Abstract
N6-methyladenosine (m6A) is the most prevalent and abundant type of internal post-transcriptional RNA modification in eukaryotic cells. Multiple types of RNA, including mRNAs, rRNAs, tRNAs, long non-coding RNAs and microRNAs, are involved in m6A methylation. The biological function of m6A modification is dynamically and reversibly mediated by methyltransferases (writers), demethylases (erasers) and m6A binding proteins (readers). The methyltransferase complex is responsible for the catalyzation of m6A modification and is typically made up of methyltransferase-like (METTL)3, METTL14 and Wilms tumor 1-associated protein. Erasers remove methylation by fat mass and obesity-associated protein and ALKB homolog 5. Readers play a role through the recognition of m6A-modified targeted RNA. The YT521-B homology domain family, heterogeneous nuclear ribonucleoprotein and insulin-like growth factor 2 mRNA-binding protein serve as m6A readers. The m6A methylation on transcripts plays a pivotal role in the regulation of downstream molecular events and biological functions, such as RNA splicing, transport, stability and translatability at the post-transcriptional level. The dysregulation of m6A modification is associated with cancer, drug resistance, virus replication and the pluripotency of embryonic stem cells. Recently, a number of studies have identified aberrant m6A methylation in cardiovascular diseases (CVDs), including cardiac hypertrophy, heart failure, arterial aneurysm, vascular calcification and pulmonary hypertension. The aim of the present review article was to summarize the recent research progress on the role of m6A modification in CVD and give a brief perspective on its prospective applications in CVD.
Keywords: m6A, cardiovascular disease, mechanism, regulation, single nucleotide polymorphism
1. Introduction
Epitranscriptomics is an emerging research field of biology, in which a recently discovered novel mechanism of post-transcriptional regulation of RNA has been suggested to play a vital role in the regulation of RNA function and to be involved in various biological processes, including disease progression (1). RNA methylation modification is the most common type of RNA modification in eukaryotes, accounting for 60% of total RNA modification. The identified RNA modifications have been found to contribute to the structural complexity of RNA and participate in multiple biological functions (2).
In the early 1970s, a novel RNA epigenetic modification, N6-methyladenosine (m6A), was first discovered and proposed in eukaryotic messenger RNA (mRNA) from Novikoff hepatoma cells (3). m6A modification is regarded as the most prevalent, reversible and dynamic eukaryotic mRNA transcriptional modification among >170 types of RNA modification, accounting for ~50% of all methylated ribonucleotides. More than 7,000 mRNAs in mammalian cells are m6A-modified, and it is estimated that m6A exists in 0.1-0.4% of adenosines (4). Recent studies have found that, in addition to mRNA, m6A also occurs in rRNA, tRNA, small nucleolar RNAs, microRNAs (miRNAs or miRs) and long non-coding RNAs (5,6). The rapid development of next-generation sequencing technology and epigenetic research have assisted in the precise assessment of the in vivo methylation state of m6A sites at single-nucleotide resolution (7). Approximately 1/4 of transcripts harbor m6A modifications, which are mainly enriched around the stop codons, within long internal exons in the 5′ and 3′ untranslated regions (UTRs) at the consensus motif RRACH (R=A or G, H=A, C or U) (8). Understanding the functionality and mechanism of m6A is essential for understanding the implications of m6A in molecular governance. The m6A modification is involved in multiple procedures throughout a number of biological processes in mammals, such as splicing regulation (9), translocation (10), RNA stability (11), translation (12) and miRNA maturation (13).
m6A is reversibly and dynamically regulated by methyl-transferase and demethylases. m6A binding proteins play the role by recognizing and binding with the m6A sites of target RNAs (14). m6A modification has become a popular research field of molecular biology, due to its crucial regulatory role in biological processes and the pathogenesis of a variety of diseases (15-18). Despite recent progress in m6A modification research, the presence and functionality of m6A remains largely unknown. Recent studies have reported the emerging roles of m6A in the development of cardiovascular diseases (CVDs). The present review focuses on the latest progress in made m6A modification research and provides an up to date summary of the association between m6A modification and CVDs, which may provide insight into m6A-related molecular biomarkers and therapeutic targets in CVD.
2. m6A methylation
Molecular mechanisms of m6A methylation
Adenosine methylation at the N6-position is regarded as the most pervasive internal post-transcriptional chemical modification in mammalian mRNA and non-coding RNAs (19,20). RNA decoration by m6A plays a fundamental role in the regulation of mRNA stability, translational efficiency and gene expression during normal cellular bioprocess or under disease conditions (21,22). m6A is essential for RNA processing during mammalian development and disease progression. m6A RNA methylation affects a variety of cellular biological processes, including splicing, processing, nuclear export, stability and decay, translation, cellular differentiation and metabolism (23,24). Dysregulated m6A modification is an important hallmark of various diseases, including cancer (25-27), neurological disorders (28,29) and osteoporosis (30). Recently, an increasing number of studies have reported the role of m6A methylation in the occurrence and development of CVD. m6A modifications are mainly enriched around the 3′UTR, near stop codons and within long internal exons at the consensus motif RRACH (R=A/G, H=A/C/U) (31).
Epitranscriptomic m6A modification is dynamically and reversibly regulated by modulators characterized as dedicated methyltransferases (writers), dedicated demethylases (erasers) and m6A binding protein (readers), according to their functions (32). The aberrant expression of methyltransferases and demethylases results in the dysregulation of m6A. Methyltransferases are responsible for the erection of m6A modification, and m6A methylation is removed by demethylases. Of note, m6A binding proteins have been shown to recognize target m6A-modified RNAs and participate in the biological process and development of human disease (33).
Writers [i.e., methyltransferase-like 3 (METTL3), METTL14 and Wilms tumor 1-associated protein (WTAP)] and erasers [i.e., fat mass and obesity-associated protein (FTO) and a-ketoglutarate-dependent dioxygenase (ALKB) homolog 5 (ALKBH5)] are responsible for catalyzing and removing m6A, respectively (34-37). The regulatory mechanisms of m6A are complex. Moreover, an increasing number of m6A modulators have been discovered, particularly methyltransferases and m6A binding proteins. m6A readers have both stimulatory and inhibitory effects on translation dynamics. YTHDF2 can accelerate the degradation of m6A-modified maternal transcripts, while YTHDF1 increases the translation efficiency of m6A-marked dynamic transcript (38-40). The site-specific m6A maps of transcripts and the role of site-specific methylation in translation warrant further investigation.
m6A methyltransferases
m6A methyltransferases are responsible for catalyzing the formation of m6A modification (18). The multicomponent methyltransferase complex consists of a METTL3/METTL14 heterodimer and various other methyltransferases (41). METTL3 has been described as the core enzyme exerting methyltransferase activity in the methyltransferase complex through its combination with S-adenosyl methionine. METTL14 acts as a second supporting enzyme to strengthen the catalytic effect of m6A RNA methylation (42,43). C-terminal arginine-glycine repeats in METTL14 are secondary RNA substrate binding sites and indispensable for METTL3-METTL14 catalytic activity (44). The heterodimer preferentially methyltransferases a GGACU motif (45).
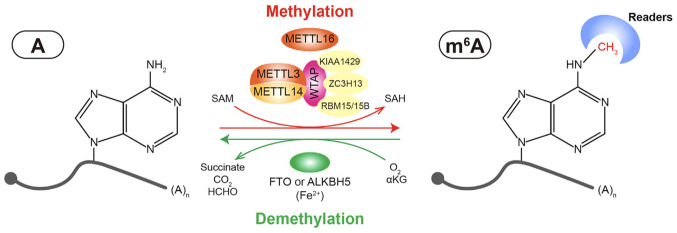
WTAP is a mammalian splicing factor responsible for the interaction with the METTL3-METTL14 complex. WTAP is critical for initiating and guiding the localization of nuclear speckles, which is required for m6A methylation activation. WTAP also regulates their recruitment to mRNA targets (46). Numerous regulatory enzymes have been found to interact with the heterodimer, such as Vir-like METTL16, KIAA1429 (47), RNA-binding motif 15 (RBM15)/RBM15B (48), Vir-like m6A methyltransferase associated (49), E3 ubiquitin-protein ligase Hakai and zinc finger CCCH domain containing protein 13 (50). The above-mentioned enzymes bind to mRNA and recruit the METTL3-METTL14 complex, guiding the heterodimer to target regions (Fig. 1).
Figure 1.
