ABSTRACT
The sugarcane (Saccharum X officinarum) is one of the most important crops used to produce sugar and raw material for biofuel in the world. One of the main causes for sucrose content and yield losses is the attack by insect. In this investigation, cry1Ac gene was introduced into sugarcane variety GT54-9(C9) using the Agrobacterium tumefaciens transformation method for transgenic sugarcane production presenting insect-resistance. The A. tumefaciens strain GV1303 including pARTcry1Ac vector was used for the production of transformed sugarcane. The Bacillus thuringiensis cry gene were successfully used to produce transgenic plants used for the improvement of both agronomic efficiency and product quality by acquiring insect resistance. PCR and Southern hybridization techniques were used to confirm the cry1Ac gene incorporation into sugarcane genome. Transformation percentage was 22.2% using PCR analysis with specific primers for cry1Ac and npt-II (Neomycin phosphotransferase) genes. The expression of cry1Ac gene was determined using reverse transcriptase polymerase chain reaction (RT-PCR), QuickStix test, and insect bioassays. Bioassays for transformed sugarcane plants showed high level of toxicity to Sesamia cretica giving 100% mortality of the larvae. Sugarcane insect resistance was improved significantly by using cry1Ac gene transformation.
KEYWORDS: Sugarcane, Agrobacterium tumefaciens, cry1Ac, borer-resistance, insect-resistance
Introduction
Sugarcane is a paramount sugar plant vastly cultivated in the subtropical and tropical regions. It supplies about eighty percent of the sugar in the world.1 Moreover, in many countries sugarcane is also considered a main raw material for the production of ethanol.2,3 Cultivated sugarcane varieties are hybrids from the cross between Saccharum spontaneium (2 n = 36–128) and Saccharum officinarum (2 n = 20–122) which represent complex aneupolyploid.4 It is an octaploid species have complex genome (x = 10 and 2 n = 80 ~ 270).5 Insect pests are an essential problem for sugarcane crop all over the world. One of the significant pests of sugarcane is lepdoptera stem borers.6 The major Lepidopteran insect pests of sugarcane are stem borer (Diatraea saccharalis) in South America, central America, the Caribbean, and the southern United States,7 root borer (Emmalocera depressalis) in India and Pakistan, sugarcane top borer (Chilo terrenellus) in Bangladesh, Thailand and Australia,8 pink borer (Sesamia inferens) in ASIA, Cambodia, China, Hong Kong, India, Mexican rice borer (Eoreuma loftini), and pink stem borer Sesamia cretica in Mediterranean basin and extends through the Middle East and Arabia to Pakistan, northern India, and northern Africa extending south to northern Kenya and northern Cameroon. These borers cause yield losses of nearly 25–30%.9
To improve economic traits in agriculture many traditional plant breeding techniques can been used but these techniques can be time consuming, especially for genomic complex crop such as sugarcane. Moreover, conventional breeding to develop insect-resistance in sugarcane is limited by the lack of resistance available in the crop germplasm. One of the effective and economic strategies for improvement the resistance of different plants to insects is introduction of insect-resistant genes including Bt genes.10 Insect resistance could be improve by using genetic engineering approaches11,12 and could help in the development of sugarcane varieties production. Bacillus thuringiensis (Bt), is a Gram positive and spore-forming bacteria. During sporulation, it produce a crystalline parasporal body that shows biocidal activity against some invertebrate orders at larval stage including dipteran, lepidopteran and coleopteran insects.13 It was first discovered in 1901 by Gill.14 There are many reports that successfully obtained insect resistant transgenic sugarcane lines through introduced Bt genes.15 A lot of other crop species has been developed through cryA(b) gene introducing; those plants such as rice,16 cotton,17 tomato,18 potato,19 corn,20 and sugarcane.21
The first research with the aim of introducing Bt gene into sugarcane to produce insect resistance plants used the cry1Ab gene.21 Lately, cry1Ac gene was introduced into sugarcane genome by Gao15 that successfully obtained insect- resistant transgenic events. Wang22 introduced both the EPSPS and cry1Ab genes into sugarcane genome and obtained transgenic lines with herbicide tolerance and insect resistance. Recently, many monocotyledonous species used Agrobacterium transformation method including rice, maize, wheat, and barley.23 The transformation by Agrobacterium have diverse advantages, including minimal DNA rearrangement in transformants, technical simplicity and the capability to transfer long fragment of DNA. Although the Agrobacterium method has been used also in sugarcane,24,25 the shortage of a reproducible result has been an obstacle to found effective transformation method for routine genetic manipulation in the crop. The present study aimed to improve the borer resistance in sugarcane plants via introducing the cry1Ac gene through Agrobacterium transformation.
Results
In order to use kanamycin as a selectable marker gene in plant transformation, suitable concentration of kanamycin that inhibits explants growth should be determined.26 Young leaves segments were placed into media containing different concentrations of kanamycin ranging from 25 up to 150 mg/l, whilst the control was placed on kanamycin free medium. The results indicated that the number of survival explants decreased with the increasing of the kanamycin concentration. The lethal kanamycin dose for the leaf segments was found to be 100 mg/L (Figure 1).
Figure 1.
