ABSTRACT
Exosomes are a type of extracellular vesicles, released from different tissues in a living individual. By virtue of their ability to be released from both the normal and diseased individual, they play an inevitable role in the diagnosis, prognosis, and therapeutic aspect of a disease. With this background, the untapped role of exosomes in the field of oral and maxillofacial region is unveiled.
KEYWORDS: Biomarkers, exosomes, extracellular vesicles, oral and maxillofacial region, therapeutics
INTRODUCTION
Human body constitutes amalgamation of tissues and organs, which are characteristically different. Communication between these cell types is by means of signal transmission, being favored by factors such as cytokines and chemokines and exosomes. Exosomes are naturally occurring nanovesicles, which can play an inevitable role in communication between the cells and the cellular function regulation under normal physiological conditions. Under unfavorable physiological conditions, the amount of exosomes secreted and their composition changes due to the alteration in cell environment, thereby increasing the intensity of the disease process by invading the immune system of the host, aggravating inflammation, mediating cancer metastasis, and more. After the demonstration of the existence of nucleic acids such as messenger ribonucleic acid (mRNA) and microRNA (miRNA), proteins, and gene products within the exosomes, which are responsible for transmitting the information between cells, the research on exosomes gained an increased momentum.[1] To overcome the limitations of treatment with stem-cell therapy, the emergence of cell-free therapy using microvesicles is rapidly evolving.
HISTORY OF EXOSOMES
Rose Johnstone in 1970 was the first to coin the term “exosomes,” which were found from sheep reticulocytes. The term “exosomes” are designated to the extracellular vesicles (EVs) released from cells by fusion of the multivesicular body (MVB) with the plasma membrane. This fusion frees intraluminal vesicles (ILVs) into the extracellular environment and these liberated particles are termed exosomes. Earlier these tiny particles were thought to be waste products liberated through shedding via plasma membrane. EVs including apoptotic bodies (50–5000 nm), microvesicles (50–1000 nm), and exosomes (30–150 nm) are of different dimensions as indicated.[2] Although the existence of extracellular membranous vesicles, which were considered as a cellular disposal of waste, was known from the past 50 years, the importance of exosomes came to light only in the past 30 years.
The increasing interest toward the research in exosomes is mainly because of
Their source of intercellular communication and intracellular mediation of macromolecules.
Their role in contribution toward the progression of several diseases pertaining to their role in transmission of lipids, proteins, mRNA, miRNA and deoxyribonucleic acid (DNA).
Their proposed role as drug vectors, as they have a cell membrane, which aids in better tolerance by the host unlike synthetic polymers
Their ability of not invoking an immune response, thereby achieving lesser chance of tissue rejection and lower risk of tumorigenesis, favors the recent explosion of research in this field.
Their role in physiological condition and their effectiveness in tissue repair and regeneration.
FORMATION AND ISOLATION OF EXOSOMES
Exosomes differ from other variants of EVs in their biogenesis. Although microvesicles are formed from budding of the cell membrane, exosomes are the result of endosomal plasma membrane invagination during the process of endosomal maturation from early to late endosomes. These late endosomes, also known as the MVBs, contain a population of ILVs that are called exosomes when released. MVBs are either transported to the cell membrane, with which they fuse and release their contents to the extracellular environment or are transported to a lysozyme and are digested.
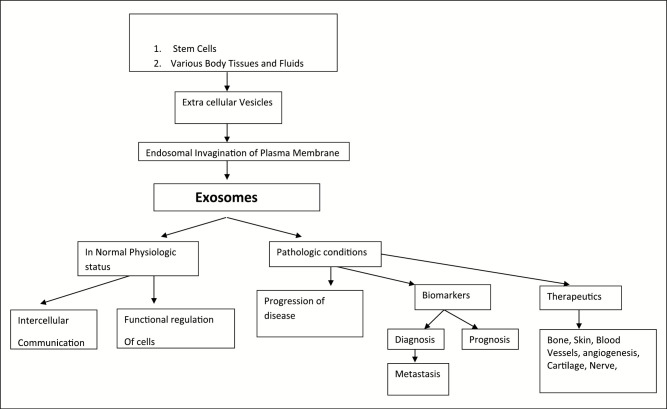
The cholesterol-rich MVB fuses with plasma membrane to release exosomes. Formation of exosomes from MVBs occurs by two identified pathways: “Endosomal Sorting Complex Required for Transport (ESCRT)-dependent mechanism” and “ESCRT-independent mechanism.”[3] The identification of exosomes is by means of their characteristic saucer-like morphology––a flattened sphere that is limited by a lipid bilayer. The exosomes, though derived from different cell types, share their common characteristic structure. In addition to the composition of exosomes that include proteins, cytokines, lipid rafts, miRNA, mRNA, and small noncoding RNAs, rRNAs, tRNAs, and occasionally DNAs, their configuration is determined by cell type of origin and they perform various functions based on origin cell-specific proteins.[3] Exosomes can be isolated from diverse body fluids including saliva, blood, urine, breast milk, semen, cerebrospinal fluid, amniotic fluid, ascetic fluid, and bile.[4] The level of exosomes secreted by cancer cells is greater than normal levels that play an inevitable role in diagnosis, development, and treatment of some cancers. Thus, the estimation of the level of exosomes in patient’s body fluids and their level as cargos presents as a potential tool in diagnosis of disease or progression of disease, therapeutic response, and overall survival. Their ability to be isolated from various body fluids makes them as a suitable candidate to play a role in liquid biopsy, which is noninvasive [Figure 1].