Reversible m6A modification on mRNA. The adenosine (A) bases reside in mRNA could be methylated to form N6-methyladenosine (m6A) by the large MTC writer complex composed of the METTL3-METTL14-WTAP core component and other regulatory cofactors or by METTL16 alone. This enzymatic reaction uses S-adenosylmethionine (SAM) as a methyl donor. m6A could be recognized by m6A binding proteins (readers) to affect mRNA fate, or could be reversibly removed by m6A eraser proteins (i.e., FTO and ALKBH5). The demethylation process requires a-ketoglutaric acid (a-KG) and molecular oxygen (O2) as co-substrates and ferrous iron (Fe2+) as a cofactor (2). METTL, methyltransferase-like; FTO, fat mass and obesity-associated protein; ALKBH5, ALKB homolog 5.
m6A demethylases
The discovery in 2011 of demethylases FTO and ALKBH5 revealed that m6A modification is dynamic and reversible (34,36). The two identified demethylases both belong to the AlkB-related family of proteins. The conserved α-ketoglutarate/iron-dependent domain is indispensable for demethylation modifications. FTO and ALKBH5 perform potent functions in the splicing, processing, stability and translation of RNA (51). The downregulation of FTO or ALKBH5 has been shown to lead to an elevated m6A modification in mRNA (34).
FTO was the first identified demethylase of m6A; it local-izes into the nucleus and has been recognized as a member of the AlkB-related family of non-heme FeII/α-KG-dependent dioxygenases. FTO has an efficient oxidative demethylation activity in targeting m6A residues in RNA (52). FTO is essential for the biological development of cardiovascular systems (52). FTO-dependent m6A demethylation can contribute to the increase or decrease of protein levels, due to the regulation of mRNA stability, and degradation and translation efficiency (53).
A number of AlkB protein family members serve as RNA methylation erasers through the oxidative demethylation of m6A marks; however, only ALKBH5 has been identified to exhibit efficient demethylation activity towards m6A in animals (54). ALKBH5 has a tight interaction with mRNA and other RNA substrates. The alanine-rich sequence and potential coiled-coil structure in the N-terminus of ALKBH5 are important for its localization. ALKBH5 can affect both the synthesis and splicing rate of mRNAs (36) (Fig. 1).
m6A RNA binding protein
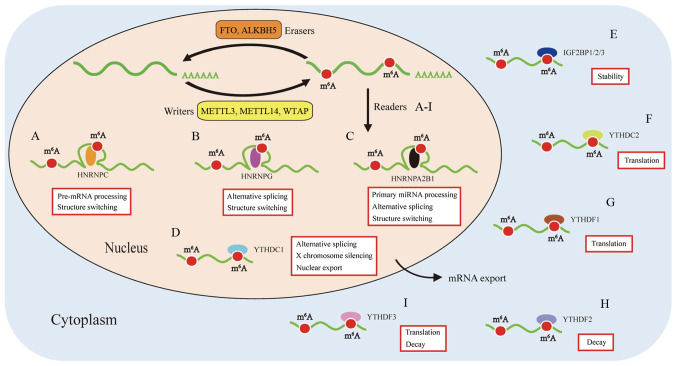
The 'reading' of m6A methylation marks is achieved through m6A RNA binding proteins (RBP), which specifically recognize target m6A-modified mRNA and subsequently play a role in the regulation of RNA splicing, fold, transport, translocation, degradation and translation. m6A RBPs are involved in the regulation of RNA metabolism and bioprocess (33). m6A-RBPs include YT521-B homology (YTH), heterogenous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) and insulin-like growth factor 2 mRNA-binding protein (IGF2BP) domain (39,55,56) (Fig. 2).
Figure 2.
The regulation of m6A modification. m6A is established by m6A methyltransferases ('writers') and removed by m6A demethylases ('erasers'). m6A readers are involved in multiple procedures of RNA metabolism through recognizing and binding to the m6A sites of RNAs. (A) HNRNPC plays an impor-tant role in the pre-mRNAs processing and structure switching. (B) HNRNPG modulates alternative splicing and structure switching. (C) HNRNPA2B1 accelerates primary miRNAs processing, alternative splicing, and structure switching. (D) YTHDC1 participates in the alternative splicing, nuclear export, and X chromosome silencing. (E) IGF2BP1/2/3 have a function to increase the stability of targeted mRNAs. (F) YTHDC2 promotes mRNAs translation. (G) YTHDF1 augments mRNAs translation. (H) YTHDF2 facilitates mRNAs decay. (I) YTHDF3 cooperates with YTHDF1 to increase mRNAs translation, and strengthens mRNAs decay mediated by YTHDF2 (33).
The YTH domain was identified in the human splicing factor YT521-B and is typically found in 174 different proteins expressed in eukaryotes, with a residue size of 100-150. It is featured by 14 invariant residues with an α-helix/β-fold to bind to RNA (57,58). The YTH domain-containing family can be divided into two subfamilies, YTHDF (YTHDF1-3) and YTHDC (YTHDC1-2) (59). YTHDF1 is known as an important m6A reader promoting the cap-independent translation regulation of m6A-modified RNA transcripts in the 5′UTR region. YTHDF1 relocates from the cytoplasm to the nucleus and initiates and augments translation in an eIF3 initiation factor-dependent manned (39). Coversely, YTHDF2 trans-ports the mRNA targets to the cytoplasmic processing body and promotes its degradation (18). Specifically, Du et al (60) reported the specific mechanism of deadenylation mediated by YTHDF2 in mammalian cells. YTHDF2 distinguishes m6A-bearing RNAs, and then interacts with CNOT1 to recruit the CCR4-NOT complex, which is critical for the demethylation of m6A-marks by CAF1 and CCR4. YTHDF3 plays a cooperative role in RNA stability and translation among proteins from the YTHDF family to influence the metabolism of m6A-methylated mRNA (24).
Nuclear binding protein YTHDC1 regulates alternative nuclear mRNA splicing by recruiting SRSF3 splicing factor and inhibiting SRSF10 binding to mRNA (61). YTHDC1 has also been found to be involved in nuclear transport (62) and gene translation silencing (48). YTHDC2 has several defined domains, including the YTH domain, R3H domain and ankyrin repeats (63). Phillip reported that YTHDC2 is a critical m6A reader involved in spermatogenesis. YTHDC2 selectively binds to m6A residues, decreases their mRNA abundance and enhances the translation efficiency of mRNAs (64).
Several proteins from the HNRNP family have been found to have the potential of recognizing m6A-modified mRNA, including HNRNPA2B1, HNRNPC and HNRNPG. HNRNPA2B1 is a nuclear m6A-reader that directly binds to nuclear transcripts, elicits regulatory effects on RNA splicing (55) and promotes primary microRNA processing (13). HNRNPC plays a regulatory role in the acceleration of pre-miRNA processing (65). HNRNPG interacts with RNA polymerase II and m6A-modified pre-mRNAs to modulate the alternative splicing and expression of target mRNAs (66).
IGF2BPs (IGF2BP1-3) belong to conserved m6A-binding proteins, whose RNA-binding domains consist of two RNA recognition motif domains and four K homology (KH) domains, with KH 3-4 being indispensable in recognizing m6A marks (67). As m6A readers, IGF2BPs are almost exclusively expressed in the cytoplasm, preferentially recognize m6A-bearing mRNAs and fortify the stability of mRNA, therefore promoting translational efficiency (56). Dysregulated IGF2BPs have been found in several aggressive cancer cells and play oncogenic roles by enhancing the stability of methylated oncogenic mRNAs (67).
Methods of assessing the methylation of m6A sites
The rapid emergence of next-generation sequencing technology has increased our understanding of epigenetic research and helped us assess the in vivo methylation state of m6A sites. The two novel m6A modification site detection technologies, m6A sequencing (m6A-seq) and m6A-specific methylated RNA immunoprecipitation (MeRIP-seq) were created. m6A-seq, presented by Dominissini et al (8), is a novel approach based on m6A antibody-mediated capture and high-throughput sequencing. m6A-Modified are highly conserved between human and mice (8). Meyer et al (68) presented the MeRIP-seq method for transcriptome-wide m6A localization, and m6A methylated RNA was immunoprecipitated, followed by next-generation sequencing. m6A-seq and MeRIP-seq identified thousands of mRNAs with m6A modification and revealed that m6A is a pervasive and dynamically reversible internal chemical modification of mRNA. However, there exist some limitations in the detection technology. This method has a relatively low accuracy in the identification of m6A modification sites in the whole transcriptome for the following reasons: First, only RNA fragments 100-200 nt long could be captured through these mapping approaches. Besides, two similar m6A sites could not be identified.
m6A-level and isoform-characterization sequencing is another m6A modification detection technique (9). Different from m6A-seq and MeRIP-seq, intact full-length transcripts, rather than fragmented RNA, were isolated and sequenced. The quantification of m6A modification helps examine differential isoform usage in transcripts with/without methylation.
Other revolutionary technological methods include photo-crosslinking-assisted m6A-seq (69), m6A individual- nucleotide-resolution cross-linking and immunoprecipitation (70) and ultraviolet (UV) CLIP (71). In these methods, UV strengthens the interaction between m6A-modified RNA and m6A antibodies, and affinity purification was performed. m6A modification sites can be detected more precisely at single-nucleotide resolution on a transcriptome-wide level. Site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography can accurately detect a single m6A modification site and the m6A modification level of the whole transcriptome. However, although highly accurate, it is also costly and time-consuming, limiting its application in m6A methylation detection (72). Other methods include MAZTER-seq, RNAmod, FunDMDeep-m6A and m6A-REF-seq (72-76). Table I summarizes the methods of m6A methylation detection (7). These findings have provided insight into the mammalian genome-wide mapping of m6A modifications and unveiled the importance of dynamic mRNA modifications on gene expression. However, numerous difficulties and challenges remain in single-nucleotide detection and quantitative sequencing, and further research is required in the future.
