Abstract
Vitamin C plays a protective role in oxidative damage by blocking the effects of free radicals. The present study investigated the mechanisms through which vitamin C partly mediates anti-apoptotic and antioxidant functions via the regulation of microRNAs (miRNAs or miRs). For this purpose, a global miRNA expression analysis on human umbilical vein endothelial cells (HUVECs) treated with vitamin C was conducted using microarrays containing human precursor and mature miRNA probes. The results revealed that there were 42 identical miRNAs among the differentially expressed miRNAs in the HUVEC group and H2O2 + vitamin C-treated HUVEC group compared to the H2O2-exposed HUVEC group, including 41 upregulated miRNAs and 1 down-regulated miRNA. Using bioinformatics analysis, differentially expressed miRNAs were investigated to identify novel target mRNAs and signaling pathways. Pathway enrichment analyses revealed that apoptosis, the mitogen-activated protein kinase (MAPK) signaling pathway, phosphoinositide 3-kinase (PI3K)/Akt signaling pathway and oxidative phosphorylation were significantly enriched. The results from western blot analysis demonstrated that the interleukin (IL)10, matrix metalloproteinase (MMP)2, cAMP-response element binding protein (CREB) and p-CREB protein expression levels in HUVECs transfected with hsa-miR-3928-5p and induced by H2O2 were significantly downregulated; the MAPK9, caspase-3 (CASP3) and p-CASP3 protein expression levels in HUVECs transfected with hsa-miR-323a-5p and induced by H2O2 were significantly downregulated. The present study therefore demonstrates that vitamin C partly exerts protective effects on HUVECs through the regulation of miRNA/mRNA axis expression.
Keywords: human umbilical vein endothelial cells, apoptosis, vitamin C, oxidative damage, H2O2
Introduction
Aging is considered the main risk factor for various diseases (1); as the aging population increases, the prevalence of several age-related chronic diseases also increases. This is mainly caused by cumulative damage in the organs and cells, thereby leading to the on-set of age-related chronic diseases. Therefore, by exploring the specific mechanisms of aging, valuable information on age-related diseases can be obtained and consequently, strategies to delay its early onset may also be developed.
Oxidative stress is a state in which there are high levels of reactive oxygen species (ROS) compared to antioxidant defenses (2), and this is a hallmark of age-related diseases, including Parkinson's disease (3), Alzheimer's disease (4), chronic inflammation (5), heart failure (6), atherosclerosis (7), kidney disease (8), certain types of cancer (9,10) and aging itself (11). Among these, vascular disease is the most common cause of mortality in the industrialized world (12,13). The normal function and integrity of vascular endothelial cells are of utmost importance for the stability of the vascular environment. The dysfunction of the vascular endothelium, characterized by morphological alterations in the vascular wall, endothelial cell inflammation and apoptosis, is considered the initial event of some diseases (14). Oxidative stress is a critical cause of vascular dysfunction, as ROS can damage cells of the vascular wall and induce vascular endothelial cell dysfunction (15). Antioxidants prevent the damage of vascular endothelial cells and are considered one of the crucial factors for the prevention of cardiovascular diseases (16).
Antioxidants are highly effective in the prevention or treatment of oxidative damage in animal models (17). Commonly used antioxidants include vitamin C, α-tocopherol and polyphenols (18). Among these, vitamin C, a recognized antioxidant, is easily accessible in daily life and has the advantages of low cost, a significant effect, easy accessibility and hydrophilicity (19). As a potent antioxidant, vitamin C plays a protective role in oxidative damage by blocking the production of free radicals (20,21). It also plays a critical role in certain types of cancer and exerts immunomodulatory effects that enhance host defenses (22). Vitamin C deficiency can lead to scurvy, a fatal disease (23). Vitamin C suppresses the expression of KRAS and BRAF, which inhibit glycolysis and the ensuing energy crisis by targeting GAPDH in colorectal cancer cells (24). In addition, high levels of vitamin C have been shown to induce the apoptosis of HT29 colon cancer and MCF7 breast cancer cells by hindering the energy flux in the tricarboxylic acid cycle and glycolysis, eventually resulting in the insufficient production of adenosine triosphosphate (25). Vitamin C can inhibit the death of human umbilical vein endothelial cells (HUVECs) induced by oxidative stress (19). Vitamin C or vitamin C-Na pre-treatment has also been shown to enhance antioxidant capacity, thus protecting H9C2 cells from heat-induced damage (26). It also improves the endothelium-dependent vasodilation of forearm resistance vessels in patients with hypercholesterolemia (27). Vitamin C reduces lipid peroxidation and enhances the antioxidant defense system, which exerts a beneficial effect on the heart by reducing oxidative stress in patients with cardiovascular disease (28). However, the molecular mechanisms of vitamin C in vascular-related diseases warrant further investigation.
MicroRNAs (miRNAs or miRs) are a family of small non-coding RNA molecules with 18-25 nucleotides that bind to the 3′-untranslated regions of target mRNAs in a sequence-specific manner, inhibiting their translation or regulating their degradation (29). The expression of miRNAs is tissue-specific, and this localized expression is crucial for their tailored roles in regionalized function and development (30). miRNAs play vital roles in the absorption of vitamin C in the intestines by modulating the post-transcriptional regulation of several vitamin transporter genes, including that of solute carrier family (SLC)23A2, SLC19A2, SLC52A3, SLC26A3 and SLC15A1 (31). Vitamin C can also regulate multiple stem cell-specific signaling pathways, such as cell adhesion molecules, fatty acid biosynthesis and hormone signaling pathways by altering miRNA expression (32).
Although vitamin C has been widely studied, the mechanisms underlying the protective effects of vitamin C on H2O2-induced HUVECs associated with vascular diseases warrant further investigation. The present study demonstrates the potential benefits of vitamin C treatment against H2O2-induced oxidative damage in HUVECs, indicating that vitamin C partly exerts protective effects against H2O2-induced oxidative damage by regulating the expression of miRNAs.