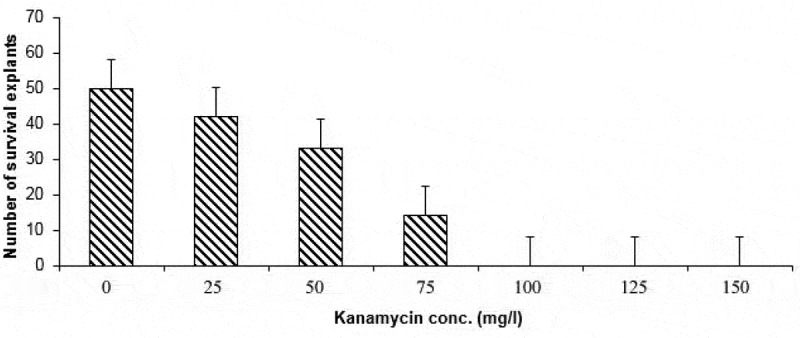
The effect of different kanamycin on the survival explants
Agrobacterium Transformation
The sugarcane variety GT54-C9 is very popular among Egyptian farmers due to its high yield and other desirable agronomic.27 Sugarcane variety GT54-9(C9) were used for Agrobacterium transformation. Young leaf explants of sugarcane variety were co-cultivated with Agrobacterium tumefaciens GV3101 harboring the binary vector pARTcry1Ac. After three days of co-cultivation, the inoculated explants were transferred to MS regeneration medium including 1 mg/l BAP and 2 mg/l NAA. After 48 hours in the dark at 28 ± 2°C, a sterile solution of strength MS medium with 300 mg/L carbenicillin was used to wash the explants then blotted briefly on sterile filter paper in the laminar flow hood. Explants were transferred to regeneration media supplemented with 100 mg/L kanamycin and 300 mg/L carbinicillin. The cultures were incubated under the regeneration conditions. After 30 days of incubation, shoots were subcultured to fresh regeneration medium with the same antibiotics in the selection plates, and were reincubated under the same conditions. Young leaves from nontransformed sugarcane were used as control. For inducing roots, regenerated shoots (about 7–10 cm) were transferred to MS medium supplemented with 60 g sucrose and 2 mg/l NAA. Regenerated plants with well-developed roots were transferred to pots containing sand, peat-moss and clay (1:1:1) and kept in a greenhouse under shadow for 15–20 days for acclimatization (Figure 2). For further analyzes, sixteen regenerated transgenic sugarcane lines were used. From a single shoot bud, a transgenic line was develop and grew on MS media containing kanamycin. Based on the morphological parameters and molecular evaluation using ISSR marker, no phenotypic abnormalities appeared in the putative transgenic plants in comparison to the untransformed control plants (data not shown).
Figure 2.
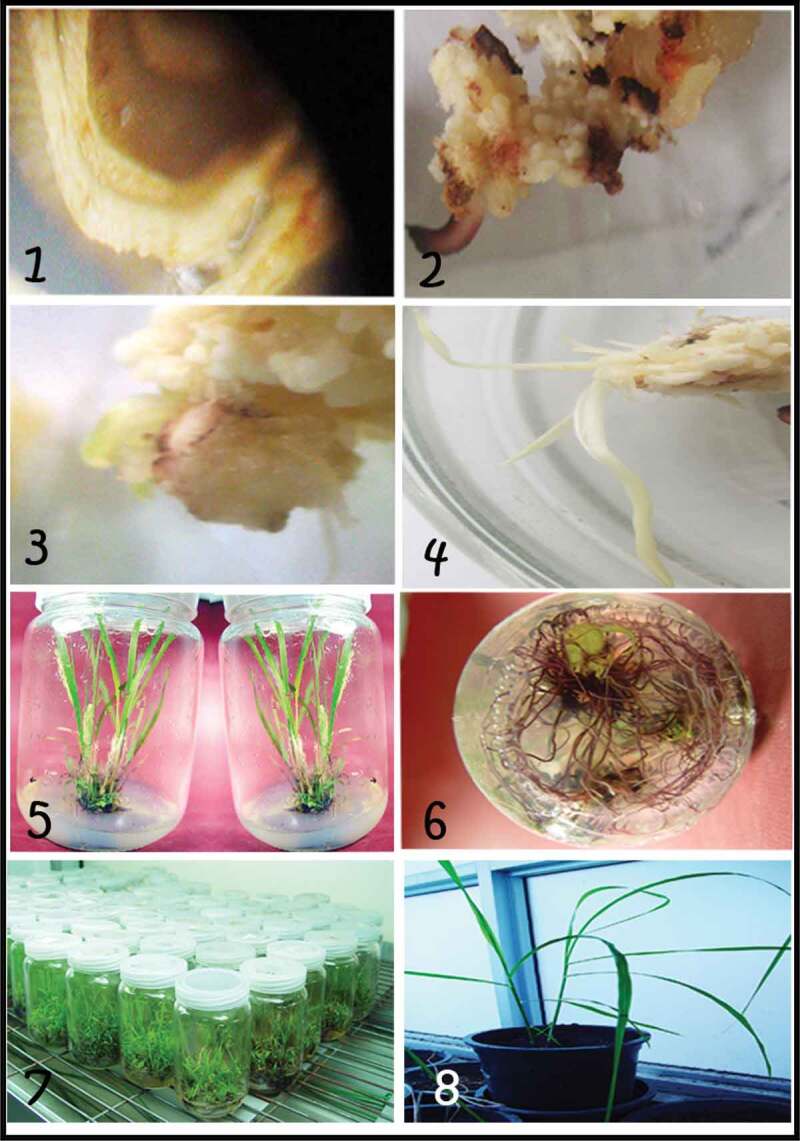
The regeneration stages of transformed sugarcane plants via direct organogenesis. (1) Leaf young explant taken from 6- to 8-month-old sugarcane (2, 3, and 4) stages of young leaf explant. (5) Shoots formation. (6) Root formation. (7 and 8) Acclimatization of transformed sugarcane plants
Detection of Transgenic Sugarcane
The transformed kanamycin resistant sugarcane shoots were used for DNA isolation. The polymerase chain reaction (PCR) was used to confirm the cry1Ac gene integration into the genetic material of the putative kanamycin resistant shoots (transgenic) of sugarcane cultivar GT54-9(C9) using nptII and cry1Ac specific primers. The selected primers were designed to amplify fragments of 250 and 497 bp of the nptII and cry1Ac genes, respectively. Out of 90 plants examined from kanamycin resistant tissues only 20 gave positive results (The PCR test showed a clear band corresponding to the relevant sequence of both primers) with a percentage of 22.2%.
Southern blot is a commonly used technique to confirm gene integration and copy numbers in transgenic plants. Southern blotting analysis was used to confirm the integration of the cry1Ac gene in the T1 sugarcane plants. A restriction enzyme, KpnI was used to digest sugarcane genome and then digested DNA was hybridized with cry1Ac specific probe that showed the integration of cry1Ac gene in sugarcane genetic material. Liner pARTcry1Ac plasmid DNA was used as positive control. Southern blotting test for transgenic plants showed bands >3256 bp molecular weight as expected (Figure 3).