Table I.
Methods for the detection of m6A methylation.
| Methods | Features |
|---|---|
| m6A-Seq | High throughput |
| MeRIP-seq | High throughput |
| m6A-LAIC-seq | High throughput, precise |
| PA-m6A-Seq | High throughput, single site |
| miCLIP | High throughput, single site |
| m6A-CLIP | High throughput, single site |
| SCARLET | High throughput, single site |
| MAZTER-seq | High throughput, single site |
| RNAmod | High throughput, single site |
| FunDMDeep-m6A | High throughput, single site |
| DART-seq | High throughput, single site |
| RNA sequencing | High throughput, single site |
| m6A-REF-seq | High throughput, single site |
3. m6A and vascular smooth muscle cell differentiation
Adipose-derived stem cells (ADSCs) are a source of mesenchymal stem cells that can be used to develop biological treatment strategies for tissue regeneration. ADSCs can differentiate into vascular smooth muscle cells (VSMCs) under certain conditions. METTL3 mRNA and protein expression are increased in the VSMC differentiation of ADSCs. The downregulation of METTL3 contributes to a decrease in the m6A modification of VSMC-specific markers, thus leading to a decline in their mRNA and protein expression. The silencing of METTL3 inhibits ADSC differentiation into VSMCs. In addition, an increased expression of paracrine factors has been observed in ADSC differentiation into VSMCs, including vascular endothelial growth factor, hepatocyte growth factor, granulocyte-macrophage colony-stimulating factor, basic fibroblast growth factor and stromal cell-derived factor-1 (77). An investigation of the role of METTL3 in the regulation of VSMC differentiation has suggested that METTL3 is involved in ADSC differentiation to VSMCs, providing a promising perspective for novel therapeutic strategies for vascular network regeneration (77).
4. m6A and cardiovascular disease
There is emerging evidence to indicate that m6A modification is closely related to the occurrence and progression of CVDs, including cardiac hypertrophy, heart failure, ischemic heart disease, aortic aneurysm, vascular calcification, pulmonary hypertension etc. Herein, the recent progress in m6A modification in CVD is briefly reviewed.
m6A and cardiac hypertrophy
Cardiac remodeling occurs in the heart following stress stimulation or injury, and it involves molecular, cellular and interstitial alterations, clinically manifested as changes in size, shape and function (78). Stress stimulation initially induces adaptive hypertrophic response to produce sufficient force to match increased wall tension or increased pressure/volume overload in cardiomyocytes (79). Currently, post-transcriptional regulation is recognized as similarly critical to transcriptional regulation in cardiac hypertrophy (80).
Dorn et al (81) demonstrated that METTL3-mediated m6A modification is significant for maintaining cardiac homeostasis and normal cardiac function and revealed increased m6A methylation in cardiomyocytes under hypertrophic stimulation. Of note, m6A peaks are specifically enriched in mRNAs encoding protein kinases and modifiers, such as mitogen-activated protein kinase kinase kinase 6, mitogen-activated protein kinase kinase kinase kinase 5 and mitogen-activated protein kinase 14. METTL3-overexpressing mice exhibited marked cardiac hyper-trophy but not accelerated dysfunction during pressure overload stress. The inhibition of m6A blocked cardiomyocyte hyper-trophy by deleting METTL3. In vivo, METTL3 knockout mice exhibited signs of failure, both morphologically and function-ally. Collectively, they demonstrated that METTL3-mediated m6A methylation is dynamic. The enhanced METTL3-mediated m6A modification caused by hypertrophic stimuli resulted to cardiac hypertrophy, whereas downregulated m6A methylation led to cardiomyocyte remodeling, highlighting the importance of the mechanism in the maintenance of cardiac homeostasis, function and adaptation to stress responses (81).
m6A and ischemic heart disease
The lysosomal-mediated degradation pathway (autophagy) is an important evolution-arily conserved degradation mechanism in eukaryotic cells that removes unnecessary and harmful parts to maintain homeostasis (82). Autophagy is associated with numerous human diseases, including CVD (83). Song et al (83) first researched the role of m6A in autophagy and discovered that m6A modification is significantly upregulated in hypoxia/reoxygenation (H/R)-treated cardiomyocytes and ischemia/reperfusion (I/R)-treated mouse hearts. The key member of the methyltransferase complex, METTL3, was abnormally upregulated in infraction heart tissue, as compared with healthy heart tissue. Consistently, an increased expression of METTL3 was identified in cardiomyocytes treated with H/R. The silencing of METTL3 increased the I/R-impaired autophagic flux and inhibited apoptosis (83).
It has been well documented that transcription factor EB (TFEB) is an important gene in lysosomal biogenesis, which also drives the expression of autophagy and lysosomal genes (84,85). Decreased TFEB mRNA stability and TFEB protein expression were previously discovered in H/R-induced cardiomyocytes. The silencing of METTL3 significantly promoted the expression and transcriptional activity of TFEB and translocation to the nucleus. TFEB knockdown inhibited the enhanced autophagy induced by the downregulation of METTL3, indicating that the mediation of autophagic flux by METTL3 is TFEB-dependent (86). The RNA binding protein heterogeneous nuclear ribonucleoprotein D (HNRNPD) was reported to bind to m6A-modified RNA (25), and an increased HNRNPD was found in patients with heart failure (87). RIP-PCR confirmed the interaction between HNRNPD and TFEB pre-mRNA. H/R further enhanced the binding (86). In accordance with previous research (88), the H/R-induced HNRNPD elevation has been shown to promote TFEB mRNA degradation, and reduce the protein expression and transcriptional activity of TFEB. Furthermore, TFEB can suppress METTL3 expression by downregulating the stability of METTL3 mRNA, thereby forming negative feedback between METTL3 and TFEB (86). On the whole, research has revealed that the METTL3-TFEB feedback loop plays an essential role in autophagy, and has laid a foundation for dynamic m6A modification in ischemic heart disease.
m6A and heart failure
There is currently no effective approach available for adverse cardiac remodeling post-ischemia. mRNA and protein expression levels in the right ventricles during heart failure are inconsistent, suggesting a role for post-transcriptional regulation in failing hearts (89). Mathiyalagan et al (90) discovered that FTO played a vital role in cardiac contractile function during homeostasis and remodeling. An increased m6A modification and significantly decreased FTO expression were found in infarct and peri-infarct regions of failing hearts, as compared with healthy heart tissues. FTO overexpression attenuated the ischemia-induced elevation in m6A modification. FTO-knockdown exhibited aberrantly increased arrhythmic events in cardiomyocytes. In vivo, FTO-overexpression significantly improved cardiac function at the chronic stage of post-myocardial infraction. Enhanced angiogenesis and alleviated fibrosis were also observed. A more rapid progression of heart failure was witnessed with a lower ejection fraction and more severe dilatation in FTO knockout mice, indicating the indispensable role of FTO in heart failure (90). Mechanistically, the Ca2+-handling transcript Serca2a has been shown to be hypermethylated in failing hearts, and FTO demethylated Serca2a and enhanced the stability of Serca2a mRNA (91) or exerted co-transcriptional regulation (38), leading to an increase in SERCA2a expression, ultimately improving cardiac contractile function. Collectively, these results provide compelling evidence that FTO-mediated m6A methylation plays an important role in cardiac function during heart failure and recommend FTO as a novel therapeutic approach (90).
Differently expressed genes of m6A methylation are involved in heart failure development (92). Hypermethylated and hypomethylated transcripts have been linked to different biological processes. Gene ontology (GO) analysis has revealed that the differentially m6A modified transcripts in heart failure are mainly involved in metabolism and cardiac signaling (92). Previous research has reported that m6A modification can affect mRNA translation efficiency by regulating ribosome occupancy (12). The Calm1 mRNA level, a member of the CaMKII signaling pathway, is unaltered during m6A modification, while Calm1 protein expression is significantly decreased in failed heart tissue, indicating that m6A methylation affects Calm1 translation rather than transcription in heart failure development. m6A-seq has revealed differentially methylated transcripts of epigenetic proteins, transcription factors and upstream regulators of signaling pathways, indicating that m6A methylation is possibly involved in the regulation of gene expression in heart failure (92). These data suggest that the modulation of m6A methylation may be a potential target for the treatment of heart failure.