Materials and methods
Culture and treatment of HUVECs
HUVECs were obtained from the Cell Bank of the Chinese Academy of Sciences and cultured in complete endothelial cell growth medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (100 U/ml; BD Biosciences). HUVECs were digested with 0.25% trypsin and 1x104 cells/ml HUVECs were seeded and cultured at 37°C under 5% CO2. In the H2O2 + vitamin C-treated HUVEC group, the HUVECs were cultured in endothelial cell growth medium containing 100 µM H2O2 (Sigma-Aldrich; Merck KGaA) for 2 h and were then cultured in endothelial cell growth medium supplemented with 200 µM vitamin C (Sigma-Aldrich; Merck KGaA) for 48 h. In the H2O2-treated HUVEC group, the HUVECs were cultured in endothelial cell growth medium containing 100 µM H2O2 for 2 h and were then cultured in endothelial cell growth medium for 48 h. HUVECs not treated with vitamin C and H2O2 were used as control.
Apoptosis assays
The HUVECs from each group were washed twice with cold phosphate-buffered saline before being resuspended in 1X Binding Buffer at a concentration of 1×106 cells/ml. The cells were stained with FITC-conjugated anti-Annexin V antibody and propidium iodide (PI) in the apoptosis kit (cat. no. 556547, BD Biosciences) for 15 min at room temperature and dark, and apoptotic cells were quantified using a FACSCalibur flow cytometer (BD Biosciences).
Measurement of ROS generation
According to the manufacturer's protocol, the ROS assay kit (S0033; Beyotime Institute of Biotechnology, Inc.) was used to measure intracellular ROS levels using the probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA). Briefly, cells treated with 100 µM H2O2 for 2 h or 100 µM H2O2 for 2 h + 200 µM vitamin C for 48 h were washed twice with PBS. The cells were then incubated with 10 µM DCFH-DA at 37°C for 30 min. After washing with PBS twice, the fluorescence of the cells was imaged using a confocal microscope (Carl Zeiss AG) with an excitation of 488 nm/emission of 529 nm. The fluorescence intensity was measured using ImageJ_v1.8.0 software.
Analysis of the expression of miRNAs
miRNAs were isolated using a miRNA isolation kit (Guangzhou Forevergen Biosciences Co., Ltd.) that specifically captures small RNAs with lengths of <200 nucleotides. The quality of the RNA samples was examined on an Agilent 2100 BioAnalyzer (Agilent Technologies, Inc.) and the yield of the RNA samples was determined using the ABI Step One Plus Real-Time PCR System (Applied Biosystems). The miRNA profile was analyzed for hierarchical clustering to produce heatmaps.
Gene ontology (GO) and pathway enrichment analyses of differentially expressed miRNAs
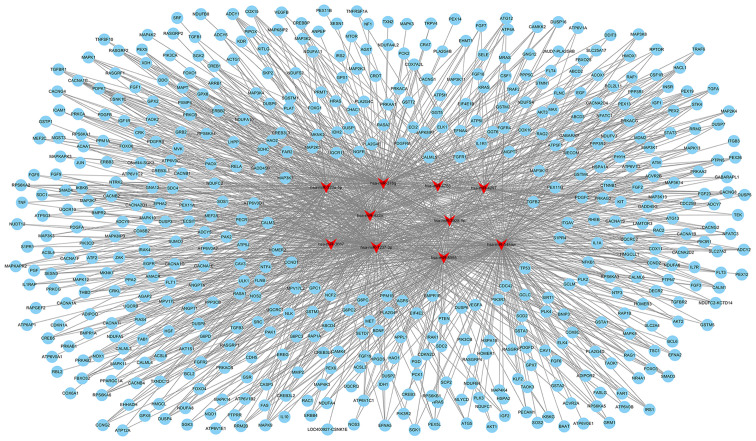
GO and pathway enrichment analyses were performed to identify biological processes that were potentially regulated by the differentially expressed miRNAs based on the GO (http://geneontology.org) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (http://www.genome.jp/kegg/pathway.html) databases. The P-value for each GO term was calculated using the right-sided hypergeometric tests. The Benjamin-Hochberg adjustment was used for multiple test correction (33,34). Those terms with a P-value <0.05 were considered to be significantly enriched. Simultaneously, the target mRNAs associated with apoptosis or oxidative metabolic signaling pathways of the top 10 differentially expressed miRNAs were predicted using TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do). The top 10 differentially expressed miRNAs and their target mRNAs associated with cell apoptosis or involved in oxidative metabolism were integrated, and regulatory networks were constructed using Cytoscape 3 software.
Reverse transcription-quantitative PCR (RT-qPCR) validation analyses of miRNAs
Total RNA was extracted from the cells and gene expression was detected by RT-qPCR. According to the manufacturer's protocol, cDNA was synthesized from total RNA using M-MLV Reverse Transcriptase (Promega Corporation). The GoTaq qPCR Master Mix (Promega Corporation) was used for qPCR. The ABI 7500 system (Applied Biosystems) was used to perform the PCR amplification, while U6 was used as an internal control. The primer information of the miRNAs is presented in Table SI.
Transient transfection
Negative control (NC) mimics, hsa-miR-323a-5p mimics and hsa-miR-3928-5p mimics were purchased from GenePharma. The sequences of the mimics were as follows: hsa-miR-323a-3p mimics sense, 5′-CAC AUU ACA CGG UCG ACC UCU-3′ and antisense, 5′-AGG UCG ACC GUG UAA UGU GUU-3′; hsa-miR-3928-5p mimics sense, 5′-UGA AGC UCU AAG GUU CCG CCU GC-3′ and antisense, 5′-AGG CGG AAC CUU AGA GCU UCA UU-3′; NC mimics sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. HUVECs were transfected with 50 nM hsa-miR-323a-3p mimics, 50 nM hsa-miR-3928-5p mimics, or 50 nM NC mimics using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. Following transfection for 48 h, the cells were incubated with 100 µM H2O2 for 2 h and harvested for western blot analysis.
