Abstract
Psoriasis is a common multifactorial autoimmune disease of the skin, and in a large percentage of patients, immune responses involve nail and joint pathology, which develop psoriatic arthritis (PsA). Historically, T helper 1 (Th1)-derived-IFN-γ was abundantly detected in psoriatic skin and its correlation with development and severity of PsO, led to an early classification of psoriasis as a Th1-mediated disease. Investigations of the cellular and molecular mechanisms of PsO pathogenesis in recent years, together with impressive results of biologics against interleukin 17 A (IL-17) have shifted focus on IL-17A. However, the contributions of IFN-γ in IL-17 induced pathology and its involvement in the development of PsA have been largely overshadowed. This review summarizes the current knowledge on IFN-γ and provides new insights on the contribution of IFN-γ to PsO and PsA disease pathogenesis and development.
Keywords: Psoriasis, Psoriatic arthritis, IFN-γ, IL-17A, Keratinocyte, Osteoclast
1. Introduction
Psoriasis (PsO) is a common skin disease affecting 2–3% of the population worldwide [1, 2]. It can be fairly diagnosed as characteristic red colored plaques with well-defined borders and silvery-white dry scale, located on elbows, knees, scalp, and in the lumbosacral area [1, 3]. Approximate 20–30% of PsO patients develop psoriatic arthritis (PsA), a chronic, immune-mediated, inflammatory arthropathy that is characterized by inflammation of the joints and entheses, including those of the axial skeleton [4–6]. The pathogenic features of PsA in the skin and joints include neutrophil infiltration of the dermis and epidermis with keratinocyte hyperplasia and synovio-entheseal inflammation which is characterized by increased angiogenesis and immune cell infiltration respectively.
IFN-γ is a member of a family of proteins originally identified by their capacity to non-specifically protect cells from viral infection [7]. This protein family is divided into three classes on the basis of structural and functional criteria as well as the ir ability to bind receptor complexes on the cell surface. Type I IFNs are primarily induced in response to viral infection of cells and have been further subdivided into groups (IFN-α/β/ω) based on their cellular origin [8]. Type II, now known as IFN-γ, is produced predominantly by T lymphocytes, NKT cells and natural killer (NK) cells following activation by immune and inflammatory stimuli rather than viral infection[7]. Type III interferon is also an important regulator of innate antifungal immunity [9]. In this review we will focus on IFN-γ because although Type I and III interferons are induced in viral immunity type II, IFN-γ is induced by IL-12 family of cytokines which are known to play a role in autoimmune diseases. In fact, the role of IFN-γ in autoimmunity has been noted since the early 1990’s by clinical observations whereby a single intradermal injection of IFN-γ in the skin of PsO patients was sufficient to induce psoriatic lesions [10, 11]. As T helper 1 (Th1)-derived-IFN-γ are abundant in PsO lesionsand non-lesional psoriatic skin (NLP) a positive correlation with development and severity of PsO led to PsO early classification as Th1-mediated disease [12–15]. Despite the initial enthusiasm the recent success of biologics targeting the IL-23/IL-17 axis has diverted attention from IFN-γ, however its contribution to disease amplification should not be underestimated. In recent years, accumulating research has demonstrated a major role of IFN-γ in disease development which encourages a reassessment of the contribution of IFN-γ to PsO and PsA pathogenesis. Herein we present some detailed analysis of the critical cellular and molecular pathways that can induced specialized cells in skin and joint tissues which are paramount for the pathogenesis and clinical manifestations as commonly observed in PsO and PsA patients.
2. IFN-γ and IL-17A signaling working separately in gene regulation
Members of the IL-12 family of cytokines induce IFN-γ expression, with IL-12 being the main inducer of IFN-γ producing Th1 cells. IL-12 is composed of the IL-12p40 subunit and the IL-12p35 subunit, and the heterodimer signals through the IL-12 receptor (IL-12R), which comprises the IL-12Rβ1 and IL-12Rβ2 subunits. IL-12 stimulates Janus kinase 2 (JAK2) and tyrosine kinase 2 (TYK2) activity, leading to phosphorylation of signal transducer and activator of transcription (STAT) family members STAT1, STAT3, STAT5 and, in particular, STAT4 homodimers [16]. Although IL-12 shares the p40 subunit with IL-23 and both signal via the IL-12Rβ2 subunit, the competitive signaling does not diminish IFN-β production as IL-23 signaling induces the expression of IFN-β in other cell types including Tregs, CD56bright NK cells, Th17 cells and non-conventional T cells such as γδ T cells [17], [18], [19].
