ABSTRACT
Preserving the natural dentition in its normal form and function is one of the major goals of endodontic treatment. Re-establishing the lost vitality and development of root maturation in immature permanent teeth with pulp necrosis/apical periodontitis is quiet challenging clinically. The very basis of regenerative endodontics relies on the concept of tissue engineering using stem cells, biomimetic scaffold, and bioactive growth factors to regenerate the pulp tissue damaged by microbial infection, injury, or developmental defects. In clinical endodontics, this approach is referred to as a “paradigm shift.” Even though repair instead of true regeneration is achieved with current protocols, it is hoped that further research in the area of stem-cell-based tissue engineering will allow for true regeneration and improved treatment outcomes. The aim of this review is to discuss about the various aspects of regenerative endodontics, current clinical protocols, and the future of pulp regeneration techniques.
KEYWORDS: Apexification, immature permanent teeth, MTA, regenerative endodontics
INTRODUCTION
Regenerative endodontics is one of the emerging treatment modalities in dentistry. Endodontic treatment for immature permanent teeth with pulp necrosis/apical periodontitis presents with a challenging clinical situation. The very basis of regenerative endodontics is the tissue engineering concept, which uses stem cells, scaffold, and growth factors to regenerate the pulp–dentin complex. These developments corroborate the attempts to preserve the natural roots, one of the major goals of endodontic treatment.
Apexification is the traditional treatment protocol for immature permanent teeth with pulp necrosis/apical periodontitis. Calcium hydroxide and mineral trioxide aggregate (MTA) are commonly used for apexification. The major drawbacks associated with calcium hydroxide are reduction in root strength, possibility of root fracture, and several treatment visits over a long period of time.[1,2] In comparison with calcium hydroxide, MTA apexification requires only short treatment time. But neither of these methods encourages root maturation, which is the thickening of root canal walls and/or closure of apex. Apexification procedure offers no possibility to re-establish the vitality of the pulp tissue, damaged by microbial infection, injury, or developmental defects. Regenerative endodontic procedures (REPs) can be considered as an alternative to apexification procedure as it offers continued root development and reinforcement of dentinal walls by hard tissue deposition, thereby providing adequate strength to the root against fracture.[3]
DEFINITION
Regenerative endodontics can be defined as “biologically based procedures designed to replace damaged tooth structures, including dentine and root structures, as well as cells of the pulp–dentine complex”.[4]
HISTORY
The scope of REPs in necrotic teeth was first analyzed by Nygaard-Ostby[5] in 1961. Iwaya et al.[6] in 2001 detailed about the treatment procedure termed revascularization that had the potential for root maturation. Revitalization was proposed as a more accurate term instead of revascularization as the tissues that regenerated in the root canal space were not only comprised of blood vessels but also both hard and soft tissues.[7] In 2004, Banchs and Trope proposed the protocol for revascularization of immature permanent teeth with apical periodontitis using triple antibiotic paste (TAP).[8] This was based on the findings observed from the experiments by Kling et al.,[9] Hoshino et al.,[10] and Nygard-Ostby and Hjortdal.[11]
TERMINOLOGY
The term “regenerative endodontics” was approved by the American Association of Endodontists (AAE) in 2007.[4] Terms such as revascularization, revitalization, and regenerative endodontics can be used interchangeably and synonymously. The term regenerative endodontic procedures cites all procedures that are directed to obtain organized repair of damaged pulp tissue and include future treatment approaches yet to unfold in regenerative endodontics.
DIFFERENCE BETWEEN PULP REVASCULARIZATION AND PULP REGENERATION
Pulp revascularization is the re-establishment of vascularity or angiogenesis in the root canal.[12] It does not suggest the repopulation of odontoblasts. Pulp regeneration does not occur in the absence of revascularization and is treated incomplete if there is no odontoblastic layer lining the dentinal surface, nociceptive as well as sympathetic and parasympathetic nerve fibers, interstitial fibroblasts, and stem/progenitor cells, which replenish the pulp cells in the newly regenerated pulp tissue.