Western blot analysis
Cells were lysed using the RIPA buffer (R0010; Solarbio) to separate the protein. The BCA working solution was used to detect the protein concentration at 562 nm using BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, 10% SDS-PAGE was used to isolate the target protein. The isolated target protein was transferred to a PVDF membrane. After sealing with 5% non-fat milk at room temperature for 1 h, primary antibodies, including anti-inter-leukin (IL)10 (1:1,000; ab34843, Abcam), anti-cAMP-response element binding protein (CREB; 1:1,000; ab31387, Abcam), anti-p-CREB (1:1,000; CST9198, Cell Signaling Technology, Inc.), anti-caspase (CASP)3 (1:1,000; CST9662, Cell Signaling Technology, Inc.), anti-cleaved-CASP3 (1:1,000; CST9662, Cell Signaling Technology, Inc.), anti-mitogen-activated protein kinase (MAPK)9 (1:1,000; CST4672, Cell Signaling Technology, Inc.), anti-matrix metalloproteinase (MMP)2 (1:1,000; ab37150, Abcam) and anti-GAPDH (1:1,000; 60004-1-1g, Proteintech) were incubated with the PVDF membrane overnight at a temperature 4°C. HRP-labeled secondary antibody (1:5,000; ab150117, Abcam) was then used to incubate the membrane for 2 h at room temperature. GAPDH was used as an internal control. The target protein levels were visualized by an enhanced chemiluminescence reagent (Vazyme Biotech Co., Ltd.). ImageJ v1.8.0 software (National Institutes of Health) was used for the semi-quantitative analysis of protein expression.
Statistical analyses
The results are shown as the means ± standard deviation from at least 3 independent experiments. Statistical signifcance was determined using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test evaluations for multiple comparisons with SPSS 19.0 statistical software. A P<0.05 was considered to indicate a statistically significant difference.
Results
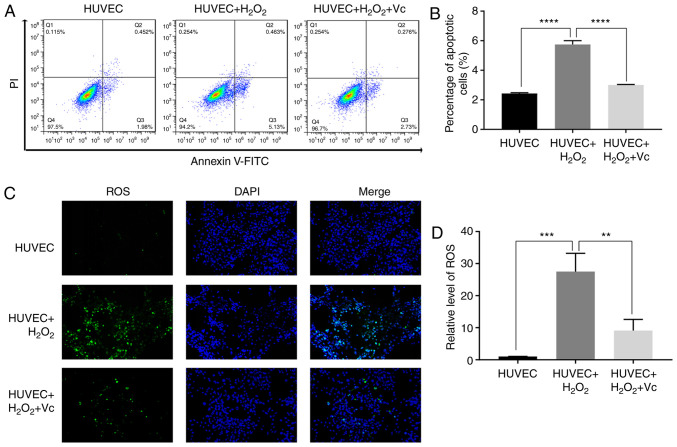
Vitamin C significantly reduces the H2O2-induced apoptosis of HUVECs
As a potent first-line antioxidant, vitamin C attenuates the production of free radicals and reduces oxidative damage (20,21). In the present study, to further investigate the antioxidant function of vitamin C, vitamin C was used to treat H2O2-induced HUVECs. The percentage of apoptotic HUVECs induced with H2O2 was approximately 6%, while vitamin C significantly reduced the percentage of apoptotic HUVECs induced by H2O2 (Fig. 1A and B). In addition, ROS assay revealed that vitamin C reduced the green fluorescence intensity in HUVECs induced with H2O2 (Fig. 1C and D). These results demonstrated that vitamin C attenuated H2O2-induced apoptosis and oxidative damage in H2O2-induced HUVECs.
Figure 1.
Vitamin C reduces apoptosis and oxidative damage in human umbilical vein endothelial cells (HUVECs) induced by H2O2. (A) The apoptosis rate of HUVECs treated with 100 µM H2O2 for 2 h or 100 µM H2O2 for 2 h + 200 µM vitamin C for 48 h was analyzed by flow cytometry. (B) Analysis of the percentage of apoptotic HUVECs in (A). (C) ROS expression level in HUVECs induced by 100 µM H2O2 for 2 h or 100 µM H2O2 for 2 h + 200 µM vitamin C for 48 h was observed using a confocal microscope. (D) Semi-quantitative analysis of ROS fluorescence intensity in (C). Data are shown as the means ± standard deviation (n=3). Statistical analysis was performed using one-way ANOVA followed by Bonferroni's post hoc test. **P<0.01, ***P<0.001, ****P<0.0001.
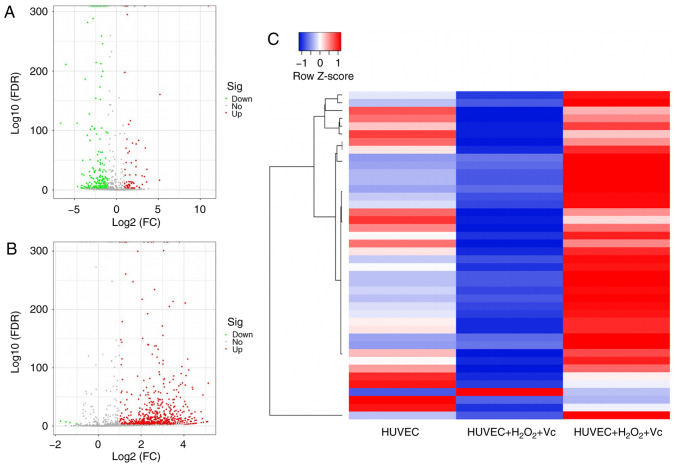
Vitamin C modulates miRNA profiles in H2O2-induced HUVECs
To identify differentially expressed miRNAs due to vitamin C treatment, a comprehensive miRNA microarray analysis of samples from H2O2-induced HUVECs with or without vitamin C treatment was conducted. These miRNAs in HUVECs were isolated after 2 h of H2O2 exposure and 48 h of vitamin C treatment. The miRNAs exhibited a significant (P<0.05) 1.5-fold difference in expression following treatment with H2O2 + vitamin C compared with the control group and H2O2 exposure group, respectively. The results revealed that there was a significant change in the expression of 287 miRNAs, including 70 upregulated miRNAs and 217 miRNAs that were downregulated in the H2O2 exposure group compared with the control group (Fig. 2A and Table SII). In addition, in the H2O2 + vitamin C treatment group, 710 (including 706 upregulated and only 4 downregulated) miRNAs were differentially expressed compared with the H2O2 treatment group (Fig. 2B and Table SIII). The analyses revealed that there were 42 identical miRNAs among the differentially expressed miRNAs induced in the HUVEC group and H2O2 + vitamin C-treated group compared to the H2O2-treated group, including 41 upregulated miRNAs and 1 downregulated miRNAs (Fig. 2C and Table SIV). Hierarchical cluster analysis using the normalized miRNA expression data confirmed that the expression of miRNAs in the H2O2 or H2O2 + vitamin C-treated HUVECs could be clearly distinguished from that in the control group (Fig. 2).