As a pleiotropic cytokine, IFN-γ regulates many cellular functions and plays a key role in innate and adaptive immunity. IFN-γ exerts its downstream influence by binding to the IFN-γ receptor (IFNGR) which results in recruitment and activation of the Janus kinases (JAK)/signal transducer and activator of transcription (STAT) signaling pathway [20]. Specifically, the IFNGR is a pre-assembled hetero tetramer made of two IFNGR1 and two IFNGR2 subunits, associated with JAK1 and JAK2 kinases, respectively [21]. JAK mediated IFNGR phosphorylation allows binding and phosphorylation of cytoplasmic STAT1, which is then translocated to the nucleus as a transcription factor and bind GAS (IFN-γ-activated site) elements present in the promoter of IFN-stimulated genes, such as interferon regulatory factor 1 (IRF1), major histocompatibility complex (MHC) class I, and CD95, thereby controlling their transcription [8] (Figure 1). Although IFN-γ and IL-17A have no apparent signaling similarities as far as the recruitment of downstream effectors and transducers is concerned, signal convergence and gene regulation is much more discrete and both IFN-γ and IL-17A signatures are detected in lesional psoriatic skin [12, 22]. As IFN-γ induces myeloid dermal dendritic cells that expand IFN-γ and IL-17A producing T cells, and more importantly Th17 cells produce both IFN-γ and IL-17A gene regulation of IFN-γ is not easily distinguishable from IL-17A in psoriasis [23, 24]. Moreover, in multiple studies the transcriptomic analysis of IFN-γ treated healthy and psoriatic skin shows a marked upregulation of IL-23 which further amplifies the IL-17A signal [8] [11]. IFN-γ treatment induced keratinocyte expression of approximately 800 genes most notably the chemokines CXCL9, CXCL10 and CXCL11, which bind to CXCR3-bearing, activated T cells and are thought to be involved in T-cell trafficking to psoriatic dermis and overlying epidermis [25]. In the same study side to side comparison of keratinocyte gene expression between IFN-γ and IL-17A stimulation also highlight the differences as well as the overlap in gene regulation. Other studies using the Reconstructed Human Epidermis (RHE) model, a full thickness epidermal skin structure, consisting of normal human-derived epidermal KCs organized into basal, spinous, granular, and cornified layers, analogously to those found in vivo have also demonstrated differences as well as the overlap in gene regulation by IFN-γ and IL-17 [26]. However, the accuracy of the human in vitro models are questionable. Comparison of the psoriatic transcriptome to the transcriptomes of cytokine-stimulated cultured keratinocytes with IL-17 and IFN-γ, revealed very little overlap, (below 6%) of the lesional psoriatic dysregulated transcriptome, emphasizing the need for more complex yet tractable experimental models of psoriasis [27].
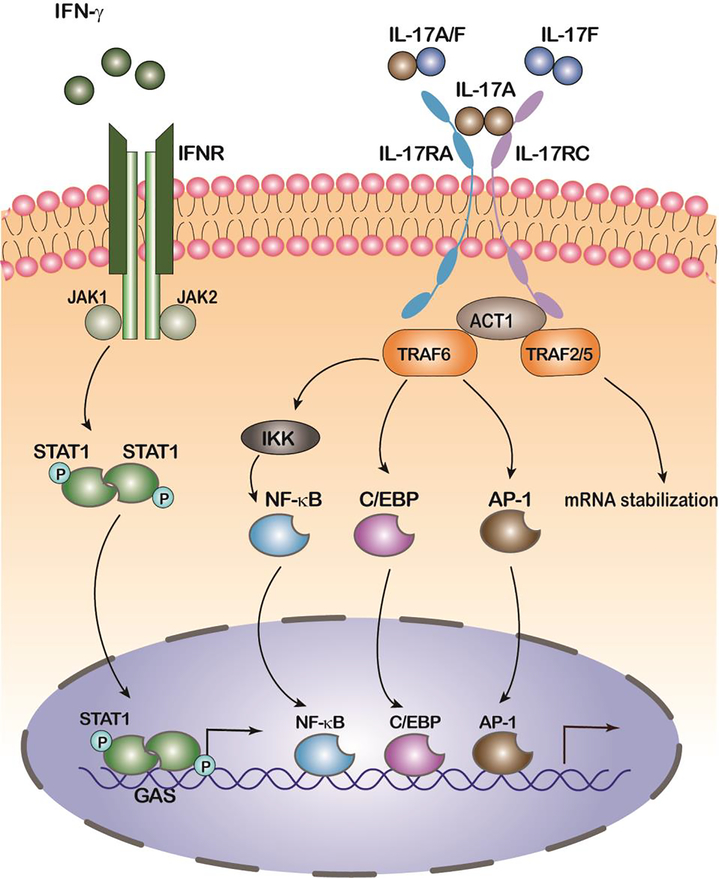
Figure 1. IFN-γ and IL-17 signaling pathways working together, separately.