CLINICAL PROTOCOL FOR REGENERATIVE ENDODONTIC PROCEDURE
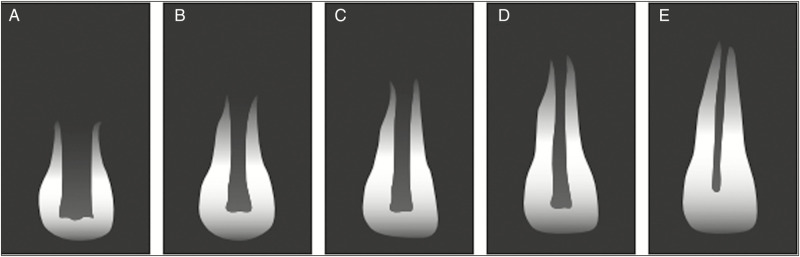
“Clinical Considerations for Regenerative Procedure” [Table 1] proposed by AAE states that regenerative endodontic therapy (RET) is advocated for teeth with pulp necrosis and immature apex.[13] On the basis of Cvek’s[14] classification for root development [Figure 1], following recommendations were proposed:
Table 1.
American Association of Endodontists clinical considerations for a regenerative procedure
| Case Selection: |
|---|
| • Tooth with necrotic pulp and an immature apex. |
| • Pulp space not needed for post/core, final restoration. |
| • Compliant patient/parent. |
| • Patients not allergic to medicaments and antibiotics necessary to complete procedure (ASA 1 or 2). |
| Informed Consent |
| • Two (or more) appointments. |
| • Use of antimicrobial(s). |
| • Possible adverse effects: staining of crown/root, lack of response to treatment, pain/infection. |
| • Alternatives: MTA apexification, no treatment, extraction (when deemed non salvageable). |
| • Permission to enter information into AAE database (optional). |
| First Appointment |
| • Local anesthesia, dental dam isolation and access. |
| • Copious, gentle irrigation with 1.5% NaOCl (20ml/canal, 5 min) followed by irrigation with saline (20ml/canal, 5 min), with irrigation needle positioned about 1 mm from root end |
| • Dry canals with paper points. |
| • Place calcium hydroxide or low concentration of triple antibiotic paste. If the triple antibiotic paste is used: 1) consider sealing pulp chamber with a dentin bonding agent (to minimize risk of staining) and 2) mix 1:1:1 ciprofloxacin: metronidazole: minocycline to a final concentration of 0.1 mg/ml. |
| • Deliver into canal system via syringe. |
| • If triple antibiotic is used, ensure that it remains below CEJ (minimize crown staining). |
| • Seal with 3-4 mm of a temporary material such as Cavit, IRM, glass-ionomer or another temporary material. Dismiss patient for 1-4 weeks |
| Second Appointment (1-4 weeks after 1st visit) |
| • Assess response to initial treatment. If there are signs/symptoms of persistent infection, consider additional treatment with antimicrobial, or alternative antimicrobial. |
| • Anesthesia with 3% mepivacaine without vasoconstrictor, dental dam isolation. |
| • Copious, gentle irrigation with 20ml of 17% EDTA. |
| • Dry with paper points. |
| • Create bleeding into canal system by over-instrumenting (endo file, endo explore) (induce by rotating a pre-curved K-file at 2 mm past the apical foramen with the goal of having the entire canal filled with blood to the level of cemento-enamel junction). Stop bleeding at a level that allows for 3-4 mm of restorative material. |
| • Place a resorbable matrix such as CollaPlug, Collacote, CollaTape or other material over the blood clot if necessary and white MTA/CaOH as capping material. A 3-4 mm layer of glass ionomer (e.g., Fuji IlLCTM, GC America, Alsip, IL) is flowed gently over the capping material and light-cured for 40s. Alternatives to MTA (such as bioceramics or tricalcium silicate cements [e.g., Biodentine®, Septodont, Lancasted, PA, USA, EndoSequence® BC RRM-Fast Set Putty, Brasseler, USA]) should be considered in teeth where there is an esthetic concern. |
| • Anterior and premolar teeth – Consider use of Collatape/Collaplug and restoring with 3 mm of RMGI followed by bonding a filled composite to the beveled enamel margin. |
| • Molar teeth or teeth with PFM crown – Consider use of Collatape/Collaplug and restoring with 3 mm of MTA, followed by RMGI or alloy. |
| Follow-up (6-, 12-, 24-months) |
| Clinical and Radiographic exam |
| • No pain, soft tissue swelling or sinus tract (often observed between first and second appointments). |
| • Resolution of apical radiolucency (often observed 6-12 months after treatment) |
| • Increased width of root walls (this is generally observed before apparent increase in root length and often occurs 12-24 months after treatment). |
| • Increased root length. |
| • Positive Pulp vitality test response |
| • Recommended yearly follow-up after the first 2 years |
| • CBCT is highly recommended for initial evaluation and follow-up visits |
Figure 1.
