ABSTRACT
Aim:
The purpose of this study was to evaluate the antimicrobial efficacy of Tylophora indica, Curcumin longa, and Phyllanthus amarus on Enterococcus faecalis biofilms formed on the tooth substrate. Sodium hypochlorite was used as a positive control. DMSO (dimethyl sulfoxide), the vehicle for the herbal extracts, was used as the negative control.
Materials and Methods:
Extracted human teeth were biomechanically prepared, vertically sectioned, placed in the tissue culture wells exposing the root canal surface to E. faecalis to form a biofilm. At the end of the third week, all groups were treated for 15 min with the test solutions and the control. The results were analyzed both quantitatively and qualitatively.
Results:
Statistical analysis was performed by using one-way analysis of variance and compared by the Mann–Whitney test using the Statistical Package for the Social Sciences (SPSS) software, version 20.0. The qualitative assay with the 3-week biofilm on the canal portion showed complete inhibition of bacterial growth for NaOCl, whereas samples treated with herbal solutions showed significant reduction of bacterial growth compared to control group, which showed 139.9 × 109 CFU/mL among the experimental herbal solutions groups. P. amarus has shown maximum bacterial count followed by C. longa and T. indica.
Conclusion:
NaOCl 5% showed maximum antibacterial activity against 3-week biofilm on tooth substrate. T. indica, P. amarus, and C. longa showed statistically significant antibacterial activity against 3-week biofilm. The use of herbal alternatives might prove to be advantageous considering the several undesirable characteristics of NaOCl.
KEYWORDS: Antibacterial, biofilm, Enterococcus faecalis, herbal irrigants
INTRODUCTION
Opportunistic pathogens in the oral microflora, which colonize the necrotic pulp tissue, are the cause of primary endodontic infections.[1] Therefore, thorough chemomechanical treatment of the root canal system is the key for the success of endodontic treatment.[2] The presence of bacteria in the root canals at the time of obturation negatively influences the final outcome.[3,4]
Despite thorough chemomechanical debridement, some bacteria might still survive leading to reinfection of the root canal. Enterococcus faecalis is one such dominant species of facultative anaerobic gram-positive cocci recovered in failed endodontic cases. Formation of biofilm, virulence factor activation, and invasion of dentinal tubules make them more resistant in an infected root canal system.
Current instrumentation techniques alone cannot guarantee the complete eradication of microbes from the root canal system. Therefore, along with the instrumentation techniques, constant irrigation is mandatory to get rid of pathogens, necrotic tissue, and other debris from the root canal. The use of irrigants enhances mechanical debridement and also disinfects the root canal system.
Along with the antibacterial property, an ideal irrigating solution should possess the ability to remove the smear layer and dissolve organic debris.[5] Studies have shown that even after complete chemomechanical debridement, a small part of flora survives in the root canal system.[6] Hence, the search for an irrigating solution that will completely remove the microflora from the root canal system is an ever-going process. Different antibiotics and antibacterials are tried and tested. Each has their shortcomings. Some are caustic and high irritant to tissue, some are toxic, and some are highly biohazardous.
Although many root canal irrigants are available, sodium hypochlorite (NaOCl) remains the most widely used irrigating solution because of its marked antimicrobial activity and the capacity to dissolve and remove organic matter from the pulp space.[5] The main drawback of NaOCl is its repugnant odor and taste along with its increased toxicity to periapical tissue, corrosiveness to instruments,[7] inability to eliminate smear layer,[8,9] and reduction in elastic modulus and flexural strength of dentin.[10]
The deleterious effects of synthetic drugs on the teeth have urged us to focus on alternative remedies such as herbal drugs. Tylophora indica is traditionally used as a folk remedy in certain regions of India.[11] Its leaves and roots have medicinal properties and are used for the management of bronchial asthma, inflammation, bronchitis, allergies, rheumatism, and dermatitis.[11]Curcumin longa, commonly known as turmeric, has been a part of Indian Ayurvedic medicine since 1900 BC to cure a wide variety of ailments.[12] Curcumin shows anti-inflammatory,[12] antioxidant,[13] anticarcinogenic,[14] antiviral,[15] and antimicrobial activity.[16,17]Phyllanthus amarus traditionally has been used for the treatment of jaundice, skin diseases such as scabby infections, offensive sores, and bruises.[18]
The purpose of this in vitro study was to evaluate the antimicrobial efficacy of T. indica, C. longa, P. amarus, and 5% NaOCl against E. faecalis biofilm formed on the tooth substrate of extracted human teeth. The NaOCl served as a positive control because the antibacterial effect of NaOCl is already proven.
