ABSTRACT
Aim and Objective:
The aim of the study was to assess the efficacy of probiotic and green tea mouthrinse on salivary pH.
Materials and Methods:
This study was conducted over a period of 1 month among 40 healthy schoolchildren aged between 6 and 8 years. The subjects who fulfilled inclusion criteria were selected and randomly divided into two groups, namely probiotic and green tea groups. Salivary pH was recorded at baseline (0 day) and at the end of the specified time using GC pH strips. Statistical analysis was done using paired t test. P < 0.05 was considered statistically significant.
Result:
The comparison of mean pH scores for green tea showed that the pH of saliva was increased in the children after rinsing with green tea (6.00–7.60) and was statistically significant (P < 0.001). Similarly, when pre- and post-mean pH was compared in the probiotic group, the pH was found to be higher in the probiotic rinse group (5.60–7.20). The results were statistically significant.
Conclusion:
The study conducted shows the beneficial effects of green tea in providing a alkaline environment, which is conducive to the oral health of children.
KEYWORDS: Dental caries, green tea, periodontal disease, probiotic, salivary pH
INTRODUCTION
Periodontal disease is characterized by complex host–parasite interactions that lead to gingival inflammation, loss of connective tissue attachment, periodontal ligament destruction, and alveolar bone resorption. It is the second most common oral disease next to dental caries, affecting 5%–30% of adult population.[1] Interaction of bacteria, diet, and host response play a major role in initiation and progression of dental caries. Saliva plays a critical role in the maintenance of optimal oral health, and thus creates an appropriate ecological balance.[1] Various components of saliva play individual roles in the properties of saliva. Salivary flow, pH, and buffering capacity play an important role in the initiation and progression of periodontal disease and dental caries. However, among these components, pH of saliva is an important component to maintain the integrity of oral cavity.[2] When the pH increases, the remineralization of tooth surface occurs because of the degree of supersaturation. The acidic pH can cause maximum incidence of dental caries. It has been well-documented that the dissolution of enamel occurs when the pH falls below critical pH of 5.5.[3] Recently, various economical and efficacious agents have been tried out in combating dental caries. Among these, green tea and probiotics are also being used. The biological properties of polyphenolic compounds found in plant foods include antioxidants and anti-inflammatory effects.[4] Green tea contains a significant amount of catechins, which are subgroup of flavonoids and play a vital role in maintaining health. Major catechins are epicatechin gallate, epicatechin, gallocatechin (GC), epigallocatechin (EGC), and EGC gallate (EGCG). GC, EGC, and EGCG possess strong bactericidal and antibacterial activity.[5] Probiotics, on the contrary, are useful microorganisms, which when administered in adequate amounts confer beneficial effects on the health of host. Commonly used probiotic strains are Lactobacillus rhamnosus GG, Lactobacillus casei, Lactobacillus reuteri, Lactobacillus plantarum, Lactobacillus brevis CD2, and Bifidobacterium species.[6] Probiotics in dentistry have proved to inhibit pathogenic biofilm formation, induction of cytoprotective proteins, reduction of inflammation, stimulation of the host immune response, and killing of pathogens through production of bacteriocins along with altering the local environment pH.[7] Hence, this study was undertaken to clinically evaluate the effect of oral use of commercially available probiotic preparation and green tea mouthrinse on salivary pH and to ascertain the efficacy of oral probiotic and green tea as a preventive tool against the development of caries.
MATERIALS AND METHODS
A purposive sample of 60 schoolchildren aged 6–8 years was included in the study. The study was conducted over a period of 1 month and was carried out at a school in Dharmapuri, Tamil Nadu, India. Informed oral and written consent was obtained from the parents of the children. Permission was sought from the principal of the respective schools. Ethical consent was obtained from the institutional ethics committee.
Inclusive criteria
Healthy children aged between 6 and 8 years without any known systemic condition
Children with written consent from their parents
Children with no active carious lesions
No history of use of antimicrobial agents or any other drugs (up to within 4 weeks)
Exclusive criteria
Children with a known history of allergy to any mouthrinse or drug
Children with any systemic illness
Children using any other commercially available probiotic products
Children using any other oral hygiene aids other than routine teeth brushing
The participants who fulfilled the inclusion criteria were selected and randomly divided into two groups, with 30 children in each group.