Stages of root development. A, B, C, D and E indicate stages 1, 2, 3, 4 and 5, respectively; 1–4 = immature teeth, 5 = mature teeth. Adapted from Figure 1 from Cvek,[14] p. 46
Immature permanent teeth with pulp necrosis at the stage 1 (less than 1/2 of root formation and open apex), stage 2 (1/2 root formation and open apex), and stage 3 (2/3 of root development and open apex) can be preferred for RET due to short root, thinned out canal walls and wide-open apex. Management of immature permanent teeth at stage 4 (nearly completed root formation and open apex) includes either RET or an apical MTA plug and a dense root canal filling because of adequate thickness and strength of the canal walls.
Immature permanent teeth with pulp necrosis requiring post and core restoration are not suitable for RET and are considered for apical MTA plug and root canal filling.
APICAL DIAMETER SIZE
The size of apical diameter is of concern as it can affect the outcome of REP in immature permanent teeth. A wide-open apex makes it easier for the mesenchymal stem cells to migrate into the root canal space, facilitating tissue ingrowth into the root canal space.[15,16] Highest clinical success rate was seen in apical diameters of 0.5–1.0 mm.[17]
AGE OF THE PATIENT
Young patients in the age group of 9–18 years are considered as suitable candidates for REPs because of increased potential for stem cell regeneration.[18] There is risk of impairing the eruption pattern of permanent teeth when REPs are undertaken in deciduous teeth.[17]
INSTRUMENTATION
Minimal or no filing of the canal is recommended in REPs to avoid weakening of the root canal walls.[19] The results of histologic and bacteriologic studies, however, indicate that some amount of mechanical debridement may also be required for the eradication of biofilm and for continued root maturation to occur.[19]
ROOT CANAL DISINFECTION
Effective root canal disinfection is crucial for the success of REPs because infection can interfere with stem cell activity and regeneration as well as the repair process.[19,20] The clinical considerations for REP advocate gentle irrigation with copious amounts of an effective irrigant such as sodium hypochlorite (NaOCl). Needle with closed end and side-vents, or EndoVac™, can be used to reduce the possibility of irrigant extrusion into the periapical space.[9] To minimize the damage to stem cells in the apical region, reduced concentrations of NaOCl are recommended (1.5% NaOCl [20 mL/canal, 5 min]).[21,22] This is followed by irrigation with saline or ethylenediaminetetraacetic acid (EDTA; 20 mL/canal, 5 min) placing the irrigation needle about 1 mm from root end. There was an increase in the survival of stem cells of the apical papilla and partial reversal of the destructive effects of NaOCl when 17% EDTA was used.[22] EDTA caused demineralization of dentine to release growth factors from the dentine matrix; improved the adhesion, migration, and differentiation of stem cells; and also enhanced the bonding of newly formed mineralized tissue to the walls of the canal.[23,24,25,26]
INTRA-CANAL MEDICAMENT
The use of triple antibiotic paste (TAP) or “3mix” consisting of minocycline, ciprofloxacin, and metronidazole is considered as an effective intra-canal medicament, due to its ability to eliminate all species of bacteria from the infected root canals.[9,14] The AAE protocol recommends the use of TAPs at a concentration that is not greater than 0.1 mg/mL.[13] At this concentration, TAP did not have any toxic effect on stem cell survival and proliferation.[27] AAE guidelines also advocate the use of calcium hydroxide. The stem cells of apical papilla showed greater attachment to root dentine when treated with calcium hydroxide than with TAP.[28] Intra-canal medicament is usually placed in the canal for a period of 3–4 weeks.
