Abstract
Purpose
ONC201 is a small molecule selective antagonist of the G protein-coupled receptor DRD2 that is the founding member of the imipridone class of compounds. A first-in-human phase I study of ONC201 was conducted to determine its recommended phase II dose (RP2D).
Experimental Design
This open-label study treated 10 patients during dose escalation with histologically-confirmed advanced solid tumors. Patients received ONC201 orally once every 3 weeks, defined as one cycle, at doses from 125 to 625 mg using an accelerated titration design. An additional 18 patients were treated at the RP2D in an expansion phase to collect additional safety, pharmacokinetic (PK), and pharmacodynamic (PD) information.
Results
No Grade >1 drug-related adverse events occurred and the RP2D was defined as 625 mg. PK analysis revealed a Cmax of 1.5 – 7.5 μg/mL (~3.9–19.4 μM), mean half-life of 11.3 hours, and mean AUC of 37.7 h.ug/L. Pharmacodynamic assays demonstrated induction of caspase-cleaved keratin 18 and prolactin as serum biomarkers of apoptosis and DRD2 antagonism, respectively. No objective responses by RECIST were achieved, however, radiographic regression of several individual metastatic lesions was observed along with prolonged stable disease (> 9 cycles) in prostate and endometrial cancer patients.
Conclusion
ONC201 is a selective DRD2 antagonist that is well tolerated, achieves micromolar plasma concentrations, and is biologically active in advanced cancer patients when orally administered at 625 mg every 3 weeks.
Keywords: ONC201, GPCR, DRD2, ISR, imipridone
INTRODUCTION
ONC201 is a selective antagonist of DRD2 that causes downstream activation of the integrated stress response (ISR) in tumor cells and is the founding member of the imipridone class of compounds that share a unique chemical core structure [1, 2]. ONC201-induced activation of the ISR via PERK-independent mechanism causes an early-stage increase in the phosphorylation of eIF2-alpha at serine 51 that attenuates global translation and up-regulates translation of the transcription factor ATF4. In turn, ATF4 induces CHOP, which is also a transcription factor that regulates several apoptosis-related genes such as the TRAIL-receptor DR5. Downstream anticancer signaling effects of ONC201 also include inactivation of the Ras effector kinases – Akt and ERK – that upregulates TRAIL gene transcription via Foxo3a to activate apoptosis in tumor cells. The ability of ONC201 to cause p53-independent induction of the immune cytokine TRAIL and apoptosis in tumor cells underpinned its initial discover as an anti-cancer compound [3]. ONC201 has exhibited pro-apoptotic anti-tumor effects, manifesting as tumor regressions or stasis, as a single agent with infrequent oral dosing in numerous subcutaneous and orthotopic advanced solid tumor models [3–6].
The safety profile of ONC201 in rat and dog toxicology studies was consistent with the preferential cytotoxicity of ONC201 in tumor over normal cells in vitro [7]. An administration schedule of once every 21 days was selected for clinical evaluation based on preclinical results that indicate sustained intratumoral PD and that infrequent dosing was sufficient for in vivo efficacy that did not significantly increase with more frequent administration. The antitumor activity of ONC201 has been shown to be saturated at a dose of 25 mg/kg in murine models, which is the human equivalent of approximately 125 mg.
Based on the favorable efficacy and safety profile of ONC201, as well as its novel mechanism of action, the clinical introduction of ONC201 in patients with advanced cancers was undertaken [8]. The primary objective of this first-in-human, phase I dose-escalation study was to determine the recommended phase II dose (RP2D) of ONC201 administered orally in patients with advanced cancers, as well as to evaluate the safety and tolerability of the drug. Secondary objectives included assessments of pharmacokinetics, pharmacodynamics and preliminary anti-tumor activity of ONC201. Dose escalation was designed to proceed from a starting dose of 125mg that is allometrically equivalent to the no-observed-adverse-event-level (NOAEL) in Sprague Dawley rats and beagle dogs, up to a maximum administered dose of 625mg that is 5-fold above the expected therapeutic dose [7].
