ABSTRACT
Cells and organisms are intrinsically prepared to effectively deal with damage caused by insults and heal themselves by triggering a plethora of stress responses including macroautophagy/autophagy. However, autophagy may become malfunctional during aging, neurodegeneration, and neurotrauma. We aimed to overcome autophagy dysfunction by refining therapeutics using multi-target approaches. Thus, we have demonstrated that modulation of autophagy with the multitarget drug NeuroHeal is neuroprotective in several neurodegeneration models in which previous autophagy modulators have failed. The key element of success is the coordinated activation of opposing forces that modulate autophagy with NeuroHeal, probably leading to the autophagy-dependent degradation of death executors such as PARP1. The precise tuning of autophagy thus allows the neuron to adapt to insults, survive and repair itself. These findings support the advent a new era of neuroprotectants that counteract neuronal damage by targeting in unison different pathways of the self-repair process, including autophagy.
KEYWORDS: Cellular resilience, endogenous mechanisms of protection, fine-tuned autophagy, multitarget, neurodegeneration, neuroheal, neuroprotection
A warrior wearing a shield is almost unbeatable. But any damage to the shield would reduce her/his possibilities to avoid accurate blows. Then it is reasonable to think that the repair of the shield will enhance the chances of survival of the warrior. This was the rationale behind our discovery of NeuroHeal. There are endogenous mechanisms of protection that neurons (and other cells) immediately engage in upon insult or damage. The fact that neurons are long-lived cells is indeed in agreement with the presence of potent endogenous mechanisms of neuroprotection that include the unfolded protein response (UPR), the ubiquitin-proteasome system (UPS), anti-apoptotic mechanisms, anti-oxidant reactions and autophagy, to cite a few. There is, however, a wealth of evidence supporting blockage dysfunction of some or several of these processes during aging and neurodegeneration – holes in the warrior’s shield. So, our workflow for the discovery of NeuroHeal was based on the search for neuroprotective drugs that reinforced endogenous neuroprotection, avoiding classical strategies in drug discovery based on the inhibition of putative pathological pathways [1]. Due to the complex nature of endogenous mechanisms of neuroprotection, as well as of neurodegenerative processes, we also abandoned the classical vision of one gene-one target for drug discovery, and stuck to systems biology approaches to gain insight into the intricate regulation of self-healing networks. NeuroHeal, a drug able to restore these pathways acting on multiple targets regulating autophagy, was thus born, and its efficacy was demonstrated on different models of neurodegeneration in adulthood.
However, neonatal neuronal death is different from what happens in adulthood, for it is essentially non-apoptotic and presents distinct dysfunction in endogenous mechanisms of neuroprotection. Would NeuroHeal be neuroprotective also in these highly dissimilar conditions? We examined whether NeuroHeal prevents the death of neonatal neurons after axotomy using a a model of peripheral nerve injury in 4-day-old rat pups. Because NeuroHeal is soluble, and can cross the blood-brain barrier and probably placental barriers, we treated pups by oral administration of the drug to nursing mothers. We observed neuroprotection of damaged neonatal motoneurons by NeuroHeal. Surprisingly, such neuroprotection was not due to the reduction of CASP3 (caspase 3) activation, but to regulation of a downstream step, leading to reduced content of the death executor PARP1. We next examined the involvement of SIRT1, which we have shown to be a target of NeuroHeal, as well as of well-known authophagy regulatory pathways. Using in vitro and in vivo genetic and pharmacological manipulations, we observed that two axes are necessary for NeuroHeal to exert neuroprotection and ameliorate the autophagy flux: SIRT1 and PI3K-AKT. Interestingly, these axes normally exert opposite effects on autophagy, because SIRT1 activates and controls autophagy flux at different sites, whereas PI3K-AKT is a general inhibitor of autophagy (Figure 1). The combined action of these opposing forces leads to low levels of autophagosome and autolysosome formation, which prevents the neuron from succumbing due to the activation of apoptosis resulting from over-activation of the autophagy machinery.
Figure 1.