Upon binding of IFN-γ, IFN-γ receptor–associated (IFNGR-associated) JAKs phosphorylate STAT1, leading to the formation of STAT1 homodimers. STAT dimers translocate to the cell nucleus and activate gene expression by binding to another class of IFN response elements, the GAS. Upon binding of IL-17A, heteromeric IL-17RA and IL-17RC recruit TRAF6, TRAF2 and TRAF5 via Act1. Activation of TRAF6 results in the triggering of NF-κB, C/EBPβ, and MAPK pathways. TRAF2 and TRAF5 transduce the IL-17 signals to stabilize mRNA transcripts of chemokines and cytokines. TRAF: TNF receptor associated factor, IKK: inhibitor of kappa B kinase.
3. IFN-γ and PsO transition to PsA
IFN-γ receptor complex is highly expressed in resident epidermal keratinocytes, which makes keratinocytes a prime target of IFN-γ signaling. Upon IFN-γ exposure, psoriatic keratinocytes show aberrant hyperproliferation and terminal differentiation and neutralization via the RAS signaling pathway [28]. In these murine experiments, neutralization of IFN-γ but not IL-17A markedly reduced KC proliferation suggesting that multiple pathways other than IL-17A could possibly be activated by IFN-γ[28]. Indeed, besides inducing keratinocyte hyperproliferation, IFN-γ also favored the keratinocyte expression of numbers of effector molecules, including adhesion molecules, cytokines, some chemokines, and their receptors and MHC pathway molecules, to accelerate PsO pathogenesis [29]. One such adhesion molecule that is induced in keratinocytes by IFN-γ but not IL-17 is CEACAM1 (Carcinoembryonic antigen-related cellular adhesion molecule 1) which as cell-surface glycoprotein that contributes to the persistence of neutrophils and thus to ongoing inflammation in psoriasis [30]. Oher groups in experiments utilizing IFN-γ and IL-17A deficient mice demonstrated that IFN-γ, but not IL-17A, is necessary for IL-21-induced epidermal hyperplasia [31]. Other variations in IFN-γ and IL-17A signaling may be critical for the initiation, development and severity of PsO as well as the transition to PsA.
IFN-γ-producing T cells were also increased in peripheral blood of PsA patients and frequencies of polyfunctional T-cells correlated significantly positively with Disease Activity in PSoriatic Arthritis (DAPSA) [32]. The polyfunctional T cells were Th1 and Th17 and interestingly, a significant proportion of synovial T-cell subsets were triple-positive for GM-CSF, TNF, and either IL-17A or IFN-γ [32]. Previous studies have also demonstrated a correlation of PsA disease with IL-17A and IFN-γ producing cells while inversely correlated with IL-10-producing B cells [33]. The frequency of circulating CD8+IL-17+ and CD8+IFNγ+ T-cells are also raised in PsA and was shown to discriminate PsA patients from psoriatic patients without joint involvement [34]. These data suggest that suggest that increased circulating levels of IFN-γ and IL-17A-producing CD8+ T-cells are linked to joint inflammation and damage in PsA. Interestingly these data have been corroborated by independent reports demonstrating that the beneficial effects of Abatacept treatment on PsA joint manifestations are accompanied by a significant reduction of the levels of circulating IFN-γ producing CD8+ T-cells [35]. In keeping with T cell involvement the Interferon gamma-induced protein 10 also known as CXCL10, which acts as a chemoattractant to T cells and promotes T cell adhesion to endothelial cells, has been closely associated with PsA that has been considered as a possible biomarker for the development of PsA among PsO patients [36]. However, other IFN-γ-inducible chemokines including CXCL9, CXCL11 and CXCL12 that result in the recruitment of monocytes/macrophages, T cells, NK cells, and dendritic cells, were also detected in PsA synovial fluid and serum [37].