Kmietczyk et al (93) explored the critical role of internal m6A modification in dilated cardiac tissue and observed an increased m6A methylation in mRNAs isolated from dilated cardiomyopathy samples. m6A methylation is highly dynamic in stress-induced cardiomyocytes. They demonstrated the opposite result to that of the study by Dorn et al (81), demonstrating that METTL3 knockdown promoted cardiac hypertrophy and remodeling in cardiomyocytes. METTL3-overexpression attenuated pathological cardiac hypertrophy (93). The different study designs may explain the contrasting data. A transgenic mouse model and a transgenic approach driven by alpha myosin heavy chain-promoter for METTL3 overexpression were used in the study by Dorn et al (81), while C57Bl6/N mice and a adeno-associated virus-based approach for METTL3 overexpression were used in the study by Kmietczyk et al (93). Further studies are required to fully elucidate the mechanisms of stress response in the heart, for the maintenance of normal cardiac homeostasis and function. The above-mentioned study (93) revealed a novel mechanism underlying stress response, with the aim of maintaining normal cardiac function. Once elucidated, the manipulations of METTL3-mediated m6A may provide a novel therapeutic strategy for the prevention of maladaptive and worsening of cardiac function. However, research to date has not elucidated the exact mechanisms underlying the regulation of cardiomyocyte growth by Mettl3 and identify the specific downstream targets.
The aforementioned research results suggested abnormal m6A methylation in failing hearts, as compared with healthy hearts, indicating that the aberrantly m6A methylation level may serve as a potential biomarker and therapeutic target by modulating m6A modulators and downstream genes.
m6A and abdominal aortic aneurysm (AAA)
AAA is a common vascular condition among the elderly with a high mortality rate (94). AAA is characterized by chronic inflammation in the tunica media and adventitia, with the upregulation of numerous cytokines activating a large amount of proteolytic enzymes, ultimately leading to the rapid expansion and rupture of AAA (95). The exact mechanisms contributing to AAA have not yet been elucidated. He et al first investigated the role of m6A modification in AAA and provided a potential epigenetic mechanism in AAA (96). They discovered that m6A modification significantly increased in AAA, as compared to healthy aortic tissues. METTL14 was shown to be associated with inflammatory infiltrates and neovascularization in AAA, and increased FTO was also involved in aberrant m6A modification. A higher m6A level was also found to be associated with a higher risk of rupture. They subsequently discovered significant correlations between the m6A modification level and the mRNA expression level of the writers, erasers and readers, indicating a tight crosstalk among these modulators. In clinical data, a higher m6A level was found to be positively associated with the AAA diameter and hematological parameters (96). Hence, abnormal m6A modification is crucial to the occurrence and progression of AAA.
Zhong et al (97) further explored METTL3-modulated methylation and the development of AAA. They revealed that the downregulation of METTL3 suppressed AAA formation in both ApoE−/− mice treated with angiotensin II, and a calcium chloride-induced mouse model. Of note, the downstream target gene they focused on, which was regulated by METTL3 in AAA, was not an mRNA, but miR-34a. Mechanistically, METTL3 mediated m6A modification and promoted miR-34a maturation from pri-miR-34a, and then miR-34a negatively regulated SIRT1 expression. Collectively, the METTL3/miR-34a/SIRT1 axis plays a role in AAA formation and may serve as a diagnostic biomarker and novel therapeutic target of AAA treatment (97).
m6A and vascular calcification
Chronic kidney disease (CKD) is a worldwide public health concern. Patients with CKD have a 2-fold higher risk of suffering from CVD and also exhibit a higher mortality rate, as compared to the healthy population (98). Patients with CKD are more likely to suffer from vascular calcification, and vascular calcification is recognized as the main cause of increased mortality from CVD (99). It has been confirmed that indoxyl sulfate (IS), a vital protein-bound uremic toxin, is associated with the development of renal and vascular progression (100). Chen et al investigated the epigenetic translation underlying IS-induced vascular calcification and noted marked in vitro, in vivo and translational evidence, indicating the essential role of METTL14 in IS-induced vascular calcification (101). A significantly elevated m6A level of total RNA was discovered in the radical arteries of patients with end-stage renal disease and preclinical calcified mouse arteries. An increased METTL14 mRNA and protein expression was detected in calcified arteries, indicating an IS-induced increase in the levels of METTL14 and METTL14, which mediated the increase in global m6A modification, which may be a hallmark of vascular calcification. In vitro, the overexpression of METTL14 caused the loss of repair function. Mechanistically, MeRIP analysis revealed that the vascular-protecting transcript Klotho mRNA was hyper-methylated in calcified arteries, resulting in the degradation and decreased mRNA expression of Klotho. Therefore, this investigation demonstrated the functional significance of METTL14 in IS-induced vascular calcification and provided proof of concept for anti-vascular calcification therapy through the modulation of METTL14 (101).
m6A and pulmonary hypertension
Pulmonary hypertension (PH) is a complex and multidisciplinary pathophysi-ological disorder defined as a resting mean pulmonary artery pressure(PAP) of >25 mmHg, as assessed by right heart catheterization (102,103). Hypoxic PH (HPH) is a progressive disease due to lung diseases and categorized as group III PH. Chronic obstructive pulmonary disease and interstitial lung diseases are common causes (2). Currently there is no specific therapy for the increased PAP and structural abnormalities of HPH (104).
Previous studies have reported that hypoxia may lead to the dysregulation of m6A methylation and may promote tumor occurrence and development. The effects of hypoxia on m6A modification are cell type-dependent (86,105-107). Little was known of m6A modification in circular RNAs (circRNAs), until thousands of m6A-modified cell-specific expressed circRNAs were identified (108). Wang et al first identified a transcriptome-wide circRNA expression profile in lung tissues from a mouse model of HPH using microarray analysis (109). The whole m6A level of circRNAs in HPH rat lung tissue was lower than that in healthy rat lung tissue. m6A abundance in circRNAs was also decreased in hypoxia in vitro. The distribution of total circRNAs, m6A-circRNAs and non-m6A cicRNAs was similar between the HPH and control groups. m6A methylation was enriched in circRNAs originating from single exons. GO and Kyoto Encyclopedia of Genes and Genomes pathway analysis identified different host genes of circRNAs with a hyper- and hypomethylated m6A level. m6A-modified cicRNAs tended to decrease in hypoxia (110).
circRNAs mostly play the role of a sponge for miRNAs, and are involved in the regulation of target miRNAs (111). A circRNA-miRNA-mRNA network was previously constructed to explore the regulation of gene expression in HPH. Key mRNAs associated with the Wnt (112) and FoxO signaling pathways (113), and miRNAs reported to be involved in HPH were filtered, and the most enriched novel circRNA Xpo6 and Tmtc3 were then identified (110). RIP-PCR confirmed that the expression of circXpo6 and circTmtc3 were significantly decreased in both hypoxia-induced pulmo-nary artery smooth muscle cells and endothelial cells (110). Collectively, the study (110) first revealed that m6A affected the stability of circRNAs, subsequently influencing the circRNA-miRNA-mRNA network, leading to the activation of the Wnt and FoxO signaling pathways and ultimately promoting HPH. The results of that study (110) suggested that m6A-modified circRNAs were associated with pulmonary hypertension. However, the research was just an expression file of circRNAs in lung tissues from HPH and control mice. The exact molecular mechanisms underlying the role of m6A methylation in HPH remain to be elucidated.
m6A-related single nucleotide polymorphisms (SNPs) and CVDs
High-throughput sequencing technology has identified millions of SNPs across multiple genomes. Distinguishing the disease-associated functional variants, which can regulate amino acids at the protein level (114), RNA secondary structure (115), RNA-protein interactions and RNA splicing (116) or editing (117) at the transcriptional or post-transcriptional level, is a major challenge. SNPs can interfere with m6A methylation by altering the RNA sequences of target sites or key flanking nucleotides (118). Putative m6A-related SNPs (m6A-SNPs), which are close to or at the exact methylation site, can disrupt m6A modification and corresponding biological processes through multiple mechanisms, due to the location of m6A-SNPs, and can subsequently affect disease progression (119). m6A-SNPs have been recognized as a fundamental class of crucial multifunctional genetic variants associated with CVDs, which is concerning. Numerous m6A-SNPs associated with CVDs have been identified using genome-wide association studies (GWAS) and the genetic functional mechanisms have been explored. These m6A-SNPs and genes may be candidates and promote novel therapeutic approaches (120,121). The present study reviewed the relevant research on m6A-SNPs and CVD.
m6A-SNPs and blood pressure (BP)
Genetic factors are significant, and heritability contributes to 40-60% of BP cases (122). A large number of SNPs for BP have been identified by a GWAS (123). Zheng et al (118) reported a comprehensive database (m6Avar), which is a useful tool for the investigation of the association between m6A-related variants and disease. A previous study first made efforts to investigate the association between m6A-SNPs and BP by excavating data from a large-scale GWAS, and demonstrated that m6A-SNP may play a pivotal role in BP regulation (124). Some identified BP-related SNPs in genes have been found to be associated with coronary artery disease (CAD) (125). The majority of m6A-SNPs were closely associated with gene expression. For example, rs9847953 and rs197922 were identified as potential functional variants and strongly associated with gene expression, ultimately contributing to the regulation of blood pressure.