MATERIALS AND METHODS
Materials used
Three herbals T. indica, P. amarus, and C. longa were collected from the medical garden of the College of Agriculture, Vellayani, Kerala, India. Fresh plant materials were washed with tap water, air dried, and then made into fine powder and stored in airtight bottles.
Extraction procedure
Soxhlet apparatus was used for the extraction of the herbals.
Soxhlet extraction
Dried powder (50g) of each extract was separated in a Soxhlet apparatus with 500 mL of 95% ethanol. The soxhelation process was carried out until the solvent remained colorless. The dark brown ethanolic extract was then filtered, and the alcohol part was removed from the extract on water bath till the volume was approximately 25 mL. The concentrated extract was stored in an airtight amber-colored container.
Bacterial strain and maintenance procedure
The bacterial strain used in this study was E. faecalis (American Type Culture Collection [ATCC] 29212) obtained from the stock culture. This was inoculated into Mueller Hinton broth, incubated at 37°C overnight and adjusted to 0.5 McFarland Standard (1 × 108 CFU/mL), and stored at 20°C.
Antibacterial activity test
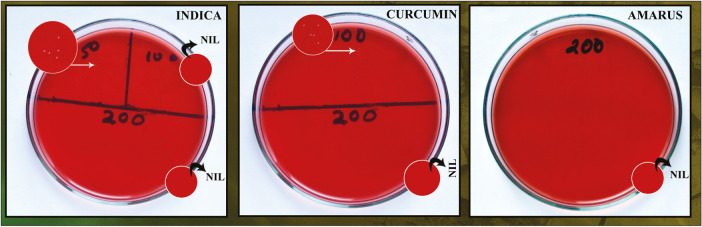
All the three extracts were made into a solution by dissolving them in 10% DMSO (dimethyl sulfoxide), and antibacterial sensitivity test was performed by agar well diffusion method. Mueller Hinton agar plates were prepared and cultures (200 µL) were spread onto agar plates. Wells of 6-mm diameter were made in the agar plates to which all the three herbal extracts were added. NaOCl was used as positive control, and DMSO was included to see if it showed any significant zone of inhibition and served as negative control [Figure 1].
Figure 1.
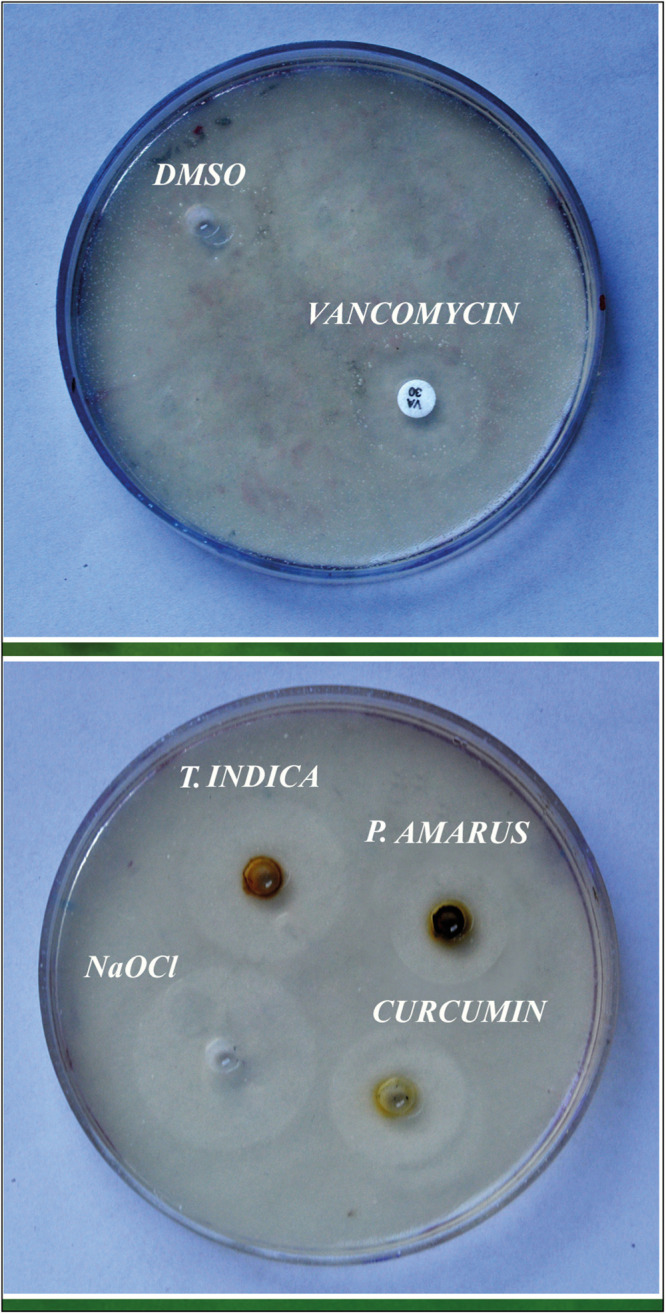
Antibacterial sensitivity test
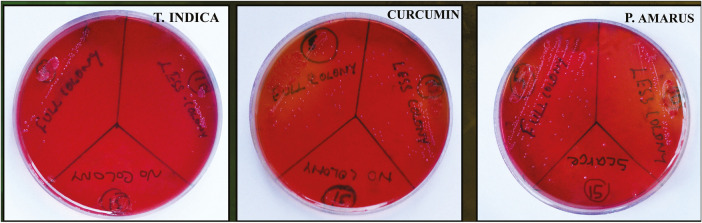
The minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) of the test solutions were determined by the tube dilution method. Double dilution was made from a higher dilution of 200 mg/mL to a lower dilution in a series of test tubes. Each tube was incubated at 37°C overnight with inoculated bacterial suspension. The MIC was regarded as the lowest concentration in the series of dilutions, which did