Group A: Probiotic group
Group B: Green tea group
Preparation of probiotic mouthrinse
Probiotic mouthrinse was prepared using commercially available probiotic product Darolac; 1g Darolac powder contains 1.25 billion freeze-dried bacterial combination comprising Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, and Saccharomyces boulardii. The sachet contents were dissolved in 10 mL of distilled water, which was used as a mouthrinse.[8]
Preparation of green tea mouthrinse
Fresh green tea (Lipton green tea bags, packing date <1 month) was procured from the local market, which is available in the form of green tea dip bags. Two percent green tea was prepared with 2g of green tea dip bag dipped in 100 mL warm water for 5 min (10 mL for each participant).[9]
Salivary pH measurements
A volume of 2 mL saliva samples was collected before the commencement of mouth rinsing, baseline, and after 1 month rinsing. Unstimulated whole saliva samples were collected using Navazesh[10] spitting method by pooling saliva for 60s and then spitting in a disposable container sitting in an upright position in a well-lit room with good ventilation. Saliva was collected in the morning between 10.00 AM and 10.30 AM to prevent any bias in the concentration of saliva due to circadian rhythm.[11] Children were also informed not to eat or drink anything (except water) 1h before saliva collection to minimize possible food debris and stimulation of saliva. Salivary pH was measured using GC pH strips. A single sheet was dipped into the saliva till it is fully wet and removed immediately. After 30s, the acid produced reacts to these pH indicators, thus leading to colorimetric change, which was compared with color code chart, and the pH value was noted.[12] After the baseline recording of salivary pH, the designated mouthrinse was dispensed to the respective groups. Group A received probiotic mouthrinse and Group B received green tea mouthrinse, and they were instructed to swish the 10 mL of mouthrinse for 1 min for 1 month between 10 AM and 11 AM, and salivary pH was measured using GC pH strips. Statistical analysis was done using paired t test. P < 0.05 was considered statistically significant.
RESULTS
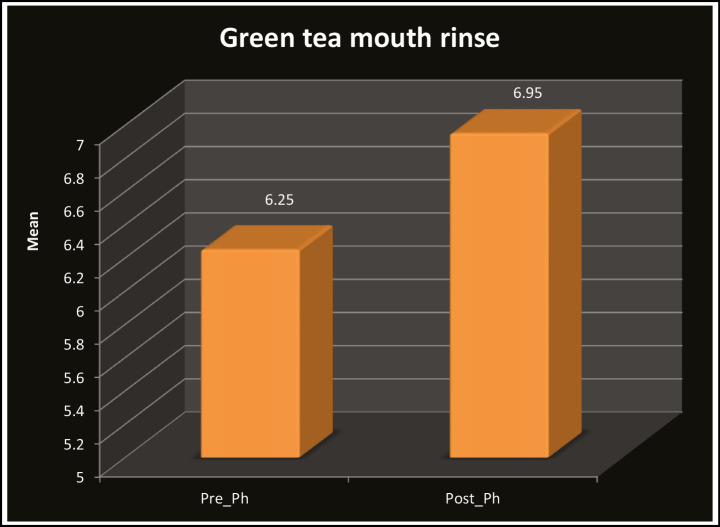
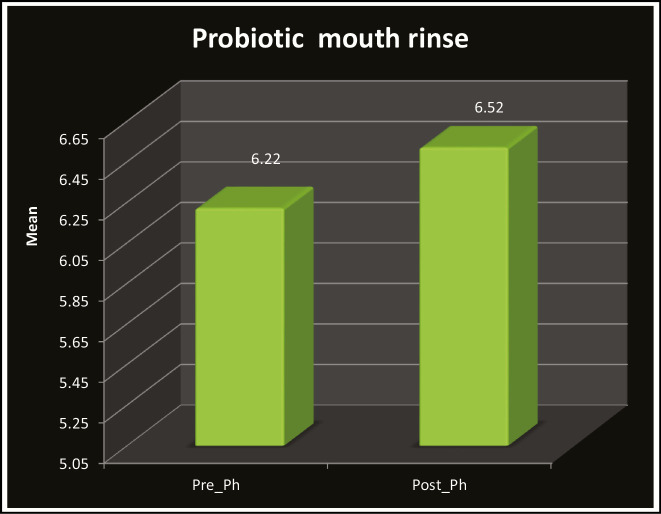
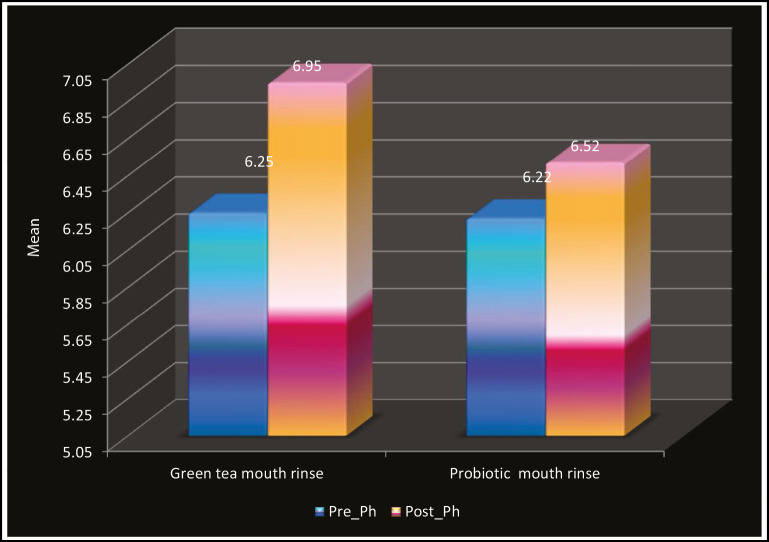
Table 1 and Graph 1 show the comparison of mean pH scores for green tea, which revealed that the pH of saliva was increased in the children after rinsing with green tea (6.00–7.60) and was statistically significant (P < 0.001). Table 2 and Graph 2 show the comparison of mean pH scores for probiotic, which revealed that the pH of saliva was increased in the children after rinsing with probiotic (5.60–7.20) and was statistically significant (P < 0.001). Similarly, when pre- and post-mean pH was compared in the green tea (6.95) and probiotic group (6.52), it was found that pH was higher in the green tea rinse group as seen in Table 3 and Graph 3. The results were statistically significant.