The FTO gene has been found to be enriched in the hypo-thalamus (126), a brain structure involved in the control of blood pressure (127). Caucasian populations with the FTO-risk genotype (AA genotype) have been shown to have a higher systolic blood, which may result from a higher sympathetic modulation of vasomotor tone (128). Another study revealed that the most common genetic variant of the FTO gene (rs9939609 A/T) (129) was not associated with blood pressure in either sex (130). Further studies are required to elucidate the role of the FTO genetic variant in blood pressure.
m6A-SNPs and coronary artery disease
CAD is the leading cause of mortality and disability worldwide, with both genetic and environmental factors playing an essential role in its pathogenesis (131). The effect of m6A-SNPs on CAD was previously explored and 304 m6A-SNPs were found to be associated with CAD (120). Some m6A-SNPs not only alter m6A methylation and local gene expression, but also regulate protein binding, indicating that m6A-SNPs may be functional variants of CAD. Among these, rs12286 was markedly associated with CAD. Mechanistically, rs12286 may influence the m6A methylation level and regulate the expression of downstream gene ADAMTS7 to exert its function (120). On the whole, these findings elucidated the role of m6A-SNPs in CAD; however, the specific regulatory mechanisms remain to be explored.
m6A-SNPs and lipid metabolism
Blood cholesterol is one of the most important heritable risk factors for CAD, and is positively associated with mortality due to CAD (132). The role of m6A modification in lipid metabolism has been reported (133). Mo et al (121) investigated the effect of m6A-SNPs on lipid levels using GWAS summary datasets of 188,578 individuals. m6A-SNP rs6859 at the 3′UTR of poliovirus receptor-related 2 was found to be significantly expressed at the genome-wide level and associated with triglycerides, total cholesterol and high/low-density lipoprotein cholesterol, suggesting that rs6859 may be a lipid metabolism-related multifunctional SNP and an important candidate for further functional studies. That study found a large amount of lipid-related m6A-SNPs (121). Further studies are required to elucidate the mechanisms.
Further large-scale GWASs are required to identify m6A-SNPs in CVD, and further technical and biological experiments through single-nucleotide gene editing of SNPs to determine the detected SNPs functionalities.
5. Potential applications of m6A in cardiovascular disease
Characteristic biomarkers play a crucial role in the prevention, diagnosis and treatment of disease. Previous studies have demonstrated that methylation serves as a potential biomarker and therapeutic target in cancer (134). Fustin et al demonstrated that m6A editing through specific METTL3 inhibition contributed to eliciting circadian period elongation and RNA processing delay (10).
m6A as a potential biomarker
A number of studies have suggested that aberrant m6A modifications affect the progression of CVD. For instance, significantly elevated METTL3-modulated m6A methylation has been found in cardiac hypertrophy, ischemic heart disease, AAA, etc. (81,86,97). In addition, innovations in the detection technology for m6A modification render it a potential diagnostic biomarker of CVD. The early detection of upregulated m6A modification may help monitor and prevent the occurrence and development of CVD. A higher FTO was detected in failing hearts, as compared with healthy hearts. The overexpression of FTO has been shown to markedly improve the cardiac function of post-myocardial infraction (90). On the whole, m6A methylation may serve as a prospective non-invasive diagnostic biomarker and be used for the diagnosis and prognosis of CVD.
m6A as a therapeutic target of CVD
Accumulated research has indicated that m6A methylation is of importance in CVD, providing novel insight into the innovative therapeutic strategy of targeting related methyltransferases, demethylases, m6A-binding proteins or m6A-modified RNA.
Total Panax notoginseng saponin (TPNS) has been reported to inhibit balloon injury-induced intimal hyperplasia and VSMC proliferation (135). Zhu et al (136) observed a reduced WTAP expression and decreased m6A modification in balloon catheter-injured rat carotid artery. Furthermore, TPNS alleviated the proliferation and migration of VSMCs through the upregulation of WTAP and downstream target p16 m6A modification. Therefore, WTAP and m6A regulatory p16 expression may serve as a novel molecular target for the assessment of arterial stenosis risk and a novel therapeutic target of arterial stenosis.
It is widely acknowledged that lipopolysaccharide (LPS) is a trigger for inflammation and metabolic diseases (137). Rao et al (138) first found that curcumin reduced the blood cholesterol level in normal animals. Lu et al discovered that the lipid metabolism disorder in the liver and increase in total cholesterol induced by LPS injection were all attenuated after dietary supplementation with curcumin (139). Mechanistically, the LPS injection increased the m6A methylation level, accompanied by an increased METTL3 level, and decreased the mRNA of ALKBH5 and FTO in the liver. Of note, curcumin affected the expression of some crucial m6A-related modulators, including METTL3, METTL14, ALKBH5, FTO and YTHDF2. Further upregulated m6A methylation in the liver was discovered in piglets that had received dietary curcumin, as compared with the LPS injection group, suggesting that the protective effect of curcumin in LPS-induced hepatic lipid metabolism may result from increased m6A RNA modification (139). The precise mechanisms of how curcumin affects gene-specific m6A modification require further investigation. Collectively, the findings indicate that m6A could be a promising therapeutic target for hyperlipidemia.
The development of m6A modulation inhibitors provides an opportunity for the treatment of CVD. For instance, Huang et al (140) identified meclofenamic acid (MA), an inhibitor of demethylase FTO. MA exhibited an efficient and selective inhibitory effect on FTO demethylation in Hela cells by competition on m6A-containing substrate binding.
Of note, Li et al (141) successfully constructed an in vivo manipulation CRISPR-Cas13b-based tool for the study of m6A-related biological functions and targeted demethylation of specific mRNA. They created a gratifying fusion protein, dm6ACRISPR, by linking a catalytically inactive enzyme to ALKBH5. dm6ACRISPR could specifically demethylate transcripts with m6A marks through the regulation of mRNA stability or translation. In addition, the manipulation tool is safe for a limited off-target effect. The decreased epidermal growth factor receptor and MYC expression edited by dm6AC-RISPR suppressed cancer cell proliferation. In the near future, dm6ACRISPR targeting RNA demethylation might be used for gene repression and regulation of cellular functions in CVD. Despite the achievements, the clinical practice of small-molecule inhibitors targeting m6A modulated enzymes is still in its infancy, and several issues remain to be resolved. First, the high selectivity in transcripts and methylated sites needs to be tackled. Methylation patterns on transcripts may be molecular markers that recruit distinct m6A readers to enter downstream metabolism, respectively. Subsequently, side-effects caused by the complex mRNAs targeted by m6A enzymes cannot be ignored. Moreover, heterogeneity in human individuals also needs to be taken into account (142).
Scientists have made numerous attempts to explore the effect of m6A modification on cell phenotypes and gene expression, aiming to develop novel approaches for the treatment diseases. Unveiling the mechanism of regulation of m6A methylation in CVD is of significance for further understanding the pathogenesis and contribute to the diagnosis of CVD and may pave the way for the development of novel special drugs targeting the altered epigenetic marks. In addition, the rapid development of m6A-seq provides a more precise localization of adenine methylation. However, the development and use of m6A-targeting drugs requires further exploitation in the future.
6. Conclusions and future perspectives
m6A methylation is a type of RNA epigenetics and the most abundant internal RNA modification type. m6A methylation is an emerging research field that is growing rapidly, especially in CVD. The present article reviewed the role of m6A and m6A-SNPs in the mechanisms of various types of CVD. The observations highlight the potential role of m6A methylation in the diagnosis of CVD by serving as an invasive biomarker, and lay the foundation for the accurate treatment of CVD.
m6A methylation presents across different species and plays a fundamental role in cardiac biological processes and the pathogenesis of CVD. An increasing number of studies have uncovered the novel dysregulation of m6A methylation as a hallmark of the development of CVDs. m6A modification promotes or inhibits the development of CVD by regulating the targeted m6A-modified mRNA levels. The dysregulation of methyltransferase, demethylases and m6A-binding proteins plays a role in the occurrence and progression of CVD. Studies have demonstrated that the regulation of these 'writers', 'erasers' and 'readers' may alleviate the progression of cardiac diseases; these modulators might provide a novel insight into potential diagnostic biomarkers or therapeutic strategies for CVD (143). Table II and Fig. 3 summarize the role of m6A modulators in CVD. Table III contains a brief introduction of m6A-related SNPs and CVD.
Table II.