4. IFN-γ and innate immunity
Despite the evident presence of T cells, the importance of myeloid cells in PsA has been documented by activation of antigen-presenting cells in PsA patients suggesting a pathogenic role for innate immunity[38]. Similarly, earlier studies where the pattern of cytokine production revealed a strong signature of TNF, IL-1β, and IL-10 in human PsA synovium have confirmed the importance of innate immunity [39]. In fact, the role of innate immune cells and especially dendritic cells which are professional antigen-presenting cells that activate T cells could play critical roles in PsO to PsA transition as they are an important source of pro-inflammatory cytokines and chemokines in PsO and PsA [40]. IFN-γ in the psoriatic skin and synovium activate APC in the early disease stage to act as an upstream cytokine in the IL-23/IL-17 axis [41]. IFN-γ is therefore responsible in programing myeloid dendritic cell and macrophages to produce CCL20 (ligand of CCR6) and secrete IL-23 and IL-1, thus favoring IL-17-producing cell recruitment and activation to further promote the cycle of inflammation [41]. Accordingly blockade of IFN-γ using a neutralizing humanized anti-IFN-γ antibody, HuZAF resulted in the downregulation of DC-derived products, including IL-23p19, IL12/23p40, CXCL9 and iNOS, further confirming IFN-γ regulation on DC activity [42].
As multiple innate immune cells contribute to the production of systemic high levels of IFN-γ which are present in psoriatic plaques and in the PsA patient synovial fluid the initial source of IFN-γ may vary. The principal source of IFN-γ other than T cells is natural killer (NK) cells, although activated macrophages and dendritic cells also secrete IFN-γ [43] (Figure 2). Elevated production of IFN-γ was observed in NK and NKT cells isolated from lesional psoriatic skin and the number of IFN-γ+ iNKT cells correlated with psoriasiform hyperplasia and the Psoriasis Area and Severity Index (PASI) [44]. Mast cells are also especially enriched in regions exposed to the external environment such as skin, gastrointestinal tract, and airways, and their number has been reported to increase in PsO [45] and inflammatory arthritis [46]. Increased production of IFN-γ may also support the activation of endothelial in dermal to allow the infiltration of immune cells from the peripheral blood into the lesional psoriatic skin [47]. Therefore, the multiple cell types of innate and lymphoid origin which serve as IFN-γ sources may account for the diversification of pathogenesis observed in PsO and the complexity of tracing the transition to PsA (Figure 2). Although neutrophil and macrophages do not secrete IFN-γ, they express cognate receptors that respond through the activation of transcriptional programs leading to cell differentiation, and immune responses. For example, CD163+ macrophages, were identified as a subpopulation of classically activated macrophages in the presence of the IFN-γ cytokine environment in PsO [48]. Further microarray analysis of in vitro monocyte-derived macrophages stimulated with IFN-γ resulted in the upregulation of genes commonly found in PsO or PsA, including STAT-1, CXCL9, HLA-DR and other inflammatory mediators including IL-23p19, IL-12/23p40, TNF, and inducible nitric oxide synthase (iNOS) [48]. Therefore, the expression levels of IFN-γ and the proximity (tissue localization) to cellular targets within skin and joint tissue may also account for the variation we observe in PsO to PsA transition.