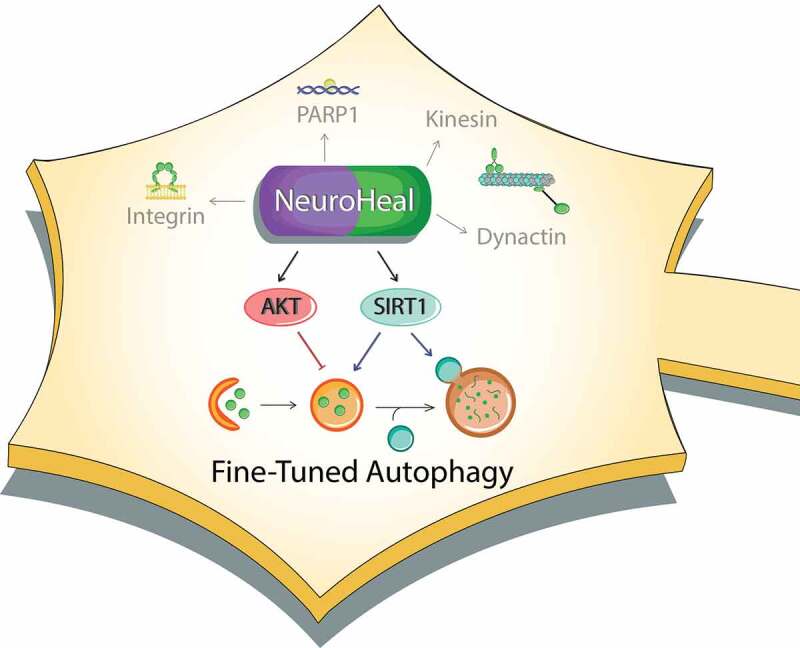
Schematic representation of NeuroHeal’s mechanism of action. NeuroHeal is multitarget modulating integrins, and cytoskeletal proteins, as well as AKT and SIRT1. The latter two act as opposing forces allowing the fine-tuning of autophagy for neuroprotection
These findings may shed light into the long-standing controversy of whether autophagy induction or inhibition ameliorates or aggravates neuronal death. The reason for disparity among studies may be the lack of control for parameters such as the state of the neurons at the starting time of the treatment, which can result in completely different results. Besides, a balanced engagement of autophagy is necessary to avoid uncontrolled outcomes. Inducing excessive autophagy leads to neuronal death by energy failure or by promoting autophagy-related (ATG) gene-dependent death (i.e. ATG5- or BECN1-dependent apoptosis). Conversely, autophagy also has non-canonical protein-degrading functions, so a complete blockade of autophagy is not a good option either. Disruption of basal autophagy thus leads to neurodegeneration, affects axonal performance, and greatly impairs daily activities such as cognition or memory fitness. Thus, although the view exists that autophagy modulation can slow down neurodegeneration, the optimal way to tackle autophagy to achieve neuroprotection is not understood. We posit that, if regulated at the right time and at the right molecular targets, autophagy will be beneficial by: i) removing nonfunctional proteins and organelles; ii) allowing neurons to readapt to new pathological scenarios, iii) degrading harmful effectors such as inflammation inducers or apoptotic mediators/executors. So, it is time to search for next-generation agents that fine-tune autophagy. We suggest that NeuroHeal is a first candidate in this list thanks to its multi-target nature.
Future studies with NeuroHeal, or other fine-tuners of autophagy, may pave the way for novel therapeutic avenues for neurodegenerative disorders, aging, and/or neurotrauma.
Aknowledgements
This study was funded by the Ministerio de Economía y Competitividad of Spain with the grant #SAF 2014-59701. We thank Elena Galea for her critical review.
Funding Statement
This work was supported by the Ministerio de Economía y Competitividad [2014-59701].
Disclosure statement
The authors declare no conflict of interest. NeuroHeal is under patent review.
Reference
- [1].Romeo-Guitart D, Marcos-DeJuana C, Marmolejo-Martínez-Artesero S, et al. Novel neuroprotective therapy with NeuroHeal by autophagy induction for damaged neonatal motoneurons. Theranostics. 2020;10(11):5154–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]