Figure 1.
Schematic representation of origin of exosomes and their function
The widely used method of exosome separation involves a series of centrifugations, often combining with sucrose gradient ultracentrifugation, which contributes to enrichment of exosomes to a higher level. The isolation can also be carried out by means of ultrafiltration based on size and also by means of high-performance liquid chromatography (HPLC)-based protocol, which results in exosome pellet, which is highly pure. The isolation of exosomes can also be carried out based on their physical properties such as size and density or based on their function. The various parameters in the process of cell culture influence the exosome cargo composition, which includes the following:[5]
Culture containers––the amount of exosomes yield from two-chamber bioreactors is amounted to be 100 times more than those from culture dishes.
Protocol for cell seeding to ensure constant reproducibility and consistency in the levels of exosomes and cargos in the isolated exosomes.
The medium used for culturing cells and the recovery of exosomes from the supernatants play an inevitable role in exosome production. The glucose levels in culture media, antibiotics, and medium components such as FBS (fetal bovine serum), which are highly protein enriched and medium components with growth factors such as epidermal growth factor (EGF) or an inhibitor of epidermal growth factor receptor (EGFR), play an important role in exosome production.
Hypoxia is a major environmental factor, which influences the production of exosomes. There will be a level of elevated secretion of exosomes under the conditions of hypoxia and it also alters the exosome cargo by bringing a change in the status of oxygenation of the cells producing exosomes.
Drugs such as forskolin, sitafloxacin, fenoterol, nitrefazole, SB218795, and pentetrazole can act as activators in the production of exosomes.
Oxidative stress and hyperthermia also play a significant role in the production of exosome and their molecular content.
Exosomes can be stored for a period of 6 months at –20°C or even lower without cryopreservative agents, without changes in size and structure of the exosomes.[6]
EXOSOMES IN HEAD AND NECK CANCER
Head and neck cancer, being the fifth most common cancer in the world, mandates an early diagnosis and is vital to reduce the mortality rate. This can be achieved by expanding the area of research on diagnostic and prognostic biomarkers to intervene in the metastatic cascade. The content of tumor-derived Exosomes (TDEs) is similar to the parental tumor cells and is shown as a part in nearly all stages of the disease. Evidence suggests that by means of intracellular communication and signal transduction TDEs can play a prominent role in the development, progression, diagnosis, and treatment of cancer. The presence of bioactive components, such as transcription factors, miRNAs, and oncogenic proteins, plays a vital role in tumorigenesis, reprogramming tumor microenvironment, immune tolerance, facilitating metastasis, and resistance to therapy. Unlike exosomes derived from healthy cells, the composition of TDEs differs in a way that they facilitate functional cargo delivery, which includes oncogenes and oncogenic proteins, which can exert biological activities. The cancer progression and metastasis is mainly mediated by the TDEs in the tumor environment owing to the presence of protein, RNA, and DNA cargo.[7] The genetic information in the tumor progression is facilitated by the abundant presence of miRNAs, mRNA, long noncoding RNA, and DNA.[8,9] The presence of protein cargoes including different oncoproteins, growth factors, and immunomodulatory molecules acts as the tumorigenesis mediators. The exosome suppresses tumor immunesurveillance by inhibiting the function of the immune cells, downregulating the activity of regulatory T cells (Tregs) and mesenchymal dental stem cells (MDSCs), interfering with the differentiation of dendritic cells (DCs), and polarization of (tumor-associated macrophages) TAMs. Exosomes derived from head and neck squamous cell carcinoma (HNSCC) promotes metastasis by increasing the proliferation of endothelial cells, epithelial mesenchymal transition (EMT), cancer associate fibroblasts (CAFs), and facilitating angiogenesis as well as stromal compartment reprogramming.[7] Thus, the HNSCC-derived exosomes play a significant role in tumor progression, thereby enhancing cancer cell growth, their invasive behavior, and their development of resistance to therapy.