PATIENTS AND METHODS
Ethics
The study was carried at the Robert Wood Johnson University Hospital/Rutgers Cancer Institute of New Jersey (CINJ) in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and was approved by relevant regulatory committees and the Institutional Review Board of CINJ. Patients provided written, informed consent for their study participation.
Patient Population
Patients of 18 years of age or older with advanced solid tumors who refused or were resistant to standard treatments, ECOG performance status of 0 or 1, and assessable disease by RECIST version 1.1 were eligible. Patients had to have finished all prior cytotoxic chemotherapy at least 4 weeks, alkylating agents at least 6 weeks, molecularly targeted agents at least 28 days, and radiotherapy at least 14 days prior to the first dose. All prior treatment-related adverse events Grade ≥ 2 except alopecia and neuropathy had to have been resolved. Patients had to have exhibited normal marrow and organ function as defined by the following parameters: absolute neutrophil count ≥ 1,500/mcL; platelets ≥ 100,000/mcL; hemoglobin ≥ 9.0 mg/dL without transfusion in 2 prior weeks; total bilirubin within normal range (for patients with liver metastases, serum bilirubin ≤ 1.5 × ULN); AST (SGOT)/ALT (SGPT) ≤ 2.5 × upper limit of normal; and measured or estimated creatinine clearance ≥ 40 mL/min/1.73 m2 for patients with creatinine levels above normal. Exclusion criteria included symptomatic brain metastases or asymptomatic brain metastases treated with steroids, prior bevacizumab treatment, prior allergic reactions to compounds similar to ONC201, uncontrolled intercurrent illnesses, combination retroviral therapy for HIV, active cardiac disease/history of cardiac dysfunction, stroke or seizures in the last 3 months, impairment of GI function that may alter absorption of ONC201, pregnancy or treatment with hematopoietic colony-stimulating growth factor ≤ 2 weeks prior to beginning treatment.
Study Design and Toxicity Assessment
The design was an open-label, dose-escalation phase I trial of monoagent ONC201 in patients with advanced, refractory solid tumors who had exhausted or refused standard treatment options for their respective indications. Capsules (125 mg) of ONC201 were provided by Oncoceutics, Inc (Philadelphia, PA) containing the dihydrochloride salt form of the compound. ONC201 was administered orally once every 21-day cycle without food intake two hours before or after drug administration. In the absence of dose-limiting toxicity, dose escalation was designed proceed to a maximum administered dose of 625mg that is 5-fold higher than the therapeutic dose in preclinical models, using the following dose increments: 125, 250, 375, 500, and 625mg. The primary objective of the study was to determine the recommended Phase II (RP2D) of ONC201 administered every three weeks that was defined was the maximum tolerated dose (MTD) or, in the absence of MTD, the maximum administered dose. The study was conducted with a single patient accelerated dose escalation design [9] that would cease if any patient experienced a Grade > I adverse event that was attributed as at least possibly-related to ONC201. In this case a traditional 3+3 dose escalation design would have been employed. With either dose escalation design, the RP2D was to be established in 6 patients in the appropriate dose cohort. Dose escalation could proceed after the previously dosed cohort completed one treatment cycle and met the criteria to proceed with the next dose level. Enrollment at each subsequent dose level required that all patients enrolled at the prior dose level completed Cycle 1 dosing and were evaluated 21 days later to assess safety. Dose levels proceeded from 125 mg to 250 mg, 375 mg, 500 mg and finally to 625 mg. Following determination of the RP2D, an expansion phase of 18 patients at the RP2D was completed to increase the robustness of the safety profile.
