysis for adverse events
Gastrointestinal symptoms were regarded as the most common adverse event that affected most of the patients. Adverse event rates (five studies): 80.3% (1128/1404 patients in delafloxacin group) compared with 78.8% (1119/1420 patients in the vancomycin + aztreonam, tigecycline, and linezolid group combined); relative risk (RR) (random effects model) 95% CI 1.01 (0.97. 1.06), P = 0.51. Hence, there was no statistical difference between the delafloxacin and the comparator group. There was also a high level of heterogeneity among studies; that is, I2 = 43%, P = 0.08 [Figure 4].[1,2,3]
Figure 4.
Forest Plot for Adverse Events
Delafloxacin versus vancomycin + aztreonam
Adverse event rates (four studies): 23.1%(157/679 patients in the delafloxacin group) compared with 29.5% (210/711 patients in the vancomycin along with aztreonam group); RR (random effects model) 95% CI 0.77 (0.60, 0.98) P = 0.03.[2,3,4,5] Therefore, significant statistical difference was concluded among the delafloxacin and vancomycin + aztreonam group. Heterogeneity was at I2 = 37% in this analysis; P = 0.19.
Delafloxacin versus tigecycline
Adverse event rates (one study): 94% (66/70 patients in the delafloxacin group) in comparison with 92% (68/74 patients in the tigecycline group); RR (random effects model) 95% CI 1.03 (0.94, 1.12), P = 0.57. Therefore no statistical difference was found between delafloxacin and tigecycline. Heterogeneity test was not applicable in this category, because there was only one randomized controlled trial.[6]
Delafloxacin versus linezolid
Adverse event rates (one study): 0% (0/69 patients in delafloxacin group) in comparison with 0.01% (1/77 patients in linezolid group); RR (random effects model) 95% CI 0.37 (0.02, 8.97), P = 0.54. Therefore no statistical difference was found between delafloxacin and linezolid. Heterogeneity test was not applicable because only one clinical trial was identified in this group.[6,7,8]
DISCUSSION
Delafloxacin is known to have a strong effect against a major range of gram-positive pathogens such as Staphylococcus species. It is especially active pathogens causing soft tissue and skin infections. Most of the uncomplicated and complicated skin infections acquired from the community are predominantly compounded by gram-positive bacterial pathogens; for example, MSSA, MRSA, and Streptococcus species. Data collated from majority of the articles mentioned in this meta-analysis also depict S. aureus to be the most common causative agent for skin infections.[13,14,15,16]
As delafloxacin is a modern antimicrobial quite effective mainly against gram-negative pathogens along with gram-positive pathogens, it can be used to treat skin infections of both common and diverse etiology. Other newer antimicrobials for the management of soft tissue and skin infections such as oritavancin, dalbavancin, tedizolid, and daptomycin are active against MRSA and a broad range of other gram-positive bacterial species responsible for causing ABSSSI. However, they do not have any activity against gram-negative bacterial species responsible for causing skin infections. Delafloxacin on the other hand is not only active against gram-positive bacterial species including MRSA, but also active against gram-negative bacterial species responsible for causing ABSSSI. Clinical trials have also proven it to be non-inferior to antimicrobials such as vancomycin and linezolid.[15,16] Previously fluoroquinolones were known to be against in vitro isolates of S. aureus, and levofloxacin was also used to treat some skin infections as well. Recent studies conducted in the United States showed that alarmingly 70% of the clinical isolates are resistant to levofloxacin. In the current era of increasing resistance of different pathogens to fluoroquinolones, it is pertinent to mention the importance of delafloxacin as a future component of antimicrobial stewardship program and potential role in therapy. A recent trial on pharmacokinetic and pharmacodynamics of 300mg IV delafloxacin showed the mean plasma concentration in humans after 30h of administration to be approximately 0.5 μg/mL. This is well above its minimal inhibitory concentration (MIC)90 of 0.01 μg/mg for S. aureus. Its concentration in plasma is also well above the MIC90 of MRSA; that is, 0.12 μg/mL. Therefore, it can be ascertained that it has favorable pharmacokinetics and pharmacodynamics properties against resistant gram-positive pathogens responsible for causing skin infections. Hence, in the current age where antimicrobial resistance is rampant, it might prove to be a relevant alternative in treating soft tissue and skin infections caused by resistant gram-positive bacterial organisms.[14,15,16]
Overall, the following meta-analysis and systematic review is suggestive of the fact that there was insignificant statistical difference between the cure rates of 300mg delafloxacin and 15mg/kg vancomycin + aztreonam. Moreover, no difference was found between 300mg delafloxacin group against both 50mg tigecycline and 600mg linezolid group. There were significant fewer adverse events in 300mg delafloxacin group in contrast with 15mg/kg vancomycin along with aztreonam and no statistical difference between the adverse events of 300mg delafloxacin versus 600mg linezolid and 50mg tigecycline. The most common treatment-related adverse event encountered by most of the patients was gastrointestinal-related events, such as diarrhea, nausea, and vomiting. Less common adverse events included hepatotoxicity. Hence, this systematic review found out delafloxacin to be non-inferior to vancomycin and aztreonam.[11,16]
Regarding adverse events, the results obtained in this review suggest delafloxacin 300mg to have a lower incidence of side effects in comparison with the 15mg/kg vancomycin + aztreonam group (RR = 0.57).[6,7,8,9] Our results also suggested 300mg delafloxacin to have no significant difference in adverse events when compared with 50mg tigecycline (RR = 0.94). Finally, no adverse events were documented in the 300mg delafloxacin when compared with the 600mg linezolid group, which had one adverse event.[9]
Therefore, it is safe to say that the efficacy of delafloxacin is not low in contrast to antimicrobials such as vancomycin, aztreonam, linezolid, and tigecycline. As no serious adverse events have been documented, and it covers the spectrum of gram-negative bacterial species as well, it will also serve as an adjunct or a replacement to aztreonam for the treatment of ABSSSI. Furthermore, the incidence of adverse events in patients taking delafloxacin was also found to be almost equal to the patients taking the aforementioned antimicrobials. This also goes in favor of delafloxacin as a useful adjunct or replacement for treating skin infections.