not permit the growth of susceptible bacteria. On freshly prepared Mueller Hinton agar plates, subcultures were made from the tubes, which do not yield any visible turbidity (growth). The agar plates were incubated at 37°C for 24h. MBC was considered as the lowest concentration of test solution that permits less than 0.1% of the original inoculum to grow on the surface medium. In each experiment, test solutions were tested in triplicate [Figure 2].
Figure 2.
Minimum bacterial concentration
The time-kill study was performed to determine the time required for killing E. faecalis (ATCC 29212) by exposing the bacteria with the bactericidal concentration of the test solutions for 30 min. At the 2-min interval, a loop full of samples was inoculated on a Mueller Hinton agar plate, incubated at 37°C for 24h, and observed for growth [Figure 3, Table 1].
Figure 3.
Time-kill study
Table 1.
Susceptibility of Enterococcus faecalis ATCC 29212 against test solutions
| Test solutions | Zone of inhibition | Minimum inhibitory concentration | Minimum bacterial concentration |
|---|---|---|---|
| Tylophora indica | 27 mm | 40 mg/mL | 120 mg/mL |
| Phyllanthus amarus | 19 mm | 180 mg/mL | 180 mg/mL |
| Curcumin longa | 26 mm | 120 mg/mL | 80 mg/mL |
| NaOCl | 29 mm | 0.25% | 0.50% |
| Vancomycin | 20 mm | 2 µg/mL | 3 µg/mL |
P < 0.001 with respect to vancomycin
Biofilm formation on tooth substrate
Single-rooted human mandibular premolars with completely formed roots were cleaned of superficial debris, calculus, and tissue tags and kept in normal saline. To obtain a standardized tooth length of 8 mm, the tooth specimens were sectioned below the cementoenamel junction with a diamond disc. Tooth specimens were checked radiographically to confer the presence of a single patent canal.
The Crown–Down technique with rotary instruments was used to instrument the root canals (ProTaper), and the canals were enlarged to an apical size F3. Two milliliters of 3% NaOCl was used as an irrigant during biomechanical preparation. All the teeth were then vertically sectioned into two halves. The concave tooth surface was minimally grounded to achieve a flat surface to enable placement in the tissue culture wells, exposing the root canal surface to E. faecalis to form a biofilm.