All toxicities were evaluated based on the Common Terminology Criteria for Adverse Events version 4. DLT was defined as a drug-related adverse event or abnormal laboratory result that occurred in the first cycle of treatment that met any of the following criteria: ≥ Grade 3 non-hematological toxicity; ≥ Grade 3 nausea, vomiting, or diarrhea that has persisted for > 72 hours despite optimal antiemetic or antidiarrheal therapy; Grade 3–4 AST/ALT in combination with a Grade 2 elevation in bilirubin; Grade 4 neutropenia lasting = 7 days; Grade 4 neutropenia and fever of > 38.5°C; Grade 3 neutropenia with > Grade 3 infection; thrombocytopenia of any grade if associated with clinically significant bleeding; Grade 4 thrombocytopenia; or Grade 4 anemia and was assessed as unrelated to disease, disease progression, inter-current illness, or concomitant medications; and is determined by the investigator to be “possibly related”, “probably related” or “definitely related” to the administration of ONC201.
Safety Assessments
Safety assessments including complete blood count, serum chemistry, and toxicity were evaluated at baseline, followed by weekly during the first 2 cycles, and then every 3 weeks afterward. Electrocardiograph monitoring was carried out just before ONC201 administration, followed by 15 minutes, 1 hour and 2 hours, after drug administration. Adverse events were graded using the CTCAE version 4.0. Disease assessments were performed using RECIST version 1.1 every 2 cycles except for prostate cancer patients, which were performed using the Prostate Cancer Clinical Trials Working Group (PCWG2) criteria in lieu of RECIST.
Pharmacokinetic Analyses
Plasma samples for PK were collected at baseline, 30 minutes, 2 hours, 4 hours, 6 hours, 24 hours, 48 hours, and 168 hours following the first dose of ONC201 and before doses prior to treatment in cycles 2–6. PK was analyzed using 50 uL of K2EDTA plasma by a validated LC-MS/MS method with positive ESI-MRM mode that has a linear range of 1 to 500 ng/mL (samples were diluted if above limit of detection). Deuterated ONC201 was used as an internal standard.
Statistical Analysis
Descriptive statistics were used for the analysis of safety and tumor response data. Non-compartmental PK analysis was performed using Phoenix® WinNonlin® Version 6.3 (Pharsight®, St. Louis, Missouri).
Pharmacodynamic Analyses
ONC201 induces apoptosis in epithelial tumor cells in preclinical models, which can be measured by caspase-cleaved cytokeratin 18 (cCK18) [10]. Antagonism of DRD2, which is a direct effect of ONC201 [11], has been shown to induce the neuroendocrine hormone prolactin that is secreted into the blood [12]. Thus, cCK18 and prolactin were selected as serum PD biomarkers to be assessed in this heterogeneous patient population.
Blood samples for PD were collected at 6 hours, days 2, 3, 8, and 15 after ONC201 treatment for cycle 1, and pre-dose on the day of drug administration for cycle 2 and 3. Serum levels of cCK18 were assessed using the M30 assay, serum levels of total cytokeratin 18 (CK18) were assessed using the M65 assay (Perviva A.B., Sweden), and serum levels of prolactin were assessed by ELISA (Human Prolactin Quantikine ELISA kit, R&D Systems, Minneapolis, Minnesota) according to the manufacturer’s instructions. Assessments of other tumor-specific markers were also evaluated per standard of care.
RESULTS
Demographics, Safety, and Determination of RP2D
Ten heavily pretreated patients with diverse advanced solid tumors were enrolled in the dose escalation phase of this study between January and July 2015 (Table 1; Supplemental Table 1). Dose cohorts ranged from the starting dose of 125 mg up to 625 mg, which was designed as the maximum administered dose that exceeds the expected therapeutic dose by 5-fold and was determined to be the RP2D. The only adverse event during the dose escalation phase that was attributed as possibly-related to ONC201 was a Grade 1 fever in one patient treated at a dose of 125mg (Table 2; Supplemental Table 2).
Table 1.
Patient demographics.
| Dose escalation cohorts (n=10) | Dose expansion (n=18) | |
|---|---|---|
| Male | 5 | 8 |
| Female | 5 | 10 |
| Age (years)* | 66.8 (47–80) | 60.1 (26–90) |
| Weight (kg)* | 76 (47.7–123) | 73 (47.7–118.9) |
| Cycles/Doses of ONC201* | 3.2 (1–4) | 3.3 (1–14) |
| Prior therapies* | 5.5 (1–10) | 6.2 (3–18) |
| Prior radiation | 5 | 12 |
| Prior surgeries | 9 | 13 |
indicate the mean with the range in parentheses.