Figure 3.
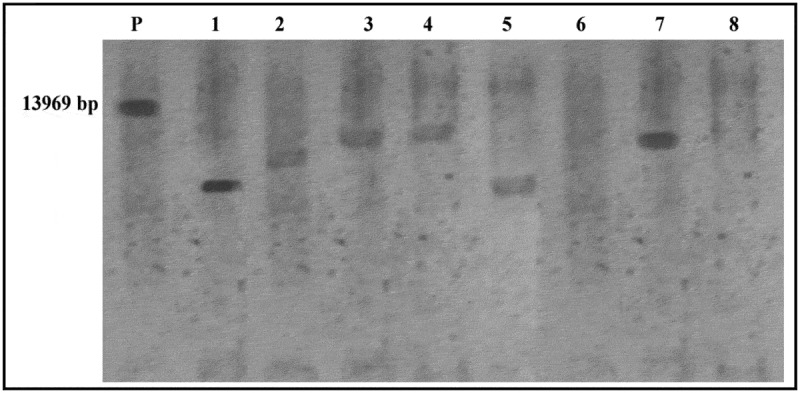
Southern blot hybridization of genomic DNA isolated from transgenic sugarcane plants, transformed by the cry1Ac gene. P; the liner pARTcryAc plasmid (positive control) and lanes 1–8 are the cry1Ac transgenic sugarcane plants
Expression of cry1Ac Gene in Transgenic Sugarcane Plants
The stable expression of the cry1Ac gene in the transgenic sugarcane lines was confirmed by using reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extract from PCR-positive putative transgenic lines and also from the nontransgenic plants (as negative control). Extracted RNA was used as a template in RT-PCR for synthesizing the cDNA followed by amplification of the cry1Ac gene with cry1Ac specific primer (Table 1). The results showed that a RT-PCR fragment with a molecular size of about 497 bp was amplified from total RNA isolated from transformed plants (Figure 4). The RT-PCR analysis for the sugarcane plants showed the occurrence of the mRNA for the cry1Ac gene in 6 out of 8 (75%) PCR-positive plants for sugarcane.
Table 1.
The primer sequences of transgenes used for confirmation of T-DNA integration in putative transformed plantlets and RT-PCR
| Genes | Sequences 5`- 3` | Expected Size |
|---|---|---|
| npt-II | F- CGCAGGTTCTCCGGCCGCTTGGG TGG | 250 bp |
| R- GACTTCGCCTTCCCTGACCGACGA | ||
| cryA1c | F- GCATCTTCGGCCCGTCCCAGTC | 497 bp |
| R- ACGCGCTCCAGGCCGGTGTTGTA |
Figure 4.
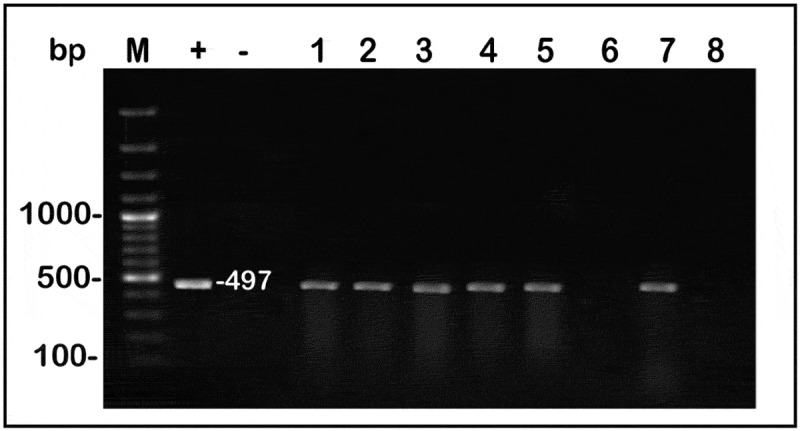
RT- PCR isolation of the cry1Ac gene of transformed sugarcane. M: 100 bp DNA ladder marker. Lanes (1, 2, 3, 4, 5, and 7) represent putative transformed Sugarcane plants, Lane (-) (nontransformed sugarcane) negative control, Lane (+) (pARTcry1Ac vector) positive control
Survey with Trip Tests for the Cry1Ac Protein
The QuickStix test was done with Cry1Ac to detect the expression of the Cry1Ac protein in eight transgenic sugarcane lines. In the strip containing lines 6 and 8, only the assay band was observed. This indicated the absence of Cry proteins and presence of analyze. It also indicates that two spurious bands are not formed due to analyze. In samples containing the cry1Ac (lines 1, 2, 3, 4, 5, and 7) both assay band and expression band were detected as shown in Figure (5). This indicated that those lines containing the Cry1Ac protein.
Figure 5.

Cry1Ac protein expression in transgenic sugarcane on QuickStix Combo strips. Lines 6 and 8 showed negative results (one band). Lines 1, 2, 3, 4, 5, and 7 showed positive results (two bands)
Bioassay
It is important to evaluate potential insect-resistant transgenic plants for insect resistance against target pest(s) at field conditions and conducting insect laboratory bioassays. Figure (6) shows the mortality percentage of Sesamia cretica caused by transgenic plants expressing cry1Ac toxin. The results indicated that the lethal concentration 50 (LC50) value for cry1Ac toxin protein from transformed sugarcane plants were 500 ppm (line 12, 15) and 300 ppm (line 5, 8, 14, 16) against the Sesamia cretica in 6 transformed plants. The mortality percentage of cry1Ac toxin expressed in all transgenic plants against Sesamia cretica were 100% with 1000 ppm compared to the negative control (Table 2). This data indicated the high expression of cry1Ac gene in the transformed sugarcane plant. The transgenic plants showed higher resistant to the target pests. However, the line 8 showed the highest toxicity to the larvae at lower concentrations followed by line 16. Therefore, these two lines are recommended for using to further experiments to control the steam borer Sesamia cretica.