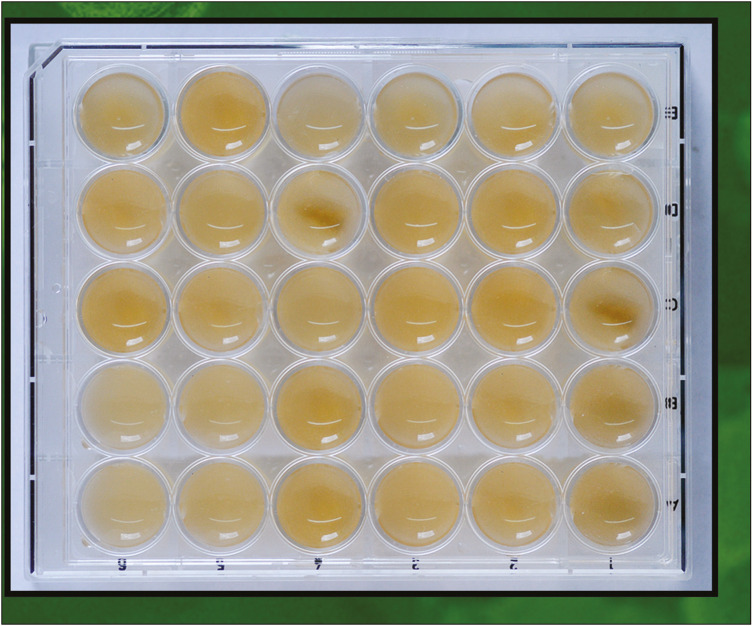
The sectioned samples were then divided into five experimental groups. Each group consisted of 10 samples each and assigned as Group 1: T. indica, Group 2: P. amarus, Group 3: C. longa, Group 4: NaOCl, and Group 5: saline (control). The samples were placed in the wells with the dimension of 20 mm diameter in culture plates. Wells containing tooth samples were inoculated with 2 mL of bacterial solution and incubated at 37°C for 3 weeks [Figure 4].
Figure 4.
Tissue culture
At the end of the third week, the cultured plates were treated for 15 min as follows: Group 1, immersed in 3 mL of T. indica (50 mg/mL in 10% DMSO), Group 2: immersed in 3 mL of P. amarus (200 mg/mL in 10% DMSO), Group 3: immersed in 3 mL of C. longa (100 mg/mL in 10% DMSO), Group 4: immersed in 3 mL of 5% NaOCl; and Group 5: immersed in 3 mL sterile saline [Figure 5, Table 2].
Figure 5.

Immersed in test solutions
Table 2.
Quantitative analysis of 3-week Enterococcus faecalis biofilm formed on tooth substrate for different groups
| Group | No. of bacteria in CFU/mL (mean ± standard deviation [SD]) |
|---|---|
| Tylophora indica | 1239 ± 22.48 |
| Phyllanthus amarus | 1858 ± 25.9 |
| Curcumin longa | 1468 ± 16.9 |
| NaOCl | 0 |
| Control | 139.9 × 109 ± 19.8 × 109 |
P < 0.001 with respect to control (Kruskal–Wallis test)
Then, the biofilm on the root canal portion was scraped and inoculated on Muller Hinton agar plates and incubated for 24h at 37°C. The quantitative analysis was performed by serial dilution method for all the groups.
Statistical analysis
One-way analysis of variance was used for statistical analysis and compared by the Mann–Whitney test using the Statistical Package for the Social Sciences (SPSS) software, version 23.0 (IBM SPSS, Chicago, IL). The criterion for statistical significance was defined as P < 0.001.
RESULTS
Table 1 shows the zone of inhibition, MIC, and MBC of test solutions for E. faecalis (ATCC 29212). All the test solutions have shown a significant zone of inhibition in the well diffusion assay when compared with vancomycin. No zone of inhibition was shown by 10% DMSO, whereas maximum inhibition was observed by 5% NaOCl followed by T. indica, C. longa, and P. amarus. Statistically, significant differences were observed between the three herbal groups (P < 0.001). NaOCl achieved 100% killing of E. faecalis at 2 min, whereas the herbals took 15 min.