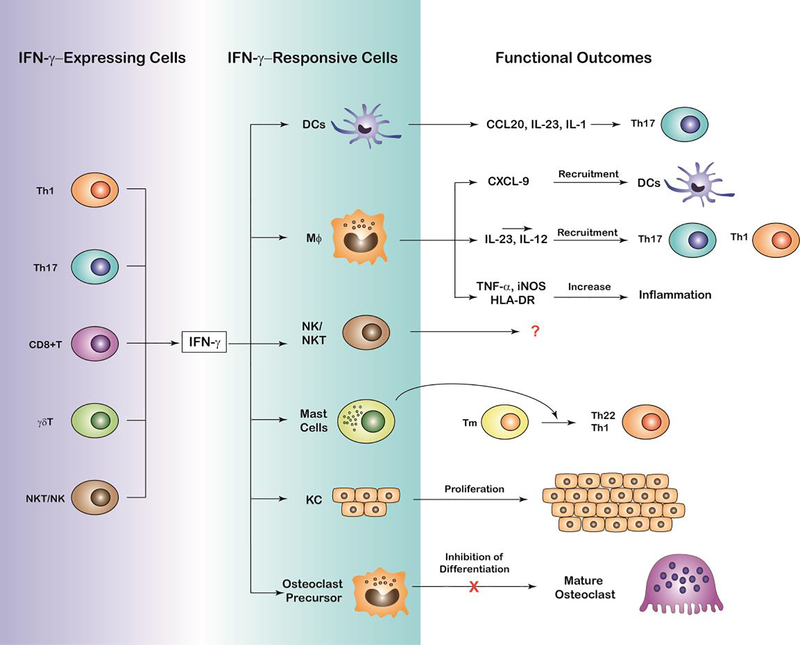
Figure 2. Cellular mechanisms of IFN-γ function and functional outcomes in PsO and PsA pathophysiology.
Schematic representation depicting IFN-γ expression by multiple cell types including T helper 1 cells (TH1), T helper 17 (Th17), CD8+ T cells, γδT and NK/NKT, cells, the target cells of IFN-γ including dendritic cells, macrophages, NK/NKT and mast cells, keratinocytes and osteoclast precursors. Functional outcomes likely to influence the pathophysiology of PsO and PsA by the interaction of IFN-γ producing and responding cells are also depicted including release of cytokines and chemokines to increase Th17 recruitment and differentiation, aberrant hyperproliferation of keratinocytes and terminal differentiation of osteoclasts likely to promote and/or inhibit skin and joint inflammation.
5. IFN-γ in synovitis and bone erosion
The majority of PsA patients develop bone erosions, leading to increased morbidity and decreased quality of life [5]. As the only specialized bone-resorbing cell, the osteoclast and the frequency of osteoclast precursors (OCPs) correlate with radiographic bone erosions and are elevated in a subset of PsA patients [49]. IFN-γ expression was shown to correlate with psoriatic arthritis and tissue destruction especially in sites of osteolysis [50]. However, this association could not be attributed to a direct effect of IFN-γ in osteoclasts or osteoclast precursors since IFN-γ is known to inhibit RANKL-mediated osteoclastogenesis by inducing rapid degradation of TRAF6, the critical RANK adapter protein, resulting in strong suppression of the RANKL-induced activation of NF-kB and JNK [51]. In keeping with the inhibitory actions of IFN-γ on RANKL-induced osteoclastogenesis long-term treatment with recombinant IFN-γ has also been shown to be efficacious in osteopetrosis treatment in humans [52]. In the same study that demonstrated the effects of IFN-γ in inhibition of osteoclast formation, surprisingly mice defective in IFN-γ have a more rapid onset of arthritis and bone resorption compared with wild-type mice [51]. This is because IFN-γ shifts monocyte precursor differentiation to macrophages rather than DC which in turn produce pro-inflammatory mediators and pro-osteoclastogenic factors such as TNF [53]. The increased inflammatory infiltrate and cytokine milieu favors RANKL-independent pathways which allows osteoclastogenesis and bone resorption to occur regardless RANKL inhibition [54]. The present results suggest that relative expression of RANKL and IFN-γ will dictate whether osteoclasts are induced or inhibited, which means that the balanced expression of these two molecules by activated T cells may be a major mechanism by which T cells control the fate of osteoclasts. Additionally, the timing of IFN-γ encounter by the osteoclasts and/or its precursors is an important determinant of its biological function during in vivo osteoclastogenesis. In the case of peripheral osteoclast precursors that have not encountered osteoclastogenic quantities of RANKL, IFN-γ rapidly induces macrophage activation and NO production for immune responses. The dominance of IFN-γ over RANKL allows macrophage activation even in the presence of RANKL expressed on circulating activated T cells. In contrast, peripheral blood macrophages exposed to RANKL may be less responsive to the IFN-γ immunoregulatory effects on macrophage activation, and may thus be better suited for bone resorbing functions. This notion is confirmed at least in vitro, where pre-osteoclasts stimulated with RANKL for 2 days are rendered resistant to the IFN-γ- induced inhibition of osteoclastogenesis, NO production, and upregulation of CD11b and RANK surface expression [55]. Despite the intriguing interplay between IFN-γ and RANKL it should be noted that the levels of RANKL increase significantly on synovial cells and infiltrating T cells in PsA and other inflammatory bone diseases to induce osteoclastogenesis despite the presence of exogenous or endogenous IFN-γ [56]. Interestingly, this resistance is not mediated by JAK-STAT1 pathway inhibition, as STAT1 phosphorylation, is preserved in the late osteoclastogenesis, suggesting that RANKL modifies downstream of STAT1 to influence IFN-γ effects [55]. Another important point is that in vivo, the presence of IFN-γ is accompanied by other cytokines such as IL-17A which also induces myelopoiesis and RANK expression therefore it is very likely to dampen the inhibitory effects of IFN-γ [57] [58]. Future studies designed to understand IFN-γ signaling in mature osteoclasts in vivo are needed to better understand inflammatory bone remodeling.