Figure 6.
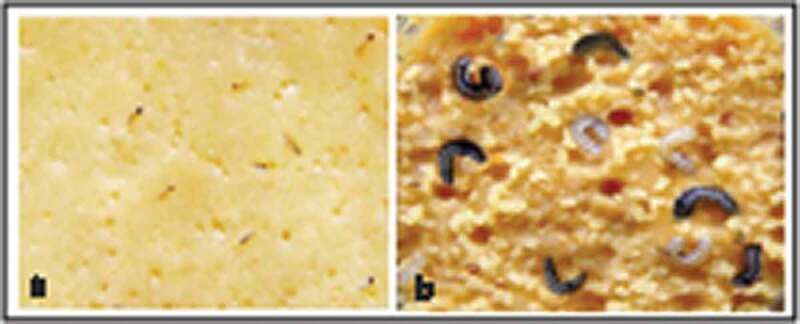
Determine the mortality percentage of Cry1Ac toxin protein against Sesamia cretica. (a) Mortality reached 100% at 1000 ppm of dried transformed sugarcane leaves compared to the (b) control
Table 2.
Mortality percentages of Sesamia cretica fed on nontransgenic (control) and six transformed sugarcane of expressing the cry1AC gene
| % mortality/Concentration (ppm) |
||||||
|---|---|---|---|---|---|---|
| Selected sugarcane lines | 1000 | 700 | 500 | 300 | 200 | Lethal concentration 50 (LC50) |
| Nontransformed | 0% | 0% | 0% | 0% | 0% | - |
| 5 | 100% | 80% | 60% | 50% | 20% | 300 ppm |
| 8 | 100% | 100% | 80% | 60% | 40% | 300 ppm |
| 12 | 100% | 70% | 50% | 40% | 20% | 500 ppm |
| 14 | 100% | 70% | 60% | 50% | 30% | 300 ppm |
| 15 | 100% | 80% | 60% | 40% | 30% | 500 ppm |
| 16 | 100% | 90% | 70% | 50% | 20% | 300 ppm |
Discussion
Transgenic plants expressing cry genes from Bacillus thuringiensis could drastically minimize the use of broad-spectrum insecticides against insect pests. Endotoxins produced by Bt strains have insecticide effect for some of the main pests of important crop plants.28 Genetically modified plants expressing Bt genes are more effective in controlling to pests than Bt formulations. In 1995, the Environmental Protection Agency (EPA) in the United States approved the commercial production and distribution of the Bt crops: corn, cotton, potato, and tobacco.29 Recently, Bt soybean varieties expressing the cry1Ac gene has been approved for commercial use in Latin America30 and Bt sugarcane CTC175 has been approved for commercial use in Brazil.31 The use of synthetic insecticides was significantly reduced by using commercialization Bt crops.32 A lot numbers of cry genes have been tested and described against main insect pests.33 Many research suggested that the toxicity of cry toxins was due to either their pore formation ability34 or the signal transduction pathway by receptor binding.35 The mode of action model of Bt genes suggest that cry toxins pass through a successive binding mechanism with several insect gut proteins leading to membrane insertion, pore formation, and toxin oligomerization.35 The cry toxin produced as protoxin in Bt bacterial cells. A high yield of an active three-domain toxin of Bt cry toxins can be produced by insect gut proteases enzymes. Both cry1A protoxin and activated toxin binds to cadherin receptor forming distinct oligomers that insert into the membrane forming lytic pores. The outcome of this double mode of action is the decreasing of possibility evolution of resistance and possibly to expand the spectrum of insect targets. For the continues use of Bt crops, it is probable that obtaining a stable expression of cry full length proteins will have the same outcome, delaying resistance and protection from a broad number of insect pests.36 The first reported of the cry1Ac gene expression using Agrobacterium transformation method was in cotton for insect resistant to bollworm (Helicoverpa armigera).37 In sugarcane, Agrobacterium mediated genetic transformation is considered to be reliable method and more efficient than direct biolistic gene transfer method.38 Agrobacterium mediated genetic transformation has traditionally been the preferred method to generate events with low transgene copy number. Standard biolistic gene transfer method, in which large quantities of whole plasmid constructs are introduced, typically result in the integration of multiple transgene copies as well as vector backbone sequences into the plant genome.39 In this investigation, sugarcane variety GT54-9(C9) was transformed with A. tumefaciens GV3101 that have pARTcry1Ac binary vector. The target gene integration and expression in the plant genomic DNA are important reasons for success of transformation as well as its inheritance in progeny plants. The stable integration of the cry1Ac gene into sugarcane DNA was assured by using the PCR and Southern blot analysis. The results showed that only twenty plants from ninety gave specific bands (250, 497 bp) corresponding to both nptII and cry1Ac specific primers, repressively about 22.2% were transformed sugarcane plants. The copy number determination of transgenes in transgenic plants is important due to the effect of the copy number on the gene expression level and genetic stability. Southern blot analysis is considered the traditional method to assess copy number of exogenous genes in transgenic plants. In this study Southern results showed the integration of cry1Ac gene in the sugarcane genetic material. However, the gene copy number was integrated in one to three position in sugarcane plants that were transformed using Agrobacterium method.24,40,41 The cry1Ac toxin demonstrated to be expressed by transgenic sugarcane plant and it remains biologically active when absorb by the target insects. Quickstix was used to quantification determination of cry proteins. RT-PCR analysis was used to confirm the expression of the cry1Ac gene. RT-PCR technique can be used to determine the presence or absence of specific transcript and the steady-state RNA levels. Falco and Silva-Filho42 used RT-PCR in transformed sugarcane plants to reveal the expression of cry1Ac gene and detected that all plants expressed mRNA of the transgene.