Therapeutic application of exosomes in HNSCC can be achieved by anchoring nanoparticles to the EVs, which aids in suppressing the communication between tumor and recipient cells. This can be achieved by various therapeutic agents such as proteins, small interfering RNA (siRNAs), miRNAs, and targeted drugs, which can be incorporated into exosomes by means of chemical-based transfection, electropolation, modification of parental cells, and also by direct incubation, which results in enhanced bioactivity and to achieve the targeted delivery in patients.
In addition to influencing the local tumor environment favoring cancer proliferation, exosomes originating from tumor cells are capable of facilitating metastasis to other organs, which is a multistep process. Exosomes play an inevitable role in the different steps of metastasis, both in progression and suppression. The exosomes separated from oral fluids in patients with oral carcinoma present differential patterns of CD9, CD69, and CD 61. There is a significantly reduced expression of CD9 in the case of metastasis to lymph node in tumors that are clinically aggressive and also in cases of malignant transformation that is microscopically diagnosed a dysplastic. The marked reduction in the expression of CD81 and CD9, even though there is a questionable rise in the CD63 expression, also appears to be an indicator of oral cancer, even in the initial disease stage.[10] Hence, the role of exosomes as biomarkers and a as therapeutic tool in HNSCC will be promising.
THERAPEUTIC APPLICATIONS OF EXOSOMES IN ORAL AND MAXILLOFACIAL REGION
Exosomes by their means of cell communication play an important role in various physiological and pathological processes. They can be an ideal biomarker for diagnosing diseases and targeted therapy as they bear the close resemblance to their parental cells, are stable in circulation, and their ease of collection from the body fluids. The structural changes, contents, and morphology of exosomes in diseases such as oral cancer, oral lichen planus, hand foot mouth disease, and Sjogren’s syndrome indicate that the characteristic aberrations in exosomes are mainly due to the change in individual’s pathophysiological condition. The oral disease progression can be influenced by means of the transmitting exosome contents, which might be characteristic to each disease process and thereby resulting in the progression of oral diseases by means of activating signaling pathways. Due to an increase in requirements of tissue regeneration in the oral and maxillofacial region, there is a rapid progression in the field of exosome- mediated tissue regeneration process thereby addressing the need to fulfil the requirements such as repairing or reconstruction of bone in areas of large defects, bone grafts for implant and periodontal surgery, and finally to restore the vitality and functionality of the tooth, lost due to dental caries. The present treatment strategies such as auto and allografts, bone regenerative growth factors, have their own limitations such as morbidity at the donor site, lower osteogenic capacity, and biosecurity concerns.[11]
Therapeutic potential of exosomes from dental pulp stem cells
Exosomes can be harvested from various tissues from the body. They are found in abundance in mesenchymal stem cells (MSCs) from bone marrow and fat tissue. Owing to the inevitable trauma encountered in the procedure for acquisition, dental pulp stem cells (DPSC) can be an alternate source of exosomes, which will be readily and easily accessible. A large number of studies indicate that T-helper 17 cell (Th17)/Treg imbalance is the prime etiological source for the development of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, graft versus host disease, and multiple sclerosis. Exosomes derived from DPSC can mitigate Th17/Treg imbalance in vitro and are more effective when compared with that of bone marrow mesenchymal stem (BMMSC)-derived exosomes. Transcription factor forkhead box P3 (FOXP3) maintains the Treg activity and the transcription factor retinoic acid related orphan receptor C gene has been identified as the prime regulator for Th17 lineage. The regulation of these transcription factors can be influenced by the exosomes derived from both BMMSC and DPSC, the latter being more powerful. The imbalance of proliferation and apoptosis of lymphocytes is also a key factor in the pathogenesis of autoimmune disease and the features of the disease weakens, once the phenomenon restores to normalcy. The immunomodulatory effects of exosomes obtained from DPSCs are stronger than those obtained from BMMSCs. This characteristic of DPSC-derived exosomes can play a significant role in cell-free therapy for various autoimmune diseases.[12]