Figure 2.
Differential miRNA expression in human umbilical vein endothelial cells (HUVECs) following exposure to H2O2 and vitamin C. (A) Significant alterations of miRNA levels in HUVECs following exposure to 100 µM H2O2 for 2 h. (B) Significant alterations in miRNA levels in HUVECs following exposure to 100 µM H2O2 for 2 h + 200 µM vitamin C for 48 h. (C) Cluster heatmap of the same miRNAs among the differentially expressed miRNAs in HUVECs induced by H2O2 or vitamin C + H2O2.
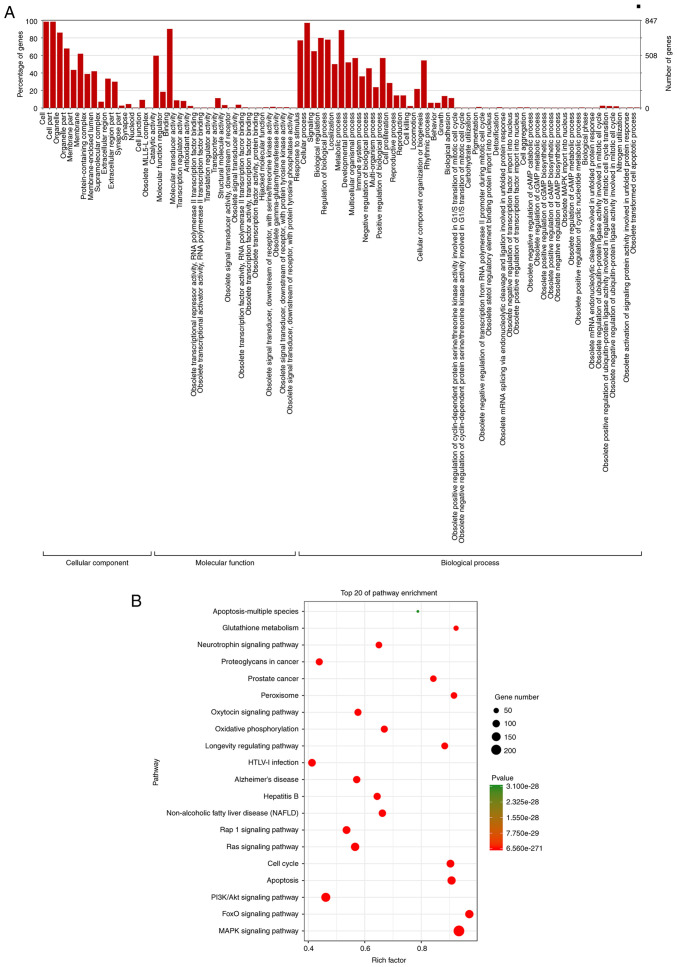
GO analysis and KEGG pathway prediction
Categorizing these miRNAs may enhance our understanding of the cellular components and biological processes regulated by the miRNAs with an altered expression in the treated HUVECs. GO enrichment analyses demonstrated that these differentially expressed miRNAs were associated with cellular components and biological processes, including cell, organelle, membrane, protein-containing complex, membrane-enclosed lumen, catalytic activity, binding, localization, metabolic processes, developmental processes, multicellular organismal processes, immune system processes, positive and negative regulation biological processes, and cellular proliferation (Fig. 3A). Pathway enrichment analyses revealed significant enrichment in apoptosis, MAPK signaling pathway, PI3KAkt signaling pathway and oxidative phosphorylation (Fig. 3B).
Figure 3.
Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway prediction. (A) GO biological pathways and (B) KEGG biological pathways were potentially affected by the same differentially expressed miRNAs following both H2O2 treatment and vitamin C + H2O2 treatment of human umbilical vein endothelial cells (HUVECs).