6. Concluding remarks
The interplay of cytokines with multiple cell types and the activation or inhibition of immune responses largely regulates autoimmunity. The effects of IFN-γ, on multiple cell types in distant tissues keratinocytes in the skin and osteoclasts in the bone suggest that IFN-γ, may regulate basic mechanisms related to PsA pathogenesis. However, elimination of Th1 cells or Th1 cytokines did not prevent the development of autoimmunity. In fact, the loss of IFN-γ, IL-12 p35, or IL-12Rγ2, all of which induce Th1 cell responses, made mice more susceptible to developing autoimmune diseases [59]. Therefore, this data suggests that IFN-γ signaling may control both activation and inhibition of immune responses that lead to different functional outcomes.
Highlights.
Levels of IFN-γ in synovium and lesional skin correlate with disease severity and therapy evaluation
IFN-γ effects directly skin resident cells (keratinocytes) and bone resident cells (osteoclasts) thereby playing critical roles in skin and joint pathology commonly observed in psoriatic arthritis.
IFN-γ is activating antigen-presenting cells early in the psoriatic cascade as an upstream cytokine of the IL-23/IL-17 axis.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81972041), Beijing Natural Science Foundation (grant number 7172112) and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT320006) to HD and by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 2R01AR062173, and a National Psoriasis Foundation Translational Research grant to IEA.
Abbreviations
- IFN-γ
Interferon γ
- IL-17A
Interleukin 17A
- PsO
Psoriasis
- PsA
Psoriatic arthritis
- DEGs
Differentially expressed genes
- STAT
signal transducer and activator of transcription
- CIA
collagen-induced arthritis
- Th
T helper
- KC
keratinocytes
- NK
Natural killer
- CEACAM1
Carcinoembryonic antigen-related cellular adhesion molecule 1
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowes MA, Suarez-Farinas M, and Krueger JG, Immunology of psoriasis. Annu Rev Immunol, 2014. 32: p. 227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestle FO, Kaplan DH, and Barker J, Psoriasis. N Engl J Med, 2009. 361(5): p. 496–509. [DOI] [PubMed] [Google Scholar]
- 3.Diani M, Altomare G, and Reali E, T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev, 2015. 14(4): p. 286–92. [DOI] [PubMed] [Google Scholar]
- 4.Ritchlin CT, Colbert RA, and Gladman DD, Psoriatic Arthritis. N Engl J Med, 2017. 376(21): p. 2095–6. [DOI] [PubMed] [Google Scholar]
- 5.Ritchlin C, Psoriatic disease--from skin to bone. Nat Clin Pract Rheumatol, 2007. 3(12): p. 698–706. [DOI] [PubMed] [Google Scholar]
- 6.Veale DJ and Fearon U, The pathogenesis of psoriatic arthritis. The Lancet, 2018. 391(10136): p. 2273–2284. [DOI] [PubMed] [Google Scholar]
- 7.Pestka S, Krause CD, and Walter MR, Interferons, interferon-like cytokines, and their receptors. Immunol Rev, 2004. 202: p. 8–32. [DOI] [PubMed] [Google Scholar]
- 8.Platanias LC, Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol, 2005. 5(5): p. 375–86. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa V, et al. , Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol, 2017. 2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fierlbeck G, Rassner G, and Muller C, Psorias is induced at the injection site of recombinant interferon gamma. Results of immunohistologic investigations. Arch Dermatol, 1990. 126(3): p. 351–5. [PubMed] [Google Scholar]
- 11.Johnson-Huang LM, et al. , A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol, 2012. 132(4): p. 1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew W, Bowcock AM, and Krueger JG, Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and "Type 1" inflammatory gene expression. Trends Immunol, 2004. 25(6): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 13.Lowes MA, et al. , Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol, 2008. 128(5): p. 1207–11. [DOI] [PubMed] [Google Scholar]