Table 2. Adverse events.
All adverse events attributed as at least possibly-related to ONC201 were Grade 1 (n=28).
| ONC201 (mg) | 125 | 250 | 375 | 500 | 625 |
|---|---|---|---|---|---|
| No of Patients | 1 | 1 | 1 | 1 | 24 |
| Pyrexia | 1 (3.6%) | 0 | 0 | 0 | 0 |
| Fatigue | 1 (3.6%) | 0 | 0 | 0 | 0 |
| Elevated amylase | 0 | 0 | 0 | 0 | 2 (7.2%) |
| Emesis | 0 | 0 | 0 | 0 | 1 (3.6%) |
| Nausea | 0 | 0 | 0 | 0 | 1 (3.6%) |
Following completion of the dose escalation phase, an additional 18 advanced cancer patients were enrolled in an expansion phase between August 2015 and February 2016 with an enrichment of prostate and endometrial cancer patients based on observations in dose escalation. The only adverse events among these 18 patients that were attributed as possibly-related to ONC201 were nausea in one patient, emesis in one patient, and increased serum amylase in two patients. All of these adverse events were Grade 1 and reversed rapidly.
Pharmacokinetics
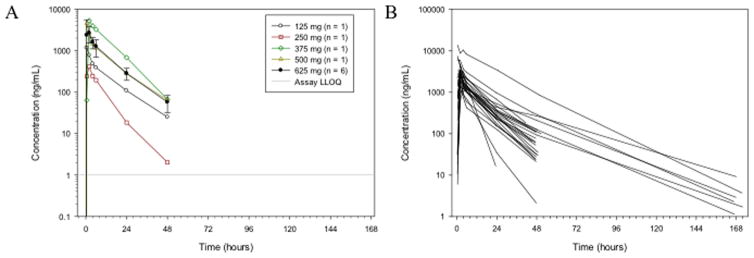
Plasma collected at serial time points during the first cycle was used to analyze systemic exposure to ONC201 and determine pharmacokinetic parameters (Figure 1; Supplemental Figure 1; Supplemental Tables 3–4). Trends of increasing exposure with dose were consistent with dose proportionality. Patients receiving 625mg ONC201 exhibited a mean half-life of 11.3 hours and achieved a mean Cmax of 3.6 ug/mL (~9.3 uM), which occurred at 1.8 hours following administration (Tmax) and surpassed the target Cmax of 1 ug/mL. The mean volume of distribution was 369 L, consistent with a large distributive volume and penetrance of target tissues observed in animals.
Figure 1. ONC201 plasma concentrations following the first dose of ONC201.
Concentrations are shown as (A) the mean for each dose cohort in dose escalation, or (B) for all individuals treated at 625 mg. Data points below the limit of detection (1ng/mL) are excluded from the plots. Error bars indicate standard deviation.
Mean AUC was 37.7 h.μg/mL and mean CL/F was 25.2 L/h, which was generally observed to be variable but consistent across all dose groups (Supplemental Figure 2A). There were no apparent relationships between drug CL/F and patient sex and age (Supplemental Figure 2B–C). Noticeable, shallow trends were observed with patient weight and BSA (Supplemental Figure 2D–E). An overall increase in CL/F was observed as weight and BSA increased. Although a slight upward trend was observed, there was no strong correlation between CL/F and CLCR (Supplemental Figure 2F).
Stronger correlations were observed with the distributive volume estimate and patient weight and BSA. An increase in volume of distribution was observed with increasing patient weight or BSA, as expected (Supplemental Figure 2G–H). Trends of decreasing exposure with increasing weight were observed in plots of Cmax/Dose and AUC/Dose versus patient weight (Supplemental Figure 2 I&J). Weight normalized CL/F was plotted versus Dose (Supplemental Figure 2K), showing a similar trend to un-normalized CL/F.