Methyltransferases/demethylases and corresponding target RNAs in cardiovascular disease and cardiac bioprocess.
| Cardiovascular diseases/bioprocess | Methyltransferases/demethylases | Expression | Target RNAs | Mechanism | (Refs.) |
|---|---|---|---|---|---|
| VSMC differentiation | METTL3 | Upregulated | - | Affecting the expression of paracrine factors | (77) |
| Heart failure | FTO | Upregulated | Calm1 | Influencing RNA–ribosome interaction and changing Calm1 protein expression | (92) |
| Heart failure | FTO | Downregulated | Serca2a | Involving in regulation of calcium and contractile function during cardiac remodeling | (90) |
| Ischemic heart disease | METTL3 | Upregulated | TFEB | Promoting the association of HNRNPD with TFEB pre-mRNA and decreasing TFEB expression, eventually impairing autophagic flux and enhancing apoptosis | (86) |
| Abdominal aortic aneurysm | METTL14, FTO | Upregulated | - | - | (96) |
| Cardiac hypertrophy and homeostasis | METTL3 | Upregulated | MAP3K6, MAP4K5, MAPK14 | Affecting kinase-regulated signaling pathway during cardiac hypertrophy | (81) |
| Vascular calcification | METTL14 | Upregulated | Klotho | METTL14 promotes the degradation of arteries preventing transcripts Klotho mRNA | (101) |
| Hypoxic pulmonary hypertension | - | Downregulated M6a level | circXpo6, circTmtc3 | M6A methylation could influence circRNA-miRNA-mRNA network | (110) |
| Arterial restenosis | WTAP | Upregulated | p16 | VSMC proliferation and migration | (136) |
| Lipid metabolism | METTL3 | Upregulated | - | - | (139) |
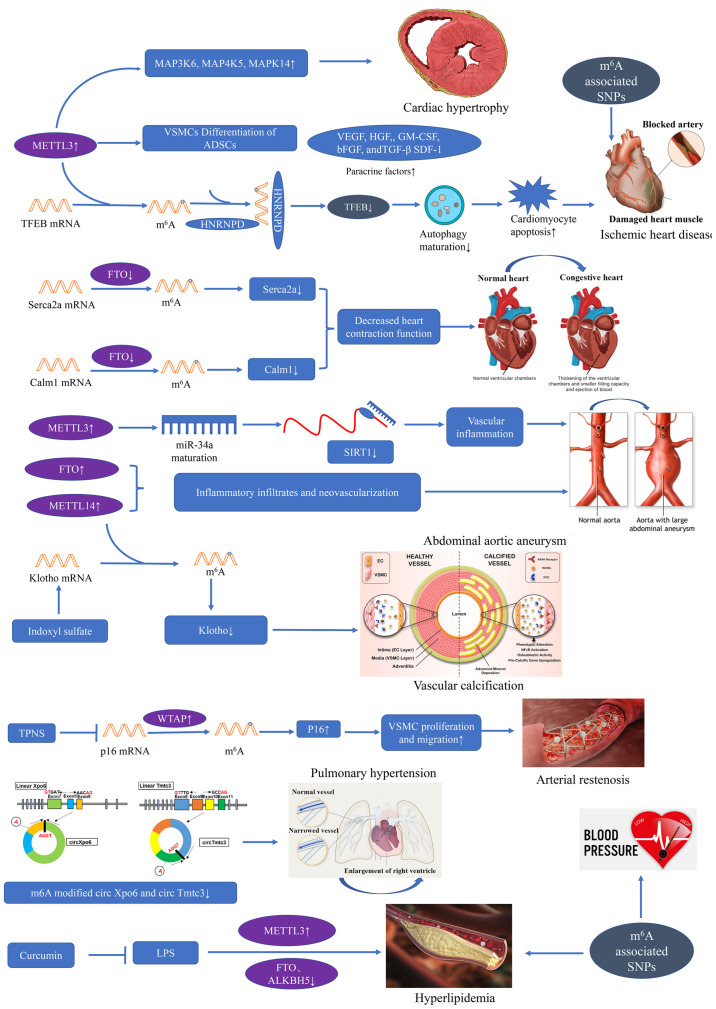
Figure 3.
m6A modulators involved in cardiovascular disease and bioprocess. METTL3-mediated m6A modification is involved in cardiac hypertrophy by affecting kinase-regulated signaling pathway. Upregulated METTL3 and increased expression of paracrine factors, including VEGF, HGF, TGF-β, GM-CSF, bFGF and SDF-1 were found in ADSCs undergoing VSMC differentiation induction. The upregulation of METTL3 promoted the association of RNA binding protein HNRNPD with TFEB pre-mRNA and decreased TFEB expression, eventually impaired autophagic flux and enhancing apoptosis in ischemic heart disease. FTO mediated decreased expression of Calm1 and Serca2a are responsible for decreased heart contraction function and heart failure. Both elevated METTL14 and FTO are associated with inflammatory infiltration and neovascularization in abdominal aortic aneurysm. In addition, METTL3 mediated miR-34a maturation from pre-miR-34a, and then miR-34a negatively regulated the expression of SIRT1. m6A-modified Klotho mRNA plays a crucial role in vascular calcification. TPNS alleviated arterial restenosis through the downregulation of m6A methylation of p16. Decreased m6A modified circ Xpo6 and circ Tmtc3 are found in hypoxic pulmonary hypertension. Elevated METTL3, decreased FTO and ALKBH5 are involved in the development of hyperlipidemia resulted from LPS. Curcumin exerts a protective effect on LPS induced abnormal lipid metabolism. m6A associated plays a role in blood pressure, hyperlipidemia and coronary artery disease.
Table III.
m6A-associated SNPs and cardiovascular disease.
Transcriptome-wide mapping of m6A in mRNA helped us catalog m6A targets and demonstrated potential epigenetic mechanisms. The functional significance of m6A methylation in physiological and biological processes is gaining appreciation and has been well described, yet studies on m6A and pathological conditions, especially in cardiovascular organs and tissues are still lacking and the precise role of m6A in CVD remains largely unknown (81). In addition, the current research on m6A methylation has species limitations, with several areas of the mechanism and function of m6A methylation still unknown. The role of different modulator-mediated types of m6A methylation in CVDs remains to be addressed. It still remains to be investigated whether there are interplay, synergistic and competitive effects between these modulators. The mechanisms of how m6A regulates gene expression are complex and differ among different cells, and more studies need to be performed to investigate the role of m6A modification in pathological conditions of CVD and the exact mechanisms in the future. Furthermore, the clinical significance of m6A in CVD should be investigated further.
To date, the majority of studies focus on the mechanism of m6A methylation; however, few studies have focused on m6A application, particularly m6A-targeting drug therapy in CVD. Future prospects need to be further explored. In addition, the detailed and precise function, as well as the specificity and sensitivity are all necessary in the development of therapeutic approaches for CVD (140). Studies on m6A-SNP and CVDs may help further explore the mechanism underlying SNPs and CVDs. More m6A-SNPs associated with CVD will be identified in the future using the emerging high-throughput data, which will broaden the current understanding of m6A-SNPs and the pathogenesis of CVD. These m6A-SNPs may become potential candidates for the detection of CVD.
In conclusion, m6A modification is the most prevalent RNA methylation type. Both m6A methylation and m6A-SNPs are closely associated with CVD. Previous studies have provided proof of concept for acting as biomarkers and therapeutic targets. More research is required to explore the mechanism of m6A in CVD and translate the existing results into clinical application.
As a newly identified type of post-transcriptional regulation, the dynamic and reversible m6A modification is the most prevalent type of internal modification of RNA methylation. m6A modification plays a significant role not only in various cellular biological processes but also in the pathogenesis of CVDs. The dysregulated m6A methylation has enhanced our understanding of epigenetic regulation in the pathogenesis in cardiovascular disorders. However, the investigation of m6A modification in CVDs remains in its infancy and a more comprehensive understanding of the biological function of m6A is needed. m6A modification may further contribute to the recognition of molecular mechanisms underlying cardio-vascular pathogenesis, and might provide novel insight into the potential biomarkers and therapeutic approaches for CVD in the future.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81970237 and 81600227).
Availability of data and materials
Not applicable.