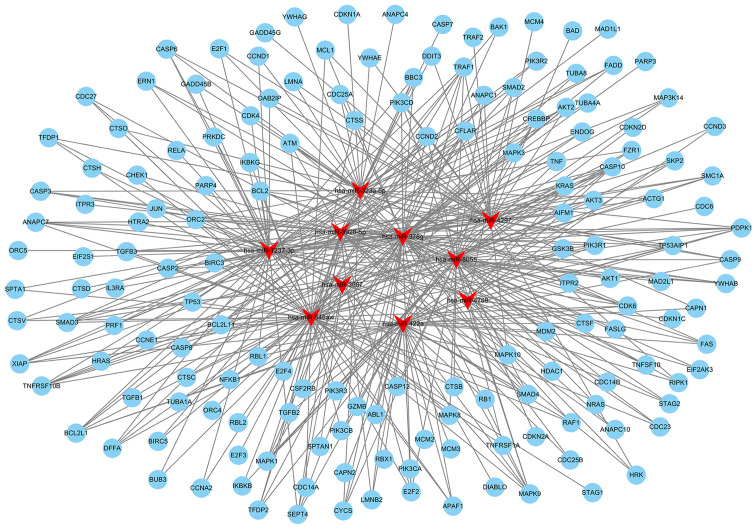
miRNA target prediction by bioinformatics analysis
The top 10 miRNAs with the most evident differences in expression were has-miR-323a-5p, hsa-miR-378g, hsa-miR-4788, hsa-miR- 4297, hsa-miR- 422a, hsa-miR-3928-5p, hsa-miR-3687, hsa-miR-1237-3p, hsa-miR-8055 and hsa-miR-548aw. The target mRNAs associated with apoptosis or oxidative metabolic signaling pathways of the top 10 differentially expressed miRNAs were predicted using TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do). The network of miRNA-targeted mRNAs was constructed according to their regulatory association. hsa-miR-323a-5p can target CASP3, CASP6, CASP9, MAPK9 and other mRNAs to regulate apoptosis; hsa-miR-422a may be associated with apoptosis by regulating PIK3CA, E2F2, FAS, or SMAD4; hsa-miR-8055 may target BCL2, FAS, TNF and other mRNAs to regulate apoptosis (Fig. 4 and Table SV). hsa-miR-3928-5p may be involved in regulating oxidative stress by targeting IL10, MMP2, or CREB; hsa-miR-378g may target ATG12, ANPEP, MAPK3, or EREG to mediate oxidative stress; hsa-miR-1237-3p may target AKT2, AKT3 and MAPK to regulate oxidative stress (Fig. 5 and Table SV).
Figure 4.
The top 10 differentially expressed miRNAs and their target mRNAs associated with cell apoptosis were integrated, and regulatory networks were constructed using Cytoscape 3 software. The red triangles represent miRNAs and the blue rounds represent the target mRNAs.
Figure 5.
The top 10 differentially expressed miRNAs and their target mRNAs involved in oxidative metabolism were integrated, and regulatory networks were constructed using the Cytoscape 3 software. The red triangles represent miRNAs and the blue rounds represent the target mRNAs.
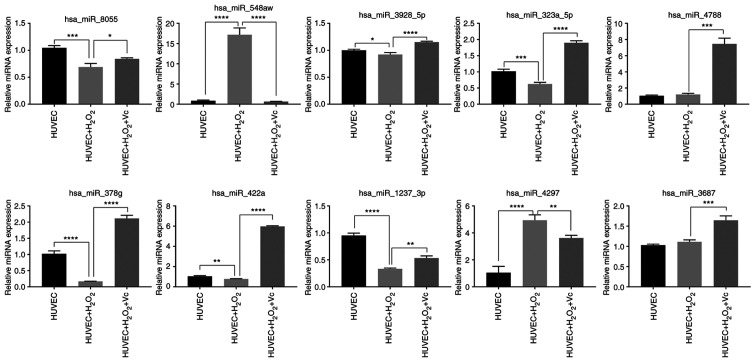
Validation of differentially expressed miRNAs
To confirm the miRNA microarray data, RT-qPCR was performed to examine the expression levels of the top 10 miRNAs from the H2O2 or H2O2 + vitamin C-treated HUVEC groups. The expression levels of hsa-miR-8055, hsa-miR-3928-5p, has-miR-323a-5p, has-miR-378g, has-miR-422a and has-miR-1237-3p detected by RT-qPCR were downregulated in the HUVECs induced by H2O2 compared to those in the control HUVECs, and were upregulated in the HUVECs treated with vitamin C and H2O2 compared to those in the HUVECs exposed to H2O2; these findings were consistent with those of the microarray analyses (Fig. 6 and Table SIV). However, the has-miR-548aw and has-miR-4297 expression levels were upregulated in the HUVECs induced by H2O2 compared to those in the control HUVECs, and were downregulated in the HUVECs treated with vitamin C and H2O2 compared to those in the HUVECs treated with H2O2; the has-miR-4788 and has-miR-3687 expression levels were upregulated in HUVECs induced by H2O2 compared to those in HUVECs; these findings were inconsistent with the results of microarray analysis. Overall, the results of RT-qPCR very-fication of the selected miRNAs were mostly consistent with the results of sequencing analysis.
Figure 6.
RT-qPCR analysis of the changes in miRNA expression in H2O2-induced and vitamin C + H2O2-treated human umbilical vein endothelial cells (HUVECs). Data are shown as the means ± standard deviation (n=3). Statistical analysis was performed using one-way ANOVA followed by Bonferroni's post-hoc test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
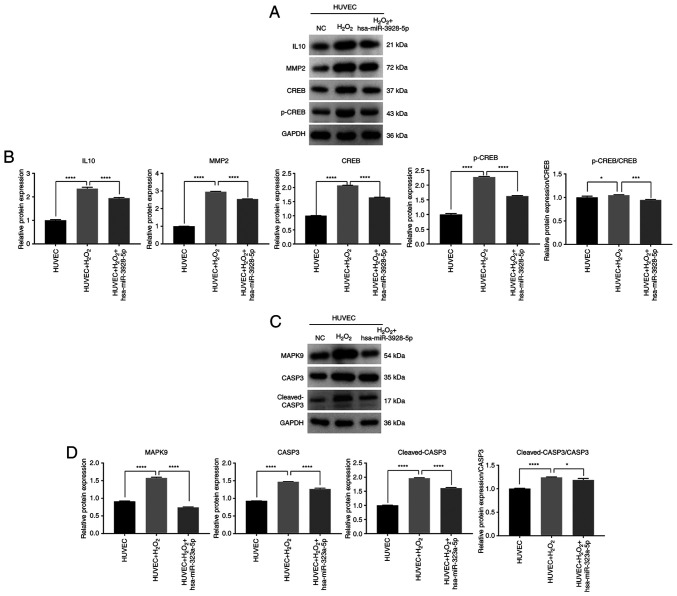
Role of has-miR-3928-5p and has-miR-323a-5p in oxidation and apoptosis of HUVECs
To further analyze the molecular mechanisms of miRNAs in apoptosis and oxidation, western blot analysis was used to analyze the expression level of mRNAs regulated by hsa-miR-3928-5p and hsa-miR-323a-5p. The results revealed that H2O2 upregulate the IL10, MMP2, CREB and p-CREB protein expression levels, and the ratio of p-CREB/CREB in HUVECs; however, the IL10, MMP2, CREB and p-CREB protein expression levels and the ratio of p-CREB/CREB in the HUVECs transfected with has-miR-3928-5p and induced by H2O2 were significantly downregulated, compared to those of HUVECs induced by H2O2 (Fig. 7A and B). Simultaneously, the results also revealed that H2O2 upregulated the MAPK9, CASP3 and cleaved-CASP3 protein expression levels and the ratio of cleaved-CASP3/CASP3 in HUVECs (Fig. 7C and D). Moreover, the MAPK9, CASP3 and cleaved-CASP3 protein expression levels and the ratio of cleaved-CASP3/CASP3 in HUVECs transfected with hsa-miR-323a-5p and induced by H2O2 were significantly downregulated, compared to those of HUVECs induced by H2O2 (Fig. 7C and D). It could thus be inferred that vitamin C may regulate the oxidation and apoptosis of HUVECs through the above-mentioned molecular mechanism.