BLOOD CLOT OR SCAFFOLD IN CANAL
Intentional provoking of the periapical tissues and resultant bleeding into the canal space initiate the formation of blood clot, which may act as a scaffold and provide adequate growth factors. Mesenchymal stem cells from the apical papilla are also introduced into the canal space, which will encourage regeneration of pulp tissue.[15] Platelet-rich fibrin or platelet-rich plasma can also be used as a scaffold because they are rich in growth factors that might promote the regeneration of pulp tissue.[29,30]
CORONAL SEAL
According to the current clinical protocols, a piece of CollaPlug (Zimmer Dental Inc, Warsaw, IN) must be placed over the blood clot. This serves as an internal matrix for placing of about 3 mm of white MTA (Dentsply, Tulsa, OK). A 3–4 mm layer of glass ionomer is then placed over the MTA followed by composite resin restoration over the glass ionomer.[9,13] MTA is popular for its biocompatibility and antibacterial properties.[31] Biodentine can be used instead of MTA.[32]
OUTCOMES
According to clinical considerations for REPs (AAE[9]), success of REPs can be defined by evaluating the treatment outcomes:
Primary or essential goal is the elimination of clinical signs and symptoms and the evidence of bony healing.
Secondary or desirable goal is to achieve continued root maturation (increased thickness of root canal wall and/or increased root length)
Tertiary goal is the re-establishment of neurogenesis or positive response to vitality testing.
RADIOGRAPHIC OUTCOMES
Chen et al.[33] described five sorts of responses for teeth treated with REPs:
Type 1—increased canal wall thickening and continued root development
Type 2—significant root development not seen (root apex is blunt and closed)
Type 3—continued root maturation but the apical foramen remains open
Type 4—presence of severe calcification or obliteration of the canal space
Type 5—formation of hard tissue barrier in the canal between the coronal MTA and the apex of the root
HISTOLOGIC OUTCOME—REGENERATION OR REPAIR?
Regeneration and repair are two forms of healing, after tissue injury. While former restores the normal tissue architecture and biologic functions by tissue similar to the original tissue, latter replaces the damaged tissue with tissue different from the original tissue with loss of biological functions.[34] The nature of tissue formed after RET is a controversial topic. The stem cells of apical papilla have the ability to survive even after pulp necrosis or apical periodontitis.[35] They along with the growth factors can migrate into the disinfected canal space and result in regeneration of pulp–dentin complex. However, in most of the histologic studies performed in humans and animals, the tissue found in the canal space after RET appeared to be cementum-like, periodontal ligament (PDL)-like, bone-like, and not pulp-like tissue.[36,37] There are some studies that have reported the presence of pulp-like tissue after RET.[27,38,39] But in those cases, the pulp was not necrotic before the treatment and there might have been residual pulp tissue left in the canal. Therefore, based on histologic findings it can be suggested that in RET there is repair of damaged tissues and not true regeneration.
RESPONSE TO VITALITY TESTING
Histologic studies in human teeth have shown that the tissue found in the canal space after RET is cementum-like, bone-like, and not pulp-like tissue.[36,37] Vital tissues being vascularized and innervated can give a positive response to pulp sensibility testing. Hence, positive response to pulp testing after RET does not always indicate regeneration of the pulp tissue.
LIMITATIONS OF REGENERATIVE ENDODONTIC THERAPY
-
(1)
Patient compliance can be of concern due to multiple clinical appointments and duration of the treatment.
-
(2)
Crown discoloration associated with minocycline in TAP[40] and MTA (gray/white).[40]
-
(3)
Histologic evidence of repair rather than true regeneration after RET.[36,37]
-
(4)
Root fractures were seen in some of the cases after RET.[41,42]
-
(5)
Cementum, PDL, and bone ingrowth into the canal space can result in internal ankylosis.[43]
-
(6)
Intra-canal calcification has been reported after revascularization.[33]
-
(7)
Age of the patient is crucial for the success of RET. It is likelier to be successful in younger patients than older individuals.
DISCOLORATION AFTER REGENERATIVE ENDODONTIC THERAPY
The discoloration of crown after regenerative endodontic treatment is of major concern. It is usually seen with the use of TAP consisting of minocycline.[44] There are reports of discoloration with calcium hydroxide also.[45] The pulp chamber can be sealed with dentine bonding agent to avoid the staining with TAP. The TAP should also remain below the cementoenamel junction. If there is any residual paste in the pulp chamber, it should be removed and wiped with cotton pellets soaked in absolute alcohol. Double antibiotic paste consisting of ciprofloxacin and metronidazole or modified TAP in which minocycline is substituted with clarithromycin[46] or fosfomycin[32] or cefuroxime[47] or Arestin[48] or cefaclor[49] have shown to avoid the staining. Biodentine can be used instead of MTA to reduce the risk of discoloration.[50]
FUTURE
Regeneration of pulp tissue is currently based on two methods: cell-free and cell-based approaches. In cell-based approach, there is isolation and ex vivo expansion of stem cells derived from host or of allogeneic nature to be seeded in the scaffold and transplanted into the root canal for regeneration.[51,52,53] Pulp-specific stem cells such as dental pulp stem cells,[54] stem cells of exfoliated deciduous teeth,[55] and stem cells of apical papilla are used. These stem cells result in regeneration of pulp-like tissue and formation of dentin-like tissue on walls of the canal.[49,50,56] However, this method has drawbacks such as difficulties in availability, isolation and expansion of stem cells, culture, handling, cost, immune rejection, government regulatory policies, and the skill of the clinician.
