ABSTRACT
Aim:
The aim of this study was to assess the efficacy of 5% topical amlexanox and 0.1% topical triamcinolone acetonide in recurrent aphthous stomatitis (RAS) management.
Materials and Methods:
Sixty adult patients of RAS of both genders were divided into two groups with each group having 30 patients. In group I, 0.1% topical triamcinolone acetonide was prescribed. In group II, 5% topical amlexanox was prescribed. Patients were recalled regularly and size of ulcer, erythema, and pain score was recorded on days 1, 3, and 5.
Results:
The mean ulcer size (mm) on day 1 in group I was 4.3 and in group II it was 4.1, on day 3 it was 3.5 in group I and in group II it was 3.6, on day 5 in group I it was 1.3 and in group II it was 1.7. The result was not statistically significant (P > 0.05). The mean pain score recorded on visual analog scale was in descending grade day by day on first, third, and fifth days. In group I, healing was seen in 29 (96.6%) patients and in 28 (93.3%) patients in group II. Partially healing was seen in 1 (3.3%) patient in each group, whereas in group II nonhealing was observed in 1 (3.3%) patient. The statistical significance was not achieved as P > 0.05.
Conclusion:
Authors found that above drugs were effective in reducing pain, size of ulcer, erythema, and improving healing in patients with recurrent aphthous stomatitis. There were better results with triamcinolone acetonide as comparison of amlexanox.
KEYWORDS: Amlexanox, recurrent aphthous stomatitis, triamcinolone acetonide
INTRODUCTION
Recurrent aphthous stomatitis (RAS) or oral ulcerations is frequently encountered among people. It is commonly known as aphthae, or canker sores. It is seen in 5%–25% of general population all over the world. It is prevalent in the second–fourth decades of life with equal gender predilection.[1] It has three subtypes namely, recurrent aphthous major, recurrent aphthous minor, and herpetiform. Among all subtypes, RAS major (MaRAS) is commonly encountered among 75%–85% of population.[2]
The origin of aphthae is from the Greek word aphthi, which refers “to set on fire” or “to inflame.” There are various causative factors for aphthous ulcers. Of which local trauma, bacterial infections, viral infection, genetic factors, nutritional deficiencies, immune, and endocrine disturbances are common one. If no other etiology can be recognized for cause of ulcer and a diagnosis of exclusion must be made, then such cases are referred to as RAS.[3]
Clinically it appears as round or oval-shaped ulcers with gray–white pseudomembranous having erythematous halos. Common site of involvement is cheeks, lips, soft palate, pharynx, and floor of the mouth. The number of ulcer may vary depending on type. It can be 1–5 in case of major, 1–10 in minor, and 10–100 in case of herpetiform ulcerations. Size of ulcers is <10 mm, whereas in case of major type it is >10 mm.[4]
Management includes use of topical agents such as topical or systemic corticosteroids, nutritional supplements, antibiotics, laser therapy, and immunomodulators.[5] Amlexanox is a commonly used drug in RAS. It is anti-inflammatory and anti-allergic in nature. 5% topical amlexanox in form of oral paste is used in patients with RAS. 0.1% topical triamcinolone acetonide is another useful drug, which imparts favorable results.[1] This study compared efficacy of 5% topical amlexanox and 0.1% topical triamcinolone acetonide in management of RAS.
MATERIALS AND METHODS
This study was carried out by the Department of Oral Medicine and Radiology. It comprised 60 adult male and female patients with RAS. Inclusion criteria were patients aged 18–48 years of either gender. Exclusion criteria were patients under 18 years of age, smokers, history of alcohol intake, anemic, and history of allergy. Ethical clearance for the study was taken from institutional ethical committee. All patients involved in study were made aware of the need of study and consent in written form was taken from them.
Relevant patient information such as name, age, and gender was recorded. They were divided into two groups of 30 patients each. In group I, 0.1% topical triamcinolone acetonide was prescribed. In group II, 5% topical amlexanox was prescribed. All patients were instructed to apply paste on the ulcers three to four times per day for a minimum of 10 min. Patients were recalled regularly and size of ulcer, erythema, and pain score was recorded on days 1, 3, and 5.
Erythema improvement was analyzed on a categorical scale as worse, same, mild, moderate, marked, and complete improvement. Visual analog scale (VAS) ranging from 1 to 10 was used to measure pain where 1 indicated no pain and 10 indicated severe pain. Healing was assessed as healed, partially healed, or not healed.