Figure 7.
Effect of hsa-miR-3928-5p and hsa-miR-323a-5p on related target mRNAs expression levels in human umbilical vein endothelial cells (HUVECs) induced by H2O2 examined by western blot analysis. (A) IL10, MMP2, CREB and p-CREB protein expression levels and the ratio of p-CREB/CREB in HUVECs under different processing conditions. (B) Semi-quantitative analysis of the protein expression levels in (A). (C) MAPK9, CASP3 and cleaved-CASP3 protein expression levels and the ratio of cleaved-CASP3/CASP3 in HUVECs under different processing conditions. (D) Semi-quantitative analysis of the protein expression levels in (C) Statistical analysis was performed using one-way ANOVA followed by Bonferroni's post-hoc test. *P<0.05, ***P<0.001, ****P<0.0001. IL, interleukin; MMP2, matrix metalloproteinase; CREB, cAMP-response element binding protein; CASP3, caspase-3.
Discussion
Vitamin C is known to be a potent antioxidant that quenches ROS and has also been demonstrated to ease vascular endothelium dysfunction in conditions, such as hyperhomocysteinemia, diabetes, hypercholesterolemia, coronary artery disease, and renovascular hypertension (27,35-38). Vitamin C induces the pluripotent differentiation of mouse embryonic stem cells via the modulation of miRNA expression (39). High Vitamin C levels result in enhanced anti-atherosclerotic and anti-senescence effects by regulating anti-inflammatory miRNAs (40,41). At present, the role of vitamin C-dependent miRNAs in the regulation of the antioxidant and antiapoptotic activities of endothelial cell remains to be fully determined. Hence, the identification of vitamin C-induced differentially expressed miRNAs is crucial for the further understanding of the specific mechanisms underlying endothelial dysfunction. In the present study, a list of differentially expressed miRNAs were identified following vitamin C treatment and it was revealed that vitamin C attenuated the apoptosis and oxidative damage of H2O2-induced HUVECs.
Simultaneously, GO analysis demonstrated that cellular components and biological processes were clearly critical to the H2O2-induced oxidation stress and apoptosis of HUVECs. Moreover, KEGG annotation demonstrated that apoptosis, the MAPK signaling pathway, PI3K/Akt signaling pathway and oxidative phosphorylation were involved in the anti-oxidative and anti-apoptotic effects of vitamin C in H2O2-induced HUVECs. Research has indicated that all MAPK inhibitors increase the O2•- levels in H2O2-induced A549 cells (42). The PI3K/Akt signaling pathway is related to survival, angiogenesis and oxidative stress under pathophysiologic conditions in ischemia (43). Klotho has been shown to weaken oxidized low-density lipoprotein (ox-LDL)-induced oxidative stress in HUVECs via the upregulation of oxidative scavengers by suppressing lectin-like ox-LDL receptor expression and activating the PI3K/Akt/eNOS pathway (44). To further investigate the potential roles of miRNAs involved in HUVECs following exposure to vitamin C, the present study analyzed the predicted target mRNAs of selected miRNAs. The present study focused on the top 10 miRNAs, including hsa-miR-323a-5p, has-miR-378g, has-miR-4788, has-miR-4297, hsa-miR-422a, hsa-miR-3928-5p, hsa-miR-3687, hsa-miR-1237-3p, hsa-miR-8055 and hsa-miR-548aw, with the most evident differences in expression. It was revealed that these miRNAs target mRNAs that regulate cell apoptosis and oxidative metabolism signaling pathways.
miR-422a can inhibit the migration and proliferation of gastric cancer cells, and can promote the metabolic transition from aerobic glycolysis to oxidative phosphorylation (45). miR-323a-3p can attenuate the apoptosis of 16HBE14o-cells stimulated with staurosporine or tunicamycin by inhibiting the CASP3 expression level (46). CASP-9/3 plays a critical role in apoptotic signaling in the mitochondria (47). The activation of CASP-9/3 mediates cell apoptosis in various cell types (48-50). In addition, 1,25-dihydroxyvitamin-D3 induces neutrophil apoptosis in periodontitis with type 2 diabetes mellitus patients via the p38/MAPK pathway (51). In this study, western blot showed that hsa-miR-323a-5p downregu-lated the protein expression levels of CASP3, Cleaved-CASP3, and MAPK9 and the ratio of the Cleaved-CASP3/CASP3 in HUVECs induced by H2O2, suggesting that hsa-miR-323a-5p obtained the antiapoptotic effects of Vitamin C by targeting CASP3 and MAPK9. In oxidative stress, MMP2 knockdown has been shown to prevent the protective effects of miR-125 inhibitor on H9C2 cells (52). MMPs play important roles in anti-inflammation; the activity of MMP-2 is increased by oxidative stress in early hypertension (53). The administration of anthocyanin suppresses the generation of ROS and attenuates naproxen-induced suppression of MMP-2 (54). IL10, a human cytokine influencing immunoregulation and inflammation, has anti-inflammatory properties and plays an important role in limiting immune responses to pathogens and oxidative stress (55). The activation of the Akt/CREB axis by stressin-1 can counteract the adverse effects of various cell stresses (56). In the present study, the results of western blot analysis revealed that hsa-miR-3928-5p downregulated the protein expression levels of IL10, MMP2, CREB and p-CREB, and the ratio of p-CREB/CREB in HUVECs induced by H2O2, suggesting that hsa-miR-3928-5p mediated the anti-inflammatory effects of vitamin C by targeting IL10, MMP2 and CREB.