- 14.Gallais Serezal I, et al. , A skewed pool of resident T cells triggers psoriasis-associated tissue responses in never-lesional skin from patients with psoriasis. J Allergy Clin Immunol, 2019. 143(4): p. 1444–1454. [DOI] [PubMed] [Google Scholar]
- 15.Austin LM, et al. , The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol, 1999. 113(5): p. 752–9. [DOI] [PubMed] [Google Scholar]
- 16.Teng MW, et al. , IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med, 2015. 21(7): p. 719–29. [DOI] [PubMed] [Google Scholar]
- 17.Li B, et al. , Dysregulation of Akt-FOXO1 Pathway Leads to Dysfunction of Regulatory T Cells in Patients with Psoriasis. J Invest Dermatol, 2019. 139(10): p. 2098–2107. [DOI] [PubMed] [Google Scholar]
- 18.Ziblat A, et al. , Interleukin (IL)-23 Stimulates IFN-gamma Secretion by CD56(bright) Natural Killer Cells and Enhances IL-18-Driven Dendritic Cells Activation. Front Immunol, 2017. 8: p. 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y, et al. , Gamma delta T cells provide a nearly sourceof interferon gamma in tumor immunity. J Exp Med, 2003. 198(3): p. 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark GR and Darnell JE Jr., The JAK-STAT pathway at twenty. Immunity, 2012. 36(4): p. 503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blouin CM and Lamaze C, Interferon gamma receptor: the beginning of the journey. Front Immunol, 2013. 4: p. 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowcock AM, et al. , In sights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum Mol Genet, 2001. 10(17): p. 1793–805. [DOI] [PubMed] [Google Scholar]
- 23.Zaba LC, et al. , Psoriasisis characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol, 2009. 129(1): p. 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson NJ, et al. , Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol, 2007. 8(9): p. 950–7. [DOI] [PubMed] [Google Scholar]
- 25.Nograles KE, et al. , Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol, 2008. 159(5): p. 1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiricozzi A,et al. , IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One, 2014. 9(2): p. e90284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudjonsson JE, et al. , Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J Invest Dermatol, 2010. 130(7): p. 1829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunderson AJ, et al. ,CD8(+) T cells mediate RAS-induced psoriasis-like skin inflammation through IFN-gamma. J Invest Dermatol, 2013. 133(4): p. 955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolk K, et al. , IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol, 2006. 36(5): p. 1309–23. [DOI] [PubMed] [Google Scholar]
- 30.Rahmoun M, et al. , Cytokine-induced CEACAM1 expression on keratinocytes is characteristic for psoriatic skin and contributes to a prolonged lifespan of neutrophils. J Invest Dermatol, 2009. 129(3): p. 671–81. [DOI] [PubMed] [Google Scholar]
- 31.Sarra M, et al. , IL-21 promotes skin recruitment ofCD4(+) cells and drives IFN-gamma-dependent epidermal hyperplasia. J Immunol, 2011. 186(9): p. 5435–42. [DOI] [PubMed] [Google Scholar]
- 32.Wade SM, et al. , Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis, 2019. 78(3): p. 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mavropoulos A, et al. , IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNgamma-producing T cells. Clin Immunol, 2017. 184: p. 33–41. [DOI] [PubMed] [Google Scholar]
- 34.Colombo E, et al. , Peripheral blood CD8+ T-cell profiles in patients with psoriatic arthritis: a cross-sectional case-control study. Eur Rev Med Pharmacol Sci, 2017. 21(22): p. 5166–5171. [DOI] [PubMed] [Google Scholar]
- 35.Scarsi M, et al. , Reduction of peripheral blood T cells producing IFN-gamma and IL-17 after therapy with abatacept for rheumatoid arthritis. Clin Exp Rheumatol, 2014. 32(2): p. 204–10. [PubMed] [Google Scholar]
- 36.Abji F, et al. , Brief Report: CXCL10 Is a Possible Biomarker for the Development of Psoriatic Arthritis Among Patients With Psoriasis. Arthritis Rheumatol, 2016. 68(12): p. 2911–2916. [DOI] [PubMed] [Google Scholar]