Pharmacodynamics
As a biomarker of apoptosis, the serum M30 and M65 assays were used to quantify serum caspase-cleaved and total cytokeratin-18 [12–17]. As expected, the patient who remained on study through 9 cycles exhibited increases in the M30 assay, but not in the M65 assay (Supplemental Figure 3). In contrast, the patient with rapid disease progression who was on study for less than one cycle exhibited increases in the M65, but not in the M30 assay. Four of the patients enrolled in the dose escalation phase of the study had an induction in the M30 assay after a single dose of ONC201, most often on day 21 post-treatment (Figure 2A; Supplemental Table 5). Given the downstream induction of TRAIL by ONC201 in preclinical models, serum TRAIL levels were also quantified (Supplemental Figure 4). Half of the patients exhibited a modest (~20%) increase in serum TRAIL that mostly peaked within the first 24 hours of drug administration.
Figure 2. Pharmacodynamic assays for apoptosis and DRD2 antagonism.
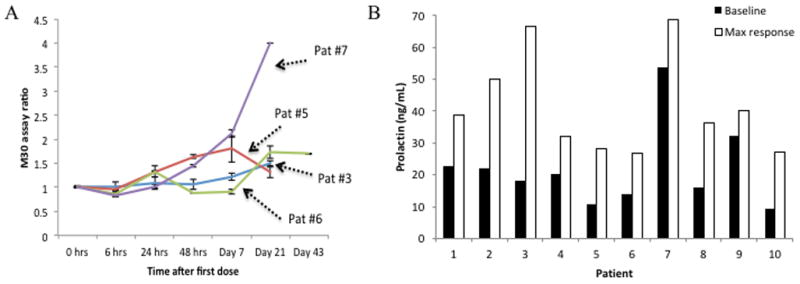
(A) M30 assay ratio in dose escalation patients who experienced induction over baseline (n=2 per sample). (B) Maximum versus baseline serum prolactin levels in dose-escalation cohort of patients.
Serum prolactin, which is induced by DRD2 antagonism in the pituitary gland, was evaluated as a surrogate marker of target engagement [12]. Twenty-two of the 25 patients evaluated in this analysis exhibited induction of prolactin over baseline. The mean peak induction of prolactin was 2.4-fold over baseline (P=0.000034) (Figure 2B and Supplemental Figure 5). Interestingly, the patient exhibiting the strongest level of prolactin induction was the endometrial cancer patient who had the reduction in lymphadenopathy. Prolactin induction appeared to be more pronounced in patients with lower baseline levels. Peak induction tended to occur at either the earliest evaluated time point, 6 hours post-dose, or much later at 14 or 21 days post-dose. Induction of prolactin did not correlate with ONC201 dose or PK parameters (Supplemental Figure 6), suggesting that DRD2 antagonism occurs at the clinical evaluated doses of ONC201 and in agreement with the dose escalation design that did not continue above 625mg.
Patient Outcomes
The overall mean number of ONC201 doses received was 3.3. Out of 28 evaluable patients, 10 patients completed at least 4 cycles, and 2 patients received at least 9 cycles (Supplemental Table 5). After 2 doses of 375mg ON201, one 72-year-old patient with advanced clear cell endometrial cancer had a mixed response with multiple nodes decreasing by >30% along with the development of new nodes. One 69-year-old patient with prostate adenocarcinoma experienced prolonged stable disease and was on study for 27 weeks.