Clear effects of cry1Ac expression were tested by the death-rate of Sesamia cretica when it was fed on transgenic Bt sugarcane. These results showed that a large amount of Bt protein was found in all sugarcane transgenic lines and that for target lepidopteran insect pests management the plants expressing cry1Ac gene could be used. The mortality percentage of cry1Ac toxin expressed in all transgenic plants against Sesamia cretica were 100% with 1000 ppm compared to the negative control. This data indicated the high expression of cry1Ac gene in the transformed sugarcane plant. The results indicated that sugarcane line 8 showed the highest toxicity to the larvae at lower concentrations followed by line 16 this may be due to the integration of only one cope number of the gene due to the result of Southern blot analysis. Many earlier researchers found that the multiple T-DNA insertions had exhibited less expression levels than single copy transgenes.43 These results also are similar to Lin et al,43 they found that bioassays with cry1Ac transformed transgenic tobacco plants showed high level of toxicity toward (Spodoptera litura) giving rate of 76.9 to 100% mortality of the larvae after 72 hr. Earlier, sugarcane cultivars, CoJ 64 and Co 86032 was modified by the cry1Ab gene.44 The percentage of cry1Ab protein in several transgenic lines ranged from 0.007% to 1.73% for total soluble protein in leaves. At the seedling stage, transgenic plants had significantly less dead rate and there was a negative relation between the dead rate and protein expression. Weng et al.45 found that only resistance was showed in transgenic sugarcane lines expressing the cry1Ac protein more than 9 ng/mg when they analyzed pest resistance of cry1Ac. A positive correlation between pest resistance and the cry1Ac content was also detected.
Conclusion
The transgenic sugarcane with cry1Ac gene that has been inserted into sugarcane genetic material by Agrobacterium transformation method, showed resistant to insects and high productivity. From different molecular analyzes confirmed that crystal protein gene is stable integrated into transgenic sugarcane genome.
Materials and Methods
Plant Material
Sugarcane variety GT54-9(C9) was obtained from Sugar Crops Research Institute, Agricultural Research Center, Giza, Egypt. Young leaf explants (apical part of the shoot) 3 cm in length of several layers of leaves taken from 6- to 8-month-old sugarcane (Saccharum officinarum cv. GT54-C9). The outer leaves were removed to expose the six inner leaves. innermost six leaves were sterilized in 70% ethanol for one min, disinfected in 40% clorox solution for 20 minutes 40% (v/v), then washed four times in sterile distilled water followed by removing the outer 6th and 5th leaves. Eight cm segments from the bases of the innermost three or four leaves were cut into small transverse sections (2–3 mm) and used as explants.
Bacterial Strains and Vector
Agrobacterium tumefaciens GV1303 strain containing pARTcry1Ac plasmid was used to transform sugarcane explants for produce insect resistance plants. The plasmid was constructed by Prof. Dr. Naglaa Abdallah from cloning the synthetic cry1Ac gene accession number AF023672.1 that was kindly provided by Dr. Pamela Green46 (it was modified for plants to achieve higher expression) into the pART27 binary vector and under the control of 35S promoter and was used for transforming sugarcane explants using Agrobacterium method (Figure 7).
Figure 7.
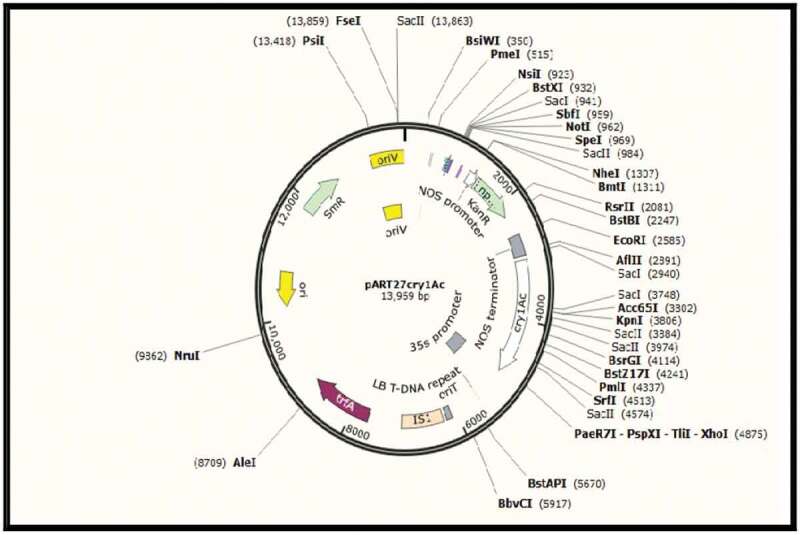
The map of pARTcry1Ac vector
Transformation and Regeneration Conditions
Young leaf explants were soaked in Agrobacterium solution for fifteen min, left to dry on a sterilized filter paper, and then co-cultivated on the shooting formation media (MS including 2 mg/l NAA and 1 mg/l BAP) for three days. After co-cultivation, the explants were transferred to the same medium supplemented with 300 mg/l carbinicillin to inhibit Agrobacterium growth in addition to 100 mg/l kanamycin for transgenic shoots selection. Developed shoots were re-cultured on the optimized elongation medium (MS supplemented 0.1 mg/l BAP and 2 mg/l Kin) to reach suitable length. The cultures were kept in the growth chamber at 28 ± 2°C under 16 hours photoperiod of 3000 Lux provided with white cool fluorescent lamps. Then the shoots were transferred to appropriate rooting media (MS including 2 mg/l NAA) and plantlets were acclimatized in the greenhouse.47
Survival Curve of Kanamycin
To set the minimal lethal dose of kanamycin, different concentrations 0, 25, 50, 75, 100, 125, 150 mg/l kanamycin was added to MS medium and 90 explants were used for each concentration. Kanamycin was sterilized using disposable filters (0.22 µn) and mixed with precooled (45–50°C) autoclaved MS medium. The percentages of explants survival (kanamycin resistant) were recorded after 21 days from culturing.