In conclusion, the present study may be an important step in obtaining a greater understanding of the mechanisms through which vitamin C exerts anti-apoptotic and antioxidant effects via miRNA signaling networks, thereby revealing the potential molecular mechanisms of vitamin C as regards the antioxidation and apoptosis of HUVEC induced by H2O2.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Guangdong Basic and Applied Basic Research Fund (Key project of Guangdong-Foshan Joint Fund) (2019B1515120044) and Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006 and FS0AA-KJ218- 1301-0010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JW, YHu and YHuang conceived and designed the experiments. JW, JingjingL, MLi, MLin, LM, XH, JianqiuL performed the experiments. JW and JingjingL analyzed the data and wrote the manuscript. JianqiuL and YHuang revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ruano L, Portaccio E, Goretti B, Niccolai C, Severo M, Patti F, Cilia S, Gallo P, Grossi P, Ghezzi A, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler. 2017;23:1258–1267. doi: 10.1177/1352458516674367. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Yong H, Przedborski S. Oxidative stress in Parkinson's disease: A mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 5.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 7.Palade F, Alexa ID, Azoicăi D, Panaghiu L, Ungureanu G. Oxidative stress in atherosclerosis. Rev Med Chir Soc Med Nat Iasi. 2003;107:502–511. In Romanian. [PubMed] [Google Scholar]
- 8.Kubrak OI, Husak VV, Rovenko BM, Poigner H, Mazepa MA, Kriews M, Abele D, Lushchak VI. Tissue specificity in nickel uptake and induction of oxidative stress in kidney and spleen of goldfish carassius auratus, exposed to waterborne nickel. Aquat Toxicol. 2012;118-119:88–96. doi: 10.1016/j.aquatox.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: An overview. Ageing Res Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int J Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge J. Free radicals in biology and medicine. J Free Radical Biol Med. 2007;1:331–332. doi: 10.1016/0748-5514(85)90140-0. [DOI] [Google Scholar]
- 12.Gibbons GH, Dzau VJ. Molecular therapies for vascular diseases. Science. 1996;272:689–693. doi: 10.1126/science.272.5262.689. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schinzari F, Tesauro M, Cardillo C. Endothelial and perivascular adipose tissue abnormalities in obesity-related vascular dysfunction: Novel targets for treatment. J Cardiovasc Pharmacol. 2017;69:360–368. doi: 10.1097/FJC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Xia L, Zhang F, Zhu D, Xin C, Wang H, Zhang F, Guo X, Lee Y, Zhang L, et al. A novel mechanism of diabetic vascular endothelial dysfunction: Hypoadiponectinemia-induced NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1556–1567. doi: 10.1016/j.bbadis.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo CX, Talal B, Taleb A, Mais E, Qilong D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic Res. 2017;51:428–438. doi: 10.1080/10715762.2017.1322205. [DOI] [PubMed] [Google Scholar]
- 19.Chen AY, Lü JM, Yao Q, Chen C. Entacapone is an antioxidant more potent than vitamin C and vitamin E for scavenging of hypochlorous acid and peroxynitrite, and the inhibition of oxidative stress-induced cell death. Med Sci Monit. 2016;22:687–696. doi: 10.12659/MSM.896462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, Cutlip RG, Always SE. Vitamin E and C supple-mentation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp Gerontol. 2010;45:882–895. doi: 10.1016/j.exger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, aging and Alzheimer's disease. Nutrients. 2017;9:690. doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 23.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uetaki M, Tabata S, Nakasuka F, Soga T, Tomita M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci Rep. 2015;5:13896. doi: 10.1038/srep13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin B, Tang S, Sun J, Zhang X, Xu J, Di L, Li Z, Hu Y, Bao E. Vitamin C and sodium bicarbonate enhance the antioxidant ability of H9C2 cells and induce HSPs to relieve heat stress. Cell Stress Chaperones. 2018;23:735–748. doi: 10.1007/s12192-018-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hyper-cholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.CIR.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 28.Karajibani M, Hashemi M, Montazerifar F, Dikshit M. Effect of vitamin E and C supplements on antioxidant defense system in cardiovascular disease patients in Zahedan, southeast Iran. J Nutr Sci Vitaminol (Tokyo) 2010;56:436–440. doi: 10.3177/jnsv.56.436. [DOI] [PubMed] [Google Scholar]
- 29.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Schug J, McKenna LB, Le Lay J, Kaestner KH, Greenbaum LE. Tissue-specific regulation of mouse microRNA genes in endoderm-derived tissues. Nucleic Acids Res. 2011;39:454–463. doi: 10.1093/nar/gkq782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Chen C, Li H, Xu J, Wu L, Yu Y, Ren S, Li H, Hua X, Yan H, et al. miR-3687 overexpression promotes bladder cancer cell growth by inhibiting the negative effect of FOXP1 on cyclin E2 transcription. Mol Ther. 2019;27:1028–1038. doi: 10.1016/j.ymthe.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolhe R, Mondal AK, Pundkar C, Periyasamy-Thandavan S, Mendhe B, Hunter M, Isales CM, Hill WD, Hamrick MW, Fulzele S. Modulation of miRNAs by vitamin C in human bone marrow stromal cells. Nutrients. 