The cell homing approach or the cell-free approach involves the use of biologic signaling molecules for the migration, proliferation, and differentiation of endogenous stem cells.[57] Kim et al.[58] were able to regenerate tooth-like structure using cell-homing approach.[58] The signaling molecules for pulp regeneration were considered to be growth factors such as vascular endothelial growth factor, basic fibroblast growth factor, platelet-derived growth factor, nerve growth factor, and bone morphogenetic protein 7. Even though the cell homing approach is simpler, economical, and readily performed by clinicians without special training, there is lack of knowledge regarding which growth factor(s) has to be used for REPs. Current clinical REPs can be categorized as cell-free approach.
CONCLUSION
REPs in immature teeth do result in resolution of signs and symptoms of apical periodontitis and promote further root development. However, evidence suggests that true regeneration of pulp–dentin complex does not occur after RET. It is hoped that further research in the field of stem-cell-based tissue engineering will allow for true regeneration and improved treatment outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rafter M. Apexification: a review. Dent Traumatol. 2005;21:1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 2.Huang GJ. Apexification: the beginning of its end. Int Endod J. 2009;42:855–66. doi: 10.1111/j.1365-2591.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 3.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard-Ostby B. The role of the blood in endodontic therapy. An experimental histological study. Acta Odontologica Scandinavia. 1961;19:324–53. [PubMed] [Google Scholar]
- 6.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 7.Huang GT-J, Lin LM. Letter to the editor: comments on the use of the term “revascularization”. J Endod. 2008;34:511. doi: 10.1016/j.joen.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Banchs F, Trope M. Revascularization of immat ,ure permanent tooth with apical periodontitis: new treatment protocol? Journal of Endodontics. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kling M, Cvek M, Mejare I. Rate and predictability of pulp revascularization in therapeutically reimplanted permanent incisors. Endodontics and Dental Traumatology. 1986;2:83–9. doi: 10.1111/j.1600-9657.1986.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Nygaard-Østby B, Hjortdal O. Tissue formation in the root canal following pulp removal. Scand J Dent Res. 1971;79:333–49. doi: 10.1111/j.1600-0722.1971.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves KM, Law AS. Regenerative endodontics. In: Hargreaves KM, Cohen S, editors. Cohen’s pathways of the pulp. 10th ed. St. Louis, MO: Mosby Elsevier; 2011. pp. 602–19. [Google Scholar]
- 13.American Association of Endodontists. AAE clinical considerations for a regenerative procedure. Availablefrom: www.aae.org/Dental Professional/ConsiderationsforRegenerativeProcedures.aspx .