PCR Conformation
The genetic material of the putative transgenic tissues were extracted via CTAB method according to Lassner.48 Two specific oligonucleotides primers for cry1Ac and npt-II genes were used to confirm the stable integration of the T-DNA into sugarcane genome in PCR reaction (Table 1). The DNA Synthesizer 392, Applied Biosystems at AGERI, ARC, Giza, Egypt was used for primers manufacturing. The PCR reaction was prepared in a 50 µl consisting of a final concentration of each of the following: 200 µM of each of dNTPs (dGTP, dCTP, dATP, and dTTP), 1 pmoles from each of the used primers, 1X PCR buffer, 0.04 U Taq DNA polymerase, 2 ng of plant DNA (as template), 2.5 mM MgCl2 and d.H2O. Amplification cycle program of the synthetic Bt gene was performed as following: 95°C for 5 min, followed by 35 cycles at 94°C for 60 sec, 55°C for 60 sec, 72°C for 60 sec, and a final extension at 72°C for 7 min. 1.5% agarose gel electrophoresis was used for PCR analysis by loading 15 µl of PCR product with 3 µl loading buffer
Southern Blotting Analysis
Total DNA was isolated from transformed sugarcane as described previously by Lassner48 method. 10 µg of DNA was digested using restriction enzyme, separated by electrophoresis (using 1% agarose gels) and transferred to Hybond NC nylon membrane (Amersham, RPN119B, Netherlands) as described by Sambrook.49 Prehybridization and hybridization conditions were in strict accordance with the manufacturer’s recommendations. PCR produced from cry1Ac gene (497 bp) was used as a probes. Biotin Chromogenic Detection kits (#K0661 & #K0662, Ferments Life Sciences, USA) was used for hybridization and detection according to instructions provided by the manufacturer.
Reverse Transcription PCR (RT-PCR) Reaction
Total RNA was extracted from PCR positive plants using SV Total RNA Isolation System (Promega, cat. #Z3100, USA). RT-PCR analysis was performed using Robust Ι RT-PCR kit, Finnzymes, Finland. The analysis were carried out on both putative transformed (PCR positive) and nontransformed plants using the cry1Ac gene specific primers (Table 1) and the PCR products were separated in 1.5% agarose gels.
Survey with Trip Tests for the Cry1Ac Protein
QuickStix Combo Kit for Cry1A and Cry2A (cat. #AS 012 LS, EnviroLogix, Portland, Oregon, USA) was used to detect the presence of cry1Ac protein in transgenic sugarcane leaves. The samples and protein extract were performed according the manufacturer’s instructions.
Two leaf punches were taken from each plant by snapping cap of eppendorf tube and were grounded by rotating pestle until fine grinding. Extraction buffer (0.5 ml) was added to the tube and mixed with the leaf tissue. QuickStix strips were dipped in the leaf and examined after 10 min for the appearance of the final bands on strip and results were recorded.
Bioassay
Sesamia cretica larvae’s obtained from a laboratory culture were reared on an artificial medium diet as method described by Dulmage.50 Transformed sugarcane plants with positive PCR results for nptII and cry1Ac were subjected for bioassay test. One gram of dried grounded sugarcane leaves was suspended in 100 ml water to give 1000 ppm and further diluted to prepare different concentrations of 1000, 700, 500, 300, and 200 ppm. A volume of 500 µl of each dilution was added onto the surface of each cup containing artificial media and three replicates from each dilution were prepared. After the toxin was completely dried and the surface become dry, 10 neonate larvae were placed on the media surface and were monitored for 96 hrs. The cups were covered with aluminum foil and left at 26°C (± 2°C). The leaves of nontransformed sugarcane plant with 500 µl water were used as negative control. The mortality was recorded every 24 hours for four days.
Statistical Analysis
For statistical analysis, ANOVA program was used for variance analysis of data. For all treatments, significance at 5% level was used to test differences among means by using Duncan51 new multiple range test as described by Snedecor and Cochran.52 Means followed by the same letter are not significantly different at p ≤ 0.05.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Raza G, Ali K, Mukhtar Z, Mansoor M, Arshad M, Asad S.. The response of sugarcane (Saccharum officinarum L.) genotypes to callus induction, regeneration and different concentrations of the selective agent (geneticin-418). Afr J Biotechnol. 2010;9:8739–47. [Google Scholar]
- 2.Fisher G, Teixeira E, Hizsnyik ET, Velthuizen HV. Land use dynamics and sugarcane production. In: Zuurbier P, Vooren JVD editors. Sugarcane ethanol: contributions to climate change mitigation and the environment. Netherlands: Wageningen Academic Publishers; 2008. p. 29–62. [Google Scholar]
- 3.Khaled KA, El-Arabi NI, Sabry NM, El-Sherbiny S. Sugarcane genotypes assessment under drought condition using amplified fragment length polymorphism. Biotechnology. 2018;17:120–27. doi: 10.3923/biotech.2018.120.127. [DOI] [Google Scholar]
- 4.Roach BT. Origin and improvement of the genetic base of sugarcane. Egan BT Ed Proc Aust Soc Sugarcane Technol. 1989;11:34–47. [Google Scholar]
- 5.Dillon SL, Shapter FM, Robert HJ, Cordeiro G, Izquierdo L, Lee SL. Domestication to crop improvement: genetic resources for Sorghum and Saccharum (Andropogoneae). Ann Bot. 2007;5:975–89. doi: 10.1093/aob/mcm192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng LX, Deng HH, Xu JL, Li Q, Zhang YQ, Jiang ZD, Zhang LH. Transgenic sugarcane plants expressing high levels of modified cry1Ac provide effective control against stem borers in field trials. Transgenic Res. 2011;20(4):759–72. doi: 10.1007/s11248-010-9456-8. [DOI] [PubMed] [Google Scholar]