Recent studies indicate that there is an increased expression of interleukin-6 (IL-6) and IL-8, the inflammatory cytokines, suggesting the strong involvement of inflammation in the progression of temporomandibular joint osteoarthritis (TMJOA). Pathological changes in chondrocytes are the result of an increased expression of inflammatory factors, and thus catabolic enzymes are upregulated in the cartilage matrix. Elevated production of matrix-degrading proteases including “MMPs (matrix metalloproteinases)” and “ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs)” results from metabolic homeostasis disruption and leads to inflammation resulting in cartilage degradation. The major pro-inflammatory cytokine, IL-1β, is involved in the destructive effects by means of an increase in the cartilage degradation and suppressing synthesis of cartilage matrix. Numerous treatment strategies are available for relieving inflammation and protecting the joint complex degradation. However, there is no definite treatment strategy for repair and regeneration of the damaged TMJ. Recently, much attention is diverted to treatment using MSC-derived exosomes. MSC-derived exosomes are known to play a significant part in the repair of damage to the tissue. “Stem Cells from Human Exfoliated deciduous tooth (SHEDs)” have advantages such as fewer ethical controversies, ready source of accessibility, collection which will be easy and invasive minimally, and high stem cell potential retention such as multipotency, cell proliferation, and immunomodulatory functions when compared to other types of MSCs. In isolates, the presence of SHED exosomes is determined using transmission electron microscopy, nanoparticle tracking analysis, and Western blotting. Exosome obtained from SHED suppresses pro-inflammatory cytokine expression in chondrocyte and on enrichment with miR-100, it suppresses inflammation by repression of (mTOR) mammalian target of rapamycin, thereby making it suitable as a treatment modality in the management of TMJOA.[13]
Exosomes from the dental pulp MSCs play a paramount role in the regenerative process; the future approach of pulp regeneration might include a conditioned medium with the exosomes onto a suitable scaffold.[14]
Exosomes from DPSCs promote the odontogenic differentiation in the recipient when cultured under conditions of odontogenic differentiation by means of activation of “MAPK (P38 mitogen activated protein kinase)” pathway. The differentiation and mineralization to cementoblasts is promoted by conditioned medium using odontoblasts. The multipotency of human DPSCs is maintained by the EVs released by the Schwann cells identified in the dental pulp.[15]
The therapeutic efficiency of exosomes is attributed by means of their characteristics such as presence of non-synthetic lipid bilayer, flexible smaller size, stability in various biological fluids, and superior biosafety profile contributed by their endogenous nature.[16]
CONCLUSION
Exosomes play a diverse role and are a vital mediators in inter cellular communication. They are present in significant amount in several body fluids. Various characteristics of exosomes, such as marked presence in various tissues both in healthy and diseased individuals, anti-inflammatory effect, immunomodulatory effect, ability to present themselves as a biomarkers in the diagnosis and prognosis of a disease process, and acting as a drug vector thereby enhancing the therapeutic efficiency, make them as a promising tool in the diagnosis, prognosis, and therapeutic aspects of various disease process. Dental surgeons have an excellent opportunity in the field of regenerative dentistry and can play an inevitable role in the therapeutic application using exosomes. Though the exosome isolation, culture characterization, and application are quite challenging, the rapidly progressing field of research can surpass these limitations, thereby making exosomes as an inevitable tool in all aspects of medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avcı E, Balci-Peynircioglu B. An overview of exosomes: from biology to emerging roles in immune response. Acta Medica. 2016;5:2–10. [Google Scholar]
- 4.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 2019;20:4684. doi: 10.3390/ijms20194684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharm Bull. 2016;6:293–9. doi: 10.15171/apb.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao C, Song F, Zheng YL, Lv J, Wang QF, Xu N. Exosomes in head and neck squamous cell carcinoma. Front Oncol. 2019;9:894. doi: 10.3389/fonc.2019.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted mir-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microrna biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and molecular features of oral fluid-derived exosomes: oral cancer patients versus healthy individuals. J Cancer Res Clin Oncol. 2016;142:101–10. doi: 10.1007/s00432-015-2005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi-Qin WA, Hui-Zhen HU, Gui-Jun SU, Jia-Jia JI, Xiang-Yi HE. The roles of exosomes related to some oral diseases. Proceedings of 2018 International Conference on Medicine Sciences and Bioengineering (ICMSB 2018) ISBN: 978-1-60595-560-5:169-77. DOI:10.12783/dtbh/icmsb2018/25429.
- 12.Ji L, Bao L, Gu Z, Zhou Q, Liang Y, Zheng Y, et al. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol Res. 2019;67:432–42. doi: 10.1007/s12026-019-09088-6. [DOI] [PubMed] [Google Scholar]
- 13.Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via mir-100-5p/mtor. Stem Cell Res Ther. 2019;10:216. doi: 10.1186/s13287-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int. 2018;2018:8973613. doi: 10.1155/2018/8973613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–15. doi: 10.1016/j.biomaterials.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadavand M, Hasni S. Exosomal biomarkers in oral diseases. Oral Dis. 2019;25:10–5. doi: 10.1111/odi.12878. [DOI] [PubMed] [Google Scholar]