Table 1.
Distribution of pre and post salivary PH in green tea mouth rinse
| N | Green tea mouthrinse | Paired t | P | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | ||||
| Pre-pH | 30 | 5.10 | 6.90 | 6.25 | 0.41 | 18.68 | 0.001** |
| Post-pH | 30 | 6.00 | 7.60 | 6.95 | 0.42 | ||
SD = standard deviation
**Highly significant
Graph 1.
Distribution of pre and post salivary PH in green tea mouth rinse
Table 2.
Distribution of pre and post salivary PH in probiotic mouth rinse group
| N | Probiotic mouthrinse | Paired t | P | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | ||||
| Pre-pH | 30 | 5.10 | 6.80 | 6.22 | 0.39 | 11.80 | 0.001** |
| Post-pH | 30 | 5.60 | 7.20 | 6.52 | 0.37 | ||
SD = standard deviation
**Highly significant
Graph 2.
Distribution of pre and post salivary PH in probiotic mouth rinse group
Table 3.
Comparison of pre and post salivary PH in green tea and probiotic mouth rinse group
| Mouthrinse | N | Mean | SD | SE | Independent t | P | |
|---|---|---|---|---|---|---|---|
| Pre-pH | Green tea mouthrinse | 30 | 6.25 | 0.41 | 0.08 | 0.32 | 0.750 |
| Probiotic mouthrinse | 30 | 6.22 | 0.39 | 0.07 | |||
| Post-pH | Green tea mouthrinse | 30 | 6.95 | 0.42 | 0.08 | 4.276 | 0.001** |
| Probiotic mouthrinse | 30 | 6.52 | 0.37 | 0.07 |
SD = standard deviation, SE = standard error
**Highly significant
Graph 3.
Comparison of pre and post salivary PH in green tea and probiotic mouth rinse group
DISCUSSION
The purpose of this study was to evaluate the effect of green tea and probiotics as mouthrinse on salivary pH in children. Salivary pH is one of the key indicators of carious process.[3] The average pH of saliva at rest is 6.8, and it varies between 6.5 and 7.0. Research suggests that it is pH, rather than sugar, which is the selective factor for caries initiation and progression. When the salivary pH is low, it promotes the growth of aciduric bacteria, which allows the acidogenic bacteria to proliferate, thereby creating an inhospitable environment for the protective oral bacteria. This allows for a shift in the environmental balance in favor of cariogenic bacteria, which further lowers the salivary pH. By controlling pH, it is possible to alter the plaque biofilms, remineralize the existing lesions, and perhaps prevent the disease altogether.[1] Baseline mean pH for green tea was 6.15, and after rinsing with green tea mouthrinse for 1 month, the salivary pH increased to 7.65. The increase in salivary pH after rinsing with green tea and black tea was also observed in a study by Srinidhi et al.,[13] and the pH rise was more in green tea intake compared to that in black tea. In this study, it was observed that green tea mouthrinse showed statistically significant results in maintaining the alkalinity of saliva compared with the baseline (P < 0.001). Studies have shown that consumption of tea reduced caries development, and the effect is attributed to its fluoride content, polyphenols, and catechin. The catechins present in green tea represent a marked effect on the pH of saliva and dental plaque. Xu et al.,[14] in their detailed study, determined the effect of EGCG on acid production by S. mutans by monitoring the glycolytic pH drop of Streptococcus mutans culture. The acid production by S. mutans cells was significantly inhibited by EGCG at subminimum inhibitory concentrations levels.[14] A study conducted by Hamilton-Miller[15] concluded that rinsing with green tea catechins for a suitable time prevents acid production and preserves pH within the normal range (7.2–7.4), which is not a favorable condition for the growth of Streptococcus mutans, and he stated that green tea possesses anticariogenic and antibacterial properties. Hirasawa et al.[16] evaluated plaque pH value at different time intervals before and after rinsing with 2% green tea for 5 min and found that the pH values were significantly higher after treatment with catechins. From this study, it was observed that in the probiotic mouthrinse group, salivary pH was increased compared to baseline, but the result was not statistically significant. A study conducted by Jindal et al.[17] also reported a statistically significant reduction in Streptococcus mutans count in saliva when probiotic powder containing combination of bacteria was used as mouthrinse for 14 days. Similarly, in this study, probiotic powder containing combination of bacteria was used as mouthrinse, and the alkalinity of saliva was maintained compared to the baseline. In this study, a patient-specific approach using GC pH strips was used to assess the salivary pH, which aids as an effective educational tool.[18] In this study, the lesser sample size was the drawback. Further, long-term clinical studies are required to evaluate the efficacy of probiotic and green tea mouthrinse on salivary pH along with microbiological evaluation.