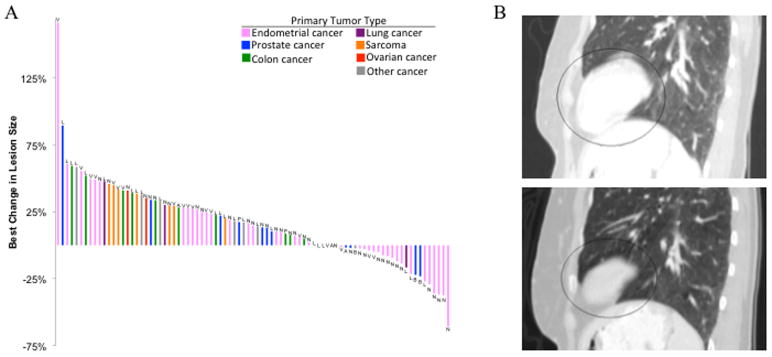
Based on these clinical observations in the dose escalation phase, the expansion phase enriched for endometrial and prostate cancer patients. A 90-year old prostate cancer patient underwent a rapid shrinking of his primary tumor and metastatic bone lesion (~25%) after only two doses of 625mg ONC201. Waterfall analysis of the 28 patients on a lesion-by-lesion basis revealed tumor regressions in prostate and endometrial cancer patients that involved lymph nodes, bone, and lung lesions (Figure 3A). One endometrial cancer patient experienced stable disease for 42 weeks and exhibited sustained regression of her metastatic lung lesion (Figure 3B).
Figure 3. Antitumor activity in metastatic lesions.
(A) Waterfall analysis on a lesion-by-lesion basis. Best change in lesion size is defined as the maximal reduction or minimal increase in sum of longest dimensions of target lesions relative to pretreatment assessment. 25/28 patients had measurable tumors at baseline and at least one post-treatment evaluation. Letters above or below bar denotes location of the lesion: V = liver; L = lung; N = lymph node; A = adrenal gland; P = primary tumor; B = bone. (B) Metastatic lung lesion of an endometrial cancer patient at baseline and after 8 doses of 625mg ONC201.
DISCUSSION
This is the first human study of the selective DRD2 antagonist, ONC201. The primary objective of the study was to determine the RP2D of oral ONC201 administered every 3 weeks to patients with solid tumors who have exhausted all treatment options. As anticipated by the preclinical safety profile of ONC201, no drug-related Grade >1 adverse events were observed in any patients despite exposure to micromolar therapeutic plasma concentrations. The safety profile of the drug allowed the study to progress rapidly without digressing from the accelerated titration design. To address the small sample size in the dose escalation phase, the safety profile of RP2D was confirmed in the 18-patient expansion phase.
The pharmacokinetic profile of ONC201 at the RP2D indicates significant absorption of the drug with oral administration that was rapid, as indicated by the 1.8 hour mean Tmax. Importantly, the PK parameters such as Cmax and AUC in the top dose cohort treated at the RP2D exceeded those associated with the antitumor efficacy in mouse models and the NOAEL in toxicology studies. While serum prolactin is a surrogate marker within inherent limitations, the results in this study indicate that ONC201 engages its target in advanced cancer patients at, and below, its recommended phase 2 dose of 625mg. Like preclinical findings, PD measurements of apoptosis with the M30 assay revealed that the effects of ONC201 were sustained over time in several patients. Serum TRAIL induction was noted in 2 patients; however, this assay is limited to the detection of serum soluble TRAIL as on-treatment tumor biopsies were not available for evaluation.
This study selected the target dose of 625 mg administered once every 3 weeks as the RP2D, which is 5-fold above the expected therapeutic dose based on preclinical models and allometric conversion. In support of this dose selection, the PK parameters exceeded thresholds derived from preclinical models and the observed PD supports target engagement at and below the RP2D. Given that the primary endpoint of the study was based on clinical safety in a group of heterogeneous and heavily-pretreated patients with aggressive cancers, it is noteworthy that some patients showed some evidence of clinical benefit. These included a patient with chemo-resistant clear cell endometrial cancer who had a mixed response, 2 patients who had alleviation of symptoms associated with sites of tumor manifestation, and 2 patients (castrate-resistant prostate and Type 2 endometrial cancers) with stable disease for > 9 cycles. Even though objective responses were not achieved by RECIST criteria, there were several instances of regressions in individual lesions of prostate and endometrial cancer patients.