RESULTS
All data were as coded and analyzed using IBM, SAS, SPSS, version 19.0 (New York, USA). Comparison of pain, erythema, and size was carried out by using Fisher’s exact test. The level of significance was set at P ≤ 0.05 at 95% confidence interval. Table 1 shows that in group I, 5% topical amlexanox was prescribed. In group I, 0.1% topical triamcinolone acetonide was prescribed. Each group had 30 patients. Table 1 shows that the mean ulcer size (mm) on day 1 in group I was 4.3 and in group II it was 4.1, on day 3 it was 3.5 in group I and in group II it was 3.6, on day 5 in group I it was 1.3 and in group II it was 1.7. The result was not statistically significant (P > 0.05). Table 2 shows that mean pain score recorded on VAS on day 1 was 5.6 in group I and in group II it was 4.2. On day 3, in group I was 3.1 and 3.9 in group II, on day 5 in group I was 1.1 and slightly higher in group II at 2.6. Table 3 shows erythema score in both the groups and it shows statistically significant difference of P < 0.05.
Table 1.
Distribution of patients
| Day | Groups | Mean | SD | P Value |
|---|---|---|---|---|
| Day 1 | Group I | 4.3 | 0.1 | 0.91 |
| Group II | 4.1 | 0.3 | ||
| Day 3 | Group I | 3.5 | 0.2 | 0.85 |
| Group II | 3.6 | 0.4 | ||
| Day 5 | Group I | 1.3 | 0.1 | 0.07 |
| Group II | 1.7 | 0.1 |
SD = standard deviation
Group I––5% amlexanox, Group II––0.1% triamcinolone acetonide
Fisher’s exact test, significant, P < 0.05
Table 2.
Comparison of pain in both groups
| Day | Groups | Mean | SD | P Value |
|---|---|---|---|---|
| Day 1 | Group I | 5.6 | 0.1 | 0.02 |
| Group II | 4.2 | 0.3 | ||
| Day 3 | Group I | 3.1 | 0.2 | 0.05 |
| Group II | 3.9 | 0.4 | ||
| Day 5 | Group I | 1.1 | 0.1 | 0.01 |
| Group II | 2.6 | 0.1 |
SD = standard deviation
Fisher’s exact test, significant, P < 0.05
Table 3.
Comparison of erythema score in both groups
| Day | Groups | Severity | P Value |
|---|---|---|---|
| Day 1 | Group I | Mild to moderate | 0.02 |
| Group II | Mild to moderate | ||
| Day 3 | Group I | Mild to moderate | 0.05 |
| Group II | Mild to moderate | ||
| Day 5 | Group I | Complete improvement | 0.01 |
| Group II | Complete improvement |
Fisher’s exact test, significant, P < 0.05
Graph 1 shows that in group I, healing was seen in 29 (96.6%) patients and in 28 (93.3%) patients in group II. Partial healing was seen in 1 (3.3%) patient in each group, whereas nonhealing was observed in 1 (3.3%) patient in II group. The difference on statistical analysiswas found to be nonsignificant (P > 0.05).
Graph 1.
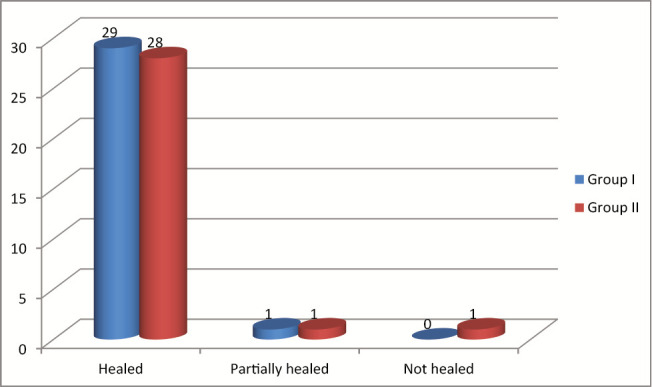
Healing of ulcer in both groups
DISCUSSION
RAS is commonly seen in middle-age people. It is characterized by pain, the intensity of which varies person to person. Clinically recurrent, painful, single, or multiple well-demarcated ulcerations are seen with peripheral red halo where healing takes place with or without scarring. It is considered to be the disease of multiple etiology.[6] The primary treatment aim for RAS is to reduce inflammation and duration of ulcer and to promote healing. No specific treatment modality exists for it. Only the severity and frequency of ulcerations can be minimized.[7]
Recent drug amlexanox acts as an inflammatory agent by inhibiting the formation and liberation of histamines and leukotrienes from mast cells, mononuclear cells, and neutrophils, thus reducing the symptoms in patients with RAS. Triamcinolone acetonide is widely used corticosteroid agent, which shows its effects by reducing inflammation.[8] This study compared efficacy of 5% topical amlexanox and 0.1% topical triamcinolone acetonide in management of RAS.
In this study, we included 60 patients of RAS. In total, 30 patients were put in each group and thus two groups were made. In group I, 5% topical amlexanox was advised for patient, whereas in group II, 0.1% topical triamcinolone acetonide was prescribed.