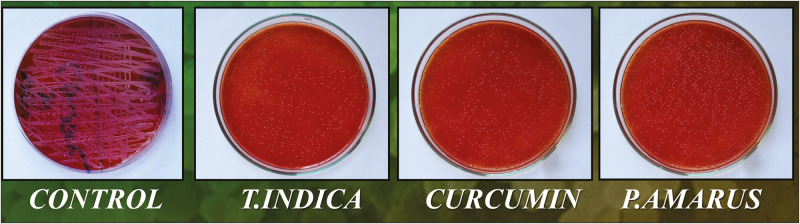
The qualitative assay with the 3-week biofilm on the canal portion showed complete inhibition of bacterial growth for NaOCl, whereas samples treated with herbals showed significant reduction of bacterial growth compared to control group, which showed 139.9 × 109 CFU/mL. In treated groups, P. amarus has shown maximum bacterial count (1468 CFU/mL). Table 2 shows the bacterial population in the quantitative assay with the 3-week biofilm for T. indica, C. longa, P. amarus, NaOCl, and control (saline-treated tooth samples) [Figure 6].
Figure 6.
Quantitative analysis results
DISCUSSION
E. faecalis is the most prevalent species in failed root canal cases and is immune to almost all antibiotics.[19,20] Enterococcus is more resistant when it is present in an infected root canal system compared to the planktonic forms. This may be due to the activation of virulence factors, biofilm formation, or their invasion into dentinal tubules. It has been reported that compared with planktonic cells, the antibiotic resistance was found to increase up to 1500 times.[21,22] Therefore, investigating the effect of an antimicrobial irrigant on planktonic cells will not satisfy the efficacy of in vivo conditions.
Studies have shown that biofilm experiments carried out on polycarbonate or glass substrate will not give a true suggestion of bacteria–substrate interaction.[23] In this study, biofilm was formed on a tooth substrate in accordance with the methodology done by Kishen et al.[24] All the groups were tested in direct contact with the biofilm formed on tooth substrate at a time interval of 6 weeks.
The concentration of the herbal solution was increased because of the fact that crude extracts have been used for experimental analysis. The effective concentration of all three herbals was found to be different. The concentration of T. indica (50 mg/mL), C. longa (100 mg/mL), and P. amarus (200 mg/mL) was found to be effective against E. faecalis. Further reduction in concentration is still feasible if purified extracts have been used; moreover, when used in vivo, the bacterial count is expected to be much less than what has been used in this study.
DMSO (10%) was used as a solvent for all the three herbals. It helps in bringing out the pure properties of all the components of the herb being used as shown.[25] Herbal alternatives showed very good antibacterial efficacy on 3-week biofilm along with the 5% NaOCl.
T. indica showed good antibacterial activity, followed by C. longa and P. amarus. The studies have shown that the principle constituents, tylophorine and O-methyl tylophorinidine have displayed both antibacterial and antifeedant activity.[26]
Turmeric components are called curcuminoids, and they mainly include curcumin (diferuloyl methane), demethoxycurcumin, and bisdemethoxycurcumin.[12] The biological effects of turmeric are mainly due to curcumin. C. longa 95% is a potent antioxidant, which also possesses anti-inflammatory, antiplatelet, cholesterol-lowering, antibacterial, and anti-fungal effects.[12,27]
P. amarus showed a statistically significant lesser antibacterial activity compared to T. indica and C. longa. The principle constituents responsible for the antibacterial property are phyllanthin and hypophyllanthin. These plants (T. indica and P. amarus) have not been tried in dentistry except turmeric.
The increasing side effects of modern medicine in recent times have prompted researchers to explore the medicinal properties of new herbals and to evaluate its application in various branches of medical science, including dentistry. Herbals, which were used in this study, had many actions such as antibacterial, anti-inflammatory, antiviral, and antifungal. In dentistry, previous studies have evaluated the irrigating potential of herbals such as curcumin, Triphala, propolis, aloe vera, and neem, but the antibacterial efficacy of T. indica and P. amarus against tooth biofilm substrate was never assessed before this study. T. indica has shown promising antibacterial efficacy compared to NaOCl. If we can extract the alkaloid, which produces the antibacterial efficacy, then we can use it as a principle element in irrigation and as an intracanal medicament.