2018;10:186. doi: 10.3390/nu10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Chen Y, Zhang H, Chen Y, Shen X, Shi C, Liu Y, Yuan W. Integrated microRNA-mRNA analyses reveal OPLL specific microRNA regulatory network using high-throughput sequencing. Sci Rep. 2016;6:21580. doi: 10.1038/srep21580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Woo J, Kim YS, Im HI. Integrated miRNA-mRNA analysis in the habenula nuclei of mice intravenously self-administering nicotine. Sci Rep. 2015;5:12909. doi: 10.1038/srep12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: An effect reversible with vitamin C therapy. Circulation. 1999;99:1156–1160. doi: 10.1161/01.CIR.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 36.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: Relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31:53–61. doi: 10.1016/S0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, et al. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci USA. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in reno-vascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 39.Gao Y, Han Z, Li Q, Wu Y, Shi X, Ai Z, Du J, Li W, Guo Z, Zhang Y. Vitamin C induces a pluripotent state in mouse embryonic stem cells by modulating microRNA expression. FEBS J. 2015;282:685–699. doi: 10.1111/febs.13173. [DOI] [PubMed] [Google Scholar]
- 40.Kim SM, Lim SM, Yoo JA, Woo MJ, Cho KH. Consumption of high-dose vitamin C (1250 mg per day) enhances functional and structural properties of serum lipoprotein to improve anti-oxidant, anti-atherosclerotic, and anti-aging effects via regulation of anti-inflammatory microRNA. Food Funct. 2015;6:3604–3612. doi: 10.1039/C5FO00738K. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi SW, Kang JS, Lee WJ, Jung KC, Kim SH, Choi YM, et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod Sci. 2010;17:1081–1089. doi: 10.1177/1933719110377663. [DOI] [PubMed] [Google Scholar]
- 42.Park WH. MAPK inhibitors, particularly the JNK inhibitor, increase cell death effects in H2O2-treated lung cancer cells via increased superoxide anion and glutathione depletion. Oncol Rep. 2018;39:860–870. doi: 10.3892/or.2017.6107. [DOI] [PubMed] [Google Scholar]
- 43.Samakova A, Gazova A, Sabova N, Valaskova S, Jurikova M, Kyselovic J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol Res. 2019;68(Suppl 2):S131–S138. doi: 10.33549/physiolres.934345. [DOI] [PubMed] [Google Scholar]
- 44.Yao Y, Wang Y, Zhang Y, Liu C. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis. 2017;16:77. doi: 10.1186/s12944-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Z, Li Z, Zhang X, Yin K, Wang W, Xu Z, Li B, Zhang L, Xu J, Sun G, et al. MiR-422a regulates cellular metabolism and malignancy by targeting pyruvate dehydrogenase kinase 2 in gastric cancer. Cell Death Dis. 2018;9:505. doi: 10.1038/s41419-018-0564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge L, Habiel DM, Hansbro PM, Kim RY, Gharib SA, Edelman JD, Königshoff M, Parimon T, Brauer R, Huang Y, et al. miR-323a-3p regulates lung fibrosis by targeting multiple profibrotic pathways. JCI Insight. 2016;1:e90301. doi: 10.1172/jci.insight.90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JK, Guo Q, Zhang XW, Wang LC, Liu Q, Tu PF, Jiang Y, Zeng KW. Aglaia odorata Lour. extract inhibit ischemic neuronal injury potentially via suppressing p53/Puma-mediated mitochondrial apoptosis pathway. J Ethnopharmacol. 2020;248:112336. doi: 10.1016/j.jep.2019.112336. [DOI] [PubMed] [Google Scholar]
- 48.Cai ZJ, Lee YK, Lau YM, Ho JC, Lai WH, Wong NL, Huang D, Hai JJ, Ng KM, Tse HF, et al. Expression of Lmna-R225X nonsense mutation results in dilated cardiomyopathy and conduction disorders (DCM-CD) in mice: Impact of exercise training. Int J Cardiol. 2020;298:85–92. doi: 10.1016/j.ijcard.2019.09.058. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim RY, Mansour SM, Elkady WM. Phytochemical profile and protective effect of Ocimum basilicum aqueous extract in doxorubicin/irradiation-induced testicular injury. J Pharm Pharmacol. 2020;72:101–110. doi: 10.1111/jphp.13175. [DOI] [PubMed] [Google Scholar]
- 50.Hirai T, Konishi Y, Mizuno S, Rui Z, Sun Y, Nishiwaki K. Differential effects of sevoflurane on the growth and apoptosis of human cancer cell lines. J Anesth. 2020;34:47–57. doi: 10.1007/s00540-019-02701-w. [DOI] [PubMed] [Google Scholar]
- 51.Tang Y, Liu J, Yan Y, Fang H, Guo C, Xie R, Liu Q. 1-25-dihydroxyvitamin-D3 promotes neutrophil apoptosis in periodontitis with type 2 diabetes mellitus patients via the p38/MAPK pathway. Medicine (Baltimore) 2018;97:e13903. doi: 10.1097/MD.0000000000013903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Zhang M, Chen W, Wang R, Ye Z, Wang Y, Li X, Cai C. LncRNA-HOTAIR inhibition aggravates oxidative stress-induced H9c2 cells injury through suppression of MMP2 by miR-125. Acta Biochim Biophys Sin (Shanghai) 2018;50:996–1006. doi: 10.1093/abbs/gmy102. [DOI] [PubMed] [Google Scholar]
- 53.Blascke de Mello MM, Parente JM, Schulz R, Castro MM. Matrix metalloproteinase (MMP)-2 activation by oxidative stress decreases aortic calponin-1 levels during hypertrophic remodeling in early hypertension. Vascul Pharmacol. 2019;116:36–44. doi: 10.1016/j.vph.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, Park YS, Paik HD, Chang HI. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br J Nutr. 2011;106:1792–1801. doi: 10.1017/S000711451100242X. [DOI] [PubMed] [Google Scholar]
- 55.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herkel J, Schrader J, Erez N, Lohse AW, Cohen IR. Activation of the Akt-CREB signalling axis by a proline-rich heptapeptide confers resistance to stress–induced cell death and inflammation. Immunology. 2017;151:474–480. doi: 10.1111/imm.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.