Quality of the included studies
All six studies finally added to this review were randomized controlled trials. Most of the studies added in this review did not clearly mention the method by which blinding of personnel and participants was performed. Furthermore, the random sequence generation bias was also unclear. However, bias of attrition was found as quite decreased in the studies. There were also differences in the primary and secondary outcome of a few studies.
Limitations
A major limitation of this review was the lack of availability of fully published articles. As delafloxacin is a comparatively new drug, there were only a few randomized controlled trials identified by authors. Of the total six studies included in this review, data from two of the studies was extracted from posters and conference abstracts. Hence, the authors had to extract data from gray literature (posters and conference abstracts that will be published in future) as well.
This review highlights the pressing need for more randomized controlled trials with higher quality that needs to be conducted in future to guide the clinicians for future treatment options of ABSSSI caused by resistant nosocomial gram-positive and negative pathogens, as delafloxacin might prove to be a useful replacement for older antimicrobials, which have already started to develop resistance against various bacterial species.
CONCLUSION
Despite the paucity of data in literature and high risk of bias in the studies, data obtained from our systematic review show both 300 and 450mg delafloxacin to be non-inferior to other oral antimicrobials such as vancomycin, aztreonam, linezolid, and tigecycline, which are routinely used to treat resistant skin and skin structure infections. In this era of antimicrobial resistance, delafloxacin might prove to be a useful antimicrobial that can be incorporated into the antimicrobial stewardship policies for different hospitals in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Russo A, Concia E, Cristini F, De Rosa FG, Esposito S, Menichetti F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect. 2016;22(Suppl 2):S27–36. doi: 10.1016/S1198-743X(16)30095-7. [DOI] [PubMed] [Google Scholar]
- 2.Kwak YG, Choi SH, Kim T, Park SY, Seo SH, Kim MB, et al. Clinical guidelines for the antibiotic treatment for community-acquired skin and soft tissue infection. Infect Chemother. 2017;49:301–25. doi: 10.3947/ic.2017.49.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulido-Cejudo A, Guzmán-Gutierrez M, Jalife-Montaño A, Ortiz-Covarrubias A, Martínez-Ordaz JL, Noyola-Villalobos HF, et al. Management of acute bacterial skin and skin structure infections with a focus on patients at high risk of treatment failure. Ther Adv Infect Dis. 2017;4:143–61. doi: 10.1177/2049936117723228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markham A. Ibalizumab: first global approval. Drugs. 2018;78:781–5. doi: 10.1007/s40265-018-0907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes P, Martens E. Antibiotics in late clinical development. Biochem Pharmacol. 2017;133:152–63. doi: 10.1016/j.bcp.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Cammarata S, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, et al. Results of a global phase 3 study of delafloxacin (DLX) compared to vancomycin with aztreonam (VAN) in acute bacterial skin and skin structure infections ( ABSSSI ) Open Forum Infect Dis. 2015;2:776. [Google Scholar]
- 7.Kemeny L, Lucasti C, Sun E, Quintas M, Lawrence L, Cammarata S. Outcomes in obese patients with acute bacterials skin and skin structure infections (ABSSSI) in a trial comparing delafloxacin to vancomycin/ aztreonam. Open Forum Infect Dis. 2015;2:S49. [Google Scholar]
- 8.Kingsley J, Mehra P, Lawrence LE, Henry E, Duffy E, Cammarata SK, et al. A randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71:821–9. doi: 10.1093/jac/dkv411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis. 2015;30:67–73. doi: 10.1016/j.ijid.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Giordano P, Pullman J, Lawrence L, Quintas M, Tseng C, Cammarata SK. Impact of delafloxacin (DLX) and vancomycin/aztreonam (VAN/AZ) on resolution of signs and symptoms of acute bacterial skin and skin structure infections (ABSSSI) Open Forum Infect Dis. 2017;4:S531–2. [Google Scholar]
- 11.Hoover R, Hunt T, Benedict M, Paulson SK, Lawrence L, Cammarata S, et al. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther. 2016;38:53–65. doi: 10.1016/j.clinthera.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Lan SH, Lai CC, Lu LC, Chang SP, Huang HT Efficacy and safety of delafloxacin in the treatment of acute bacterial skin and skin structure infections: a systematic review and meta-analysis of randomized controlled trials Infect Drug Resist. 2019;12:1415–23. doi: 10.2147/IDR.S202160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, et al. PROCEED Study Group. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72:3471–80. doi: 10.1093/jac/dkx329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Sader HS, Rhomberg PR, Flamm RK. In vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrob Agents Chemother. 2017;61:e02609–16. doi: 10.1128/AAC.02609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Righi E, Carnelutti A, Vena A, Bassetti M. Emerging treatment options for acute bacterial skin and skin structure infections: focus on intravenous delafloxacin. Infect Drug Resist. 2018;11:479–88. doi: 10.2147/IDR.S142140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorgensen SCJ, Mercuro NJ, Davis SL, Rybak MJ. Delafloxacin: place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect Dis Ther. 2018;7:197–217. doi: 10.1007/s40121-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]