CONCLUSION
Within the limitations of this study, it was concluded that 5% NaOCl showed maximum antibacterial activity against 3-week biofilm on tooth substrate. T. indica, P. amarus, and C. longa showed statistically significant antibacterial activity against 3-week biofilm. Compared to P. amarus, T. indica and C. longa showed more pronounced results. The antibacterial potential of T. indica and C. longa observed in this study opens new perspectives for its use as a root canal medicament and irrigating solution. The use of herbal alternatives might prove to be advantageous considering the several undesirable characteristics of NaOCl. In this study, crude extracts of all the herbals had been taken, they are expected to show much more promising results if the concentrated form of principle constituents has been extracted from the corresponding herbals. Further research is needed to know their interactions with other materials and its side effects to be used as an irrigating agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tronstad L, Sunde PT. The evolving new understanding of endodontic infections. Endod Topics. 2003;6:57–77. [Google Scholar]
- 2.Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119–25. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomes BP, Lilley JD, Drucker DB. Clinical significance of dental root canal microflora. J Dent. 1996;24:47–55. doi: 10.1016/0300-5712(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 4.Schirrmeister JF, Liebenow AL, Pelz K, Wittmer A, Serr A, Hellwig E, et al. New bacterial compositions in root-filled teeth with periradicular lesions. J Endod. 2009;35:169–74. doi: 10.1016/j.joen.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Gomes BP, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J. 1996;29:235–41. doi: 10.1111/j.1365-2591.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 7.Spangberg L, Engström B, Langeland K. Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856–71. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- 8.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1:238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001;34:120–32. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Parekh J, Chanda S. In vitro screening of antibacterial activity of aqueous and alcoholic extracts of various Indian plant species against selected pathogens from Enterobacteriaceae. Afr J Microbiol Res. 2007;1:092–9. [Google Scholar]
- 12.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric. J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 13.Kunchandy E, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–40. [Google Scholar]
- 14.Frank N, Knauft J, Amelung F, Nair J, Wesch H, Bartsch H. No prevention of liver and kidney tumors in Long-Evans cinnamon rats by dietary curcumin, but inhibition at other sites and of metastases. Mutat Res. 2003;523-524:127–35. doi: 10.1016/s0027-5107(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh F. Analytical microbiology. New York: Academic Press; 1963. p. 125. [Google Scholar]
- 16.Anderson AM, Mitchell MS, Mohan RS. Isolation of curcumin from turmeric. J Chem Ed. 2000;77:3. [Google Scholar]
- 17.Leal PF, Braga ME, Sato DN, Carvalho JE, Marques MO, Meireles MA. Functional properties of spice extracts obtained via supercritical fluid extraction. J Agric Food Chem. 2003;51:2520–5. doi: 10.1021/jf0260693. [DOI] [PubMed] [Google Scholar]
- 18.Oluwafemi F, Debiri F. Antimicrobial effect of Phyllanthus amarus and Parquetina nigrescens on Salmonella typhi. Afr J Biomed Res. 2008;11:215–9. [Google Scholar]
- 19.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 21.Mah TFC, O Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;8:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 22.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 23.Mcbain AJ, Gilbert, Allison DG. Biofilms and biocides: are there implications for antibiotic resistance? Rev Environ Sci Technol. 2003;2:141–6. [Google Scholar]
- 24.Kishen A, George S, Kumar R. Enterococcus faecalis-mediated biomineralized biofilm formation on root canal dentine in vitro. J Biomed Mater Res A. 2006;77:406–15. doi: 10.1002/jbm.a.30622. [DOI] [PubMed] [Google Scholar]
- 25.de la Torre JC. Biological actions and medical applications of dimethyl sulfoxide. Ann NY Acad Sci. 1983;411:1–403. [PubMed] [Google Scholar]
- 26.Krishna Reddy B, Balaji M, Uma Reddy P, Sailaja G, Vaidyanath K, Narasimha G. Antifeedant and antimicrobial activity of T. indica. Afr J Biochem Res. 2009;3:393–97. [Google Scholar]
- 27.Naz S, Jabeen S, Ilyas S, Manzoor F, Aslam F, Ali A. Antibacterial activity of Curcuma longa varieties against different strains of bacteria. Pak J Bot. 2010;42:455–62. [Google Scholar]