- 37.Antonelli A, et al. , High values of Th1 (CXCL10)and Th2 (CCL2) chemokines in patients with psoriatic arthtritis. Clin Exp Rheumatol, 2009. 27(1): p. 22–7. [PubMed] [Google Scholar]
- 38.Candia L, et al. , Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? J Rheumatol, 2007. 34(2): p. 374–9. [PubMed] [Google Scholar]
- 39.Ritchlin C, et al. , Patterns of cytokine production in psoriatic synovium. J Rheumatol, 1998. 25(8): p. 1544–52. [PubMed] [Google Scholar]
- 40.Mahil SK, Capon F, and Barker JN, Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin Immunopathol, 2016. 38(1): p. 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kryczek I, et al. , Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol, 2008. 181(7): p. 4733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harden JL, et al. , Humanized anti-IFN-gamma(HuZAF)in the treatment of psoriasis. J Allergy Clin Immunol, 2015. 135(2): p. 553–6. [DOI] [PubMed] [Google Scholar]
- 43.Munder M, et al. , Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med, 1998. 187(12): p. 2103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kono F,et al. , Interferon-gamma/CCR5 expression in invariant natural killer T cells and CCL5 expression in capillary veins of dermal papillae correlate with development of psoriasis vulgaris. Br J Dermatol, 2014. 170(5): p. 1048–55. [DOI] [PubMed] [Google Scholar]
- 45.Ackermann L, et al. , Mast cells in psoriatic skin are strongly positive for interferon-gamma. Br J Dermatol, 1999. 140(4): p. 624–33. [DOI] [PubMed] [Google Scholar]
- 46.Nigrovic PA and Lee DM, Mast cells in inflammatory arthritis. Arthritis Res Ther, 2005. 7(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroder K, et al. , Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol, 2004. 75(2): p. 163–89. [DOI] [PubMed] [Google Scholar]
- 48.Fuentes-Duculan J, et al. , A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol, 2010. 130(10): p. 2412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchlin CT, et al. , Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest, 2003. 111(6): p. 821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canete JD, et al. , Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann Rheum Dis, 2000. 59(4): p. 263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takayanagi H, et al. , T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature, 2000. 408(6812): p. 600–5. [DOI] [PubMed] [Google Scholar]
- 52.Key LL Jr., et al. , Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med, 1995. 332(24): p. 1594–9. [DOI] [PubMed] [Google Scholar]
- 53.Delneste Y, et al. , Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood, 2003. 101(1): p. 143–50. [DOI] [PubMed] [Google Scholar]
- 54.Adamopoulos IE and Mellins ED, Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol, 2015. 11(3): p. 189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W, O'Keefe RJ, and Schwarz EM, Exposure to receptor-activator of NFkappaB ligand renders pre-osteoclasts resistant to IFN-gamma by inducing terminal differentiation. Arthritis Res Ther, 2003. 5(1): p. R49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotake S,et al. ,Activate dhuma n T-cell sdirectl yinduc eosteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum, 2001. 44(5): p. 1003–12. [DOI] [PubMed] [Google Scholar]
- 57.Adamopoulos IE,et al. ,Interleukin-17 Aupregulate srecepto ractivato rof NF-kappaB on osteoclast precursors. Arthritis Res Ther, 2010. 12(1): p. R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adamopoulos IE,et al. ,IL-17 Agene transfe rinduce sbon elo ssand epidermal hyperplasia associated with psoriatic arthritis. Ann Rheum Dis, 2015. 74(6): p. 1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pate l DDa ndKuchro o VK,Th 17Ce llPathway inHuman Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity, 2015. 43(6): p. 1040–51. [DOI] [PubMed] [Google Scholar]