CONCLUSION
The study conducted shows beneficial effects of green tea and probiotic mouthrinse in providing a alkaline environment, which is conducive to oral health of children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hurlbutt M, Novy B, Young D. Dental caries: a pH-mediated disease. CA Dent Hyg J. 2010;25:9–15. [Google Scholar]
- 2.Young WG. The oral medicine of tooth wear. Aust Dent J. 2001;46:236–50. doi: 10.1111/j.1834-7819.2001.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 3.Shetty C, Hegde MN, Devadiga D. Correlation between dental caries with salivary flow, pH, and buffering capacity in adult South Indian population: an in-vivo study. Int J Res Ayurveda Pharm. 2013;4:219–23. [Google Scholar]
- 4.Groppo FC, Bergamaschi Cde C, Cogo K, Franz-Montan M, Motta RH, de Andrade ED. Use of phytotherapy in dentistry. Phytother Res. 2008;22:993–8. doi: 10.1002/ptr.2471. [DOI] [PubMed] [Google Scholar]
- 5.Goenka P, Sarawgi A, Karun V, Nigam AG, Dutta S, Marwah N. Camellia sinensis (tea): implications and role in preventing dental decay. Pharmacogn Rev. 2013;7:152–6. doi: 10.4103/0973-7847.120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G. The use of probiotic strains in caries prevention: a systematic review. Nutrients. 2013;5:2530–50. doi: 10.3390/nu5072530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal V, Kapoor S, Shah N. Role of “live microorganisms” (probiotics) in prevention of caries: going on the natural way towards oral health. Ind J Multidiscip Dent. 2012;2:491–6. [Google Scholar]
- 8.Thakkar PK, Imranulla M, Naveen Kumar PG, Prashant GM, Sakeenabi B, Sushanth VH. Effect of probiotic mouthrinse on dental plaque accumulation: a randomized controlled trial. Dent Med Res. 2013;1:7–12. [Google Scholar]
- 9.Neturi RS, Srinivas R, Simha VB, Sree SY, Shekhar CT, Kumar SP. Effects of green tea on Streptococcus mutans counts—a randomised control trail. J Clin Diagn Res. 2014;8:ZC128–30. doi: 10.7860/JCDR/2014/10963.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 11.Jain I, Jain P. Comparative evaluation of antimicrobial efficacy of three different formulations of mouth rinses with multi-herbal mouth rinse. J Indian Soc Pedod Prev Dent. 2016;34:315–23. doi: 10.4103/0970-4388.191409. [DOI] [PubMed] [Google Scholar]
- 12.Coulter C, Walsh LJ. Saliva testing: good practice, good sense. Stepwise. 2006;1:32–5. [Google Scholar]
- 13.Srinidhi PB, Basha S, Naveen Kumar P, Prashant GM, Sushanth VH, Imranulla M. Effect of two different commercially available tea products on salivary pH: a randomized double blinded concurrent parallel study. Dent Med Res. 2014;2:39–42. [Google Scholar]
- 14.Xu X, Zhou XD, Wu CD. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother. 2011;55:1229–36. doi: 10.1128/AAC.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton-Miller JMT. Anti-cariogenic properties of tea (Camellia sinensis) J Med Microbiol. 2001;50:299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 16.Hirasawa M, Takada K, Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Res. 2006;40:265–70. doi: 10.1159/000092236. [DOI] [PubMed] [Google Scholar]
- 17.Jindal G, Pandey RK, Agarwal J, Singh M. A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Eur Arch Paediatr Dent. 2011;12:211–5. doi: 10.1007/BF03262809. [DOI] [PubMed] [Google Scholar]
- 18.Tayab T, Rai K, Kumari V, Thomas E. Effect of chewing paneer and cheese on salivary acidogenicity: a comparative study. Int J Clin Pediatr Dent. 2012;5:20–4. doi: 10.5005/jp-journals-10005-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]