Authors' contributions
YQin, LL, EL, JH, GY, DW, YQiao and CT contributed to the conception and design of the study. YQin, LL, EL and JH searched the relevant literature. YuQ wrote the manuscript. GY, YQiao and DW provided advice and were responsible for revising the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stellos K. The rise of epitranscriptomic era: Implications for cardiovascular disease. Cardiovasc Res. 2017;113:e2–e3. doi: 10.1093/cvr/cvx030. [DOI] [PubMed] [Google Scholar]
- 2.Huang H, Weng H, Chen J. m6A modification in coding and non-coding RNAs: Roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m(6)A and U-tail. Cell. 2014;158:980–987. doi: 10.1016/j.cell.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Bhat SS, Bielewicz D, Jarmolowski A, Szweykowska- Kulinska Z. N6-methyladenosine (m6A): Revisiting the old with focus on new, an arabidopsis thaliana centered review. Genes (Basel) 2018;9:596. doi: 10.3390/genes9120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao W, Qi X, Liu L, Ma S, Liu J, Wu J. Epigenetic regulation of m6A modifications in human cancer. Mol Ther. 2020;19:405–412. doi: 10.1016/j.omtn.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 9.Molinie B, Wang J, Lim KS, Hillebrand R, Lu ZX, Van Wittenberghe N, Howard BD, Daneshvar K, Mullen AC, Dedon P, et al. m(6)A-LAIC-seq reveals the census and complexity of the m(6)A epitranscriptome. Nat Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5′ UTR m(6) A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XY, Zhang J, Zhu JS. The role of m6A RNA methylation in human cancer. Mol Cancer. 2019;18:103. doi: 10.1186/s12943-019-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, Wang SY, Baltissen MPA, Jansen PWTC, Rossa M, et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol. 2017;24:870–878. doi: 10.1038/nsmb.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visvanathan A, Somasundaram K. mRNA traffic control reviewed: N6-Methyladenosine (m6A) takes the driver's seat. Bioessays. 2017 Dec 4; doi: 10.1002/bies.201700093. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 19.Tong J, Flavell RA, Li HB. RNA m6A modification and its function in diseases. Front Med. 2018;12:481–489. doi: 10.1007/s11684-018-0654-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, Han D, Dominissini D, Dai Q, Pan T, He C. High-resolution N(6)- methyladenosine (m(6) A) map using photo-crosslinking-assisted m(6) A sequencing. Angew Chem Int Ed Engl. 2015;54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, He C. Dynamic RNA modifications in posttranscriptional regulation. Mol Cell. 2014;56:5–12. doi: 10.1016/j.molcel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S, Wang JZ, Chen D, He YT, Meng N, Chen M, Lu RX, Chen XH, Zhang XL, Yan GR. An oncopeptide regulates m6A recognition by the m6A reader IGF2BP1 and tumorigenesis. Nat Commun. 2020;11:1685. doi: 10.1038/s41467-020-15403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, Li J, An P, Lu L, Luo N, et al. m6A-induced lncRNA RP11 triggers the dissemi-nation of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, Brönneke HS, Dietrich MO, Jordan SD, Saletore Y, Elemento O, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042–1048. doi: 10.1038/nn.3449. [DOI] [PubMed] [Google Scholar]
- 29.Du K, Zhang L, Lee T, Sun T. m(6)A RNA methylation controls neural development and is involved in human diseases. Mol Neurobiol. 2019;56:1596–1606. doi: 10.1007/s12035-018-1138-1. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang Y, Li J, Sheng R, Deng P, Wang Y, et al. Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9:4772. doi: 10.1038/s41467-018-06898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: Implications for RNA processing. Mol Cell Biol. 1985;5:2298–2306. doi: 10.1128/MCB.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roignant JY, Soller M. m6A in mRNA: An ancient mechanism for fine-tuning gene expression. Trends Genet. 2017;33:380–390. doi: 10.1016/j.tig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Shi Y, Shen H, Xie W. m6A-binding proteins: The emerging crucial performers in epigenetics. J Hematol Oncol. 2020;13:35. doi: 10.1186/s13045-020-00872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao QJ, Sang L, Lin M, Yin X, Dong W, Gong Y, Zhou BO. Mettl3-Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell Res. 2018;28:952–954. doi: 10.1038/s41422-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltrans-ferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slobodin B, Han R, Calderone V, Vrielink JAFO, Loayza-Puch F, Elkon R, Agami R. Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell. 2017;169:326–337.e12. doi: 10.1016/j.cell.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi J, Ieong KW, Demirci H, Chen J, Petrov A, Prabhakar A, O'Leary SE, Dominissini D, Rechavi G, Soltis SM, et al. N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat Struct Mol Biol. 2016;23:110–115. doi: 10.1038/nsmb.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m(6)A methyltransferase. J Mol Evol. 2002;55:431–444. doi: 10.1007/s00239-002-2339-8. [DOI] [PubMed] [Google Scholar]
- 42.Śledź P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schöller E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, Meister G. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA. 2018;24:499–512. doi: 10.1261/rna.064063.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, et al. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang C, Klukovich R, Peng H, Wang Z, Yu T, Zhang Y, Zheng H, Klungland A, Yan W. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3′-UTR mRNAs in male germ cells. Proc Natl Acad Sci USA. 2018;115:E325–E333. doi: 10.1073/pnas.1717794115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, Yeo GS, Meyre D, Golzio C, Molinari F, Kadhom N, et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet. 2009;85:106–111. doi: 10.1016/j.ajhg.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell. 2018;172:90–105.e23. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.A Alemu E, He C, Klungland A. ALKBHs-facilitated RNA modifications and de-modifications. DNA Repair (Amst) 2016;44:87–91. doi: 10.1016/j.dnarep.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Theler D, Kaminska KH, Hiller M, de la Grange P, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM, Allain FH, Stamm S. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoilov P, Rafalska I, Stamm S. YTH: A new domain in nuclear proteins. Trends Biochem Sci. 2002;27:495–497. doi: 10.1016/S0968-0004(02)02189-8. [DOI] [PubMed] [Google Scholar]
- 59.Liao S, Sun H, Xu C. YTH domain: A family of N6-methyladenosine (m6A) readers. Genomics Proteomics Bioinformatics. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kretschmer J, Rao H, Hackert P, Sloan KE, Höbartner C, Bohnsack MT. The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5′-3′ exoribonuclease XRN1. RNA. 2018;24:1339–1350. doi: 10.1261/rna.064238.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zarnack K, König J, Tajnik M, Martincorena I, Eustermann S, Stévant I, Reyes A, Anders S, Luscombe NM, Ule J. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell. 2013;152:453–466. doi: 10.1016/j.cell.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell JL, Wächter K, Mühleck B, Pazaitis N, Köhn M, Lederer M, Hüttelmaier S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70:2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleo-tide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, Winkler R, Nir R, Lasman L, Brandis A, et al. Deciphering the 'm6A Code' via antibody-independent quantitative profiling. Cell. 2019;178:731–747.e16. doi: 10.1016/j.cell.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Liu Q, Gregory RI. RNAmod: An integrated system for the annotation of mRNA modifications. Nucleic Acids Res. 2019;47:W548–W555. doi: 10.1093/nar/gkz479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang SY, Zhang SW, Fan XN, Zhang T, Meng J, Huang Y. FunDMDeep-m6A: Identification and prioritization of functional differential m6A methylation genes. Bioinformatics. 2019;35:i90–i98. doi: 10.1093/bioinformatics/btz316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Chen LQ, Zhao YL, Yang CG, Roundtree IA, Zhang Z, Ren J, Xie W, He C, Luo GZ. Single-base mapping of mA by an antibody-independent method. Sci Adv. 2019;5:eaax0250. doi: 10.1126/sciadv.aax0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin J, Zhu Q, Huang J, Cai R, Kuang Y. Hypoxia promotes vascular smooth muscle cell (VSMC) differentiation of adipose-derived stem cell (ADSC) by regulating Mettl3 and paracrine factors. Stem Cells Int. 2020;2020:2830565. doi: 10.1155/2020/2830565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 79.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 81.Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, Hanna JH, van Berlo JH, Accornero F. The N6-Methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139:533–545. doi: 10.1161/CIRCULATIONAHA.118.036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song H, Pu J, Wang L, Wu L, Xiao J, Liu Q, Chen J, Zhang M, Liu Y, Ni M, et al. ATG16L1 phosphorylation is oppositely regulated by CSNK2/casein kinase 2 and PPP1/protein phos-phatase 1 which determines the fate of cardiomyocytes during hypoxia/reoxygenation. Autophagy. 2015;11:1308–1325. doi: 10.1080/15548627.2015.1060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pastore N, Brady OA, Diab HI, Martina JA, Sun L, Huynh T, Lim JA, Zare H, Raben N, Ballabio A, Puertollano R. TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy. 2016;12:1240–1258. doi: 10.1080/15548627.2016.1179405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao E, Czaja MJ. Transcription factor EB: A central regulator of both the autophagosome and lysosome. Hepatology. 2012;55:1632–1634. doi: 10.1002/hep.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song H, Feng X, Zhang H, Luo Y, Huang J, Lin M, Jin J, Ding X, Wu S, Huang H, et al. METTL3 and ALKBH5 oppositely regu-late m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15:1419–1437. doi: 10.1080/15548627.2019.1586246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Misquitta CM, Iyer VR, Werstiuk ES, Grover AK. The role of 3′-untranslated region (3′-UTR) mediated mRNA stability in cardiovascular pathophysiology. Mol Cell Biochem. 2001;224:53–67. doi: 10.1023/A:1011982932645. [DOI] [PubMed] [Google Scholar]