Given the benign safety profile of the drug established in this trial, other clinical studies are exploring more frequent ONC201 dose regimens. The signs of anti-tumor activity and absence of any meaningful side effects in this trial indicate that ONC201 may offer clinical benefit without imposing the typical toxicities of anticancer therapies. Several ongoing clinical trials are investigating the activity of ONC201 in advanced cancers that exhibit preclinical sensitivity to the compound and where the mechanism of action is relevant: glioblastoma, pheochromocytoma, endometrial cancer, non-Hodgkin’s lymphoma, multiple myeloma, and acute myeloid leukemia.
In conclusion, this study demonstrates that ONC201 is very well tolerated at the RP2D of 625mg, achieves its anticipated PK profile, and exhibits signs of activity in patients with advanced solid tumors that warrants further investigation in several ongoing advanced cancer clinical trials [8].
Supplementary Material
Table 3.
Pharmacokinetic parameters for 625 mg ONC201 (N=24).
| Cmax (ug/mL) | Tmax (h) | Tlag (h) | AUClast (h.ug/mL) | λZ (h−1) | t1/2 (h) | AUC (h.ng/mL) | VZ/F (L) | CL/F (L/h) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 3.6 | 1.8 | 0.02 | 37.0 | 0.076 | 11.3 | 37.7 | 369 | 25.19 |
| SD | 2.6 | 0.9 | 0.08 | 41.6 | 0.046 | 5.2 | 41.6 | 193 | 14.22 |
Translational Relevance.
There is a need for safe and effective therapies that meet the challenges of treating patients with advanced cancers. ONC201 is an orally active small molecule that selectively antagonizes the G protein-coupled receptor (GPCR) DRD2. Downstream of target engagement, the mechanism of action of ONC201 involves the integrated stress response and inactivation of Akt/ERK signaling, leading to upregulation of the pro-apoptotic immune cytokine TRAIL. Preclinical studies have determined that ONC201 has prolonged anti-proliferative and pro-apoptotic effects against a broad range of tumor cells but not normal cells. The first-in-human clinical trial of ONC201 in advanced solid tumors confirmed that ONC201 is exceptionally well-tolerated at the recommended phase II dose of 625 mg every three weeks that exceeds targeted thresholds for dose, pharmacokinetics, and pharmacodynamics. Early signs of clinical benefit were observed in advanced prostate and endometrial cancer patients.
Acknowledgments
The authors thank Lee Schalop, MD for his careful review of the manuscript and his constructive suggestions.
Footnotes
Conflicts of Interest: JEA, MS, and WO are employees of Oncoceutics. WSE-D, JEA, MS, WO, and JB have stock or stock options in Oncoceutics.
References
- 1.Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO, Borthakur G, Mu H, Zeng Z, Tabe Y, Allen JE, Wang Z, Ma W, Lee HC, Orlowski R, Sarbassov DD, Lorenzi PL, Huang X, Neelapu SS, McDonnell T, Miranda RN, Wang M, Kantarjian H, Konopleva M, Davis RE, Andreeff M. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Science Signaling. 2016;9:ra17-ra17. doi: 10.1126/scisignal.aac4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kline CLB, Van den Heuvel APJ, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Science Signaling. 2016;9:ra18-ra18. doi: 10.1126/scisignal.aac4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W, Zhou JY, Wu GS, El-Deiry WS. Dual Inactivation of Akt and ERK by TIC10 Signals Foxo3a Nuclear Translocation, TRAIL Gene Induction, and Potent Antitumor Effects. Science translational medicine. 2013;5:171ra117-171ra117. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JE, Krigsfeld G, Patel L, Mayes PA, Dicker DT, Wu GS, El-Deiry WS. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer. 2015;14:99. doi: 10.1186/s12943-015-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen JE, Prabhu VV, Talekar M, van den Heuvel A, Lim B, Dicker DT, Fritz JL, Beck A, El-Deiry WS. Genetic and pharmacological screens converge in identifying FLIP, BCL2 and IAP proteins as key regulators of sensitivity to the TRAIL-inducing anti-cancer agent ONC201/TIC10. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-14-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishizawa J, Kojima K, Chachad D, Ruvolo PP, Ruvolo VR, Jacamo R, Dilip A, Mu H, Zeng Z, Matre P, Allen JE, Neelapu SS, McDonnell TJ, Miranda RN, Kwak LW, Kantarjian HM, Konopleva M, Davis RE, Andreeff M. ONC201 Induces p53-Independent Apoptosis and Cell Cycle Arrest in Hematological Malignancies and Leukemic Stem/Progenitor Cells By Inducing ER Stress and mTOR Inhibition. 2014 [Google Scholar]
- 7.Allen JE, Crowder R, E-DW First-In-Class Small Molecule ONC201 Induces DR5 and Cell Death in Tumor but Not Normal Cells to Provide a Wide Therapeutic Index as an Anti-Cancer Agent. PloS one. 2015;10:e0143082. doi: 10.1371/journal.pone.0143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JE, Kline CLB, Prabhu VV, Wagner J, Ishizawa J, Madhukar N, Lev A, Baumeister M, Zhou L, Lulla A, Stogniew M, Schalop L, Benes C, Kaufman HL, Pottorf RS, Nallaganchu BR, Olson GL, Al-Mulla F, Duvic M, Wu GS, Dicker DT, Talekar MK, Lim B, Elemento O, Oster W, Bertino J, Flaherty K, Wang ML, Borthakur G, Andreeff M, Stein M, El-Deiry WS. Discovery and clinical introduction of first-in-class imipridone. 2016;ONC201 doi: 10.18632/oncotarget.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. Journal of the National Cancer Institute. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 10.Markianos M, Hatzimanolis J, Lykouras L. Neuroendocrine responsivities of the pituitary dopamine system in male schizophrenic patients during treatment with clozapine, olanzapine, risperidone, sulpiride, or haloperidol. European archives of psychiatry and clinical neuroscience. 2001;251:141–146. doi: 10.1007/s004060170049. [DOI] [PubMed] [Google Scholar]
- 11.Madhukar NS, Elemento O, Benes CH, Garnett MJ, Stein M, Bertino JR, Kaufman HL, Arrillaga-Romany I, Batchelor TT, Schalop L, Oster W, Stogniew M, Andreeff M, El-Deiry W, Allen JE. D2-like dopamine receptor antagonism by ONC201 identied by confluence of computational, recepto binding and clinical studies. Annual Meeting of the American Association for Cancer Research (AACR); 2016. [Google Scholar]
- 12.Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Biven K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer research. 2004;64:1751–1756. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- 13.Biven K, Erdal H, Hagg M, Ueno T, Zhou R, Lynch M, Rowley B, Wood J, Zhang C, Toi M, Shoshan MC, Linder S. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis: an international journal on programmed cell death. 2003;8:263–268. doi: 10.1023/a:1023672805949. [DOI] [PubMed] [Google Scholar]
- 14.Demiray M, Ulukaya EE, Arslan M, Gokgoz S, Saraydaroglu O, Ercan I, Evrensel T, Manavoglu O. Response to neoadjuvant chemotherapy in breast cancer could be predictable by measuring a novel serum apoptosis product, caspase-cleaved cytokeratin 18: a prospective pilot study. Cancer investigation. 2006;24:669–676. doi: 10.1080/07357900600981307. [DOI] [PubMed] [Google Scholar]
- 15.Kramer G, Schwarz S, Hagg M, Havelka AM, Linder S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. British journal of cancer. 2006;94:1592–1598. doi: 10.1038/sj.bjc.6603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno T, Toi M, Biven K, Bando H, Ogawa T, Linder S. Measurement of an apoptotic product in the sera of breast cancer patients. European journal of cancer. 2003;39:769–774. doi: 10.1016/s0959-8049(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 17.Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung cancer. 2007;56:399–404. doi: 10.1016/j.lungcan.2007.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.