- 14.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 15.Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–8. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010;16:3023–31. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y, Wang X, Zhu J, Su C, Yang Y, Meng L. Influence of apical diameter on the outcome of regenerative endodontic treatment in teeth with pulp necrosis: a review. J Endod. 2018;44:414–31. doi: 10.1016/j.joen.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Estefan BS, El Batouty KM, Nagy MM, Diogenes A. Influence of age and apical diameter on the success of endodontic regeneration procedures. J Endod. 2016;42:1620–5. doi: 10.1016/j.joen.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D. Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod. 2014;40:291–5. doi: 10.1016/j.joen.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Fouad AF, Nosrat A. Pulp regeneration in previously infected root canal space. Endod Topics. 2013;28:24–37. [Google Scholar]
- 21.Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–15. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Martin DE, De Almeida JF, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–5. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Galler KM, Buchalla W, Hiller KA, Federlin M, Eidt A, Schiefersteiner M, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41:363–8. doi: 10.1016/j.joen.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–41. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, et al. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J. 2016;49:581–90. doi: 10.1111/iej.12492. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi N, Yamauchi S, Nagaoka H, Duggan D, Zhong S, Lee SM, et al. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod. 2011;37:390–7. doi: 10.1016/j.joen.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013;39:138–44. doi: 10.1016/j.joen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Kitikuson P, Srisuwan T. Attachment ability of human apical papilla cells to root dentin surfaces treated with either 3mix or calcium hydroxide. J Endod. 2016;42:89–94. doi: 10.1016/j.joen.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod. 2011;37:265–8. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Bakhtiar H, Esmaeili S, Fakhr Tabatabayi S, Ellini MR, Nekoofar MH, Dummer PM. Second-generation platelet concentrate (platelet-rich fibrin) as a scaffold in regenerative endodontics: a case series. J Endod. 2017;43:401–8. doi: 10.1016/j.joen.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review–part II: leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Yoldaş SE, Bani M, Atabek D, Bodur H. Comparison of the potential discoloration effect of bioaggregate, biodentine, and white mineral trioxide aggregate on bovine teeth: in vitro research. J Endod. 2016;42:1815–8. doi: 10.1016/j.joen.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45:294–305. doi: 10.1111/j.1365-2591.2011.01978.x. [DOI] [PubMed] [Google Scholar]
- 34.Majno G, Joris I. Cells, tissues, and disease. 2nd ed. Oxford, London, UK: Oxford University Press; 2004. [Google Scholar]
- 35.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torabinejad M, Faras H, Corr R, Wright KR, Shabahang S. Histologic examinations of teeth treated with 2 scaffolds: a pilot animal investigation. J Endod. 2014;40:515–20. doi: 10.1016/j.joen.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Nosrat A, Kolahdouzan A, Hosseini F, Mehrizi EA, Verma P, Torabinejad M. Histologic outcomes of uninfected human immature teeth treated with regenerative endodontics: 2 case reports. J Endod. 2015;41:1725–9. doi: 10.1016/j.joen.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endod. 2012;38:864–8. doi: 10.1016/j.joen.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu E, Jong G, Partridge N, Rosenberg PA, Lin LM. Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod. 2012;38:1293–7. doi: 10.1016/j.joen.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010;36:536–41. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu E, Ricucci D, Albert J, Alobaid AS, Gibbs JL, Huang GT, et al. Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J Endod. 2013;39:1078–83. doi: 10.1016/j.joen.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Saoud TM, Mistry S, Kahler B, Sigurdsson A, Lin LM. Regenerative endodontic procedures for traumatized teeth after horizontal root fracture, avulsion, and perforating root resorption. J Endod. 2016;42:1476–82. doi: 10.1016/j.joen.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Andreasen JO, Bakland LK. Pulp regeneration after non infected and infected necrosis, what type of tissue do we want? A review. Dent Traumatol. 2012;28:13–8. doi: 10.1111/j.1600-9657.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36:1086–91. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 45.Nagata JY, Soares AJ, Souza-Filho FJ, Zaia AA, Ferraz CC, Almeida JF, et al. Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod. 2014;40:778–83. doi: 10.1016/j.joen.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 46.Mandras N, Roana J, Allizond V, Pasqualini D, Crosasso P, Burlando M, et al. Antibacterial efficacy and drug-induced tooth discolouration of antibiotic combinations for endodontic regenerative procedures. Int J Immunopathol Pharmacol. 2013;26:557–63. doi: 10.1177/039463201302600232. [DOI] [PubMed] [Google Scholar]
- 47.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45:942–9. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 48.Krastl G, Allgayer N, Lenherr P, Filippi A, Taneja P, Weiger R. Tooth discoloration induced by endodontic materials: a literature review. Dent Traumatol. 2013;29:2–7. doi: 10.1111/j.1600-9657.2012.01141.x. [DOI] [PubMed] [Google Scholar]
- 49.Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47–50. [PubMed] [Google Scholar]
- 50.Marconyak LJ, Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42:470–3. doi: 10.1016/j.joen.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, et al. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2013;2:521–33. doi: 10.5966/sctm.2012-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCS) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SG, Zhou J, Solomon C, Zheng Y, Suzuki T, Chen M, et al. Effects of growth factors on dental stem/progenitor cells. Dent Clin North Am. 2012;56:563–75. doi: 10.1016/j.cden.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89:842–7. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]