- 88.Gratacós FM, Brewer G. The role of AUF1 in regulated mRNA decay. Wiley Interdiscip Rev RNA. 2010;1:457–473. doi: 10.1002/wrna.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su YR, Chiusa M, Brittain E, Hemnes AR, Absi TS, Lim CC, Di Salvo TG. Right ventricular protein expression profile in end-stage heart failure. Pulm Circ. 2015;5:481–497. doi: 10.1086/682219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y, Agarwal N, Jha D, Zhang S, Kohlbrenner E, Chepurko E, et al. FTO-Dependent N6-Methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139:518–532. doi: 10.1161/CIRCULATIONAHA.118.033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berulava T, Buchholz E, Elerdashvili V, Pena T, Islam MR, Lbik D, Mohamed BA, Renner A, von Lewinski D, Sacherer M, et al. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. Eur J Heart Fail. 2020;22:54–66. doi: 10.1002/ejhf.1672. [DOI] [PubMed] [Google Scholar]
- 93.Kmietczyk V, Riechert E, Kalinski L, Boileau E, Malovrh E, Malone B, Gorska A, Hofmann C, Varma E, Jürgensen L, et al. m6A-mRNA methylation regulates cardiac gene expression and cellular growth. Life Sci Alliance. 2019;2:e201800233. doi: 10.26508/lsa.201800233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 95.Reeps C, Pelisek J, Seidl S, Schuster T, Zimmermann A, Kuehnl A, Eckstein HH. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology. 2009;76:243–252. doi: 10.1159/000228900. [DOI] [PubMed] [Google Scholar]
- 96.He Y, Xing J, Wang S, Xin S, Han Y, Zhang J. Increased m6A methylation level is associated with the progression of human abdominal aortic aneurysm. Ann Transl Med. 2019;7:797. doi: 10.21037/atm.2019.12.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhong L, He X, Song H, Sun Y, Chen G, Si X, Sun J, Chen X, Liao W, Liao Y, Bin J. METTL3 induces AAA development and progression by modulating N6-methyladenosine-dependent primary miR34a processing. Mol Ther Nucleic Acids. 2020;21:394–411. doi: 10.1016/j.omtn.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85:142–150. doi: 10.1038/ki.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao XS, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10:111–119. doi: 10.2215/CJN.04730514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J, Ning Y, Zhang H, Song N, Gu Y, Shi Y, Cai J, Ding X, Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034. doi: 10.1016/j.lfs.2019.117034. [DOI] [PubMed] [Google Scholar]
- 102.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 104.Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Pelletier A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131:493–498. doi: 10.1164/arrd.1985.131.4.493. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat commun. 2019;10:4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fry NJ, Law BA, Ilkayeva OR, Holley CL, Mansfield KD. N6-methyladenosine is required for the hypoxic stabilization of specific mRNAs. RNA. 2017;23:1444–1455. doi: 10.1261/rna.061044.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, Xing Y, Giallourakis CC, Mullen AC. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Zhu MC, Kalionis B, Wu JZ, Wang LL, Ge HY, Chen CC, Tang XD, Song YL, He H, Xia SJ. Characteristics of circular RNA expression in lung tissues from mice with hypoxiainduced pulmonary hypertension. Int J Mol Med. 2018;42:1353–1366. doi: 10.3892/ijmm.2018.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Su H, Wang G, Wu L, Ma X, Ying K, Zhang R. Transcriptome-wide map of m6A circRNAs identified in a rat model of hypoxiamediated pulmonary hypertension. BMC Genomics. 2020;21:39. doi: 10.1186/s12864-020-6462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baarsma HA, Königshoff M. 'WNT-er is coming': WNT signalling in chronic lung diseases. Thorax. 2017;72:746–759. doi: 10.1136/thoraxjnl-2016-209753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 114.Haraksingh RR, Snyder MP. Impacts of variation in the human genome on gene regulation. J Mol Biol. 2013;425:3970–3977. doi: 10.1016/j.jmb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 115.Mao F, Xiao L, Li X, Liang J, Teng H, Cai W, Sun ZS. RBP-Var: A database of functional variants involved in regulation mediated by RNA-binding proteins. Nucleic Acids Res. 2016;44:D154–D163. doi: 10.1093/nar/gkv1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu X, Hurst LD. Determinants of the usage of splice-associated cis-Motifs predict the distribution of human pathogenic SNPs. Mol Biol Evol. 2016;33:518–529. doi: 10.1093/molbev/msv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramaswami G, Deng P, Zhang R, Anna Carbone M, Mackay TFC, Billy Li J. Genetic mapping uncovers cis-regulatory land-scape of RNA editing. Nat Commun. 2015;6:8194. doi: 10.1038/ncomms9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, Miao Y, Zuo Z, Ren J. m6AVar: A database of functional variants involved in m6A modification. Nucleic Acids Res. 2018;46:D139–D145. doi: 10.1093/nar/gkx895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang N, Ying P, Tian J, Wang X, Mei S, Zou D, Peng X, Gong Y, Yang Y, Zhu Y, et al. Genetic variants in m6A modification genes are associated with esophageal squamous-cell carcinoma in the Chinese population. Carcinogenesis. 2020;41:761–768. doi: 10.1093/carcin/bgaa012. [DOI] [PubMed] [Google Scholar]
- 120.Mo XB, Lei SF, Zhang YH, Zhang H. Detection of m6A-asso-ciated SNPs as potential functional variants for coronary artery disease. Epigenomics. 2018;10:1279–1287. doi: 10.2217/epi-2018-0007. [DOI] [PubMed] [Google Scholar]
- 121.Mo X, Lei S, Zhang Y, Zhang H. Genome-wide enrichment of m6A-associated single-nucleotide polymorphisms in the lipid loci. Pharmacogenomics J. 2019;19:347–357. doi: 10.1038/s41397-018-0055-z. [DOI] [PubMed] [Google Scholar]
- 122.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJC. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45:80–85. doi: 10.1161/01.HYP.0000149952.84391.54. [DOI] [PubMed] [Google Scholar]
- 123.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. doi: 10.1038/s41588-018-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mo XB, Lei SF, Zhang YH, Zhang H. Examination of the associations between m6A-associated single-nucleotide polymorphisms and blood pressure. Hypertens Res. 2019;42:1582–1589. doi: 10.1038/s41440-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 125.Lee JY, Lee BS, Shin DJ, Woo Park K, Shin YA, Joong Kim K, Heo L, Young Lee J, Kyoung Kim Y, Jin Kim Y, et al. A genome-wide association study of a coronary artery disease risk variant. J Hum Genet. 2013;58:120–126. doi: 10.1038/jhg.2012.124. [DOI] [PubMed] [Google Scholar]
- 126.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 128.Pausova Z, Syme C, Abrahamowicz M, Xiao Y, Leonard GT, Perron M, Richer L, Veillette S, Smith GD, Seda O, et al. A common variant of the FTO gene is associated with not only increased adiposity but also elevated blood pressure in French Canadians. Circ Cardiovasc Genet. 2009;2:260–269. doi: 10.1161/CIRCGENETICS.109.857359. [DOI] [PubMed] [Google Scholar]
- 129.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marcadenti A, Fuchs FD, Matte U, Sperb F, Moreira LB, Fuchs SC. Effects of FTO RS9939906 and MC4R RS17782313 on obesity, type 2 diabetes mellitus and blood pressure in patients with hypertension. Cardiovasc Diabetol. 2013;12:103. doi: 10.1186/1475-2840-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. 2011;365:2098–2109. doi: 10.1056/NEJMra1105239. [DOI] [PubMed] [Google Scholar]
- 132.Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 133.Yadav PK, Rajvanshi PK, Rajasekharan R. The role of yeast m6A methyltransferase in peroxisomal fatty acid oxidation. Curr Genet. 2018;64:417–422. doi: 10.1007/s00294-017-0769-5. [DOI] [PubMed] [Google Scholar]
- 134.Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, Yuan W, Kan Q, Sun Z. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 2019;12:121. doi: 10.1186/s13045-019-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang W, Chen G, Deng CQ. Effects and mechanisms of total Panax notoginseng saponins on proliferation of vascular smooth muscle cells with plasma pharmacology method. J Pharm Pharmracol. 2012;64:139–145. doi: 10.1111/j.2042-7158.2011.01379.x. [DOI] [PubMed] [Google Scholar]
- 136.Zhu B, Gong Y, Shen L, Li J, Han J, Song B, Hu L, Wang Q, Wang Z. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m6A modulation. Biomed Pharmacother. 2020;124:109935. doi: 10.1016/j.biopha.2020.109935. [DOI] [PubMed] [Google Scholar]
- 137.Nakarai H, Yamashita A, Nagayasu S, Iwashita M, Kumamoto S, Ohyama H, Hata M, Soga Y, Kushiyama A, Asano T, et al. Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: Potential link with metabolic complications. Innate Immun. 2012;18:164–170. doi: 10.1177/1753425910393370. [DOI] [PubMed] [Google Scholar]
- 138.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutri. 1970;100:1307–1315. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 139.Lu N, Li X, Yu J, Li Y, Wang C, Zhang L, Wang T, Zhong X. Curcumin attenuates lipopolysaccharide-induced hepatic lipid metabolism disorder by modification of m6 A RNA methylation in piglets. Lipids. 2018;53:53–63. doi: 10.1002/lipd.12023. [DOI] [PubMed] [Google Scholar]
- 140.Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J, Chen Z, Chen F, Xie G, Ling Y, Peng Y, Lin Y, Luo N, Chiang CM, Wang H. Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. Nucleic Acids Res. 2020;48:5684–5694. doi: 10.1093/nar/gkaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 2018;20:1349–1360. doi: 10.1038/s41556-018-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paramasivam A, Vijayashree Priyadharsini J, Raghunandhakumar S. N6-adenosine methylation (m6A): A promising new molecular target in hypertension and cardiovascular diseases. Hypertens Res. 2020;43:153–154. doi: 10.1038/s41440-019-0338-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.