- 7.Rossato JAS Jr., Fernandes OA, Mutton MJR, Higley LG, Madaleno LL. Sugarcane response to two biotic stressors: diatraea saccharalis and Mahanarva fimbriolata. Int Sugar J. 2011;113:453–55. [Google Scholar]
- 8.Goebel FR, Way M. Investigation of the impact of Eldana saccharina (Lepidoptera: pyralidae) on sugarcane yield in field trials in Zululand. Proc S Afr Sug Technol Ass Durban S Afr. 2003;7:256–65. [Google Scholar]
- 9.Kalunke MR, Archana MK, Babu KH, Prasad DT. Agrobacterium-mediated transformation of sugarcane for borer resistance using Cry1Aa3 gene and one-step regeneration of transgenic plants. Sugar Tech. 2009;11(4):355–59. doi: 10.1007/s12355-009-0061-1. [DOI] [Google Scholar]
- 10.Karthikeyan A, Valarmathi R, Nandini S, Nandhakumar M. Genetically modified crops: insect resistance. Biotechnology. 2012;11:119–26. doi: 10.3923/biotech. [DOI] [Google Scholar]
- 11.Basso MF, da Cunha BADB, Ribeiro AP, Martins PK, de Souza WR, de Oliveira NG, Nakayama TJ, Augusto Das Chagas Noqueli Casari R, Santiago TR, Vinecky F. Improved genetic transformation of sugarcane (Saccharum spp.) embryogenic callus mediated by Agrobacterium tumefaciens. Curr Protoc Plant Biol. 2017;2:221–39. doi: 10.1002/cppb.20055. [DOI] [PubMed] [Google Scholar]
- 12.Anunanthini P, Kumar SR, Sathishkumar R. Factors affecting genetic transformation efficiency in sugarcane. In: Mohan C, editor. Sugarcane biotechnology: challenges and prospects. Cham (Switzerland): Springer International Publishing; 2017. p. 61–73. [Google Scholar]
- 13.Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- 14.Gill SS, Cowles E, Pietrantonio PV. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–36. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Yang Y, Wang C, Guo J, Zhou D, Wu Q, Su Y, Xu L, Que Y. Transgenic sugarcane with a cry1Ac gene exhibited better phenotypic traits and enhanced resistance against sugarcane borer. Plos One. 2016;11:e0153929. doi: 10.1371/journal.pone.0153929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye GY, Yao HW, Shu QY, Cheng X, Hu C, Xia YW, Gao MW, Altosaar I. High levels of stable resistance in transgenic rice with a cry1Ab gene from Bacillus thuringiensis Berliner to rice leaves folder, Cnaphalocrocis medinalis (Guenee) under field conditions. Crop Prot. 2003;22:171–78. doi: 10.1016/S0261-2194(02)00142-4. [DOI] [Google Scholar]
- 17.Stewart SD, Adamczyk JJ, Knighten KS, Davis FM. Impact of Bt cottons expressing one or two insecticidal proteins of Bacillus thuringiensis Berliner on growth and survival on noctuid (Lepidoptera) larvae. J Econ Entomol. 2001;94:752–60. doi: 10.1603/0022-0493-94.3.752. [DOI] [PubMed] [Google Scholar]
- 18.Delannay X, LaVallee BJ, Proksch RK, Fuchs RL, Sims SR, Greenplate JT, Marrone PG, Dodson RB, Augustine JJ, Layton JG, et al. Field performance of transgenic tomato plants expressing the Bacillus thuringinesis var. kurstaki insect control plant. Bio Technol. 1989;7:1265–69. [Google Scholar]
- 19.Perlak FJ, Stone TB, Muskopf YM, Peterson LJ, Parker GB, Mcpherson SA, Wyman J, Love S, Reed G, Biever D, et al. Genetically improved potatoes: protection from damage by colorado potato beetles. Plant Mol Biol. 1993;22:313–21. doi: 10.1007/BF00014938. [DOI] [PubMed] [Google Scholar]
- 20.Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson L, Desai N, Hill M, Kadwell S, et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Bio Technol. 1993;11:194–200. [Google Scholar]
- 21.Arencibia A, Vazquez RI, Prieto D, T´ Ellez P, Carmona ER, Coego A, Hernandez L, De la Riva´ GA, Selman-Housein G. Transgenic sugarcane plants resistant to stem borer attack. Mol Breed. 1997;3:247–55. doi: 10.1023/A:1009616318854. [DOI] [Google Scholar]
- 22.Wang WZ, Yang BP, Feng XY, Cao ZY, Feng CL, Wang JG, Xiong GR, Shen LB, Zeng J, Zhao TT. Development and characterization of transgenic sugarcane with insect resistance and herbicide tolerance. Front Plant Sci. 2017;8:1535. doi: 10.3389/fpls.2017.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan SA, Hanif Z, Irshad U, Ahmad R, Yasin M, Chaudhary FM, Afroz A, Javed MT, Rashid U, Rashid H. Genetic transformation of sugarcane variety HSF-240 with marker gene GUS. Int J Agric Biol. 2013;15:1258–64. [Google Scholar]
- 24.Arencibia AD, Carmona ER, Tellez P, Chan MT, Yu SM, Trujillo LE, Oramas P. An efficient protocol for sugarcane (Saccharum spp L.) transformation mediated by Agrobacterium tumefaciens. Transgenic Res. 1998;7:213–22. doi: 10.1023/A:1008845114531. [DOI] [Google Scholar]
- 25.Enriquez-Obregon GA, Vazquez-Padron RI, Pieto-Samsonov DL, Dela Riva GA, Selman-Housein G. Herbicide-resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta. 1998;206:20–27. doi: 10.1007/s004250050369. [DOI] [Google Scholar]
- 26.Colby SM, Meredith CP. Kanamycin sensitivity of cultured tissue of Vitis. Plant Cell Rep. 1990;9:237–40. doi: 10.1007/BF00232291. [DOI] [PubMed] [Google Scholar]
- 27.Khalil SM. Regeneration via somatic embryogenesis and microprojectile-mediated co-transformation of sugarcane. Arab J Biotech. 2002;5:19–32. [Google Scholar]
- 28.Gatehouse JA. Biotechnological prospects for engineering insect-resistant plants. Plant Physiol. 2008;146(3):881–87. doi: 10.1104/pp.107.111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbas MST. Genetically engineered (modified) crops (Bacillus thuringiensis crops) and the world controversy on their safety. Egypt J Biol Pest Control. 2018;28:52. doi: 10.1186/s41938-018-0051-2. [DOI] [Google Scholar]