Shrivastava et al.[9] conducted a study in which 40 adult patients of RAS were prescribed 0.1% topical triamcinolone acetonide and 5% topical amlexanox. Parameters such as erythema, ulcer size, pain scores, and ulcer healing were evaluated on first, third, and fifth days. It was found that 90% of patients on triamcinolone acetonide showed complete improvement as compared to 65% of patients on amlexanox. Pain reduction was clearly evident from first to fifth days. There was 90% reduction in patients on triamcinolone acetonide as compared to 70% in the patients of amlexanox group. The mean ulcer size also showed reduction from first to fifth days in both groups (F = 18.611 and P = 0.000).
A significant reduction was observed in pain score recorded on VAS in both groups. Group I showed more reduction in pain as compared to amlexanox group. Both groups also showed reduction in size of ulcer; however, the difference between groups was not significant as it showed that P > 0.05.
Abbasi et al.[10] in their double-blind clinical trial study assessed efficacy of amlexanox and Adcortyl in patients with RAS. The factors for assessment were pain, lesion size, and tingling on first-, third-, fifth-, and seventh-day follow-ups. Authors found that the pain score, tingling, and lesion size observed on similar days between amlexanox and Adcortyl groups showed no significant difference at all. Reduction in the variables assessed was significant between days 1–3, 3–5, and 5–7 days in both groups (P < 0.001).
There was significant healing on day 5 in both groups. However, triamcinolone acetonide was found to be better as compared with amlexanox, which revealed one case of nonhealing. Chalkoo et al.[11] in their study on 36 patients of recurrent aphthous ulcers assessed pain score, ulcer size, and tingling sensation. Patients in group I were administered with triamcinolone 0.1% (Abbott laboratories, Illinois, USA, 0.1% oral paste), and patients in group II were administered with amlexanox 5% (Macleods pharmaceuticals, Mumbai, India, 5% oral paste) four times daily for 1 week. The patients were followed at days 0, 3, 5, and 7 and scores were assessed using VAS. Subsequent follow-up visits at third, fifth, and seventh days showed reduction of pain and ulcer size significantly at P < 0.01 in both groups. On seventh day of treatment, no patient reported of any pain. Smaller sample size in this study was one of the limitations and other being that only two drugs could be compared. Side effect of drugs was not assessed in this study.
CONCLUSION
Both the drugs used in this study were effective in reducing pain, size of ulcer, erythema, and improving healing in patients with recurrent aphthous stomatitis. However, the results were better with triamcinolone acetonide as compared with amlexanox indicating its effectiveness in treating RAS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Belenguer-Guallar I, Jiménez-Soriano Y, Claramunt-L,ozano A. Treatment of recurrent aphthous stomatitis. A literature review. J Clin Exp Dent. 2014;6:e168–74. doi: 10.4317/jced.51401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montgomery-Cranny JA, Wallace A, Rogers HJ, Hughes SC, Hegarty AM, Zaitoun H. Management of recurrent aphthous stomatitis in children. Oral Med. 2015;42:564–72. doi: 10.12968/denu.2015.42.6.564. [DOI] [PubMed] [Google Scholar]
- 3.Ofluoglu D, Ergun S, Warnakulasuriya S, Namdar-Pekiner F, Tanyeri H. An evaluation of the efficacy of a topical gel with triester glycerol oxide (TGO) in the treatment of minor recurrent aphthous stomatitis in a Turkish cohort: a randomized, double-blind, placebo-controlled clinical trial. Med Oral Patol Oral Cir Bucal. 2017;22:e159–66. doi: 10.4317/medoral.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58:281–97. doi: 10.1016/j.cden.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal H, Singh MP, Nahar P, Mathur H, Gv S. Efficacy of low-level laser therapy in treatment of recurrent aphthous ulcers: a sham controlled, split mouth follow up study. J Clin Diagn Res. 2014;8:218–21. doi: 10.7860/JCDR/2014/7639.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes CC, Gomez RS, Zina LG, Amaral FR. Recurrent aphthous stomatitis and helicobacter pylori. Med Oral Patol Oral Cir Bucal. 2016;21:e187–91. doi: 10.4317/medoral.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera C. Essentials of recurrent aphthous stomatitis. Biomed Rep. 2019;11:47–50. doi: 10.3892/br.2019.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrivastava K, Naidu G, Deshpande A, Handa H, Raghuvanshi V, Gupta M. Comparative evaluation of the efficacy of topical amlexanox 5% oral paste and triamcinolone acetonide 0.1% oral paste in the treatment of Recurrent Aphthous Stomatitis (RAS) J Indian Acad Oral Med Radiol. 2018;30:235–40. [Google Scholar]
- 10.Abbasi F, Raoof M, Khatami R, Shadman N, Borjian-Boroojeni F, Nazari F. Effectiveness of amlexanox and adcortyl for the treatment of recurrent aphthous ulcers. J Clin Exp Dent. 2016;8:e368–72. doi: 10.4317/jced.52540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalkoo AH, Wani BA, Sharma P, Jan T. An evaluation of the efficacy of amlexanox and triamcinolone topical paste in the treatment of recurrent aphthous stomatitis. Int J Contemp Med Res. 2018;5:I20–2. [Google Scholar]