- 30.Marques LH, Santos AC, Castro BA, Storer NP, Babcock JM, Lepping MD, Sa V, Moscardini VF, Rule DM, Fernandes OA. Impact of transgenic soybean expressing Cry1Ac and Cry1F proteins on the non-target arthropod community associated with soybean in Brazil. Plos One. 2018;13(2):e0191567. doi: 10.1371/journal.pone.0191567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianotto AC, Rocha MS, Cutri L, Lopes FC, Dal’Acqua W, Hjelle JJ, Lirette RP, Oliveira WS, Sereno ML. The insect-protected CTC91087-6 sugarcane event expresses Cry1Ac protein preferentially in leaves and presents compositional equivalence to conventional sugarcane. GM Crops Food. 2019;10:208–19. doi: 10.1080/21645698.2019.1651191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferre J, van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann Rev Entomol. 2002;47:501–33. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 33.Crickmore N, Baum J, Bravo A, Lereclus D, Narva K, Sampson K, Chnepf E, Sun M, Zeigler DR. 2018. Bacillus thuringiensis toxi nomenclature. Bacterial Pesticidal Protein Resource Center. https://www.bpprc.org. [Google Scholar]
- 34.Soberón M, Gill SS, Bravo A. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci. 2009;66:1337–49. doi: 10.1007/s00018-008-8330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Candas M, Griko NB, Taussig R, Bulla LA Jr. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci. 2006;103:9897–902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bravo A, Gómez I, Conde J, Muñoz-Garay C, Sánchez J, Zhuang M, Gill SS, Soberón M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim Biophys Acta. 2004;1667:38–46. doi: 10.1016/j.bbamem.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Soberón M, Monnerat R, Bravo A. Mode of action of cry toxins from Bacillus thuringiensis and resistance mechanisms. In: Gopalakrishnakone P, Stiles B, Alape-Girón A, Dubreuil JD, Mandal M, editors. Microbial toxins, toxinology. Dordrecht (The Netherlands): Springer Nature; 2016. p. 15–27. doi: 10.1007/978-94-007-6725-6_28-1. [DOI] [Google Scholar]
- 38.Wu H, Awan FS, Vilarinho A, Zeng Q, Kannan B, Phipps T, McCuiston J, Wang W, Caffall K, Altpeter F. Transgene integration complexity and expression stability following biolistic or Agrobacterium-mediated transformation of sugarcane. In Vitro Cell Dev Biol Plant. 2015;51:603–11. doi: 10.1007/s11627-015-9710-0. [DOI] [Google Scholar]
- 39.Jayaraj J, Liang GH, Muthukrishnan S, Punja ZK. Generation of low copy number and stably expressing transgenic creeping bentgrass plants using minimal gene cassette bombardment. Biol Plant. 2008;52:215–21. doi: 10.1007/s10535-008-0048-x. [DOI] [Google Scholar]
- 40.Benedict J, Altman D. Commercialization of transgenic cotton expressing insecticidal crystal protein. In: Jenkins JN, Saha S, editors. Genetic improvement of cotton: emerging technologies. Enfield (UK): Science Publishers; 2001. p. 137–201. [Google Scholar]
- 41.Hobbs SLA, Kpodar P, DeLong CMO. The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol. 1990;15:851–64. doi: 10.1007/BF00039425. [DOI] [PubMed] [Google Scholar]
- 42.Falco MC, Silva-Filho MC. Expression of soybean proteinase inhibitors in transgenic sugarcane plants: effects on natural defense against Diatraea saccharalis. Plant Phys Biochem. 2003;41:761–66. doi: 10.1016/S0981-9428(03)00100-1. [DOI] [Google Scholar]
- 43.Lin CH, Chen YY, Tzeng CC, Tsay HS, Chen JC. Expression of a Bacillus thuringiensis cry1C gene in plastid confers high insecticidal efficacy against tobacco cutworm - a Spodoptera insect. Bot Bull Acad Sin. 2003;44:199–210. [Google Scholar]
- 44.Arvinth S, Arun S, Selvakesavan RK, Srikanth J, Mukunthan N, Ananda Kumar P, Premachandran MN, Subramonian N. Genetic transformation and pyramiding of aprotinin-expressing sugarcane with cry1Ab for shoot borer (Chilo infuscatellus) resistance. Plant Cell Rep. 2010;29(4):383–95. doi: 10.1007/s00299-010-0829-5. [DOI] [PubMed] [Google Scholar]
- 45.Weng LX, Deng HH, Xu JL, Li Q, Wang LH, Jiang ZD, Zhang HB, Li QW, Zhang LH. Regeneration of sugarcane elite breeding lines and engineering of strong stem borer resistance. Pest Manage Sci. 2006;62:178–87. doi: 10.1002/ps.1144. [DOI] [PubMed] [Google Scholar]
- 46.Rocher EJ, Vargo-Gogola TC, Diehn SH, Green PJ. Direct evidence for rapid degradation of Bacillus thuringiensis toxin mRNA as a cause of poor expression in plants. Plant Physiol. 1998;117:1445–61. doi: 10.1104/pp.117.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dessoky SE, Ismail RM, Abdelhadi AA, Abdallah NA. Establishment of regeneration and transformation system of sugarcane cultivar GT54-9 (C9). GM Crops. 2011;2(2):126–34. doi: 10.4161/gmcr.2.2.17288. [DOI] [PubMed] [Google Scholar]
- 48.Lassner MW, Peterson P, Yoder JI. Simultaneous amplification of multiple DNA fragments by polymerase chain reaction in the analysis of transgenic plants and their progeny. Plant Mol Biol Rep. 1989;7:116–28. doi: 10.1007/BF02669627. [DOI] [Google Scholar]
- 49.Sambrook J, Fritschi EF, Maniatis T. Molecular cloning: a laboratory manual. New York (NY): Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Dulmage HT. Production of delta-endotoxin by 18 isolates of Bacillus thuringiensis, serotype 3, in 3 fermentation media. J Invert Pathol. 1971;18:353–58. doi: 10.1016/0022-2011(71)90037-1. [DOI] [PubMed] [Google Scholar]
- 51.Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 52.Snedecor GW, Cochran WG. Statistical methods. 6th ed. Ames, IA: Lowa State University; 1967. [Google Scholar]


